Abstract
To avoid high systemic doses, strategies involving antigen-specific delivery of cytokine via linked antibodies or antibody fragments have been employed. Targeting cancer-associated peptides presented by MHC molecules (pepMHC) increases the number of potential target antigens, and takes advantage of cross-presentation on tumor stroma and in draining lymph nodes. Here, we use a soluble, high-affinity single-chain T cell receptor Vα-Vβ (scTv), to deliver cytokines to intracellular tumor-associated antigens presented as pepMHC. Since typical wild-type TCRs exhibit low affinity (Kd =1–100μM or more), we used an engineered TCR, m33, that binds SIY/Kb with nanomolar affinity (Kd =30nM). We generated constructs consisting of m33 scTv fused to murine IL-2, IL-15, or IL-15/IL-15Rα (IL-15 linked to IL-15Rα sushi domain, called “superfusion”). The fusions were purified with good yields, and bound specifically to SIY/Kb with high affinity. Proper cytokine folding and binding were confirmed, and the fusions were capable of stimulating proliferation of cytokine-dependent cells, both when added directly and when presented in trans, bound to cells with the target pepMHC. The m33 superfusion was particularly potent and stable, and represents a promising design for targeted anti-tumor immunomodulation.
Keywords: high affinity TCR, TCR-cytokine fusion, immunokine, IL-2, IL-15/IL-15Ralpha
Introduction
Cancer is caused by mutations that result in inadequately restrained proliferation. The cellular immune system is thought to be involved in identifying and eliminating such cells that present either mutated-self antigens, or other proteins that are not mutated but upregulated aberrantly. In line with these processes, many tumor-associated antigens have been identified as peptides that are bound to class I or class II products of the major histocompatibility complex (MHC). The complexes between peptide and MHC class I or II molecules (pepMHC) serve as the ligands for T cells (reviewed in 1–4).
Although T cells specific for tumor-associated pepMHC are often present in cancer patients, those T cells fail to eliminate the tumor. As many of the characterized tumor-associated antigens are simply overexpressed self antigens, the T cell repertoire that survives negative selection (tolerance) in the thymus may consist of T cells with low functional avidity, or T cells that have been specifically anergized by peripheral tolerance mechanisms. Accordingly, a major hope of tumor immunotherapy is to overcome anergy or tolerance of the cellular immune response without becoming destructive to vital tissues and organs.
One strategy to enhance the activity of T cells against tumors involves treatment with immunostimulatory cytokines, such as IL-2 and IL-15. IL-2 and IL-15 stimulate activation and proliferation of T cells and NK cells by binding to cell surface receptors made up of unique alpha chains (IL-2Rα and IL-15Rα, respectively) combined with shared beta and gamma chains (IL-2/IL-15Rβ and γc, reviewed in 5). Recombinant IL-2 has been used to enhance cellular immunity in humans,6 but treatment strategies involving systemic IL-2 at the high doses required for beneficial anti-cancer effects have been plagued by severe toxicity.7 Furthermore, IL-2 promotes activation-induced cell death (AICD) of T cell clones,8 and it is required for the maintenance of regulatory T cells (Treg,9), a property that could be counter-productive in the generation of a robust response to cancer. For these reasons, IL-15 has potential advantages10, 11 in that it promotes survival of T lymphocytes 5, and it can reverse tolerance of CD8+ T cells.12 Although IL-15 has been shown to be considerably less toxic than IL-2 in murine models of vascular leak syndrome,11 IL-15 also requires high doses for effectiveness in vivo.13, 14 Interestingly, mutant forms of IL-2 engineered for increased binding affinity to IL-2Rα have been generated, and were characterized to behave more similarly to IL-15 in terms of T cell stimulation properties.15
IL-15 has been shown to be highly active in a “trans” presentation mode, where IL-15Rα on the surface of a cell can bind and present IL-15 for binding to another cell that expresses the receptor complex, IL-15/IL-2Rβ and the common γc chain.16 Several studies have also shown that soluble IL-15Rα can stabilize IL-15, thereby enhancing its biological activity through binding to the IL-15Rβ/γc.17–21 Surface presentation of IL-15 on the surface of cytokine-secreting, IL-15Rα+ tumors leads to potent anti-tumor NK cell responses and complete rejection of the tumors in B cell- and T cell-negative mice.22 Similarly, IL-15 fused to the truncated, N-terminal sushi domain of IL-15Rα was able to drive NK cell proliferation and reduce tumor metastases in an animal model.23–26 The affinity of IL-15 and IL-15Rα sushi domain is high (Kd~10−9 M 26), and this non-covalent interaction has been exploited to create soluble dimeric and heterodimeric targeting agents by fusion of the cytokine and the sushi domain separately to binding proteins.27 Previous studies have shown evidence that the inclusion of the IL-15Rα domain provides enhanced activity for IL-15.26, 28 The hyperagonist IL-15/IL-15Rα fusion given either as a soluble protein23, 24 or as an adenoviral gene therapy agent29 given 1 or 3 days after i.v. injection of cancer cells has been shown to reduce the number of metastases and prolong survival in mice.
