Abstract
Background
Arterial hypertension is a major risk factor that can lead to complication of peripheral vascular disease due, in part, to endothelial dysfunction. Because sodium nitrite (SN) can be converted to nitric oxide (NO), which counteracts endothelial dysfunction, we explored the effect of nitrite on neovascularization following hind-limb ischemia in different models of hypertension (HT).
Methods and results
Chronic delivery of angiotensin-II (Ang-II, 400ng/Kg/min) or N(omega)-nitro-L-arginine-methyl-ester (L-NAME, 0.1g/L) were used for a two week period to induce hypertension. Mice were subjected to femoral artery ligation induced-ischemia in the hind-limb followed by treatment with SN (50mg/L) for 2-weeks. SN significantly reduced systolic arterial blood pressure in mice receiving Ang-II and L-NAME, but had no effect in sham animals. After 2 weeks, blood flow and microangiography showed 60%±1.0 recovery in sham compared to 40%±1.3 in HT mice. Importantly, sham and HT mice treated with SN showed a 100% blood flow recovery associated with normalization in capillary density. The inhibition of xanthine-oxido-reductase (allopurinol) or VEGFR (SU-5416) prevented the neovascularization in HT mice treated with SN. Cyclic GMP (cGMP) content in the hind-limb was significantly increased in mice treated with SN compared to non-treated mice. Nitrite/nitrate content was only increased in the sham group treated with SN. Immunoprecipitation and Western blot analysis revealed an increase in eNOS/Akt/VEGFR phosphorylation in skeletal muscle from mice treated with SN compared to non-treated mice.
Conclusion
Our findings indicate that SN therapy rescues the neovascularization and blood flow recovery in ischemic hind-limb of sham and HT mice likely through the Akt/NO/cGMP and VEGFR pathways.
Keywords: Hypertension, neovascularization, ischemia, Angiotensin II, L-NAME, sodium nitrite, peripheral ischemia
Introduction
Arterial hypertension is a major risk factor that can lead to complication of peripheral vascular disease.[35] Previous studies showed that hypertension in human and animal models is associated with endothelial dysfunction and microvascular rarefaction.[2] These data suggest that hypertension contributes to alterations of vascular growth patterns. Therefore, understanding the pathophysiological mechanisms regulating angiogenesis would provide valuable insight into mechanisms of therapeutic angiogenesis for preventing or treating chronic tissue ischemia that occurs in cardiovascular diseases such hypertension.
Nitric oxide (NO) is produced by NO-synthase (NOSs) in the endothelium and regulates vascular reactivity,[29] which is altered in hypertension.[37] NO is also critical in angiogenesis, as evidenced by the reduction in angiogenesis in mice lacking the gene encoding for eNOS and, the enhanced angiogenesis response in animals treated with NO donors.[5,13,40] Vascular endothelial growth factor (VEGF) regulates many cellular and molecular events in the neovascularization process such endothelial cells migration and proliferation.[11,30] It is well known that shear stress releases NO, which is also an important factor in the mechanism of angiogenesis. Moreover, there are reciprocal interactions between VEGF and NO in the mechanisms of angiogenesis.[57,12]
Nitrite has previously been used as a marker of NO formation in tissues and plasma.[13,26] Recently nitrite was recognized as a beneficial storage form of NO in blood and tissues that initiates rapidly reduced to NO that initiates cytoprotective signaling in pathological states like kidney ischemia/reperfusion injury.[54,40] Recent studies described the ability of nitrite to enhance angiogenesis in the ischemic hind-limb of normotensive mice.[32] Since our goal is to determine the role of nitrite therapy in impaired angiogenesis related to deficiency of NO associated with hypertension, we chose models of hypertension where NO pathway is impaired. It has been shown by our group and others that mice infused with angiotension II or L-NAME display a significant impairment in NO pathway. Therefore, to accomplish our goal, we infused mice with angiotensin II or L-NAME to induce hypertension with impairment in NO pathway and angiogenesis. In these models, we test the hypothesis that sodium nitrite therapy would enhance ischemia-induced angiogenesis.
Methods
Animal Model and Surgery
Sixty male C57BL/6J (8–10 weeks old) were used in the present study. These studies are conformed to the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and the Tulane University Institutional Animal Care approved the protocol. Mice were infused with Ang II (400 ng/kg/min)[21] or received L-NAME (100 mg/L) for two weeks.[32] Then hind-limb ischemia model was induced in the following groups by ligation of the proximal part of the left femoral artery, as previously described [32,49,3] for two weeks in: 1) sham; 2) sham + sodium nitrite (50 mg/L in the drinking water) [51]; 3) L-NAME (eNOS inhibitor); 4) L-NAME (in the drinking water) + sodium nitrite ± (allopurinol “xanthine oxidase” or SU-5416 “VEGFR inhibitor” dissolved in DMSO); 5) Ang II (mini-osmotic pump); and 6) Ang II + sodium nitrite ± (allopurinol or SU-5416 i.p. injection). Hypertension and hind-limb ischemia were induced on the same day and lasted for a period of two weeks.
Blood Pressure
Systolic Blood Pressure (SBP) was measured by tail-cuff plethysmography (Softron, BP-98A), before treatment and then once a week as previously described.[21] All mice were trained to the tail cuff measurements one week before starting experiments. Measurements were performed at the same time, always at 10 am the morning, with the same number of measurements (10 trials) for a particular single measurement. The temperature at the time of measurement was the same for all groups of mice. The tail cuff procedure accurately measures SBP but underestimates diastolic blood pressure. Also, changes in time of day; ambient conditions; operator handling of each animal; or subtle behavioral differences between groups, strains, or individual animals can introduce experimental variability to the measurement.
