Abstract
Many studies address the influence of the gut microbiome on the immune system, but few dissect the effect of T cells on gut microbiota and mucosal responses. We have employed larval thymectomy in Xenopus to study the gut microbiota with and without the influence of T lymphocytes. Pyrosequencing of 16S rRNA genes was used to assess the relative abundance of bacterial groups present in the stomach, small and large intestine. Clostridiaceae was the most abundant family throughout the gut, while Bacteroidaceae, Enterobacteriaceae and Flavobacteriaceae also were well represented. Unifrac analysis revealed no differences in microbiota distribution between thymectomized and unoperated frogs. This is consistent with immunization data showing that levels of the mucosal immunoglobulin IgX are not altered significantly by thymectomy. This study in Xenopus represents the oldest organisms that exhibit class switch to a mucosal isotype and is relevant to mammalian immunology, as IgA appears to have evolved from IgX based upon phylogeny, genomic synteny, and function.
Keywords: microbiota, thymectomy, T cells, pyrosequencing, evolution
1. Introduction
The thymus is the primary T lymphoid organ of vertebrates from sharks to mammals (Cooper et al., 1966; Criscitiello et al., 2010). In humans, a small deletion on chromosome 22 in DiGeorge syndrome often results in an absent or hypoplastic thymus, with resulting loss of T-mediated responses (reviewed in (Weidberg et al., 2011a)). The hairless “nude” strain of mouse has an absent or greatly degenerated thymus due to a mutation in the Foxn1 gene (Weidberg et al., 2011b). These mice do have B cells but T cells are very few. Due to the lack of both cytotoxic and helper T cells, nude or thymectomized mice have abolished allograft and mixed leucocyte reactions, proliferative responses to classical T cell mitogens, and antibody responses against T-dependent antigens (Saftig and Klumperman, 2009).
The mucosal immune system forms the largest vertebrate immune compartment and is mediated by specialized cells and immunoglobulins, such as plasma cells producing secretory IgA in birds and mammals. IgA production in the gut is not constitutive, shown by the absence of both IgA and IgA-secreting plasma cells in the lamina propriaof germ-free mice (Crabbe et al., 1970). There are many studies investigating the humoral mucosal immune responses of mammals lacking T cells, but they have yielded mixed results (Ebersole et al., 1979; Lindner et al., 2012). However, a picture is emerging of a significant T cell independent mechanism of gut IgA management of mutualistic flora (Macpherson et al., 2000; Macpherson and Slack, 2007). Gut IgA-producing plasma cells in mammals employ tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS) usually associated with innate phagocytes (Fritz et al., 2012). Interestingly, B cells of lower vertebrates have been found to have strong phagocytic activity (Li et al., 2006). The B cell phagocytic activity is consistent with an emerging theme of primitive, innate, T-independent, IgA switched B cells in the gut, although this has never been actually tested in a lower tetrapod. This gap in our knowledge prompted our assessment of the T-dependence of humoral mucosal immunology in a phylogenetically relevant model species.
The African clawed frog Xenopus laevis belongs to the tongue-less frog family Pipidae. It is a choice model for ontogeny and phylogeny of both humoral and cell mediated immunity. Xenopus shares a common ancestor with mammals 350 million years ago and links them to the more ancient vertebrates where the adaptive immune system arose (reviewed in (Korolchuk et al., 2011)). The ability to perform thymectomy on transparent early stage Xenopus tadpoles made this frog an ideal model species to query the thymic dependent management of gut microbiota and mucosal antibody responses from a fundamental point in vertebrate humoral immunity (reviewed in (Horton et al., 1998)).
This present study is the first investigation determining the gut bacterial populations of an amphibian using 454-pyrosequencing of the 16S rRNA gene. In addition, we examined the flora of normal and thymectomized frogs and the ability of thymectomized frogs to make mucosal antibody responses. IgX has been functionally associated with mucosal responses and (in contrast to IgY) was found in thymectomized frogs (Du et al., 2012; Mussmann et al., 1996a). However, evolutionarily it has been thought to be closer to IgM (Mussmann et al., 1996b). T-independent responses are known from Xenopus serum (Tochinai, 1976), but here we aimed to determine the effect of thymectomy upon the gut flora, the mucosal and the systemic IgX responses. We also evaluated the relationship of amphibian IgX to mammalian IgA, in hopes of resolving ambiguity as to the origins and natural history of the class of antibody that manages vast numbers of mutualistic microbes and is the first defense of the barriers breached by most pathogens.
2. Methods
2.1 Animals
Xenopus laevis was used as a model for the tetrapod vertebrate immune system. Outbred frogs were initially purchased from Xenopus Express (Brooksville FL). Subsequent generations were bred in-house using human chorionic gonadotropin hormone to prime for egg and sperm maturation (Sigma-Aldrich, St. Louis MO). Frogs were maintained at the Texas A&M Comparative Medicine Program facility. They were housed in two separate but similar recirculating rack systems (Techniplast, Buguggiate Italy) on a 12-hour light cycle. Frogs were moved from an antigen free system to a “DNP-KLH exposed” system upon first immunization. Adults were fed a sinking pellet and tadpoles a powder diet (Xenopus Express). All husbandry, surgery, and immunization protocols were approved by the Texas A&M Institutional Animal Care and Use Committee (AUP 2008–33). Post-metamorphosis frogs were micro-chipped (Avid, Norco CA) and assigned to gut microbiota harvest or immunization protocols.
2.2 Thymectomy
Frogs were thymectomized nine days post-fertilization through microscopic cauterization, adapting the protocol devised by Horton (Horton and Manning, 1972). The surgery was performed under a dissecting microscope using a micro-cautery apparatus originally designed for insect stylectomy (http://aphidzapper.com), delivering a VHF pulse of 10 millisecond duration and power of 10 watts via an abraded tungsten wire to the target tissue. Tadpoles were anesthetized using a 300mg/L MS222 (tricaine methanesulfonate, Argent, Redmond WA) bath for 2–10 minutes, before placing on wet cool cheesecloth over a grounded metal plate stage. The thymus was then burned with one pulse on each side. The thymus is located bilaterally caudal and medial to the eyes and lateral to the dark central nervous system (Figure 1A). The tadpole was transferred to an ice bath immediately after thymic ablation to cool the animal for three seconds before being allowed to recover in an aerated tank with but 80mg/L carbenicillin (and no MS-222) to prevent superficial infections. After 24 hours tadpoles were transferred back to the primary recirculating Xenoplus systems (with no antibiotic). Three days after surgery tadpoles were monitored for any re-growth of the thymus by visual inspection for the melanized organ under the microscope (approximately 20% of frogs that recover from surgery). The monitoring continued every 4–6 days for one month post-surgery. Tadpoles with thymic regrowth due to incomplete thymectomy were euthanized.
