Abstract
Aim
To determine whether inpatient diabetes management and education with improved transition to outpatient care (IDMET) improves glycemic control after hospital discharge in patients with uncontrolled type 2 diabetes (T2DM).
Methods
Adult inpatients with T2DM and HbA1c≥7.5% (11 mmol/mol) admitted for reasons other than diabetes to an academic medical center were randomly assigned to IDMET vs. usual care (UC). Linear mixed models estimated treatment-dependent differences in the change in HbA1c (measured at 3, 6, and 12 months) from baseline to 1 year follow-up.
Results
Thirty-one subjects had mean age 55 ± 12.6 years, with mean HbA1c of 9.7 ± 1.6% (82 ± 18 mmol/mol). Mean inpatient glucose was lower in the IDMET than in the UC group (176 ± 66 vs. 195 ± 74 mg/dl [9.7 vs. 10.8 mmol/l]), p=0.001. In the year after discharge, the average HbA1c reduction was greater in the IDMET compared to the UC group by 0.6% (SE 0.5%, [7 (SE 5) mmol/mol], p=0.3). Among patients newly discharged on insulin, the average HbA1c reduction was greater in the in the IDMET than in the UC group by 2.4% (SE 1.0%, [25 (SE 11) mmol/mol] p=0.04).
Conclusions
Inpatient diabetes management (IDMET) substantially improved glycemic control one year after discharge in patients newly discharged on insulin; patients previously treated with insulin did not benefit.
Keywords: inpatient diabetes management, diabetes care management strategy, inpatient to outpatient transitions in care
Introduction
Up to one-third of patients with diabetes are hospitalized annually, usually for reasons other than uncontrolled diabetes [1]. Much of the research in this area has focused on appropriate inpatient glycemic management. It has now been established that notwithstanding the controversy over intensive glycemic control in ICU patients [2], good glycemic control remains an important goal among all hospitalized patients [3] and can be achieved safely in general medical and surgical settings [4, 5].
From a health systems perspective, hospitalization may also offer an opportunity to impact long-term glycemic control. Hospital admission identifies diabetes patients at highest risk for uncontrolled diabetes, complications, and costs [1]. As a group, hospitalized diabetes patients generate 40–50% of the total national inpatient and outpatient costs associated with diabetes in the United States [6]. Better glycemic control is associated with lower hospital admission rates and decreased inpatient costs [7]. The hospital setting, with its concentration of resources, may thus present an opportunity to improve long-term glycemic control and diabetes care as well as lowering post-discharge healthcare costs [8–11] The one randomized trial in this field showed improvement in post-discharge glycemic control after inpatient diabetes management, but followed patients for 3 months only [12]. In observational trials with longer follow-up, hospital admission to address uncontrolled diabetes has been associated with improved post-discharge glycemic control measured by HbA1c [13, 14], but these results must be interpreted cautiously, for there is a tendency for HbA1c levels to decline in the year following a hospital admission even in the absence of intervention [15]. While these studies support the proposal that that hospital admission may serve as a “window of opportunity” to improve long-term diabetes care [8, 9], there may be drawbacks to this approach, such as the potential risk that intensification of diabetes medication regimens may increase the rate of hypoglycemia after discharge; in addition, the marginal benefit of diabetes teaching in the inpatient setting, when patients are coping with acute illness, is not clear.
The effect of inpatient diabetes management and education aimed at improving long-term glycemic control when patients with uncontrolled diabetes are admitted for non-metabolic reasons has not been studied in a randomized trial. We performed a randomized trial to test the hypothesis that focused inpatient diabetes management, education, and discharge planning would improve glycemic control in patients with type 2 diabetes and hemoglobin A1c (HbA1c) ≥7.5% (58 mmol/mol) in the year following discharge.
Methods
Study Design
The study was a 12-month non-blinded randomized trial conducted on general medical and surgical wards of a tertiary care hospital (Massachusetts General Hospital, Boston, MA). The objective was to determine whether an inpatient diabetes management, education, and discharge transition (IDMET) program, including medication titration and augmented diabetes self-management education while subjects were hospitalized, followed by facilitated transition to outpatient care, could improve long term glycemic control in inpatients with HbA1c≥7.5% (58 mmol/mol), compared to usual care. The study was approved by the Partners Healthcare Institutional Review Board and overseen by a data safety monitor. Preliminary results were presented previously [16].
Subjects
Patients were recruited from non-ICU medical and surgical units between April 2009 and May 2010. Potentially eligible patients were initially identified by screening newly admitted patients; methods have been previously published [17]. Participants were at least 18 years of age and had a previous diagnosis of type 2 diabetes with outpatient prescription of oral hypoglycemic medication or insulin and HbA1c ≥7.5% (58 mmol/mol) within one year prior to admission or random blood glucose greater than 200 mg/dl (11 mmol/l) at admission. Participants were excluded if they had uncontrolled diabetes as the primary reason for admission, anemia, recent blood transfusion or need for blood transfusion (which would interfere with interpretation of HbA1c), liver or kidney disease, presence of hypoglycemia unawareness, life expectancy of less than one year, or were pregnant, unable or unwilling to self-administer insulin, were not eating, or did not speak English. Additionally, patients were excluded if they had an endocrinology consult in the course of usual inpatient care prior to study enrollment.