As many therapeutic agents have toxic side effects on non-cancerous tissues, “targeted therapies” that aim at shuttling an agent with greater selectivity to the cancer cells are in development. The leading examples involve monoclonal antibodies that recognize tumor cell surface antigens as a strategy to deliver chemotherapeutic agents, toxins, or radioisotopes directly to the site of tumors.30–32 Inspired by the past experience with IL-2 therapy, anti-tumor monoclonal antibody fragments have been fused to IL-2. Notably, IL-2 fused to a scFv of the L19 antibody (which binds the EDB domain of fibronectin)33 has entered clinical trials as a therapeutic, and early clinical data have been promising.34 Antibody- or scFv-targeted versions of other immunostimulatory cytokines have also been constructed, including IL-15, IL-12, GM-CSF, TNF-α, and IFN-α.35–38 Of note, IL-15 fused to the scFv L19 showed significant specific anti-cancer properties in murine subcutaneous and metastatic tumor models.36 In addition, IL-15 linked to the IL-15Rα sushi domain has been fused to scFv36 directed toward fibroblast activation protein (FAP); treatment with this construct resulted in more efficient control of murine lung metastases than untargeted IL-15 and IL-15Rα sushi domain, or scFv36 fused to IL-15 alone.39
However, while recent evidence suggests that some T cells can be activated at the site of a tumor,40 it is clear that most anti-tumor T cells are instead first activated in the draining lymph nodes, where potential cancer antigens are carried through the antigen presentation system, primarily dendritic cells.41–44 In the lymph node, antigens are presented to T cells as peptides bound to products of the major histocompatibility complex (MHCs). It would therefore be useful if the pro-inflammatory cytokine IL-15 could be directly delivered to both the tumor and the draining lymph nodes. To accomplish this, a soluble T cell receptor (TCR) with specificity for a tumor pepMHC antigen could be used together with the soluble cytokine. This strategy can take advantage of the many identified tumor-associated antigens that have been identified as pepMHC complexes on tumor cells. These epitopes, which are often associated with changes related to immortalization, may also be cross-presented on tumor stroma, which is vital for tumor support and survival.43, 45, 46
A major limitation of the TCR-based approach is that the affinities of wild-type TCR are low (Kd =1–500 μM, reviewed in 47), and the affinities of anti-tumor antigen TCRs have been reported to be even lower.48 In fact, some naturally occurring TCR:pepMHC interactions are so weak as to not be measurable by standard methods.49 These affinities may be too low to effectively direct a soluble therapeutic, although enhanced activity in reducing human lung metastases in nude mice was seen by when IL-2 was fused to a soluble, murine three-domain single-chain TCR (VαVβCβ) raised against a human tumor-associated pepMHC (p53/A2).50, 51 The same three-domain TCR has recently been fused to IL-15 and, separately, the IL-15Rα sushi domain, which enhanced pepMHC binding by relying on the IL-15/IL-15Rα interaction to give a bivalent soluble TCR-containing construct.27 However, strategies that use wild-type TCRs (i.e. that do not involve in vitro engineering steps for affinity maturation) are unlikely to yield the types of affinities that have shown success in antibody-antigen systems as cancer therapeutics. It has been shown to be advantageous to engineer interactions for improved affinity beyond antibody:antigens, including cytokines and their receptors, or TCRs and their pepMHC ligands.52, 53