Laser Doppler Measurement of Hind-limb Blood Flow
Hind-limb blood flow measurements over the region of interest were performed before surgery, immediately after surgery, and serially over 2 weeks with a MoorLDI-Laser (Moor Instruments). The blood flow of right and left hind-limbs was assess by scanning the same lower abdomen and limbs of the mice with a laser Doppler blood flow meter as previously reported.[3,50]
X-Ray Quantification of Hind-limb Angiogenesis
Vessel density was assessed by high-definition microangiography at the end of the treatment period, as previously described.[3,25] Briefly, mice were anesthetized and contrast media (barium sulfate, 1 g/mL) was injected through a catheter introduced into the abdominal aorta. Vessel density quantification was determined using Multi Gauge – FUJIFILM.
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blot analysis was performed as previously described.[3,7,8,52] We used Western blot analysis to assess the phosphorylation and total levels of Akt, eNOS and VEGFR, in the ischemic and non-ischemic hind-limb, using specific antibodies anti-eNOS or anti-Akt or anti-VEGFR antibodies at the dilution (1:1000). The quantification of Western blot was determined using Fujifilm-Multi Gauge software.
Colorimetric Determination of Nitrite, Nitrate and cGMP
Nitrite, Nitrate and cGMP levels were measured in hind-limb muscle from all mice. The mice were sacrificed, the hind-limb muscles were immediately harvested and snap frozen in liquid nitrogen. The cGMP measurements were performed using a sandwich enzyme-linked immunosorbent assay (ELISA; Cayman Chemical, Ann Arbor, MI) according to the manufacturer instructions and as previously described.[48,43] Sham values were considered as 100%. All data from other groups were normalized to the sham values. For nitrite and nitrate measurements, we used chemiluminescence as previously described.[32]
Immunohistochemistry
Immunohistochemistry was performed as previously described.[3,7,52,6] Hind-limb tissues were stained with α-actin, von Willebrand factor (Cell signaling) antibodies, and secondary rabbit anti mouse coupled to Alexa Fluor (Molecular Probes). Cellular nuclei were counterstained with DAPI (Molecular Probes). After two weeks, mice were anesthetized and perfused with formalin for 45 min. Hind-limb muscles were then harvested, sectioned at 5 μm using a cryostat and mounted on slides for immunohistochemistry. We used primary antibodies: rabbit polyclonal anti-human factor VIII (DAKO) to detect endothelial cells; mouse monoclonal anti-smooth muscle actin (clone 1A4; Sigma) to detect smooth muscle cells. Goat anti-mouse and chicken anti-rabbit Alexa-Fluor 594 antibodies were used as secondary antibodies. The capillary density was then determined by counting the number of capillaries in each section of muscle.
Antibodies
Phosphorylated VEGF receptor 2 (Tyr951, dilution 1:1000), total VEGF receptor, total Akt, phosphorylated Akt (Ser476, dilution 1:1000), total eNOS, phosphorylated eNOS (Ser1177, dilution 1:1000), von Willebrand factor (dilution 1:1000) and α-smooth muscle actin (dilution 1:1000) were purchased from Sigma, Cell Signaling Technology and Promega.
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons between groups in term of systolic blood pressure, blood flow, vascular density, staining cumulative data, cGMP, nitrite and nitrate in the tissue and Western blot analysis data were analyzed by using posthoc Bonferroni t tests and Student’s t test with a minimum of P< 0.05 to reach significance. Also One-way or 2-way ANOVA was used to compare each parameter when there were 2 independent groups. Values of P<0.05 were considered significant.
RESULTS
Effects of Sodium Nitrite on Systolic Blood Pressure
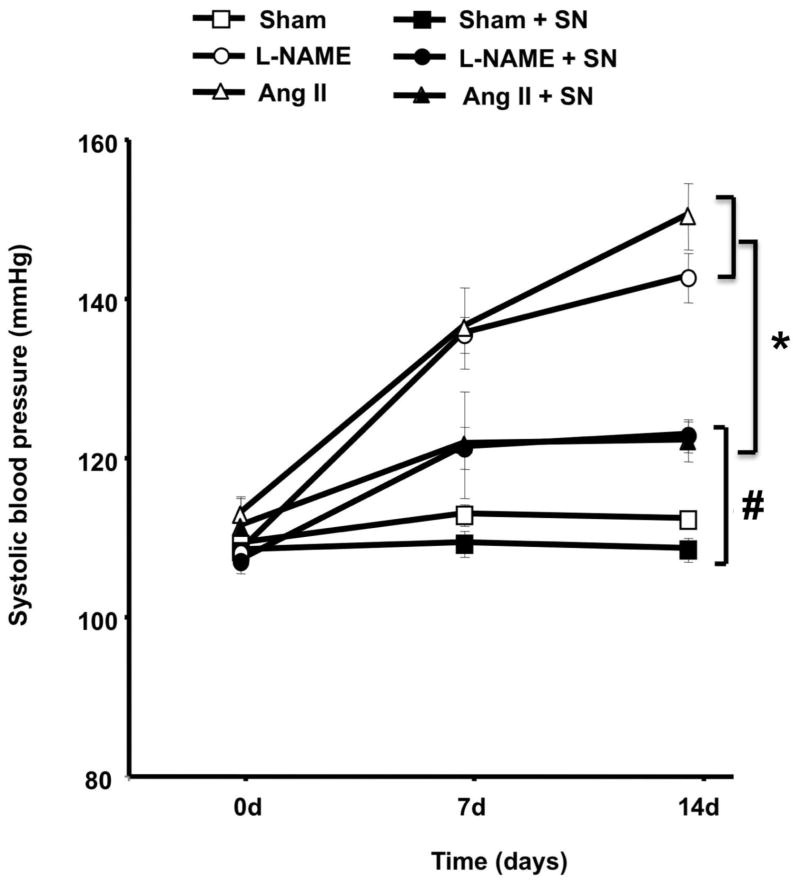
Systolic blood pressure was increased significantly in mice receiving angiotensin II (Ang II) infusion or L-NAME treatment compared to sham. Hypertensive mice treated with sodium nitrite (SN) had significantly reduced systolic blood pressure (Figure 1). The treatment of sham animal with SN did not alter systolic blood pressure (Figure 1).