Interventions
After obtaining informed consent, participants were assigned by a computer-generated algorithm that randomly allocated subjects in blocks of four to inpatient diabetes management, education, and improved discharge transition (IDMET) or to usual care. IDMET participants were evaluated by an endocrinologist and a nurse practitioner (NP) certified diabetes educator (CDE). The intervention included three components:
-
!!
Diabetes management: the endocrinologist recommended diabetes medications and followed the patient for the duration of the inpatient stay.
-
!!
Diabetes education: The NP CDE provided diabetes education, focusing on diabetes survival skills and insulin teaching, when needed, in a 60 to 90 minute visit, occasionally spread over two visits if necessary. These sessions used a standardized flip chart to cover the ABCs of diabetes, testing of blood sugar with patient return demonstration, keeping daily records, drawing up and injecting insulin, signs, symptoms, and management of hypo- and hyperglycemia, sick day rules, diet, foot and skin care, and insulin and exercise, if appropriate. Physician and nurse practitioner visits were documented using standard templates.
-
!!
Discharge Transition: IDMET patients received diabetes discharge medication instructions printed on a single page template outlining the regimen and suggested changes for alterations in meals and activity. In addition, a brief summary of the diabetes medication changes, the hospital course, and the diabetes discharge medication instructions were sent to primary care physician.
Usual care (UC) subjects received whatever care was deemed appropriate by the non-study care team and diabetes education as provided by non-specialist physicians and nurses caring for the patient. The usual care team was not precluded from consulting endocrinology if desired. Both IDMET and UC participants had nutrition counseling provided by inpatient dietitians and completed study survey instruments.
At discharge, subjects were advised to resume diabetes care with their regular physicians but were encouraged to call for help with diabetes-related problems if their usual physician was not available. All subjects received a telephone call from the study coordinator one week after discharge for safety monitoring and to promote retention; these calls did not alter management or involve physician contact since there were no safety concerns.
Outcomes and Follow-up
HbA1c was measured at baseline and 3, 6, and 12 months at the MGH laboratory, one of the reference labs for the National Glycohemoglobin Standardization Program (NGSP), or in laboratories that used NGSP, DCCT-aligned standardized assays. The primary outcome was average change in HbA1c over the course of one-year follow-up after hospital discharge. Secondary outcomes included glycemic control during admission, differences in diabetes distress, measured by Problem Areas in Diabetes [18] scores at baseline and six months, and readmission rates.
Medical histories, comorbidities, and medications were obtained from the chart and confirmed by patient interview. Race and ethnicity were self-reported. Height, weight, blood pressure, and survey instruments were recorded and completed at the initial hospitalization. Patient satisfaction was measured by a five-item instrument with satisfaction rated from 1 (least) to 10 (most) for each question. Diabetes-specific emotional impact was measured by the 20-item Problem Area in Diabetes (PAID) Scale [18].
All subjects were asked to return for follow-up at 3, 6, and 12 months after discharge to the MGH Diabetes Research Center to meet with the study coordinator for HbA1c testing and measurement of weight and blood pressure and to report severe hypoglycemia (defined as requiring assistance, coma, or seizure), update medical history and medications, and complete the PAID questionnaire at 6 months. Additional outpatient post-discharge diabetes self-management education was tracked in both groups. Subjects who were unable to return for follow-up could complete HbA1c testing at a local NGSP-compliant laboratory and update rates of hypoglycemia, medical history, medications, and complete instruments over the telephone. All MGH hospital readmissions were captured, and subjects were asked to report admissions to outside hospitals at follow-up visits or telephone calls.
Sample Size
Using a two-sample t-test, we estimated that a sample size of 36 patients was required to have 80% power to detect a 0.75% difference between the baseline and follow-up HbA1c values between treatment groups with a two-tailed P value of 0.05, assuming mean HbA1c of 8.78% (72 mmol/mol) and SD of 0.76% (8 mmol/mol).
Statistical methods
We determined differences in characteristics between IDMET and usual care groups using t-tests for continuous variables, rank-sum tests for highly skewed continuous variables, and Fisher Exact tests for categorical variables. We planned that analyses would be stratified according to whether patients were or were not treated with insulin prior to admission (called “previously treated with insulin” or “insulin-naïve”). We used linear mixed models to test the effect of the intervention on HbA1c levels. The models included fixed effects of treatment group (IDMET vs. usual care), visit (0, 3, 6, and 12 months as categorical levels), and the treatment times visit interaction and random participant-specific intercepts and slopes over follow-up time. Linear contrasts were used to estimate the treatment-dependent difference in the change from baseline to each visit individually and on average over all three follow-up visits. All tests were two-tailed with 98.3% confidence intervals reported for the visit-specific estimates based on a Bonferroni correction for multiple comparisons and a 95% confidence interval for the average effect of treatment. Equivalent models were used to analyze the full study cohort and the insulin-naïve subgroup. Analyses were intention-to-treat, with subjects analyzed according to their assigned treatment group rather than treatment exposure. We did not impute missing HbA1c data to avoid introducing bias.
