Abstract
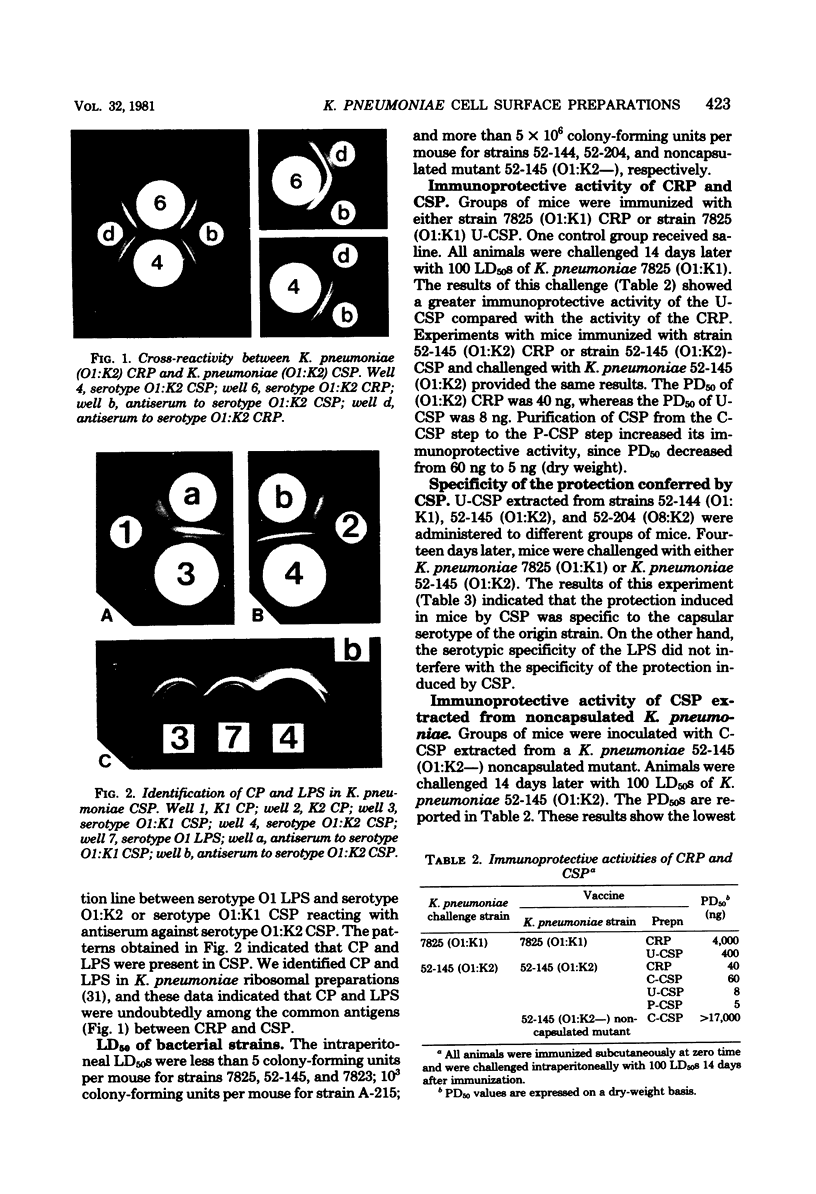
Cell surface preparations and ribosomal preparations were extracted from Klebsiella pneumoniae. Agar gel diffusion with antisera to cell surface preparations or ribosomal preparations indicated common antigenic components among the preparations. Lipopolysaccharide and capsular polysaccharide were identified in the cell surface preparations. These results and the previous identification of lipopolysaccharide and capsular polysaccharide in ribosomal preparations suggest that these antigens are responsible for the immunochemical cross-reactivity observed among these two bacterial extracts. Active protection could be induced in mice by these two preparations. On a dry-weight basis, cell surface preparations provided better immunoprotective activity than did ribosomal preparations. However, the 50% protective dose of both preparations is practically the same on the basis of their capsular polysaccharide content. These results are consistent with the hypothesis that the immunoprotective moiety of ribosomal preparations is the contaminating cell surface antigens. Furthermore, the low level of nucleotidic components detected in purified cell surface preparations led us to infer that the immunoprotective activity of capsular polysaccharide may not be dependent on the adjuvant activity of ribonucleic acid. The involvement of capsular polysaccharide in the immunoprotective capacity of cell surface preparations is demonstrated either by using a degradation of this antigen by K. pneumoniae bacteriophage K2-associated glycanase or by using a preparation extracted from a noncapsulated mutant of K. pneumoniae. Nevertheless, the low protective ability of purified capsular polysaccharides is in contrast to its greater activity when induced in bacterial cell surface preparations. The protective activity of K. pneumoniae capsular polysaccharide may be dependent on its association with other surface antigenic components present in cell surface preparations or may be dependent on its native form in the bacterial cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L., Davis C. E. Immunochemical characterization of the "native" type III polysaccharide of group B Streptococcus. J Exp Med. 1976 Feb 1;143(2):258–270. doi: 10.1084/jem.143.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Véron M., Tinelli R. Vaccin acellulaire de Pseudomonas aeruginosa. I. Préparation et activité. Ann Microbiol (Paris) 1976 Feb-Mar;127A(2):247–259. [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect Immun. 1977 Feb;15(2):533–538. doi: 10.1128/iai.15.2.533-538.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanges R., Robert D., Content Y., Nis G. Study of the immunogenicity of ribosomes and ribosomal RNA extracted from K. pneumoniae and S. pneumoniae. Arzneimittelforschung. 1980;30(1A):142–172. [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Parkes L., McNelis R. M., Gotschlich E. C. Protection against group B meningococcal disease. I. Comparison of group-specific and type-specific protection in the chick embryo model. J Exp Med. 1976 Aug 1;144(2):319–329. doi: 10.1084/jem.144.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan L. C., Sandford P. A., Conrad H. E. The structure of the serotype 2 capsular polysaccharide of Aerobacter aerogenes. Biochemistry. 1967 Sep;6(9):2755–2767. doi: 10.1021/bi00861a016. [DOI] [PubMed] [Google Scholar]