Figure 1.
Effect of sodium nitrite (SN) treatment on blood pressure in normotensive (sham, n=10) and hypertensive mice induced by chronic infusion of angiotensin II (Ang II, n=10) or L-NAME treatment, n=10; *P < 0.05 for Ang II or L-NAME vs. Ang II + SN or L-NAME + SN; #P < 0.05 for Ang II and L-NAME vs. sham, sham + SN, L-NAME + SN, Ang II + SN; &P<0.05 for L-NAME + SN or Ang II + SN vs. sham with and without SN.
Effects of Sodium Nitrite on Blood Flow Recovery in Ischemic Hind-limb from Normotensive and Hypertensive Mice
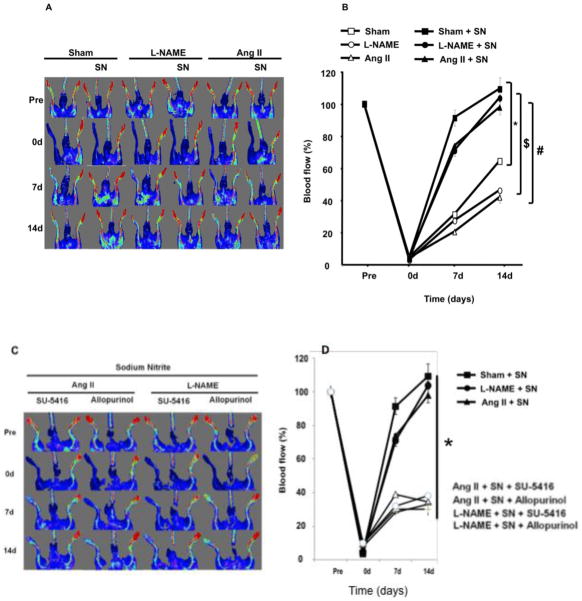
Blood flow was assessed by Doppler-flow before, just after surgery, and then once a week for two weeks in sham ± SN, L-NAME ± SN and Ang II ± SN mice (Fig. 2A&B). After femoral artery ligation, blood flow was significantly reduced to less than 2% of the value before ligation in all groups associated with a problem in hind-limb movement within the first two days. After two weeks, blood flow was significantly lower in the ischemic hind-limb from hypertensive mice compared to sham (Figure 2). Interestingly, as shown in Figure 2B, quantitative analysis of the laser Doppler imaging revealed a significant increase in blood flow recovery in the ischemic hind-limb from hypertensive and normotensive mice treated with SN in comparison with non treated mice.
Figure 2.
Blood flow analysis in the hind-limb of hypertensive (angiotensin II “Ang II” or L-NAME) and normotensive mice (sham) treated with or not treated with sodium nitrite (SN); 2A: Blood flow measured with MoorLDI-Laser in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN) before and just after surgery, and once a week for 2 weeks (this image is representative of n=10); 2B: Alternative strategy for measuring Blood flow with MoorLDI-Laser in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN) before and just after surgery, and once a week for 2 weeks; n=10; *P < 0.05 for sham + SN, Ang II + SN and L-NAME + SN vs. sham; $P < 0.05 statistically significant for sham + SN, Ang II + SN and L-NAME + SN vs. Ang II or L-NAME; #P<0.05 for sham + SN, L-NAME + SN, Ang II + SN vs. L-NAME or Ang II; 2C–D: Blood flow measurements in hypertensive mice treated with sodium nitrite and allopurinol (xanthine-oxido-reductase inhibitor, 100 mg/kg, i.p.) or SU-5416 (VEGF receptor antagonist, 50 mg/kg, i.p.).
The inhibition of xanthine-oxido-reductase (allopurinol, 100 mg/kg, i.p.) or the VEGFR (SU-5416, 50 mg/kg, i.p.) prevented recovery of flow in the ischemic hind-limb of hypertensive mice treated with sodium nitrite (Figure 2C, D).