DCCT HbA1c units (%) were converted to International Federation of Clinical Chemistry (IFCC) units (mmol/mol) using the standard equation: IFCC-HbA1c (mmol/mol) = [DCCT-HbA1c (%) − 2.15] × 10.929. DCCT unit standard deviations and standard errors were multiplied by 10.929 for conversion to IFCC units. Because the conversion equation does not allow calculation of IFCC units for low values of HbA1c, change variables were determined by taking the difference between the IFCC values of the estimates. All analyses were conducted using SAS software, version 9.1 (SAS Institute, Cary, North Carolina).
Results
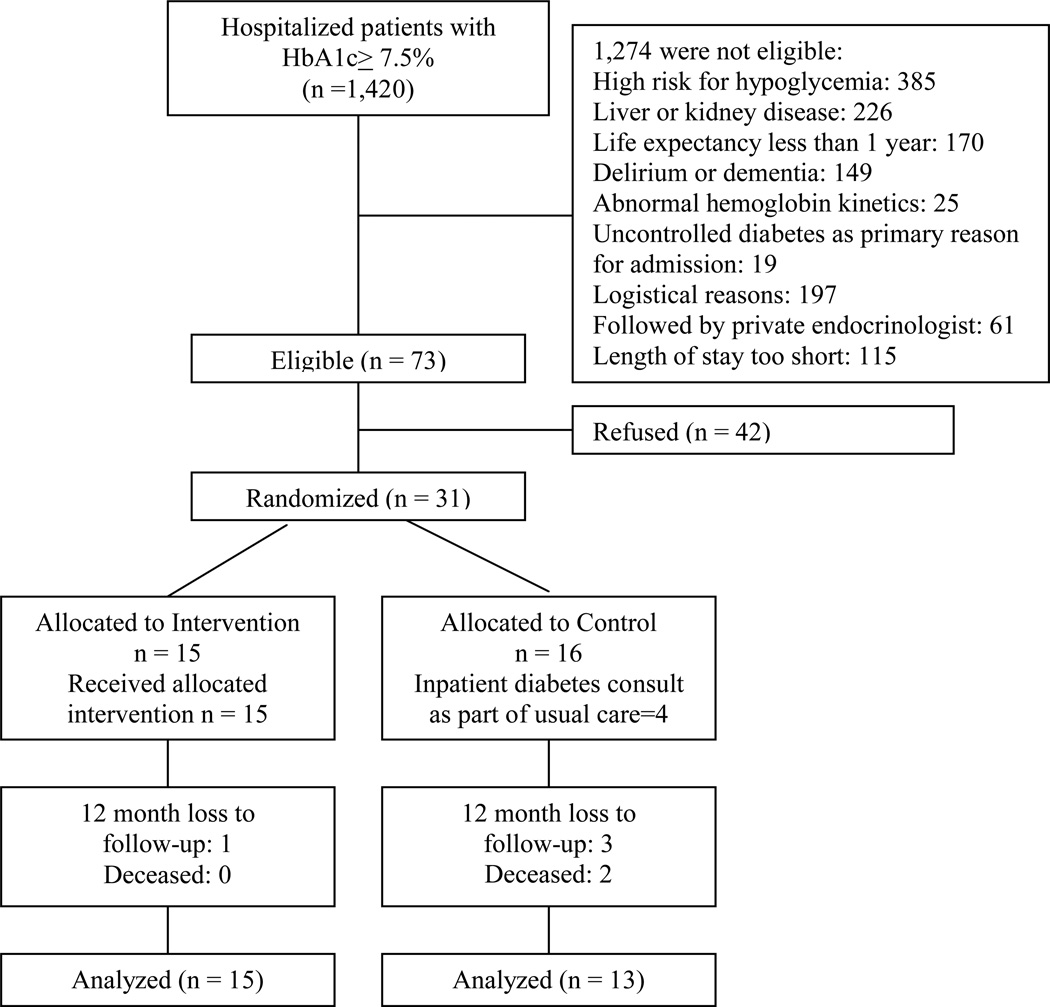
Of the 1,420 initially screened patients who met HbA1c eligibility criteria, the majority (n=745) were too ill to participate in the trial, while others (n=300) were excluded if they could not speak English, had an endocrinology consult prior to screening, or resided out-of-state. Overall, 73 patients were eligible for the trial, and 31 (42%) consented to enroll (see CONSORT diagram, Figure 1). Enrollment was closed after 13 months of recruitment.
Figure 1. Consort Diagram.
All 15 subjects assigned to the IDMET group received the intervention. Most of the insulin-naïve patients in this group initiated insulin in the hospital. One initiated insulin with the help of the study team the day following discharge, and one, a 39 year-old woman with BMI of 65 kg/m2, started exenatide at the time of discharge. Four of the 16 subjects assigned to usual care had endocrinology consults in the hospital and initiated insulin which was continued at discharge, as allowed by the protocol. Of the usual care group participants who did not have endocrinology consult, there was no contamination by IDMET interventions (i.e. no UC subjects shared a room and/or overheard IDMET interventions).