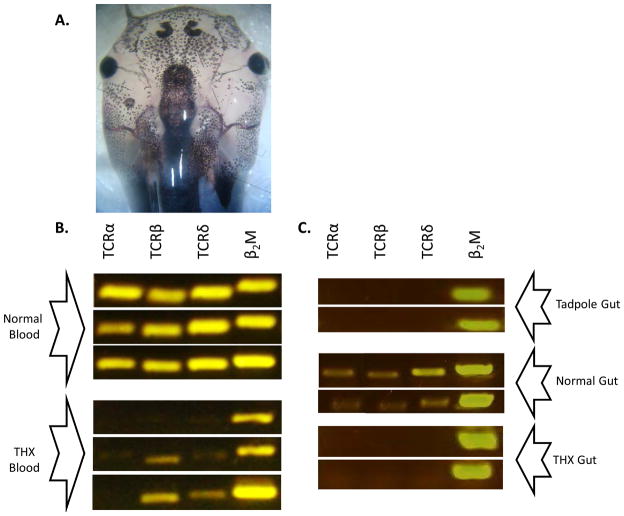
Figure 1. Larval thymectomy greatly depletes TCRα and δ message from the adult frogs.
A. Unilateral thymectomy at day 9 shows absence of naturally melanized thymus on the left compared to intact organ on the right. Image captured at day 20 at 3X magnification. Experimental frogs were bilaterally thymectomized. B. PCR contrasting levels of TCRα, β and δ to β2-microglobulin in peripheral blood of three intact post-metamorphic frogs and three thymectomized post metamorphic frogs. Results of 40 cycles of amplification. C. RT-PCR comparing the same amplicons from intestine of nine-day tadpoles (the age of thymectomy, gut of 10 animals pooled per row) and two intact post-metamorphic frogs and two thymectomized post-metamorphic frogs.
2.3 PCR validation of thymectomy
Frogs thymectomized as tadpoles were checked for the presence of TCR α, β, and δ mRNA using PCR at least six months post-surgery (they undergo metamorphosis in the second month, Supplemental Table 1). The more exposed tarsal veins associated with digits two and three were bled for 100–500 μL with 1 mL syringes and 28 gauge needles. Additional checks for TCR expression were performed on the gut of nine-day tadpoles, the gut of thymectomized adult, and the gut of normal adult. PCR was performed on cDNA prepared from RNA prepared from whole peripheral blood, due to the small volumes of blood collected using RNAeasy preparations (Qiagen, Valencia CA) with on-column genomic DNA digestion. First strand cDNA was synthesized using random hexamer priming with Superscript III (Invitrogen, Carlsbad CA) according to the manufacturer’s protocol. PCR amplification was performed using oligonucleotide primers designed for constant domain genes of TCR α, β, δ and β2M (Supplemental Table 2). Gotaq polymerase (Promega, Madison, WI) and 50 ng template were used during these PCR for 35 cycles for blood, 2 μg template for 39 cycles for intestine. Primer sets for TCR α and β2m annealed at 52 °C and β and δ at 58 °C.
2.4 DNA preparation and 16S rRNA gene sequencing
Gut contents were sampled from normal and thymectomized frogs at three anatomical sites: stomach, small intestine and large intestine (as shown in Supplemental Figure 1). Bolus and chyme were scraped from longitudinally opened organs and collected as ~300 μL samples for DNA isolation via phenol-chloroform-isoamyl alcohol extraction (Suchodolski et al., 2004). Bacterial tag-encoded FLX-titanium amplicon pyrosequencing (bTEFAP) was performed similarly as described previously at the Research and Testing Laboratory, Lubbock, TX, USA (Handl et al., 2011), but based on the V4–V6 region (E. coli position 530–1100) of the 16S rRNA gene, with primers forward 530F and reverse 1100R (Supplemental Table 2). Briefly, the DNA concentration was determined using a Nanodrop spectrophotometer (Nyxor Biotech, Paris France). A 100 ng (1 μl) aliquot of each DNA sample was used for a 50 μl PCR reaction. HotStarTaq Plus Master Mix Kit (Qiagen, Valencia CA) was used for PCR under the following conditions: 94°C for 3 min followed by 32 cycles of 94°C for 30 sec; 60°C for 40 sec and 72°C for 1 min; and a final elongation step at 72°C for 5 min. A secondary PCR was performed for FLX (Roche, Nutley NJ) amplicon sequencing under the same conditions by using designed special fusion primers with different tag sequences as: LinkerA-Tags-530F and LinkerB-1100R. The use of a secondary PCR prevents amplification of any potential bias that might be caused by inclusion of tag and linkers during initial template amplification reactions. After secondary PCR, all amplicon products from the different samples were mixed in equal volumes, and purified using Agencourt Ampure beads (Agencourt, Danvers MA).
2.5 Gut flora analysis
Raw sequence data were screened, trimmed, filtered, denoised, and chimera depleted with default settings using the QIIME pipeline version 1.4.0 (http://qiime.sourceforge.net) (Caporaso et al., 2010) and with USEARCH using the OTU pipeline (www.drive5.com). Operational taxonomic units (OTUs) were defined as sequences with at least 97% similarity using QIIME. For classification of sequences on a genus level the naïve Bayesian classifier within the Ribosomal Database Project (RDP, v10.28) was used. The compiled data were used to determine the relative percentages of bacteria for each individual sample, six frogs by three samples each for a total of eighteen. To account for unequal sequencing depth across samples subsequent analysis was performed on a randomly selected subset of 1800 sequences per sample. This number was chosen to avoid exclusion of samples with lower number of sequence reads from further analysis. Alpha diversity (i.e., rarefaction) and beta diversity measures were calculated and plotted using QIIME. Differences in microbial communities between disease groups were investigated using the phylogeny-based unweighted Unifrac distance metric. This analysis measures the phylogenetic distance among bacterial communities in a phylogenetic tree, and thereby provides a measure of similarity among microbial communities present in different biological samples.
2.6 IgX phylogenetics
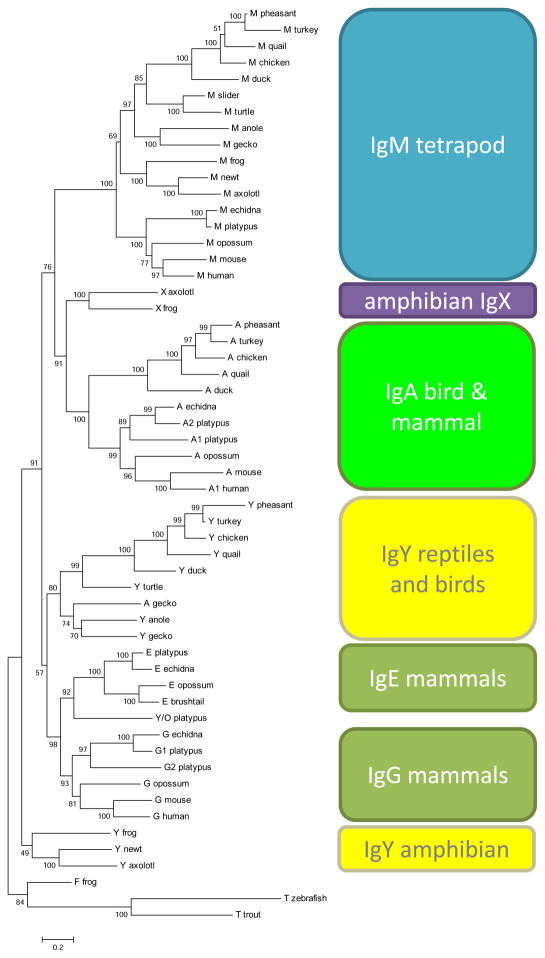
Amino acid sequences of tetrapod immunoglobulin heavy chain constant regions were compiled and aligned in BioEdit with ClustalW employing gap opening penalties of 10 and gap extension penalties of 0.1 for pairwise alignments and then 0.2 for multiple alignments with the protein-weighting matrix of Gonnett or Blossum (Hall, 1999; Tamura et al., 2007). These alignments were then heavily modified by hand. MEGA was used to infer the phylogenetic relationships of the immunoglobulin heavy chain constant genes. Evolutionary distances were computed using the Dayhoff matrix (Schwarz and Dayhoff, 1979) and 509 column positions in the 56 selected sequences. Several tree-building algorithms were employed, including a consensus neighbor-joining tree made from 1000 bootstrap replicates.