Effects of Sodium Nitrite on Blood Vessel Density in Ischemic Hind-limb from Normotensive and Hypertensive Mice
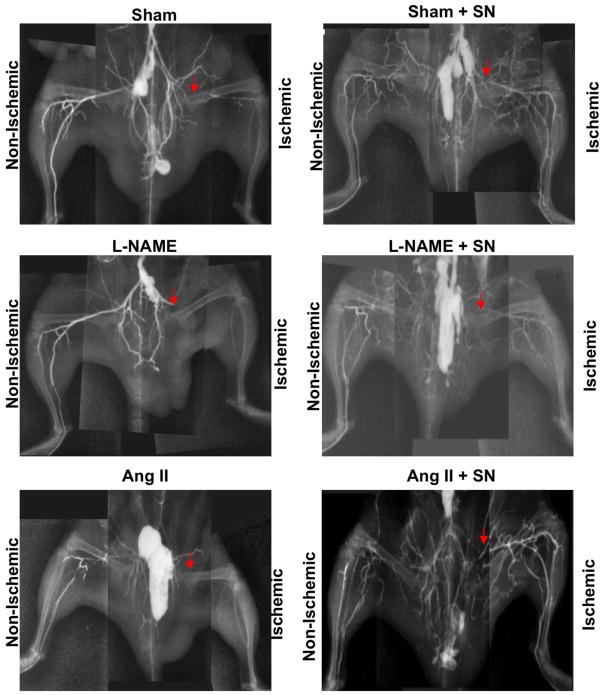
Vessel density was evaluated by high-definition microangiography at the end of the treatment period. We injected contrast media (barium sulfate, 1 g/ml) into the abdominal aorta, and angiogram from the right and left hind-limbs were then examined using digital X-ray (Fig. 3). After two weeks, vessel density in sham mice was significantly lower in the ischemic hind-limb compared to the non-ischemic hind-limb. However, in sham treated with SN, the ischemic hind-limb vessel density was not changed compared to non-ischemic hind-limb. In the group receiving L-NAME or Ang II, vessel density was reduced compared to sham (Figure 3). Mice treated with L-NAME or Ang II and then treated with SN displayed a similar pattern vessel density compared to sham mice (Figure 3).
Figure 3.
Microangiography determined at the end of the treatment period in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN). Contrast media (barium sulfate, 1 g/ml) was injected into the abdominal aorta, and then angiogram from of the right and left hind-limb was assessed with digital X-ray transducer acquired images. Images were then assembled to obtain a complete view of the hind-limb vasculature; image is representative of n=5.
Effects of Sodium Nitrite on Capillary Density in Ischemic Hind-limb from Normotensive and Hypertensive Mice
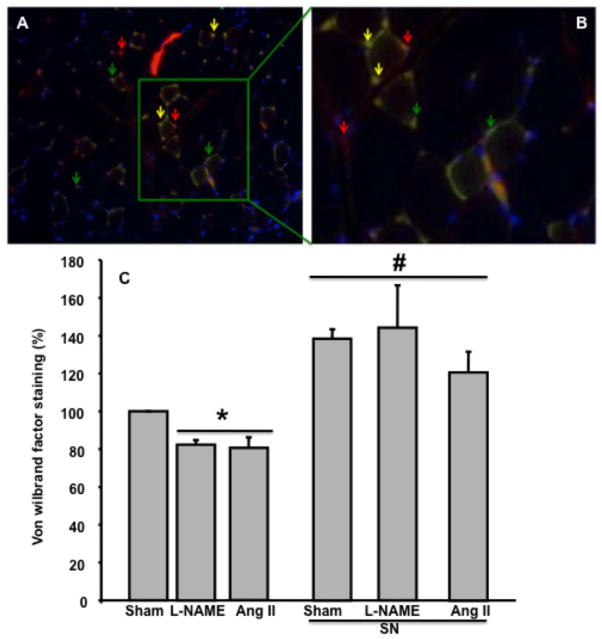
Blood flow and micro-angiographic data were confirmed by capillary density analysis using Von Willebrand factor staining at the end of the treatment period (Figure 4A,B). Data showed a significant reduction in Von Willebrand factor staining in hypertensive mice, which was rescued with SN treatment (Figure 4A,B). Capillary density was reduced in the ischemic hind-limb from hypertensive mice compared to sham (Figure 4C). Sham and hypertensive mice treated with SN displayed significant increases in capillary density compared to non-treated mice (Figure 4C).
Figure 4.
A: Example of overlapping immunostaining image for Von willebrand Factor (green arrow), α-actin (red arrow), Dapi (blue arrow), and over-lap between Von willebrand and α-actin (yellow arrow) in mouse hind-limb; B: Amplification of a portion from image A; C: Effect of sodium nitrite (SN) treatment on the capillary density in sham and hypertensive mice induced by chronic infusion of angiotensin II (Ang II) or L-NAME. Capillary density was assessed by von Willebrand immunostaining, n=5; *P < 0.05 for sham vs. L-NAME treatment and Ang II; #P < 0.05 for sham, L-NAME and Ang II treated with sodium nitrite vs. sham, L-NAME and Ang II.
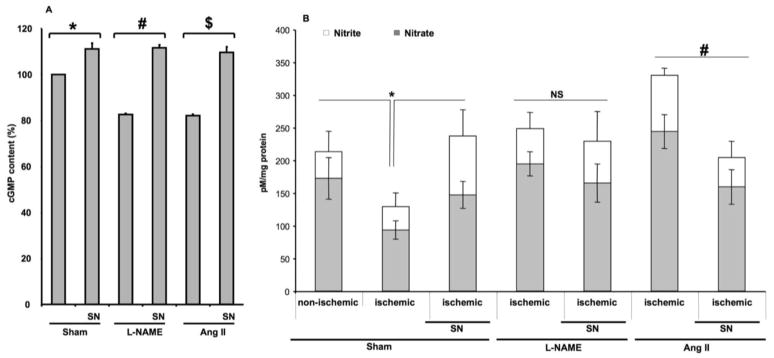
Effects of Sodium Nitrite on cGMP and Nitrite/Nitrate Content in Ischemic Hind-limb from Normotensive and Hypertensive Mice
Colorimetric determination of nitrite, nitrate and cGMP analysis in hind-limb ischemic muscle lysates was performed in all groups. The content of cGMP was decreased in ischemic hind-limb from mice receiving L-NAME or Ang II compared to sham. Mice treated with SN showed significantly increased cGMP content in sham and hypertensive mice (Figure 5A). The treatment with SN significantly enhanced nitrite and nitrate levels (marker of nitric oxide) in ischemic hind-limb from sham, while no effect was observed in mice treated with L-NAME (Figure 5B). In mice infused with Ang II, sodium nitrite treatment decreased nitrite and nitrate levels (Figure 5B).