There were no differences in baseline characteristics between groups (Table 1). Participants had very poor glycemic control with mean HbA1c greater than 9.5% (80 mmol/mol) in both groups. Insulin use prior to hospital admission was not statistically significantly different between groups. Over 50% of both groups had diagnosed cardiovascular disease.
Table 1.
Baseline Characteristics
| IDMETi Intervention |
IDMETii Insulin-naïve |
Usual Care Control |
|
|---|---|---|---|
| Entire sample, n | 15 | 9 | 16 |
| Age, years, mean (SD) | 57.2 (11.3) | 57.6 (13.7) | 52.87 (13.7) |
| Female sex, n (%) | 9 (60.0) | 5 (55) | 6 (38) |
| White race, n (%) | 12 (86) | 7 (78) | 12 (86) |
| Hispanic, n (%) | 1 (7) | 0 | 1 (6) |
| BMI, kg/m2, mean (SD) | 36 (12) | 34 (4) | 33 (6) |
| Self-reported duration of diabetes, years, mean (SD) | 10 (7) | 8 (6.7) | 12 (8) |
| Self-reported duration of diabetes, years, median (IQR) | 10 (5–18) | 8 (4–10) | 11.5 (7–15) |
| Admission diagnosesiii | |||
| Acute coronary syndrome, n (%) | 2 (13) | 2 (22) | 2 (12) |
| CHF, n (%) | 2 (13) | 0 | 4 (25) |
| Other cardiac, n (%) | 5 (33) | 2 (22) | 2 (12) |
| Lower extremity cellulitis or ulcer, n (%) | 2 (13) | 1 (11) | 2 (12) |
| Pneumonia, n (%) | 1 (7) | 1 (11) | 1 (6) |
| Other, n (%) | 3 (20) | 3 (33) | 5 (31) |
| Comorbidities | |||
| Cardiovascular disease, n (%) | 8 (53) | 5 (55) | 10 (62) |
| Depressive disorder, n (%) | 4 (26.7) | 3 (33) | 4 (25) |
| Glycemia, all subjects | |||
| HbA1c, mean (SD) DCCT (%) IFCC (mmol/mol) |
9.6 (1.6) 82 (17) |
9.5 (1.5) 81 (16) |
9.7 (1.7) 83 (19) |
| Insulin use prior to admission, n (%) | 6 (36) | none | 11 (69) |
Inpatient diabetes management and education
The subset of the inpatient diabetes management and education group who were not prescribed insulin prior to hospital admission
Percentages do not sum to 100 because of rounding error
Inpatient mean glucose levels were lower in the intervention than in the usual care group (Table 2). Episodes of inpatient hypoglycemia less than 60 mg/dl (3.3 mmol/l) were infrequent and equivalent. Ninety-three percent of the IDMET group and 50% of the usual care group (p=0.01) were treated with basal-bolus insulin in the hospital, including all of the usual care patients who had off-study endocrinology consults. One subject in the intervention group and three subjects in the usual care group were lost to follow-up.
Table 2.
Results
| IDMET Intervention |
Usual Care Control |
Intervention effect (SE)iv |
P valuev | |
|---|---|---|---|---|
| Inpatient parameters, n | 15 | 16 | ||
| Inpatient diabetes consult | 15 (100%) | 4 (25%) | <0.0001 | |
| Basal-bolus regimen during admission | 14 (93%) | 8 (50%) | 0.01 | |
| Mean hospital glucose, mean (SD)vi mg/dl mmol/l |
178 (66) 9.7 (3.6) |
195 (74) 10.8 (4.1) |
0.001 | |
| Hypoglycemia< 60 mg/dl (3.3 mmol/l), n (%) | 3 (20) | 1 (7) | 0.6 | |
| Patient satisfaction at discharge, mean (SD)vii | 9.5 (0.8) | 9.5 (0.8) | 0.9 | |
| Length of stay, days, median (IQR) | 5 (3–10) | 6 (4–8) | 0.7 | |
| Glycemic results in all subjects | ||||
| HbA1c change baseline to 3 months, mean (SD) DCCT, % |
−1.7 (1.5) | −0.9 (1.0) | −0.8 (0.7) | 0.2 |
| HbA1c change baseline to 6 months, mean (SD) DCCT, % |
−1.3 (2.3) | −0.4 (1.1) | −0.7 (0.7) | 0.3 |
| HbA1c change baseline to 12 months, mean (SD) DCCT, % |
−0.6 (2.1) | −0.2 (1.1) | −0.6 (0.8) | 0.5 |
| Average intervention effect, mean (SE) DCCT,% IFCC, mmol/molviii |
−0.6 (0.5) −7 (5) |
0.3 | ||
| Glycemic results in insulin-naïve subjects, n | 9 | 5 | ||
| HbA1c change baseline to 3 months, mean (SD) DCCT, % |
−2.2 (1.4) | −2.3 (2.9) | −1.4 (0.9) | 0.12 |
| HbA1c change baseline to 6 months, mean (SD) DCCT, % |
−2.6 (1.9) | −0.6 | −4.9 (1.4) | 0.004 |
| HbA1c change baseline to 12 months, mean (SD) DCCT, % |
−1.1 (2.2) | 0.8 (1.1) | −3.6 (1.3) | 0.01 |
| Average intervention effect DCCT, % IFCC, mmol/molv |
−2.4 (1.0) −25 (11) |
0.04 | ||
| Problem Areas in Diabetes Score, n | 15 | 16 | ||
| PAID score at baseline | 39 (18) | 41 (14) | 0.7 | |
| PAID change | −13 (17) | −4 (15) | 0.3 | |
| Severe hypoglycemia, n patients / n events | 1 / 3 | 2 / 3 | ||
| Number of patients readmitted, n (%)ix | 5 (33) | 8 (50) | 0.3 |
The intervention effect is the difference between groups using used a linear mixed model to test the effect of the intervention on HbA1c levels accounting for repeated measures. See methods for details.