2.7 Immunizations
For immunization, two routes of administration were used. Four frogs received intracoelomic (IC, frogs have no peritoneal cavity) and four oral (PO) immunization. We used an oral gavage needle to deliver conjugated dinitrophenol- keyhole limpet hemocyanin (DNP-KLH, Calbiochem, San Diego CA) for mucosal immunization as previously described for X. laevis (Du et al., 2012). There were two normal and two thymectomized frogs in each group for a total of eight frogs. Frogs orally immunized received 2.5 mg DNP/KLH with 10μg cholera toxin as adjuvant three times, each at weekly intervals. Animals in the intracoelomic group received 200μg of antigen with equal volume (200μl) of Freund’s complete adjuvant once and Freund’s incomplete adjuvant twice, at weekly intervals.
2.8 Lymphocyte isolation and culture
Three weeks after the last boost, we euthanized frogs with an MS-222 overdose and decapitation. Spleens were removed and cells dissociated by scraping the organs over a wire mesh inundated with amphibian-adjusted PBS. The intestine was excised below the stomach and above the rectum. We removed mucous, chime and fecal matter by physically scraping and flushing the organ with PBS. The intestine was cut into smaller pieces (approximately 0.5 cm2) and placed in PBS. We then added 2% collagenase (Calbiochem, San Diego CA) in amphibian PBS to aid in isolation of the intestinal lymphocytes. We allowed the intestine to digest for 120 minutes at room temperature. It was vortexed initially and once every thirty minutes. The intestine was strained through a 100μm nylon cell strainer (BD Falcon, San Jose CA) at the end of the 120 minutes. Lymphocytes were isolated from the washed supernatant of the intestine and from the cells isolated from the spleen with Lymphocyte Separation Medium (Mediatech, Manassas VA). All intestine and spleen cells isolated were counted (Supplemental Figure 2) and cultured in twenty-four well plates containing 250μl of L-15 media with 10% fetal calf serum at a density of 2.3×106 cells/ml for the spleen cells and 1.0×106 cells/ml for the intestinal cells. Cultures were incubated at humidified 28°C and 5% CO2. On the third day, supernatant was collected and new media was added to maintain a volume of 250μL per well. On the sixth day, supernatant was once again collected and pooled with the day three collections.
2.9 Enzyme-linked Immunosorbent Assay (ELISA)
We used ELISAs to determine the total (non-antigen specific) and antigen-specific levels of amphibian mucosal antibody isotype IgX produced in response to DNP-KLH. Serial dilutions from 1/10 to 1/1011 were made from the supernatants of the spleen and intestinal cultures. For the total immunoglobulin ELISAs, we added 100μL of each dilution to the well of a plate and incubated for one hour at 37°C. The plate was then washed 2X with 200μL PBS and 200μL of 2% casein in PBS was added as blocking solution. The plate was allowed to sit overnight at 4°C, and was then washed 3X with 200μL PBS. We added mouse anti-Xenopus IgX monoclonal 4110B3 (kind gifts of Martin Flajnik, University of Maryland at Baltimore and Louis Du Pasquier, University of Basel), to each well to obtain a volume of 100μl (Hsu and Du Pasquier, 1984). The plate was allowed to incubate at room temperature for one hour and then was washed 4X with 200μl PBS with 0.05% Tween-20 (PBS-T, Sigma, St. Louis MO). The wells received 100μl of anti-mouse IgG peroxidase conjugated secondary antibody (Sigma) and were incubated for one hour at room temperature. We then washed the plate 4X with 200μl PBS-T and 1X with PBS. A 3, 3′, 5, 5′-tetramethylbenzidine substrate solution was then added to each well and the reaction was allowed to take place for three minutes before being stopped with 2M H2SO4. Plates were read at an optical density of 450nm in a BioRad iMark Microplate Reader (Hercules CA). We used the 104 dilution of supernatant for all trials. The antigen-specific ELISAs used a similar protocol, except 100 μl of 10 μg/ml DNP-KLH was added to each well for initial coating. After blocking overnight, serial dilutions of the sample supernatant were added and allowed to incubate for two hours at 37°C. The remaining protocol was the same as that for total IgX. We assayed wells in triplicate, showed the standard error of the mean and employed a student’s t-test.
3. Results
3.1. Larval thymectomy depletes T cells in the adult
To study the role of T cells in the management of gut bacterial communities and mucosal humoral immunity from a fundamental standpoint in vertebrate evolution, we used the frog larval thymectomy model. We performed thymectomies largely executed as in the original studies (Horton and Manning, 1972), but two days later in development than in the original protocol due to slightly slower development of the larvae in our system. No frogs used in the thymectomized group showed any thymic regrowth after cauterization (using microscopic inspection). Thymectomy greatly diminished the detectable number of TCRα transcripts using constant region PCR in the adult frog. TCR β and δ amplicons could be detected in some frogs, but at a much lower frequency than in frogs that had not undergone surgery (Figure 1B). Regardless, larval bilateral thymectomy almost completely ablated expression of TCRα, showing that the classic αβ T cell compartment has been removed from the experimental gut model.
3.2. Pyrosequencing reveals diverse gut bacterial flora in amphibian
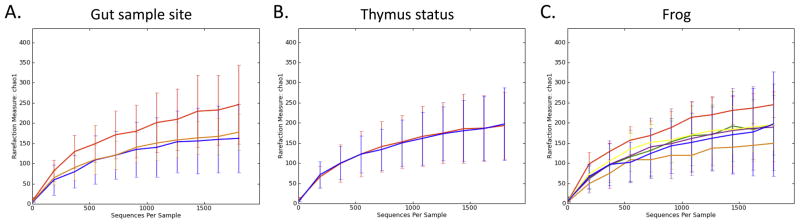
DNA was prepared from frog luminal contents at three distinct locations in the gastrointestinal tract, which is relatively simple in poikilothermic frogs and reptiles as they have lower metabolic rates compared to most mammals and birds (Supplemental Figure 1). Pyrosequencing of the 16S rRNA gene resulted in a total of 51,992 quality sequencing tags (mean, range: 2888mean, range: 1870–5314). Figure 2 illustrates the rarefaction curves for all samples at 1800 sequences, suggesting sufficient coverage. Although rare OTUs may have been identified with a higher sequencing depth, the number of sequence tags used here is deemed adequate to allow comparison of the beta diversity between samples (Kuczynski et al., 2010). Samples taken from Large intestine showed the richest diversity of flora. Individual frogs showed distinct microbiota composition (Figure 3). Four frogs yielded similar rarefaction curves, whereas one non-thymectomized frog (N321) showed more diversity and one non-thymectomized (N571) less. Averaged thymectomized samples gave a very similar rarefaction plot as those from normal animals. These data allowed us to gain an initial molecular description of the base amphibian gastrointestinal microbiota and look for differences between anatomic sites and surgical groups.