Figure 5.
5A: Effect of sodium nitrite (SN) on cGMP level in ischemic hind-limb muscle from sham ± SN and hypertensive mice induced by chronic infusion of angiotensin II (Ang II) ± SN or L-NAME ± SN, n=5; *P < 0.05 for sham vs. sham + SN; #P < 0.05 for L-NAME vs. L-NAME + SN; $P < 0.05 for Ang II vs. Ang II + SN; 5B: Effect of sodium nitrite (SN) on nitrite (white square) and nitrate (grey square) levels in ischemic hind-limb from sham ± SN and hypertensive mice induced by chronic infusion of angiotensin II (Ang II) ± SN or L-NAME treatment ± SN, n=5; *P < 0.05 for ischemic sham vs. non-ischemic and ischemic + SN; #P < 0.05 for Ang II vs. Ang II + SN.
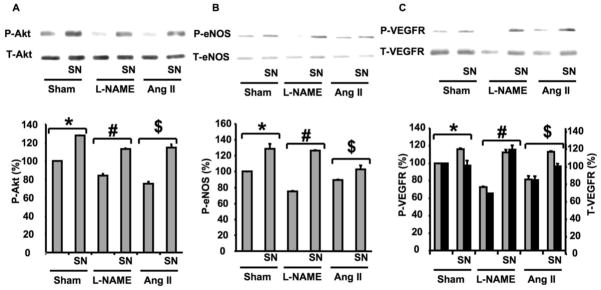
Effects of Sodium Nitrite on Akt, eNOS and VEGF Receptor Phosphorylation in Ischemic Hind-limb from Normotensive and Hypertensive Mice
Muscle lysates were immunoprecipitated with total anti-bodies for Akt, eNOS and VEGF receptor and then blotted with specific antibodies for phosphorylated Akt, eNOS and VEGF receptor phosphorylation. Akt and eNOS phosphorylation were significantly reduced in hypertensive mice (L-NAME and Ang II) compared to sham, while total Akt and total eNOS were similar in all groups (Figure 6A; B). In contrast, sham and hypertensive mice treated with SN displayed an increase in Akt and eNOS phosphorylation compared to non-treated mice (Figure 6A; B). Figure 6C illustrates in sham an increase in VEGF receptor phsophorylation in response to SN; while total VEGF receptor expression was similar. VEGF receptor phosphorylation and expression were down regulated in hypertensive animals; and treatment with SN normalized VEGF receptor phosphorylation and expression level (Figure 6C).
Figure 6.
Effect of sodium nitrite (SN) treatment on Akt/eNOS and VEGF receptor expression in ischemic hind-limb from sham and hypertensive mice. 6A: Immunoprecipitation, Western blots analysis and quantitative data showing phosphorylated and expression of Akt in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN), n=5; *P < 0.05 for sham vs. sham + SN; #P < 0.05 for L-NAME vs. L-NAME + SN; $P < 0.05 for Ang II or Ang II + SN vs. sham (total expression of Akt was similar in all groups); 6B: Immunoprecipitation, Western blots analysis and quantitative data showing phosphorylated and total expression of eNOS in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN), n=5; *P < 0.05 for sham vs. sham + SN; #P < 0.05 for L-NAME vs. L-NAME + SN; $P < 0.05 for Ang II or Ang II + SN vs. sham (total expression of eNOS was similar in all groups); 6C: Immunoprecipitation and Western blots analysis and quantitative data showing phosphorylated, expression of VEGF receptor (VEGFR) in all groups (sham ± SN, Ang II ± SN and L-NAME ± SN), n=5; *P < 0.05 for sham vs. sham + SN; #P < 0.05 for L-NAME vs. L-NAME + SN; $P < 0.05 for Ang II or Ang II + SN vs. sham.
Discussion
The present study provides evidence supporting the beneficial effect of sodium nitrite (SN) as a therapy to improve neovascularization and eventually blood flow recovery in the ischemic hind-limb of normotensive and hypertensive mice.
It is well established that nitric oxide (NO) is an important regulator of vascular homeostasis.[24,23,14,46] Nitric oxide is an autocrine and paracrine signaling molecule with a very short lifetime.[15] Importantly, NO can also be stabilized in blood and tissue by oxidation to nitrate and nitrite and can then be reduced back to NO under physiological and pathological conditions.[19] In most hypertensive animal models, the bioavailability of NO is markedly reduced mainly because of an increase in oxidative stress.[35,9]
Hypertension was induced by either chronic inhibition of endothelial NO-synthesis (eNOS) or infusion of Angiotensin II (Ang II).[18,20] The treatment of hypertensive mice with the SN significantly lowered systolic blood pressure, whereas no effect was observed in sham animals. Our data are in agreement with previous results showing that nitrite therapy reduced pulmonary hypertension and hypertension-induced by L-NAME.[58,34,27] These data strongly suggest the beneficial therapeutic role of nitrite in the reduction of hypertension.