P value using t-test for normally distributed continuous variables, rank-sum test for non-normally distributed continuous variables, Fisher exact test for number of readmissions, and value from linear mixed model for intervention effect, with 98.3% confidence intervals for individual timepoints and 95% confidence interval for average effect. See methods for details.
Mean hospital glucose was the mean of all point-of-care glucose tests during the index admission.
Measured by a 5-item instrument with satisfaction rated from 1 (least) to 10 (most) satisfied.
IFCC value was calculated by using the absolute difference between HbA1c values converted into IFCC units, because direct conversion of low values of HbA1c is not possible using the conversion equation. See Methods, last paragraph, for details.
There was also no difference in total number diabetes-related readmissions.
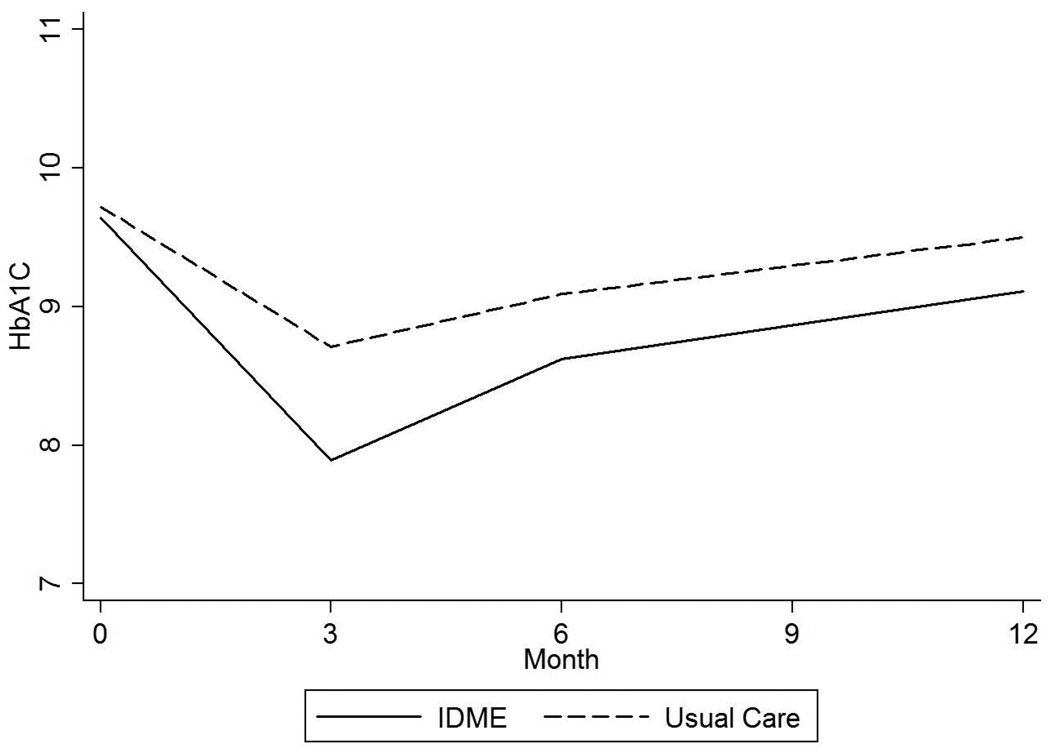
After discharge, the average intervention effect over 12 months of follow up was a 0.63% (SE 0.56%; 7 [SE 5] mmol/mol) HbA1c reduction in the IDMET compared with the usual care arm (p=0.3). The maximum difference between groups was seen at six months (mean HbA1c change −1.28% [SD 2.33] vs. −0.36% [SD 1.09]) with a return to near-baseline values at 12 months in both groups (Table 2 and Figure 2).