Figure 2. Rarefaction analysis of 16S rRNA gene sequences obtained from frog gastrointestinal content.
The analysis was performed on a randomly selected subset of 1800 sequences per sample. Lines represent the average of each sample type. A. stomach = orange, large intestine = red, and small intestine = blue. B. normal (no surgery) = blue and thymectomized = red. C. frog N011 = red, N321 = blue, T258 = green, T606 = yellow, T339 = brown, and N571 = orange.
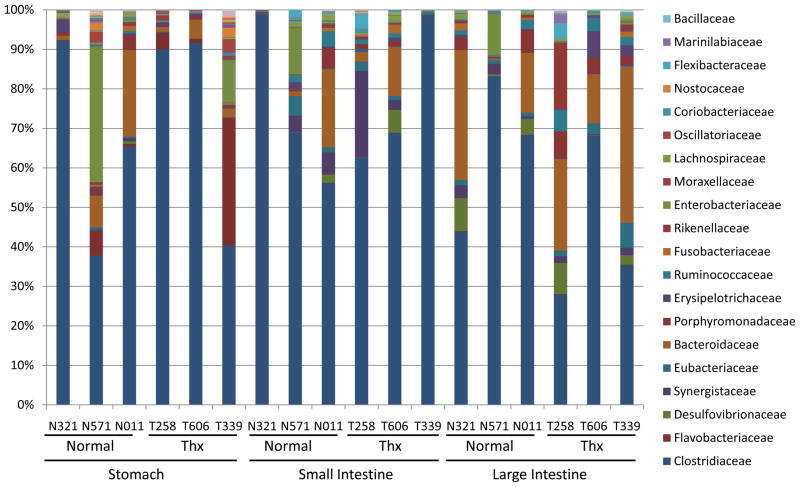
Figure 3. Bacterial families in the gastrointestinal tract of Xenopus laevis.
Familial distribution is shown with different colors from individual samples, grouped by thymus status from each of the three sampled positions in the gastrointestinal tract. Families with at least 1% representation in any sample are listed at the right. Complete taxonomic data is in Supplemental Table 2. Clostridiaceae were the predominant family, and no differences in bacterial groups between normal and thymectomized frogs were observed.
3.3. Frog gut flora is anatomically distinct but not altered by T cells
Many of the prokaryotic groups that dominate the human flora are also major components in the frog flora. Clostridiaceae dominated this amphibian community, and Bacteroidaceae and Enterobacteriaceae were abundant in our sequencing (Figure 3, Supplemental Table 3). In contrast to terrestrial mammalian flora, the environmental Flavobacteriaceae constituted nearly a third (32.34%) of the stomach flora of one frog and comprised 6.20% and 4.20% in two other individuals. The Synergistaceae, Desulfovibrionaceae, Erysipelotrichaceae, Ruminococcaceae, Rikenellaceae and Porphyromonadaceae also were substantial contributors (over 5% in at least one sample) to the X. laevis microbiota.
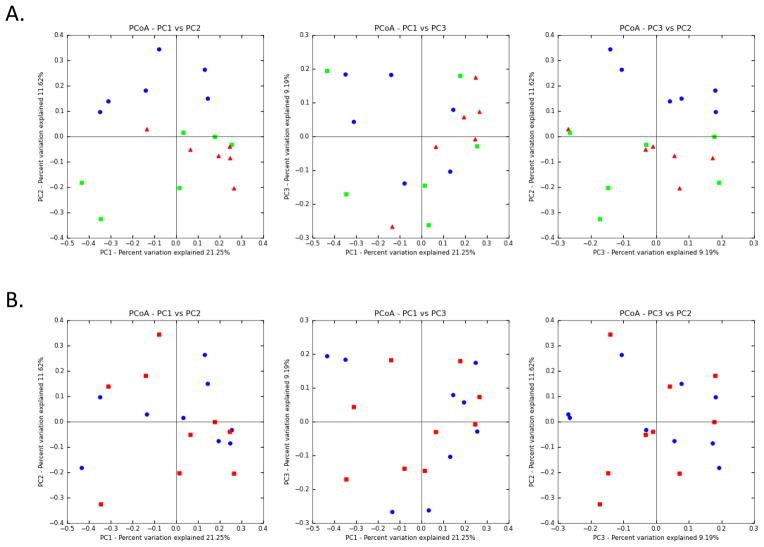
The PCoA plots based on the unweighted UniFrac distance metric indicated that the X. laevis stomach is composed of distinct microbial communities compared to the small and large intestine (Figure 4A). This was most pronounced in the greater representation of Flavobacteriaceae in the stomach compared to the more distal sites. Oscillatoriaceae cyanobacteria and Enterobacteriaceae were in greater abundance in the stomach. This latter group includes the common gram negative sometimes pathogens Salmonella, Escherichia coli, Klebsiella, Shigella and Yersinia pestis more commonly associated with the mammalian intestine. Synergistaceae was found in the intestines more than the stomach, particularly in the small intestine of thymectomized frog #258. However, PCoA plots based on the unweighted UniFrac did not reveal differences between small and large intestinal microbiota and, importantly, between normal and thymectomized frogs (Figure 4B). The similar microbiological findings in the guts of normal and T cell depleted frogs prompted investigations of the mucosal humoral immune compartment in this model.
Figure 4. Principal Coordinates Analysis (PCoA) of unweighted UniFrac distances of 16S rRNA gene sequencing.
The analysis was performed on a randomly selected subset of 1800 sequences per sample. A. Stomach (blue circles) samples separated from small (green square) and large (red triangle) intestine, indicative of distinct flora. B. No such separation was seen between non-thymectomized (blue circle) and thymectomized (red square) samples.
3.4 Amphibian IgX is likely orthologous to mammalian IgA
In order to assess the T-dependence of humoral immunity in the frog alimentary canal, we wanted to be more confident of the mucosal immunoglobulin in this amphibian. As more immunogenetic data has recently become available from reptile, bird, and ancestral groups of mammals (Supplemental Table 3), we revisited the phylogenetic relationships of tetrapod antibody classes to see if there was now convergence of expression and functional data suggesting IgX as the mucosal isotype. Entire immunoglobulin heavy chain C region sequences from diverse vertebrates were used to determine the relationship between amphibian IgX and mammalian IgA (Figure 5). Sequences were aligned and manually adjusted to maintain domain alignment between isotypes having either three or four constant domains (Supplemental Figure 3). The resulting trees showed that IgX did not cluster closest to IgM. Unlike any past phylogenetic analyses, these data show that IgX and IgA share a common ancestor earlier than either IgX and IgA does with IgM with high statistical support (91% of 1000 bootstrap replications). This finding provided confidence in assaying IgX in the frog as an ortholog as well as a functional analog of mammalian IgA in mucosal immunity.