Here, we generated the first soluble cytokine fusions with a high-affinity single-chain TCR (Vα-linker-Vβ, hereafter referred to as scTv, by analogy with scFv of antibodies54). We used a high-affinity mutant of the 2C TCR (Kd for SIY/Kb = 30 μM) called m33 that has a 1000-fold higher affinity for the model antigen SIY/Kb (Kd = 30 nM55, 56). The stabilized scTv m33 is analogous to an antibody-based scFv in size and affinity, but by recognizing a pepMHC antigen is able to target intracellular antigens such as those displayed on tumor cells or cross-presenting tumor stroma. We generated fusions of the high-affinity scTv to IL-2 (IL-2:m33), IL-15 (m33:IL-15), and an IL-15 hyperagonist construct including the sushi domain of IL-15Rα (m33 superfusion). The fusion proteins were expressed and purified in good yield, and exhibited proper binding specificities and affinities. The fusions were also capable of mediating immunostimulatory function (proliferation) both directly and in trans. The m33 superfusion that consisted of a stabilized, high-affinity scTv linked to IL-15 and the IL-15Rα sushi domain, exhibited proliferation-stimulatory activity even higher than soluble IL-15 cytokine alone.
Materials and Methods
Peptides, Antibodies, and Reagents
SIY and OVA peptides were synthesized by standard F-moc chemistry at the Macromolecular Core Facility at Pennsylvaina State University College of Medicine (Hershey, PA) and purified by reverse-phase HPLC. Rat anti-murine IL-2 and biotinylated rat anti-murine IL-2 detecting a separate epitope were purchased from BD Pharmingen (San Diego, CA). Biotinylated polyclonal rabbit anti-murine IL-15 antibody, Mouse IL-15 ELISA kit, and carrier-free murine IL-2 and IL-15 were purchased from eBiosciences (San Diego, CA). Polyclonal goat anti-murine IL-15Rα was purchased from R&D Systems (Minneapolis, MN). Biotinylated polyclonal rabbit anti-goat was purchased from AbCam (Cambridge, MA). The pDisplay mammalian expression vector and streptavidin linked to Alexa 647 were purchased from Invitrogen (Carlsbad, CA). Tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) and detergent were purchased from ATCC (Manassas, VA). Vent DNA polymerase, BglII and NotI restriction enzymes, Calf Intestine Alkaline Phosphatase, T4 DNA ligase, and PNGase F were purchased from New England Biolabs (Ipswich, MA).
Cell lines
MC57 fibrosarcoma cells and B16 melanoma cells (from Hans Schreiber’s lab at the University of Chicago), CTLL-2 cytokine-dependent murine T cell line (from ATCC, Manassas, VA), and T2-Kb TAP-deficient cells were cultured in complete RPMI media, consisting of RPMI 1640 supplemented with HEPES, pH 7.0, L-glutamine, penicillin, streptomycin, β-mercaptoethanol, and 10% fetal bovine serum. For CTLL-2, the media was additionally supplemented with 10% TStim (culture supernatant from rat T cells stimulated with con A) from BD Biosciences (San Jose, CA). HEK-293F cells were cultured at 37°C with 5% CO2 in serum-free Freestyle 293 media (both from Invitrogen, Carlsbad, CA).