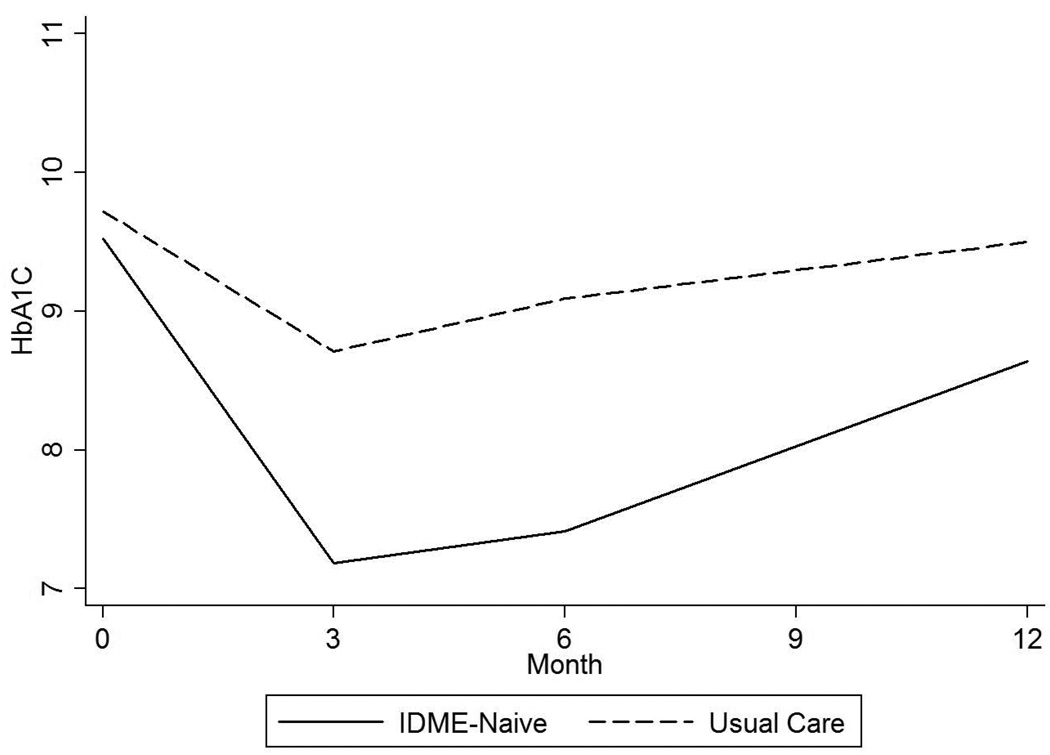
Figure 2.
a. HbA1c up to 12 months after hospital discharge in all subjects.
Linear mixed model including fixed effects of treatment group, treatment times visit interaction, and random participant-specific intercepts and slopes over time; P value for overall difference between groups=0.3.
b. HbA1c up to 12 months after hospital discharge in patients not treated with insulin prior to hospital admission (“insulin-naïve”).
Linear mixed model including fixed effects of treatment group, treatment times visit interaction, and random participant-specific intercepts and slopes over time; P value for overall difference between groups=0.04.
Among insulin-naïve patients (those not prescribed insulin prior to the hospital admission), the average intervention effect was HbA1c decline of 2.35% (SE 1.03%, 25 [SE 11] mmol/mol, p=0.04;) over 12 months following hospital discharge. The magnitude and persistence of the effect on glycemia was greater in this subgroup than in subjects previously treated with insulin, with HbA1c of −2.57 ± 1.87% at 6 months and −1.07 ± 2.17% at 1 year (Table 2 and Figure 2).
The emotional impact of diabetes measured by PAID scores appeared to show greater improvement in the IDMET compared to the usual care group from baseline to 6 months (declining 13 ± 17 versus 4 ± 15) but the difference was not statistically significant (Table 2). Patient satisfaction, outpatient hypoglycemia, and readmissions were not affected by the intervention. The rate of post-discharge diabetes self management education was similar in both groups (three subjects in the IDMET group, one who was insulin-naïve at admission, and four in the usual care group, two who were insulin-naïve at admission). There was no overall change in weight in the entire group or in the insulin-naïve subgroup (including or excluding the exenatidetreated patient, who lost over 60 lbs; data not shown).
Discussion
This is the first randomized trial to study the impact of inpatient diabetes management, education, and discharge transition planning on glycemic control up to one year after hospital discharge. While the intervention had little impact in patients treated with insulin prior to hospital admission, it was beneficial in insulin-naïve patients. Most of these patients initiated insulin, learned to administer and manage it, and had the dose titrated while they were in the hospital; their transition back to primary care was facilitated by clear communication with the patient and the primary care physician. We conclude that using hospital admission for medication initiation and titration and patient education is likely to yield large dividends when initiating a new injectable therapy, usually insulin, but offers little marginal benefit for long-term glycemic control in insulin-treated patients.
Initiating insulin therapy in the primary care setting is resource-intensive [19] and remains challenging for both patients and providers [20, 21]. Many patients who start insulin in the outpatient setting often fail to achieve therapeutic targets [19]. Consequently, leveraging the time and resources available in the hospital may be one strategy to help overcome these outpatient barriers to insulin initiation and titration in patients who are hospitalized for other reasons.
We observed a waning of the intervention effect by month 12 of follow-up. There was minimal post-discharge contact other than research coordinator follow-up visits in both groups by design, since the goal was to test the utility of an inpatient intervention with transfer to usual outpatient care. The waning effect we observed suggests that any inpatient intervention may need to be coupled with post-discharge follow-up care, or more effective transfer of care between inpatient and outpatient providers, to sustain any impact over time. These results are consistent with inpatient interventions targeting other chronic, complex, and costly conditions, such as congestive heart failure and smoking cessation. In general, effective and cost-effective programs for these conditions have identified patients while hospitalized and then, crucially, sustained the intervention for one to several months post-discharge [22–25]. Thus, a strategy of using the hospital admission to motivate change while facilitating linkage to post-discharge care would likely help to strengthen the intervention effect in new insulin users, and it may be more effective than an inpatient intervention in patients who were treated with insulin prior to admission.