Figure 5. Amphibian IgX is orthologous to IgA of birds and mammals.
Neighbor joining phylogenetic tree of the constant regions of diverse tetrapod immunoglobulin heavy chains, with the fish mucosal isotype IgZ/T included as an outgroup. Numbers at nodes show bootstrap support for each bifurcation after 1000 replications. The alignment used to build this is available as Supplemental Figure 2.
3.5 Thymectomy does not impede mucosal antibody production
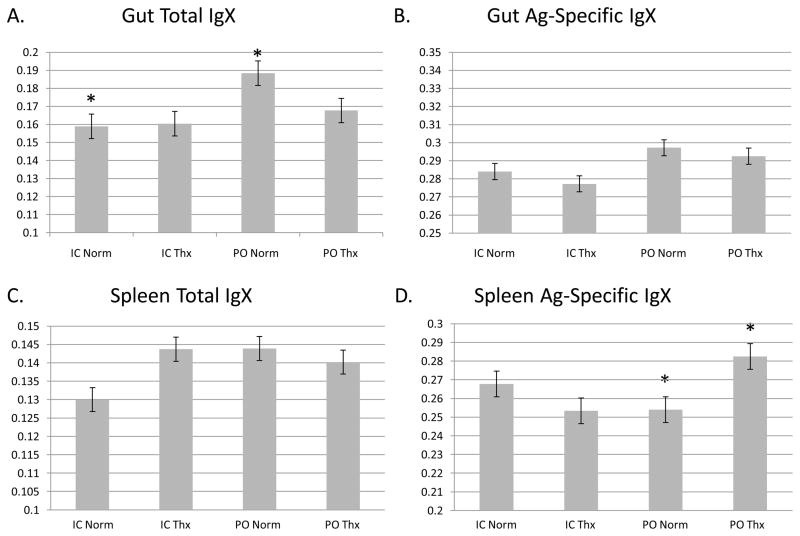
Normal and thymectomized frogs distinct from those assayed for gut flora were immunized with DNP-KLH either intracoelomically or orally (Figure 6). B cells were harvested and cultured from spleen and gut of these animals, and ELISAs were performed for both total IgX and antigen-specific IgX on the supernatant. Oral immunization elicited significantly (p=0.025) more total IgX from intestinal B cells than intra-coelomic delivery, but no significant difference was seen from the spleen cells or between B cells from normal and thymectomized animals. When DNP-KLH specific IgX was assayed, the only significant (p=0.013) difference seen was an increase in specific IgX from orally immunized splenocytes from thymectomized frogs versus orally immunized spleen cells from normal frogs. Thus larval thymectomy does not appear to retard the frogs’ ability to make total IgX, or IgX specific to this hapten-carrier conjugate.
Figure 6. Thymectomy does not retard induction of mucosal IgX response.
ELISA for the IgX isotype on supernatant of lymphocytes cultured from spleen or intestine of frogs immunized to DNP-KLH. Units of the Y axes are absorbance at 450nm. A. Oral (PO) gives a significantly greater total IgX response in the gut than intracoelomic (IC) immunization (p=0.025, marked by *), but no significant difference was seen with thymectomized frogs. No significance was seen monitoring neither antigen-specific IgX in the gut (B.) nor total IgX in the spleen (C.) D. Antigen specific IgX actually increased from spleen cells with thymectomy (p=0.013, marked by*).
4. Discussion
4.1. Frog gut flora
Molecular sequencing techniques have surpassed culture methods of investigating gastrointestinal microbiota due to their increased sensitivity (and the majority of unculturable genera present there) (Nocker et al., 2007). The amplification and subsequent sequencing of the 16S rRNA gene allows the identification of bacterial groups present in the GI tract of humans and other animal species (Huse et al., 2008; Nordentoft et al., 2011; van Kessel et al., 2011).
High throughput sequencing techniques had not been applied to the gut microbiota in any amphibian model, and X. laevis’s use in developmental, cell-and immuno-biology made it an obvious first candidate (Robert and Ohta, 2009). High-throughput 16S rRNA sequencing has been used to analyze the anti-fungal cutaneous bacterial populations in a salamander (Lauer et al., 2008). The most comprehensive culture based studies of amphibian gut flora have been performed in the leopard frog (Rana pipiens) (Gossling et al., 1982). Similar to the present work in Xenopus, Rana was found to have many Clostridiaceae, Eubacteriaceae, and Bacteroidaceae, and hibernating frogs at lower temperatures had a significant shift of flora to dominant Pseudomonodaceae (Banas et al., 1988). Using the pyrosequencing based approach described here we were able to identify >60 families of bacteria in the Xenopus gut that to our knowledge have not been described previously in amphibians. These include the known decomposers of plant polymers Marinilabiaceae (Detkova et al., 2009), the potential pathogens in the guts of humans and fish Porphyromonadaceae (Mulder et al., 2009), insect endosymbiont Sphingobacteriaceae (Zhou et al., 2009) and the known fermenters of fish microbiota Verrucomicrobiaceae (Rawls et al., 2006).
The frogs in our gut flora analysis all spent their lives in the same aquatic animal room descended from the same outbred founders just one generation before. They all received the same prepared diet. Although not sterilized, this homogenous, consistent feed is certainly in stark contrast to the varied, inconsistent, and microbe rich diet wild Xenopus would consume in Africa. Yet we saw great individual variance in their gastrointestinal flora, as has been described in humans (Eckburg et al., 2005) and dogs (Suchodolski et al., 2004). These communities are undoubtedly temporally dynamic as well within the individual animals. This work in X. laevis provides a reference for anatomically discrete gut microbial communities in an omnivorous amphibian. This is the Class of vertebrates that gave rise to the amniotic reptiles, birds and mammals, and first employed immunoglobulin heavy chain istotype switching to a mucosal isotype.
4.2 T cell influence on mucosal immunity
Formative studies in nude (athymic) mice found that the poorly developed Peyer’s patches lacking substantial germinal centers and low IgA levels of this rodent model could be largely restored by thymic grafts and the resulting T cell population (Guy-Grand et al., 1975). Yet loss of T cell function was not found to dramatically alter the cultivable gastrointestinal microbiota in these mice (Brown and Balish, 1978). As our understanding of T cell help, class switch recombination and mucosal immunity have improved in the decades since this work; much energy has focused on the relationship between the adaptive immune system and the gut flora. This has extended to hypotheses of the two coevolving and even rationale for the original genesis of the adaptive system (Lee and Mazmanian, 2010; McFall-Ngai, 2007).