Construction of gene fusions of scTv m33 and immunostimulatory cytokines
The gene for scTv m33,56 Vα-linker-Vβ, where the linker sequence is (GGGGS)4, was amplified by PCR from the pET22b E. coli expression vector, including stabilizing mutations.57 The cDNA for murine IL-2 and IL-15 were purchased from Open Biosystems (Thermo Scientific, Huntsville, AL). A codon-optimized sequence for the murine IL-15Rα sushi domain (amino acids 34–103 of Isoform 1, UniProt accession #Q60819), a linker with the sequence GG(SGG)6, and murine IL-15 was purchased from GenScript (Piscataway, NJ). The scTv m33 was linked to each cytokine construct using splicing by overlap extension (SOE PCR, 58), using a unique Gly-Ser linker (GGGSGGGGSGSGGGSGGGGS) as the overlap. Each gene fusion was amplified with a BglII restriction site at the 5′ end, and a His6 tag for purification, two stop codons, and a NotI restriction site at the 3′ end, and then cloned into pDisplay (Invitrogen, Carlsbad, CA) using BglII and NotI, noting that use of these sites removes the c-myc epitope and the platelet derived growth factor receptor (PDGFR) transmembrane domain included in the vector, allowing for soluble expression. The pDisplay vector includes G418 resistance for selection in mammalian cells, and includes the human CMV promoter for constitutive expression, the murine Ig-κ leader sequence for targeting to the secretory pathway, and an N-terminal HA epitope tag present in the mature protein.
Production and purification of m33:cytokine fusions
HEK-293F cells were transfected with pDisplay plasmid containing each scTv:cytokine fusion, respectively, using 293-Fectin (Invitrogen, Carlsbad, CA) per manufacturer’s instructions. Selection was carried out in Freestyle-293 media containing 250 μg/mL G418 antibiotic (Mediatech, Manassas, VA). Transfected cells were expanded to 300 mL cultures in 1 L baffled flasks shaking at 120 rpm at 37°C with 5% CO2 and allowed to grow for 4–10 days, depending on the optimal expression time for each construct. After the expression period, the cultures were spun to remove the cells, and the supernatant was collected and incubated stirring at 4°C overnight with Ni-NTA beads. The beads were collected on a sintered glass funnel, washed in phosphate-buffered saline (PBS), pH 7.4, and protein was eluted with 0.5M imidazole in PBS. The eluted protein was purified by size exclusion chromatography using a Superdex 200 10/300 column (GE Healthcare Life Sciences, Piscataway, NJ). Purified protein concentration was estimated by UV absorbance at 280 nM (molar extinction coefficients estimated from primary sequence as 48,165 for IL-2:m33, 51,270 for IL-15:m33 and m33:IL-15, and 60,000 for the m33 superfusion; absorbance measured using a Cary 50 Bio UV-Vis Spectrophotometer, Varian, Palo Alto, CA). Proteins were analyzed by SDS-PAGE, and small test injections of purified fusion constructs and carrier-free cytokines over the Superdex 200 column were compared to size standards from Bio-Rad (Hercules, CA). To evaluate the size of the purified protein without glycosylation, m33:IL-15 was incubated with PNGase F according to manufacturer’s instructions and analyzed by SDS-PAGE.
Production and purification of SIY/Kb streptavidin tetramers
H2-Kb soluble heavy chain containing a C-terminal biotinylation signal peptide and human β2-microglobulin light chain were expressed as inclusion bodies in Escherichia coli. Kb was biotinylated in vivo by co-induction of biotin ligase in the same cells, resulting in a biotin tag at the C-terminus of the heavy chain as described.46 Both chains were solubilized in urea, and refolded together in vitro in the presence of excess SIY peptide. Folded complexes in 20mM tris, pH 8.0 were purified by anion exchange chromatography using HiTrap Q columns (GE Healthcare, Piscataway, NJ). MHC complexes were incorporated into fluorescent tetramers for staining and dissociation experiments by adding streptavidin-phycoerythrin (BD Pharmingen, San Diego, CA) stepwise to the purified, biotinylated MHCs to a final 1:4 molar ratio.