Most current studies suggest that the hospital admission is not being used to address diabetes care when patients with uncontrolled diabetes are admitted for other reasons. Admission diabetes medication regimens are discontinued in over 10% of hospitalized patients without initiation of new therapy at the time of discharge; this practice is associated with increased one year mortality [26]. In one large multicenter study, 32% of hospitalized veterans with HbA1c greater than 8% (64 mmol/mol) had no change in medication regimen or timely follow-up after discharge [27]. While discharge planning is a fundamental mechanism by which inpatient changes in the medical regimen may be translated into post-discharge improvements in diabetes care, it receives little attention [28]. Taken together with the results of this study, it is likely that certain target populations would derive long-term benefit from more coordinated inpatient diabetes care.
Our findings should be interpreted in the context of several limitations. The study design tested the clinical effectiveness of a care strategy, but the rigor imposed by a patient-level randomized controlled trial study design posed several challenges. Maintaining a usual care control group was difficult: many eligible participants had endocrinology consults in the course of usual care before screening and after trial enrollment, leading to high exclusions and a 25% crossover rate from the usual care group (crossovers biased the results to the null hypothesis of no effect). Although there was no difference between post-discharge diabetes self-management education between groups, the fact that four usual care subjects had DSME after discharge may also have limited our ability to detect a difference between groups. (We did not query pre-admission history of diabetes self-management education.) The low eligibility (5%) and participation (42%) rate of eligible patients was to some degree related to the constraints of trial eligibility criteria rather than those that would prevail in clinical care. We used more stringent criteria than would pertain in clinical care or quality improvement program implementation in order to maximize safety due to limited post-discharge follow-up and to promote HbA1c interpretability. The process of informed consent for what could be considered a care improvement intervention allowed for patient refusal. We did not formally collect reasons for refusal, but many refusers stated they were not interested because they were in pain, overwhelmed by the hospitalization, did not want to start insulin, and/or did not want to follow up after discharge. Many of the patients who declined to participate would not necessarily have refused if their own clinical care team had recommended insulin initiation. Conversely, many of the patients who were ineligible to participate because of logistical reasons, such as residence out of state and comorbidities that limited interpretability of HbA1c, might have been good candidates for this intervention if it had been delivered in the course of clinical care. A more pragmatic and less stringent trial design might have circumvented some of these limitations and been more appropriate for testing a care strategy [29]. These caveats notwithstanding, the participation and eligibility rates in this trial were similar to those of other inpatient interventions for chronic conditions that have required outpatient follow-up [30]. Because appropriate insulin initiation has such a large effect, it is possible to demonstrate benefit even with small sample sizes, but given that this was a single-center pilot study with a small sample size, these findings should be confirmed in a larger multicenter trial.
In conclusion, we found that inpatient diabetes management, education, and improved discharge transition planning improved glycemic control up to 12 months after hospital discharge in insulin-naïve patients with uncontrolled diabetes but did not affect post-discharge glycemic control in patients treated with insulin prior to hospital admission. Realizing the “window of opportunity to improve diabetes care when patients with diabetes are hospitalized for other reasons” [8] may prove more challenging than previously anticipated, especially among insulin-treated patients. These results also suggest that the effect of any intervention may be enhanced, and be more widely applicable, by using hospitalization to identify high-risk patients and then to link them to early post-discharge follow-up. As global payment mechanisms contingent on quality of care and population management predominate, centralizing the provision of specialty services to overcome barriers to insulin initiation while patients are hospitalized for other reasons may be a cost-effective strategy to target high-risk diabetes patients. Further study will be required to determine whether this is the case.
Acknowledgements
We are grateful to Emily Boykin APRN CDE, formerly of the MGH Diabetes Center, Tiffany Soper APRN CDE of the MGH Diabetes Center, and Richard Pompei RN of MGH for help with initiation and conduct of the trial and to Hui Zheng PhD of MGH for statistical consultation.
This study was funded by and DJW is supported by an NIDDK Career Development Award (K23 DK 080 228). DMN is funded by the Charlton Fund for Innovative Diabetes Research.
Abbreviations
- HbA1c
Hemoglobin A1c
- IFCC
International Federation of Clinical Chemistry
- IDMET
Inpatient diabetes management, education, and discharge transition program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors declare that they have no conflict of interest.
Preliminary results of this trial were published in abstract form at the Endocrine Society conference, ENDO 2011, in Boston, MA on June 4, 2011 and presented orally as part of a presentation titled “Effect of inpatient diabetes management on outpatient control: Implications for inpatient staff” at the American Diabetes Association Scientific Sessions in Philadelphia, PA on June 12, 2012.