Femoral artery ligation was performed in all groups of mice. After two weeks, angiogenesis and blood flow recoveries were greater in sham compared to hypertensive mice indicating an alteration in angiogenesis development. These data are in agreement with previous studies, showing that neovascularization was altered in hypertensive animal models.[22,33,56] Angiotensin II has a differential effect on angiogenesis. A non-hypertensive dose was shown to promote angiogenesis,[53,38] while hypertensive doses of Ang II reduce angiogenesis as reported in the present and previous studies.[56,36] These data indicate that the physiological effects of Ang II is to enhance angiogenesis whereas, the pathological effect is to reduce angiogenesis. Sham and hypertensive mice treated with SN displayed normalization in hind-limb vascularization associated with 100% blood flow recovery. It was reported that normotensive mice treated with SN had more rapid restoration of blood flow in the ischemic hind-limb.[32] These findings are similar to our results, suggesting that continuous nitrite therapy is an effective strategy for enhancing vascularization and eventually tissue perfusion. It has reported that aldosterone inhibits angiogenesis in response to ischemia.[31] Indeed in the present study infusion of Ang II and L-NAME both support increases in aldosterone levels.[42,47] The beneficial effect of sodium nitrite therapy could be through the inhibition of aldosterone secretion or through down stream signaling to aldosterone. Future studies are needed to demonstrate the relationship between sodium nitrite and aldosterone in terms of neovascularization in response to ischemia.
To extend our data on the effect of sodium nitrite therapy by its conversion into nitric oxide in our model, mice were treated with allopurinol, an inhibitor of xanthine-oxido-reductase that reduces nitrite to nitric oxide. Allopurinol did not alter systolic blood pressure in L-NAME and Ang II groups treated with sodium nitrite (data not shown). The inhibition of xanthine-oxido-reductase or VEGF receptor prevents neovascularization in hypertensive mice treated with sodium nitrite. These data indicate that the beneficial effect of sodium nitrite is to enhance neovascularization in response to ischemia is mediated by xanthine-oxido-reductase and VEGF receptor. We do not have direct evidence to show reduced nitrite-nitric oxide formation in the tissues, but indirect evidence is supported by the attenuation in ischemia-induced neovascularization in the presence of allopurinol.
The occurrence of vascular rarefaction has been well recognized in hypertensive patients and animal models.[44,45,4] Our data suggest that ischemic hind-limb from mice treated with L-NAME or Ang II display a significant reduction in capillary density compared to sham. Sham and hypertensive mice treated with SN show a significant increase in capillary density, indicating that SN is a potent promoter of vascular growth.
It is well established that NO acts through two distinctive signaling pathways, either modulating protein function by S-nitrosation or via binding to the heme of guanylate cyclase, the principal NO receptor. Previous studies show that NO is critical in the process of vascularization.[39,16] In the present study, we demonstrated that cGMP, the downstream signaling mediator of NO, was reduced in hypertensive mice compared to sham suggesting that the NO pathway is impaired in hypertension, as previously reported.[9,41] The sham and hypertensive mice treated with SN had significantly enhanced cGMP levels in the ischemic hind-limb. These data are not in agreement with previous studies, showing no change in cGMP levels in mice treated with nitrite.[32,10]
We used another strategy to determine that increased NO was present in the ischemic hind-limb of mice treated with SN. We measured nitrite and nitrate content in ischemic hind-limb muscle from all mice. These results revealed a significant increase in nitrite and nitrate content in sham mice treated with sodium nitrite. Surprisingly, ischemic hind-limb muscle from mice infused with L-NAME did not show an effect, while Ang II group had a high level of nitrite and nitrate content compared to sham and the group of mice treated with L-NAME. In addition, Ang II-induced hypertensive mice treated with SN displayed a reduced level of nitrite and nitrate content compared to Ang II alone. Therefore, it is important to determine in the future whether Ang II and L-NAME could increase nitrite and nitrate content through the induction of iNOS as previously reported.[1]
To emphasize the role of the NO pathway, we measured the phosphorylation of Akt, eNOS and the VEGF receptor. Western blot analysis and immunoprecipitation studies revealed that Akt, eNOS and VEGR phosphorylation were significantly reduced in ischemic hind-limb from hypertensive mice compared to sham. These data are in agreement with previous studies and suggest that Akt/eNOS/VEGF receptor phosphorylation pathway is impaired in hypertension.[55,28] Hypertension has a differential effect on Akt/eNOS and VEGR expression. Our data show that Akt and eNOS expression were similar in all groups, while VEGFR expression was significantly reduced in hypertensive mice. Sham and hypertensive mice treated with SN had restored expression and phosphorylation of Akt/eNOS and VEGFR. These results are with agreement in the concept that Akt/eNOS and VEGFR are important factors in angiogenesis.[5,17]
In this study, we demonstrated that sodium nitrite treatment accelerates angiogenesis and eventually blood flow recovery in the ischemic hind-limb from normotensive and hypertensive mice, by modulating the eNOS, Akt and VEGF receptor pathways. These data provide a new insight in the knowledge of the therapeutic effect of sodium nitrite in the induction of angiogenesis in ischemic tissue, in hypertension.
Clinical Perspective
Arterial hypertension is a progressive disease associated with endothelial dysfunction, and is a major risk factor for coronary artery disease and subsequent myocardial infarction. Thirty five to fifty five percent of patients with peripheral artery disease are hypertensive. It has been shown that chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis in normotensive mice. In the present study, we demonstrated that hypertensive mice treated with sodium nitrite have significantly reduced systolic blood pressure and accelerates angiogenesis and blood flow recovery, probably mediated through VEGF/eNOS/Akt and cGMP pathway. Our data suggest that sodium nitrite could provide novel treatment for arterial hypertension and ischemic diseases by enhancing neovascularization.