We turned to the major biological model “between” zebrafish and mammals to ask questions about the influence of T cells in the mucosal immune compartment. This choice affords a comparative view of what the first immune system with both MHC-restricted T cells and a humoral response capable of class switch to a mucosal isotype was like in the ancestral tetrapod 300 million years ago. Thymectomy in this animal is an established model system for removal of the T cell compartment (Gravenor et al., 1995; Horton et al., 1998; Horton et al., 1996, 1997; Nagata and Cohen, 1983), but this study is the first to rigorously test the adults for T cell receptor expression by PCR. We assayed TCRβ, δ, and α, as TCRγ has been shown to be expressed early in the X. laevis thymus (Chida et al., 2011). Initially we were dismayed by some constant region (not necessarily indicative of functional rearrangement) TCR mRNA expression from peripheral blood of adults in which we visually scored the thymectomies to be perfectly clean. But perhaps this is to be expected, as TCRβ constant region message has been shown from bone marrow derived lymphocytes (Soloff et al., 1995) and even functional TCRδ and TCRγ transcripts have been found in nude mice (Spiess et al., 1993). Despite these observed low levels of constant domain nucleic acid expression, we are confident that the scrupulous culling of tadpoles with incomplete surgeries and molecular diagnostics of mature animals yielded frogs with no functional αβ T cell compartment (and γδ as well). Monoclonal antibodies have confirmed this absence at the cellular level of receptor expression in this model (Horton et al., 1994).
The possibility of extra-thymic T cell development was explored in Xenopus by including PCR controls of gut tissue at the time of thymectomy in addition to adult gut from normal and thymectomized frogs (Figure 1). Extra-thymic T cell development has been described in mouse gut (Guy-Grand et al., 1991) and may even be stimulated by thymic ablation (Guy-Grand et al., 2003). Highly organized mucosal lymphoid structures such as Peyer’s patches do not exist in poikilothermic vertebrates such as Xenopus (Shields, 2000), yet a suggestion of gut T lymphopoiesis has been described from bony fish (Rombout et al., 2011). Tadpoles showed no TCR expression in gut at the age of thymectomy, nor was TCR expression seen in adult gut of larval thymectomized frogs. While extrathymic routes of T cell development are possible in frog our data suggest that these are at best minor relative to thymic for gut seeding, and that thymectomy does not force extrathymic developmental programs.
Some “natural” gut IgA in mouse has been thought to be from T-independent B-1 cells (Kroese et al., 1995). The specificity of gut IgA was later shown to be less “natural” and actually driven by specific antigens of the gut microbial symbionts and food (Macpherson et al., 2000). Moreover, use of TCRβ/δ double knock-out transgenic mice ensured that T cells from thymus or elsewhere were not responsible for this phenomenon, nor was any “bystander” contribution of their lymphokines (Wetzel, 1989). They further showed that this response was at least in part due to B1 peritoneal cells in mice (Macpherson et al., 2000). These findings seem consistent with the lack of effect that thymectomy has on mucosal IgX in the present study. Yet in mammals there is plenty of evidence for T-dependence in gut IgA too. Most human IgA-switched plasma cells in the lamina propria show evidence of somatic hypermutation, presumably from a germinal center event with T cell help (Dunn-Walters et al., 1997a; Dunn-Walters et al., 1997b). Moreover, most (~80%) plasma cells in the gut were found to be antigen specific and not poly-reactive (Benckert et al., 2011). AID transgenic mice defective somatic hypermutation but still competent to make IgA exhibited greater colony counts of small intestinal flora, germinal center hyperplasia and susceptibility to Yersinia enterocolitica (Wei et al., 2011). This suggests T-dependent, germinal center processes do shape the gut flora through the specific IgA generated against it in mice. Deep sequencing of IgA rearrangements in CD3−/− mice showed evidence for T-dependent somatic hypermutation in aged mice, thus the relatively young age of the frogs in this study could be a factor (Lindner et al., 2012). We did not find evidence corroborating such T dependences in the amphibian, but recognize that this is one relatively small study in a captive population.
4.3 Evolution of mucosal antibody isotypes and IgA
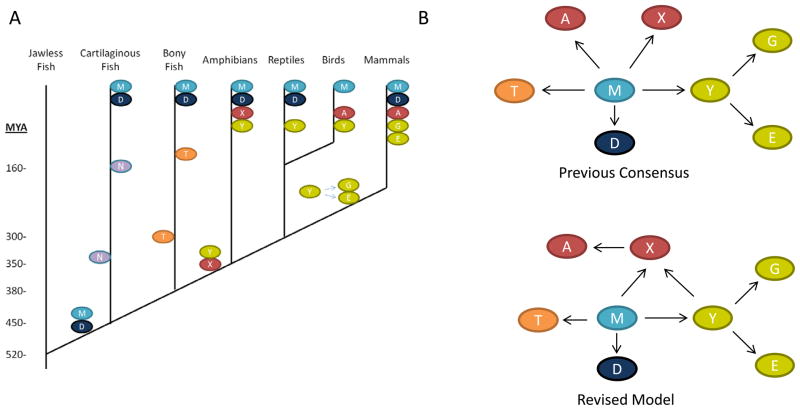
Recent data now allow more rigorous studies of the natural history of tetrapod immunoglobulin genes. Sequence similarities and predicted structural resemblance to IgM, originally suggested that IgX might be the functional analog, but not the ortholog of IgA (Mussmann et al., 1996a; Mussmann et al., 1996b). The IgA of Aves appears to be a mucosal functional analog of mammalian IgA (Mansikka, 1992) and there is high sequence identity that suggests orthology (Choi et al., 2010). However, there are four C domains in avian IgA suggesting a deletion occurred to yield the mammalian IgA of three (Aveskogh and Hellman, 1998). The incomplete evolutionary loss of the Cα2 domain present in IgX and avian IgA could have given rise to the hinge region in mammalian IgA before the divergence of monotremes and the therian marsupial and placental mammals (Vernersson et al., 2010).
Although IgX has been hypothesized to be an ortholog of IgA (Du et al., 2012; Sun et al., 2012) the phylogenetic analysis described here is the first to lend experimental support to the notion. The availability of more diverse tetrapod immunoglobulin sequences allowed us to make this analysis. The CH1 and CH2 encoding exons of the IgX gene may have been derived from the IgY encoding locus and the CH3 and CH4 from the IgM encoding gene (Deza et al., 2007; Wei et al., 2009). These relationships will need to be retested as more amphibian, reptile, bird and non-placental mammal genomes are sequenced. However, this scenario is consistent with the known synteny of the C region encoding genes in the immunoglobulin heavy chain loci of known tetrapod genomes (Supplemental Figure 4), where IgX/A is in between the genes encoding the IgM/D and IgY encoding loci that birthed it. The locus in mammals has undergone duplicative expansions giving rise to subfunctionalization of IgG and IgE from IgY and a proliferation of sub-isotypes (Warr et al., 1995). Thus, the resulting IgY/IgM chimera IgX gave rise to (or perhaps should be synonymous with) IgA in endothermic vertebrates. This appears to be the second time vertebrate evolution has produced a dedicated mucosal immunoglobulin isotype (Figure 7), the first being IgZ/T that is unique to some teleost fish (Danilova et al., 2005; Hansen et al., 2005; Zhang et al., 2010). IgX/A is the first mucosal isotype whose expression is controlled via AID mediated class switch recombination, as IgZ/T is produced via deletional RAG-mediated V(D)J recombination similar to the rearrangement at the α/δ T cell receptor locus (Flajnik, 2005; Salinas et al., 2011).
Figure 7. Model of immunoglobulin natural history with mucosal IgX/A emerging in early tetrapods.