Author Contributions
DW conceived of the project and designed the study with assistance from DN and ML. DW, CB, EC, and ML executed the study. SR managed the data and assisted with some statistical analyses. DW performed the remainder of the data analysis. DW drafted the manuscript. All authors reviewed and edited the manuscript. DW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Menzin J, Korn JR, Cohen J, Lobo F, Zhang B, Friedman M, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264–275. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 3.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27:553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 Surgery) Diabetes Care. 2011;34:256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. RAndomized Study of Basal Bolus Insulin Therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial) Diabetes Care; 2007;30:2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 6.Gandra SR, Lawrence LW, Parasuraman BM, Darin RM, Sherman JJ, Wall JL. Total and component health care costs in a non-Medicare HMO population of patients with and without type 2 diabetes and with and without macrovascular disease. J Manag Care Pharm. 2006;12:546–554. doi: 10.18553/jmcp.2006.12.7.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control: A managed care perspective. Diabetes Care. 2001;24:51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Roman SH, Chassin MR. Windows of opportunity to improve diabetes care when patients with diabetes are hospitalized for other conditions. Diabetes Care. 2001;24:1371–1376. doi: 10.2337/diacare.24.8.1371. [DOI] [PubMed] [Google Scholar]
- 9.Braithwaite SS, Magee M, Sharretts JM, Schnipper JL, Amin A, Maynard G. The case for supporting inpatient glycemic control programs now: the evidence and beyond. J Hosp Med. 2008;3:6–16. doi: 10.1002/jhm.350. [DOI] [PubMed] [Google Scholar]
- 10.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15:353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 11.Levetan CS, Passaro M, Jablonski K, Kass M, Ratner RE. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21:246–249. doi: 10.2337/diacare.21.2.246. [DOI] [PubMed] [Google Scholar]
- 12.Koproski J, Pretto Z, Poretsky L. Effects of an intervention by a diabetes team in hospitalized patients with diabetes. Diabetes Care. 1997;20:1553–1555. doi: 10.2337/diacare.20.10.1553. [DOI] [PubMed] [Google Scholar]
- 13.Raccah D, Hanaire-Broutin H, Sert-Langeron C, Brin S, Chabrier G, Fontaine PM, et al. Insulin initiation in type 2 diabetic patients admitted in hospital in France and follow-up at 1 year. Diabetes Metab. 2006;32:244–250. doi: 10.1016/s1262-3636(07)70275-6. [DOI] [PubMed] [Google Scholar]
- 14.Gobl CS, Dobes B, Luger A, Bischof MG, Krebs M. Long-term impact of a structured group-based inpatient-education program for intensive insulin therapy in patients with diabetes mellitus. Wien Klin Wochenschr. 2010;122:341–345. doi: 10.1007/s00508-010-1398-x. [DOI] [PubMed] [Google Scholar]
- 15.Wei NJ, Grant RW, Nathan DM, Wexler DJ. Effect of hospital admission on glycemic control one year after discharge. Endocr Pract. 2012:1–22. doi: 10.4158/EP11309.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wexler DJ, Beauharnais CC, Regan S, Nathan DM, Cagliero E, Larkin ME. Impact of inpatient diabetes management and education on glycemic control 12 months after discharge. Abstract presented at Endocrine Society; June 4, 2011; Boston, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauharnais CC, Larkin ME, Zai AH, Boykin EC, Luttrell J, Wexler DJ. Efficacy and cost-effectiveness of an automated screening algorithm in an inpatient clinical trial. Clin Trials. 2012 doi: 10.1177/1740774511434844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 19.Hayward RA, Manning WG, Kaplan SH, Wagner EH, Greenfield S. Starting insulin therapy in patients with type 2 diabetes: effectiveness, complications, and resource utilization. JAMA. 1997;278:1663–1669. [PubMed] [Google Scholar]
- 20.Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56:e418–e424. [PMC free article] [PubMed] [Google Scholar]
- 21.Karter AJ, Subramanian U, Saha C, Crosson JC, Parker MM, Swain BE, et al. Barriers to insulin initiation: The translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733–735. doi: 10.2337/dc09-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 23.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 24.Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008;168:1950–1960. doi: 10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 26.Lipska KJ, Wang Y, Kosiborod M, Masoudi FA, Havranek EP, Krumholz HM, et al. Discontinuation of antihyperglycemic therapy and clinical outcomes after acute myocardial infarction in older patients with diabetes. Circ Cardiovasc Qual Outcomes. 2010;3:236–242. doi: 10.1161/CIRCOUTCOMES.109.887620. [DOI] [PubMed] [Google Scholar]
- 27.Griffith ML, Boord JB, Eden SK, Matheny ME. Clinical inertia of discharge planning among patients with poorly controlled diabetes mellitus. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3216. [DOI] [PubMed] [Google Scholar]
- 28.Cook CB, Seifert KM, Hull BP, Hovan MJ, Charles JC, Miller-Cage V, et al. Inpatient to outpatient transfer of diabetes care: Planning for an effective hospital discharge. Endocr Pract. 2009;15:263–269. doi: 10.4158/EP.15.3.263. [DOI] [PubMed] [Google Scholar]
- 29.Roland M, Torgerson DJ. What are pragmatic trials? BMJ. 1998;316:285. doi: 10.1136/bmj.316.7127.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]