Acknowledgments
Sources of Funding
We acknowledge grant support from National Institutes of Health (HL095566 - Dr. Matrougui; P20RR017659 - Dr. Navar; HL26371; HL080482 – Dr. Kevil; HL097111 – Dr. Trebak).
Footnotes
Disclosures
None.
References
- 1.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. 01.RES.0000147365.86159.f5 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357 (Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 90(7):985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33 (4):998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 5.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995;73 (7):333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- 6.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WM. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29(6):802–808. doi: 10.1161/ATVBAHA.109.186189. ATVBAHA.109.186189 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57 (6):1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008;295 (1):H69–76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci. 2009;14:1–18. doi: 10.2741/3228. 3228 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Cauwels A, Buys ES, Thoonen R, Geary L, Delanghe J, Shiva S, Brouckaert P. Nitrite protects against morbidity and mortality associated with TNF- or LPS-induced shock in a soluble guanylate cyclase-dependent manner. J Exp Med. 2009;206(13):2915–2924. doi: 10.1084/jem.20091236. jem.20091236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin SY, Pandey KN, Shi SJ, Kobori H, Moreno C, Navar LG. Increased activity and expression of Ca(2+)-dependent NOS in renal cortex of ANG II-infused hypertensive rats. Am J Physiol. 1999;277 (5 Pt 2):F797–804. doi: 10.1152/ajprenal.1999.277.5.F797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105 (18):2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 13.Davies CA, Rocks SA, O’Shaughnessy MC, Perrett D, Winyard PG. Analysis of nitrite and nitrate in the study of inflammation. Methods Mol Biol. 2003;225:305–320. doi: 10.1385/1-59259-374-7:305. 1-59259-374-7-305 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–911. doi: 10.1111/j.1440-1681.2007.04638.x. CEP4638 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A. 2008;105(32):11430–11435. doi: 10.1073/pnas.0800700105. 0800700105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98(5):2604–2609. doi: 10.1073/pnas.041359198 98/5/2604 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner SM, Kemp PA, Bennett T, Palmer RM, Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquarters vasoconstriction in Brattleboro rats. Eur J Pharmacol. 1992;213 (3):449–451. doi: 10.1016/0014-2999(92)90636-i. [DOI] [PubMed] [Google Scholar]
- 19.Gigante B, Morlino G, Gentile MT, Persico MG, De Falco S. Plgf−/−eNos−/− mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia. FASEB J. 2006;20(7):970–972. doi: 10.1096/fj.05-4481fje. fj.05-4481fje [pii] [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298(1):F150–157. doi: 10.1152/ajprenal.00477.2009. 00477.2009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53(2):351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. HYPERTENSIONAHA.108.124511 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humar R, Zimmerli L, Battegay E. Angiogenesis and hypertension: an update. J Hum Hypertens. 2009;23(12):773–782. doi: 10.1038/jhh.2009.63. jhh200963 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Ignarro LJ. Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep. 1999;19 (2):51–71. doi: 10.1023/a:1020150124721. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84 (24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110(16):2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. 01.CIR.0000145142.85645.EA [pii] [DOI] [PubMed] [Google Scholar]
- 26.Jensen FB. The role of nitrite in nitric oxide homeostasis: a comparative perspective. Biochim Biophys Acta. 2009;1787(7):841–848. doi: 10.1016/j.bbabio.2009.02.010. S0005-2728(09)00058-9 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Kanematsu Y, Yamaguchi K, Ohnishi H, Motobayashi Y, Ishizawa K, Izawa Y, Kawazoe K, Kondo S, Kagami S, Tomita S, Tsuchiya K, Tamaki T. Dietary doses of nitrite restore circulating nitric oxide level and improve renal injury in L-NAME-induced hypertensive rats. Am J Physiol Renal Physiol. 2008;295 (5):F1457–1462. doi: 10.1152/ajprenal.00621.2007. [DOI] [PubMed] [Google Scholar]
- 28.Kawahara S, Umemoto S, Tanaka M, Umeji K, Matsuda S, Kubo M, Matsuzaki M. Up-regulation of Akt and eNOS induces vascular smooth muscle cell differentiation in hypertension in vivo. J Cardiovasc Pharmacol. 2005;45(4):367–374. doi: 10.1097/01.fjc.0000157454.60939.43. 00005344-200504000-00015 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Kiefer FN, Misteli H, Kalak N, Tschudin K, Fingerle J, Van der Kooij M, Stumm M, Sumanovski LT, Sieber CC, Battegay EJ. Inhibition of NO biosynthesis, but not elevated blood pressure, reduces angiogenesis in rat models of secondary hypertension. Blood Press. 2002;11 (2):116–124. doi: 10.1080/08037050211256. [DOI] [PubMed] [Google Scholar]
- 30.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50(1):49–59. 035001049. [PubMed] [Google Scholar]