A. Simplified phylogeny of vertebrates showing approximate emergence times of heavy chain isotypes. B. This analysis suggests that the isotype previously described as IgX in amphibians may be orthologous to IgA of warm blooded vertebrates (model adapted from M. Flajnik’s chapter of Fundamental Immunology, W. Paul editor). In addition to the isotypes extant in man, the immunoglobulin light chain-less IgNAR of cartilaginous fish and the mucosal IgZ/T of bony fish is also shown.
4.4 Conclusions
In the frog we find evidence for an ancient, T-independent, humoral mucosal response. We defined the gut flora of the model amphibian X. laevis but found the bacterial communities of the stomach, small and large intestine to be unaffected by thymectomy. More representative phylogenetic analysis of the relationship between amphibian IgX and IgA of amniotic tetrapods shows their orthology, explaining the origin of human’s most abundant immunoglobulin. Therefore we conclude that the T cell independent IgA pathway is likely an ancient mechanism to manage the microbial symbionts of the gut and other mucosal surfaces. More comparative studies must resolve the (convergent?) functional relationship between the two vertebrate mucosal isotypes: IgZ/T and IgX/A.
Supplementary Material
Acknowledgments
This work was supported by the NIH through a K22 award to MFC (AI56963) and a graduate student grant from the Texas A&M College of Veterinary Medicine to SM. AEG was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through Grant Number 5 T35 RR019530-07. The authors appreciate the expert training in tadpole thymectomy provided by Martin Flajnik.
Footnotes
We have no conflicts of interest.
Contributor Information
Sara Mashoof, Email: smashoof@cvm.tamu.edu.
Anna Goodroe, Email: aegoodroe@cvm.tamu.edu.
Christina C. Du, Email: christinacdu@gmail.com.
Jeannine O. Eubanks, Email: jeubanks@cvm.tamu.edu.
Natalie Jacobs, Email: njacobs@cvm.tamu.edu.
Jörg M. Steiner, Email: jsteiner@cvm.tamu.edu.
Ian Tizard, Email: itizard@cvm.tamu.edu.
Jan S. Suchodolski, Email: jsuchodolski@cvm.tamu.edu.
Michael F. Criscitiello, Email: mcriscitielllo@cvm.tamu.edu.
References
- Aveskogh M, Hellman L. Evidence for an early appearance of modern post-switch isotypes in mammalian evolution; cloning of IgE, IgG and IgA from the marsupial Monodelphis domestica. Eur J Immunol. 1998;28:2738–2750. doi: 10.1002/(SICI)1521-4141(199809)28:09<2738::AID-IMMU2738>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Banas JA, Loesche WJ, Nace GW. Classification and distribution of large intestinal bacteria in nonhibernating and hibernating leopard frogs (Rana pipiens) Applied and environmental microbiology. 1988;54:2305–2310. doi: 10.1128/aem.54.9.2305-2310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. The Journal of clinical investigation. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JF, Balish E. Gastrointestinal microecology of BALB/c nude mice. Applied and environmental microbiology. 1978;36:144–159. doi: 10.1128/aem.36.1.144-159.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida AS, Goyos A, Robert J. Phylogenetic and developmental study of CD4, CD8 alpha and beta T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis. Dev Comp Immunol. 2011;35:366–377. doi: 10.1016/j.dci.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Suh KS, Choi DE, Lim BJ. Morphometric analysis of podocyte foot process effacement in IgA nephropathy and its association with proteinuria. Ultrastruct Pathol. 2010;34:195–198. doi: 10.3109/01913121003648402. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123:75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Laboratory investigation; a journal of technical methods and pathology. 1970;22:448–457. [PubMed] [Google Scholar]
- Criscitiello MF, Ohta Y, Saltis M, McKinney EC, Flajnik MF. Evolutionarily conserved TCR binding sites, identification of T cells in primary lymphoid tissues, and surprising trans-rearrangements in nurse shark. J Immunol. 2010;184:6950–6960. doi: 10.4049/jimmunol.0902774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- Detkova EN, Zaichikova MV, Kevbrin VV. Physiology and biochemistry of alkaliphilic anaerobic hydrolytic bacterium Alkaliflexus imshenetskii. Mikrobiologiia. 2009;78:310–316. [PubMed] [Google Scholar]
- Deza FG, Espinel CS, Beneitez JV. A novel IgA-like immunoglobulin in the reptile Eublepharis macularius. Dev Comp Immunol. 2007;31:596–605. doi: 10.1016/j.dci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Du CC, Mashoof SM, Criscitiello MF. Oral immunization of the African clawed frog (Xenopus laevis) upregulates the mucosal immunoglobulin IgX. Veterinary immunology and immunopathology. 2012;145:493–498. doi: 10.1016/j.vetimm.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Walters DK, Boursier L, Spencer J. Hypermutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur J Immunol. 1997a;27:2959–2964. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
- Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of human IgVH genes indicates that ileal lamina propria plasma cells are derived from Peyer’s patches. Eur J Immunol. 1997b;27:463–467. doi: 10.1002/eji.1830270217. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Taubman MA, Smith DJ. Thymic control of secretory antibody responses in the rat. J Immunol. 1979;123:19–24. [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF. The last flag unfurled? A new immunoglobulin isotype in fish expressed in early development. Nat Immunol. 2005;6:229–230. doi: 10.1038/ni0305-229. [DOI] [PubMed] [Google Scholar]
- Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, Martin A, Casellas R, Philpott DJ, Girardin SE, McCoy KD, Macpherson AJ, Paige CJ, Gommerman JL. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossling J, Loesche WJ, Nace GW. Large intestine bacterial flora of nonhibernating and hibernating leopard frogs (Rana pipiens) Applied and environmental microbiology. 1982;44:59–66. doi: 10.1128/aem.44.1.59-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravenor I, Horton TL, Ritchie P, Flint E, Horton JD. Ontogeny and thymus-dependence of T cell surface antigens in Xenopus: flow cytometric studies on monoclonal antibody-stained thymus and spleen. Dev Comp Immunol. 1995;19:507–523. doi: 10.1016/0145-305x(95)00030-w. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Azogui O, Celli S, Darche S, Nussenzweig MC, Kourilsky P, Vassalli P. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–341. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D, Griscelli C, Vassalli P. Peyer’s patches, gut IgA plasma cells and thymic function: study in nude mice bearing thymic grafts. J Immunol. 1975;115:361–364. [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS microbiology ecology. 2011;76:301–310. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci U S A. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J, Horton T, Ritchie P, Gravenor I, Gartland L, Cooper M. Developmental and Comparative Immunology. Wageningen; The Netherlands: 1994. Use of monoclonal antibodies to demonstrate absence of T cell development in early-thymectomized Xenopus. [Google Scholar]
- Horton JD, Horton TL, Dzialo R, Gravenor I, Minter R, Ritchie P, Gartland L, Watson MD, Cooper MD. T-cell and natural killer cell development in thymectomized Xenopus. Immunol Rev. 1998;166:245–258. doi: 10.1111/j.1600-065x.1998.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Horton JD, Manning MJ. Response to skin allografts in Xenopus laevis following thymectomy at early stages of lymphoid organ maturation. Transplantation. 1972;14:141–154. doi: 10.1097/00007890-197208000-00001. [DOI] [PubMed] [Google Scholar]