Detection of folded cytokine by ELISA
HEK-293F cells alone or transfected with a scTv:cytokine fusion gene were maintained in culture for 2–10 days, and the supernatants were harvested for analysis by cytokine ELISA, either murine IL-2 (BD Pharmingen, San Diego, CA) or murine IL-15 (eBiosciences, San Diego, CA), as per the manufacturer’s instructions. Briefly, the capture antibody was diluted in PBS and incubated in the wells of Immulon 2HB plates (Thermo Fisher Scientific, Pittsburgh, PA) overnight at 4°C. The wells were blocked with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBS, then washed with PBST (0.1% Tween-20 in PBS), and incubated with the cell supernatants or cytokine standards. The plate was washed three times with PBST, then incubated with a biotinylated detection antibody in dilution solution (0.3% BSA, 0.1% Tween-20 in PBS). Next, the plate was washed three times with PBST, and incubated with avidin or streptavidin linked to horseradish peroxidase. Finally, the plate was washed three times with PBST and developed with TMB substrate (KPL, Gaithersburg, MD). Development was stopped by adding an equal volume of 1N H2SO4 per well as TMB substrate solution. Absorbance at 450 nm in each well was read using an ELx800 universal microplate reader (Bio-Tek Instruments, Winooski, VT).
Binding and detection of m33:cytokine fusion by flow cytometry
For detection of peptide-specific binding of the scTv portion of the fusion molecules, T2-Kb cells were loaded with 1 μM SIY or OVA peptide in unsupplemented RPMI 1640 for two hours at 37°C. The cells were washed into FACS buffer (1% bovine serum albumin and 0.02% sodium azide in PBS), and stained at 4°C with or without 1 μM purified fusion protein. The cells were washed, and the cytokine portion was detected as follows: biotinylated anti-IL-15 followed by streptavidin-phycoerythrin for m33:IL-15; biotinylated anti-IL-2 followed by streptavidin-phycoerythrin for IL-2:m33; and goat anti-IL-15Rα followed by biotinylated rabbit anti-goat and streptavidin-Alexa 647 for m33 superfusion. All stains were done at 4°C, and cells were washed with FACS buffer between each step and before analysis.
For detection of cytokine binding to cytokine receptors, MC57 or CTLL-2 cells were incubated at 4°C with varying concentrations of purified fusion protein in FACS buffer. The cells were washed in FACS buffer, and then were stained with 30 nM SIY/Kb streptavidin-phycoerythrin tetramer at 4°C. The cells were washed with FACS buffer before analysis.
Flow cytometry data were collected on an Accuri C6 (Accuri Cytometry Inc., Ann Arbor, MI) or a FACS Canto (BD Biosciences, San Jose, CA), and data was analyzed using FCS Express (De Novo Software, Los Angeles, CA).
CTLL-2 proliferation assays
CTLL-2 cells were rested overnight at 37 °C, 5% CO2 in complete RPMI media with no added cytokines. The cells were washed and freshly re-suspended at 50,000 cells per well in a 96-well plate in 100 μL complete RPMI media containing various concentrations of IL-2, IL-15, IL-2:m33, m33:IL-15, or m33 superfusion. The cells were cultured for 48 hours, and then 10μL MTT was added per well, and the cells were incubated at 37 °C, 5% CO2 for three more hours, and then 100 μL per well detergent was added, and the plate was incubated at room temperature overnight. Absorbance at 570 nm in each well was read using an ELx800 universal microplate reader (Bio-Tek Instruments, Winooski, VT).
Values for EC50 were obtained by using a least squares method to fit absorbance data at 570 nm (y) as a function of concentration (x) to the general equation describing a sigmoidal curve:
Results
Construction of m33:cytokine fusions
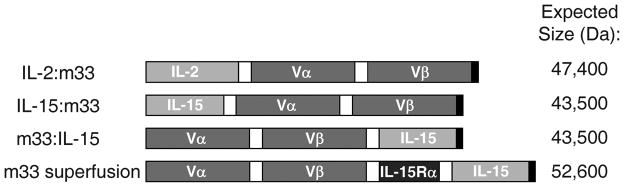
To evaluate the potential of high affinity TCRs as targeting molecules for immunomodulators, we constructed gene fusions consisting of cytokines linked to the m33 scTv (see Fig. 1). Linkers consisted of glycine and serine repeats of 20 amino acids in length, and each linker was designed to be unique in both nucleotide and amino acid sequence within each molecule for ease of cloning. The scTv m33 sequence included stabilizing mutations identified for improved expression of the 2C TCR in a scTv format,59 and the CDR3α mutations that conferred high affinity for SIY/Kb.56 Efforts to express and refold the fusions in the same E. coli system that provided high yields of the m33 scTv55 were unsuccessful (data not shown). The fusions were thus cloned into a mammalian expression vector and transfected into HEK293 cells. Yields of IL-2:m33 from the HEK293 cells were more than 10mg/L culture. In this system, initial results with IL-15 expressed at the N-terminus of the protein, and the scTv expressed at the C-terminus (IL-15:m33) showed that the fusion was produced in poor yields. Expression in the opposite orientation, with the m33 scTv at the N-terminus (m33:IL-15), resulted in improved yields of 0.2–1 mg/L culture and was thus adopted for further study (although the binding and function for both orientations of that fusion were identical, data not shown).