- Geyer H., Stirm S., Himmelspach K. Immunochemical properties of oligosaccharide-protein conjugates with Klebsiella-K2 specificity. I. Specificity and crossreactivity of anti-conjugate versus anti-bacterial antibodies. Med Microbiol Immunol. 1979 Jan 24;165(4):271–288. doi: 10.1007/BF02152925. [DOI] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B., Jodal U., Hanson L. A. Studies on antibody response and tolerance to E, coli K antigens in immunized rabbits and in children with urinary tract infection. Int Arch Allergy Appl Immunol. 1973;44(2):260–273. doi: 10.1159/000230935. [DOI] [PubMed] [Google Scholar]
- Kaijser B., Vahlne G. Escherichia coli K antigen. Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):271–288. [PubMed] [Google Scholar]
- Kasper D. L., Baker C. J., Baltimore R. S., Crabb J. H., Schiffman G., Jennings H. J. Immunodeterminant specificity of human immunity to type III group B streptococcus. J Exp Med. 1979 Feb 1;149(2):327–339. doi: 10.1084/jem.149.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Goroff D. K., Baker C. J. Immunochemical characterization of native polysaccharides from group B streptococcus: the relationship of the type III and group B determinants. J Immunol. 1978 Sep;121(3):1096–1105. [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Bartlett J. G. Quantitative determination of the antibody response to the capsular polysaccharide of Bacteroides fragilis in an animal model of intraabdominal abscess formation. J Infect Dis. 1977 Dec;136(6):789–795. doi: 10.1093/infdis/136.6.789. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical characterization of surface antigens of Bacteroides melaninogenicus. Rev Infect Dis. 1979 Mar-Apr;1(2):263–277. doi: 10.1093/clinids/1.2.263. [DOI] [PubMed] [Google Scholar]
- Michel F. B., Dussourd D'Hinterland L., Bousquet J., Pinel A. M., Normier G. Immuno-stimulation by a ribosomal vaccine associated with a bacterial cell wall adjuvant in humans. Infect Immun. 1978 Jun;20(3):760–769. doi: 10.1128/iai.20.3.760-769.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miler J. M., Spilsbury J. F., Jones R. J., Roe E. A., Lowbury E. J. A new polyvalent Pseudomonas vaccine. J Med Microbiol. 1977 Feb;10(1):19–27. doi: 10.1099/00222615-10-1-19. [DOI] [PubMed] [Google Scholar]
- Pieroni R. E., Broderick E. J., Bundeally A., Levine L. A simple method for the quantitation of submicrogram amounts of bacterial endotoxin. Proc Soc Exp Biol Med. 1970 Mar;133(3):790–794. doi: 10.3181/00379727-133-34565. [DOI] [PubMed] [Google Scholar]
- Riottot M. M., Fournier J. M., Jouin H. Direct evidence for the involvement of capsular polysaccharide in the immunoprotective activity of Klebsiella pneumoniae ribosomal preparations. Infect Immun. 1981 Jan;31(1):71–77. doi: 10.1128/iai.31.1.71-77.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riottot M., Fournier J. M., Pillot J. Capsular serotypic specificity of the protection conferred on mice by Klebsiella pneumoniae ribosomal preparations. Infect Immun. 1979 May;24(2):476–482. doi: 10.1128/iai.24.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Johnson W. Immunogenicity of ribosomal vaccines isolated from group A, type 14 Streptococcus pyogenes. Infect Immun. 1975 Jun;11(6):1195–1202. doi: 10.1128/iai.11.6.1195-1202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. The effect of mycobacterial RNA on the primary antibody response of mice to bovine globulin. J Immunol. 1972 Aug;109(2):217–221. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]