- Horton TL, Ritchie P, Watson MD, Horton JD. NK-like activity against allogeneic tumour cells demonstrated in the spleen of control and thymectomized Xenopus. Immunology and cell biology. 1996;74:365–373. doi: 10.1038/icb.1996.64. [DOI] [PubMed] [Google Scholar]
- Horton TL, Ritchie P, Watson MD, Horton JD. NK cell evolution: studies on Xenopus. Biochemical Society transactions. 1997;25:263S. doi: 10.1042/bst025263s. [DOI] [PubMed] [Google Scholar]
- Hsu E, Du Pasquier L. Studies on Xenopus immunoglobulins using monoclonal antibodies. Mol Immunol. 1984;21:257–270. doi: 10.1016/0161-5890(84)90096-8. [DOI] [PubMed] [Google Scholar]
- Huse SM, Dethlefsen L, Huber JA, Mark Welch D, Relman DA, Sogin ML. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS genetics. 2008;4:e1000255. doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O’Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nature cell biology. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese FG, Ammerlaan WA, Deenen GJ, Adams S, Herzenberg LA, Kantor AB. A dual origin for IgA plasma cells in the murine small intestine. Advances in experimental medicine and biology. 1995;371A:435–440. doi: 10.1007/978-1-4615-1941-6_91. [DOI] [PubMed] [Google Scholar]
- Kuczynski J, Costello EK, Nemergut DR, Zaneveld J, Lauber CL, Knights D, Koren O, Fierer N, Kelley ST, Ley RE, Gordon JI, Knight R. Direct sequencing of the human microbiome readily reveals community differences. Genome biology. 2010;11:210. doi: 10.1186/gb-2010-11-5-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer A, Simon MA, Banning JL, Lam BA, Harris RN. Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. The ISME journal. 2008;2:145–157. doi: 10.1038/ismej.2007.110. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- Lindner C, Wahl B, Fohse L, Suerbaum S, Macpherson AJ, Prinz I, Pabst O. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med. 2012;209:365–377. doi: 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Current opinion in gastroenterology. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- Mansikka A. Chicken IgA H chains. Implications concerning the evolution of H chain genes. J Immunol. 1992;149:855–861. [PubMed] [Google Scholar]
- McFall-Ngai M. Adaptive immunity: care for the community. Nature. 2007;445:153. doi: 10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- Mulder IE, Schmidt B, Stokes CR, Lewis M, Bailey M, Aminov RI, Prosser JI, Gill BP, Pluske JR, Mayer CD, Musk CC, Kelly D. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79. doi: 10.1186/1741-7007-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Du PL, Hsu E. Is Xenopus IgX an analog of IgA? Eur J Immunol. 1996a;26:2823–2830. doi: 10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Wilson M, Marcuz A, Courtet M, Du PL. Membrane exon sequences of the three Xenopus Ig classes explain the evolutionary origin of mammalian isotypes. Eur J Immunol. 1996b;26:409–414. doi: 10.1002/eji.1830260221. [DOI] [PubMed] [Google Scholar]
- Nagata S, Cohen N. Specific in vivo and nonspecific in vitro alloreactivities of adult frogs (Xenopus laevis) that were thymectomized during early larval life. Eur J Immunol. 1983;13:541–545. doi: 10.1002/eji.1830130705. [DOI] [PubMed] [Google Scholar]
- Nocker A, Burr M, Camper AK. Genotypic microbial community profiling: a critical technical review. Microbial ecology. 2007;54:276–289. doi: 10.1007/s00248-006-9199-5. [DOI] [PubMed] [Google Scholar]
- Nordentoft S, Molbak L, Bjerrum L, De Vylder J, Van Immerseel F, Pedersen K. The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T-RFLP and 454 pyrosequencing. BMC microbiology. 2011;11:187. doi: 10.1186/1471-2180-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238:1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombout JH, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish & shellfish immunology. 2011;31:616–626. doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nature reviews. Molecular cell biology. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R, Dayhoff M. Matrices for detecting distant relationships. In: MD, editor. Atlas of protein sequences. National Biomedical Research Foundation; 1979. pp. 353–358. [Google Scholar]
- Shields JW. The functional evolution of GALT: a review. Lymphology. 2000;33:47–57. [PubMed] [Google Scholar]
- Soloff RS, Wang TG, Lybarger L, Dempsey D, Chervenak R. Transcription of the TCR-beta locus initiates in adult murine bone marrow. J Immunol. 1995;154:3888–3901. [PubMed] [Google Scholar]
- Spiess S, Kuhrober A, Schirmbeck R, Arden B, Reimann J. Diversity of functional T-cell receptor delta-chain transcripts from bone marrow cells of athymic nude mice. Immunology. 1993;78:252–259. [PMC free article] [PubMed] [Google Scholar]
- Suchodolski JS, Ruaux CG, Steiner JM, Fetz K, Williams DA. Application of molecular fingerprinting for qualitative assessment of small-intestinal bacterial diversity in dogs. Journal of clinical microbiology. 2004;42:4702–4708. doi: 10.1128/JCM.42.10.4702-4708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wei Z, Li N, Zhao Y. A comparative overview of immunoglobulin genes and the generation of their diversity in tetrapods. Dev Comp Immunol. 2012 doi: 10.1016/j.dci.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tochinai S. Demonstration of thymus-independent immune system in Xenopus laevis. Response to polyvinylpyrrolidone. Immunology. 1976;31:125–128. [PMC free article] [PubMed] [Google Scholar]
- van Kessel MA, Dutilh BE, Neveling K, Kwint MP, Veltman JA, Flik G, Jetten MS, Klaren PH, Op den Camp HJ. Pyrosequencing of 16S rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.) AMB Express. 2011;1:41. doi: 10.1186/2191-0855-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernersson M, Belov K, Aveskogh M, Hellman L. Cloning and structural analysis of two highly divergent IgA isotypes, IgA1 and IgA2 from the duck billed platypus, Ornithorhynchus anatinus. Mol Immunol. 2010;47:785–791. doi: 10.1016/j.molimm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunology today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- Wei Z, Wu Q, Ren L, Hu X, Guo Y, Warr GW, Hammarstrom L, Li N, Zhao Y. Expression of IgM, IgD, and IgY in a reptile, Anolis carolinensis. J Immunol. 2009;183:3858–3864. doi: 10.4049/jimmunol.0803251. [DOI] [PubMed] [Google Scholar]
- Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Developmental cell. 2011a;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annual review of biochemistry. 2011b;80:125–156. doi: 10.1146/annurev-biochem-052709-094552. [DOI] [PubMed] [Google Scholar]
- Wetzel GD. Interleukin 5 regulation of peritoneal Ly-1 B lymphocyte proliferation, differentiation and autoantibody secretion. Eur J Immunol. 1989;19:1701–1707. doi: 10.1002/eji.1830190926. [DOI] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, LaPatra SE, Bartholomew J, Sunyer JO. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Huang H, Meng K, Shi P, Wang Y, Luo H, Yang P, Bai Y, Zhou Z, Yao B. Molecular and biochemical characterization of a novel xylanase from the symbiotic Sphingobacterium sp. TN19. Applied microbiology and biotechnology. 2009;85:323–333. doi: 10.1007/s00253-009-2081-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.