- 31.Kobayashi N, Fukushima H, Takeshima H, Koguchi W, Mamada Y, Hirata H, Machida Y, Suzuki N, Yokotsuka F, Tabei K, Kobayashi E, Fukuda N, Ishimitsu T. Effect of eplerenone on endothelial progenitor cells and oxidative stress in ischemic hindlimb. Am J Hypertens. 2010;23(9):1007–1013. doi: 10.1038/ajh.2010.91. ajh201091 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Jr, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105(21):7540–7545. doi: 10.1073/pnas.0711480105. 0711480105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurosaka M, Suzuki T, Hosono K, Kamata Y, Fukamizu A, Kitasato H, Fujita Y, Majima M. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomed Pharmacother. 2009;63(9):627–634. doi: 10.1016/j.biopha.2009.01.001. S0753-3322(09)00015-8 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Lara AR, Erzurum SC. A urinary test for pulmonary arterial hypertension? Am J Respir Crit Care Med. 2005;172(3):262–263. doi: 10.1164/rccm.2505009. 172/3/262 [pii] [DOI] [PubMed] [Google Scholar]
- 35.le Noble FA, Stassen FR, Hacking WJ, Struijker Boudier HA. Angiogenesis and hypertension. J Hypertens. 1998;16 (11):1563–1572. doi: 10.1097/00004872-199816110-00001. [DOI] [PubMed] [Google Scholar]
- 36.Lee JS, Kim JH. The role of activated hepatic stellate cells in liver fibrosis, portal hypertension and cancer angiogenesis. Korean J Hepatol. 2007;13(3):309–319. doi: 10.3350/kjhep.2007.13.3.309. 200709309 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Lee PC, Salyapongse AN, Bragdon GA, Shears LL, 2nd, Watkins SC, Edington HD, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277 (4 Pt 2):H1600–1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Kondo T, Numaguchi Y, Kobayashi K, Aoki M, Inoue N, Okumura K, Murohara T. Role of bradykinin, nitric oxide, and angiotensin II type 2 receptor in imidapril-induced angiogenesis. Hypertension. 2008;51(2):252–258. doi: 10.1161/HYPERTENSIONAHA.107.097394. HYPERTENSIONAHA.107.097394 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25(5):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. 01.ATV.0000161048.72004.c2 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. nrd2466 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Luscher TF, Bock HA. The endothelial L-arginine/nitric oxide pathway and the renal circulation. Klin Wochenschr. 1991;69 (13):603–609. doi: 10.1007/BF01649323. [DOI] [PubMed] [Google Scholar]
- 42.Muldowney JA, 3rd, Davis SN, Vaughan DE, Brown NJ. NO synthase inhibition increases aldosterone in humans. Hypertension. 2004;44(5):739–745. doi: 10.1161/01.HYP.0000143852.48258.f1 01.HYP.0000143852.48258.f1 [pii]. [DOI] [PubMed] [Google Scholar]
- 43.Ng WH, Moochhala S, Yeo TT, Ong PL, Ng PY. Nitric oxide and subarachnoid hemorrhage: elevated level in cerebrospinal fluid and their implications. Neurosurgery. 2001;49(3):622–626. doi: 10.1097/00006123-200109000-00016. discussion 626–627. [DOI] [PubMed] [Google Scholar]
- 44.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99(8):1873–1879. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paiardi S, Rodella LF, De Ciuceis C, Porteri E, Boari GE, Rezzani R, Rizzardi N, Platto C, Tiberio GA, Giulini SM, Rizzoni D, Agabiti-Rosei E. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin Hemorheol Microcirc. 2009;42 (4):259–268. doi: 10.3233/CH-2009-1195. [DOI] [PubMed] [Google Scholar]
- 46.Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertens. 2008;17(1):70–75. doi: 10.1097/MNH.0b013e3282f34b02 00041552-200801000-00012 [pii]. [DOI] [PubMed] [Google Scholar]
- 47.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141 (10):3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. 0167-4889(93)90006-B [pii] [DOI] [PubMed] [Google Scholar]
- 49.Senthilkumar A, Smith RD, Khitha J, Arora N, Veerareddy S, Langston W, Chidlow JH, Jr, Barlow SC, Teng X, Patel RP, Lefer DJ, Kevil CG. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27 (9):1947–1954. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 50.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113 (1):118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 51.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296 (5):H1281–1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 52.Su J, Lucchesi PA, Gonzalez-Villalobos RA, Palen DI, Rezk BM, Suzuki Y, Boulares HA, Matrougui K. Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28 (8):1432–1438. doi: 10.1161/ATVBAHA.108.167205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamarat R, Silvestre JS, Kubis N, Benessiano J, Duriez M, deGasparo M, Henrion D, Levy BI. Endothelial nitric oxide synthase lies downstream from angiotensin II-induced angiogenesis in ischemic hindlimb. Hypertension. 2002;39 (3):830–835. doi: 10.1161/hy0302.104671. [DOI] [PubMed] [Google Scholar]
- 54.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18(2):570–580. doi: 10.1681/ASN.2006050450. ASN.2006050450 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Suzuki J, Yamawaki H, Sharma VK, Sheu SS, Berk BC. Losartan metabolite EXP3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor receptor-2 in endothelial cells: angiotensin II type 1 receptor-independent effects of EXP3179. Circulation. 2005;112(12):1798–1805. doi: 10.1161/CIRCULATIONAHA.104.509760. 112/12/1798 [pii] [DOI] [PubMed] [Google Scholar]
- 56.You D, Cochain C, Loinard C, Vilar J, Mees B, Duriez M, Levy BI, Silvestre JS. Hypertension impairs postnatal vasculogenesis: role of antihypertensive agents. Hypertension. 2008;51 (6):1537–1544. doi: 10.1161/HYPERTENSIONAHA.107.109066. [DOI] [PubMed] [Google Scholar]
- 57.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92 (3):308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 58.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. CIRCULATIONAHA.109.891077 [pii] [DOI] [PubMed] [Google Scholar]