Figure 1. Gene constructs fusing high affinity scTv to immunostimulatory cytokines.
Immunostimulatory cytokines were fused to the scTv m33 with high affinity for the SIY/Kb pepMHC complex. Domains were linked by soluble Gly-Ser stretches of 20 amino acids in length (depicted by white boxes), and each gene included a C-terminal His6 tag for ease of purification (depicted by black boxes). The expected protein size of each construct is given.
In addition to the fusion of m33 to IL-15 alone, we constructed a fusion that included both IL-15 and the IL-15Rα sushi domain, which was purified at a yield of over 12 mg/L expression culture. Previous studies suggested that soluble IL-15Rα can enhance the effectiveness of IL-15,17–20 and expression of IL-15 as a fusion with the IL-15Rα sushi domain resulted in a hyperagonist construct.23, 24, 26, 29 All constructs described in the present report contain the murine cytokine proteins in order to test them in fully murine systems.
Expression, purification, and characterization of m33:cytokine fusion proteins
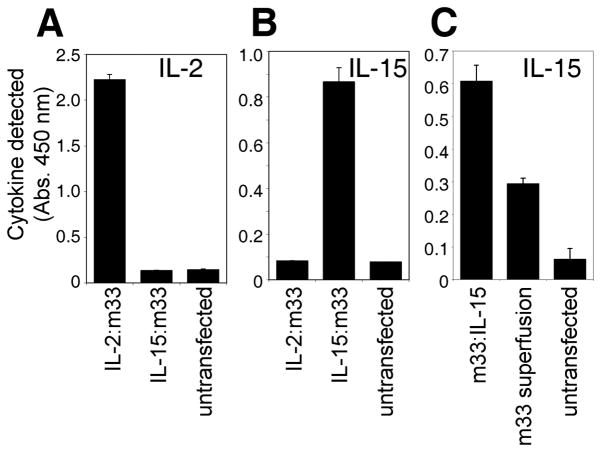
Transfected 293F cells were incubated in shake flasks for 4–10 days, depending on the optimal period for expression for each construct. Western blots of supernatants with an anti-His antibody (data not shown) verified that transfected cells were expressing the recombinant proteins of approximately the expected sizes (see below). To confirm proper folding of the cytokine portion of the scTv fusion proteins, we analyzed supernatants by cytokine ELISAs (Fig. 2A–C). IL-2:m33 was detected specifically by IL-2 sandwich ELISA (Fig. 2A), but not by IL-15 sandwich ELISA (Fig. 2B), and the reverse was true for IL-15:m33. The m33:IL-15 fusion consistently yielded a stronger signal by IL-15 ELISA than the m33 superfusion (Fig. 2C). We speculate that this may be a consequence of some IL-15 epitopes, recognized by the polyclonal antibody used for detection, being blocked sterically by association with the IL-15Rα sushi domain in the superfusion.29, 60
Figure 2. Detection of scTv:cytokine fusion proteins secreted from HEK293 cells.
Sandwich ELISA cytokine assays detecting the cytokine domains of the fusion proteins secreted into the culture supernatant of transfected HEK-293F cells. (A) IL-2 detected in 6 day culture supernatant. (B) IL-15 detected in 6 day supernatant. (C) IL-15 detected in 8 day supernatant.
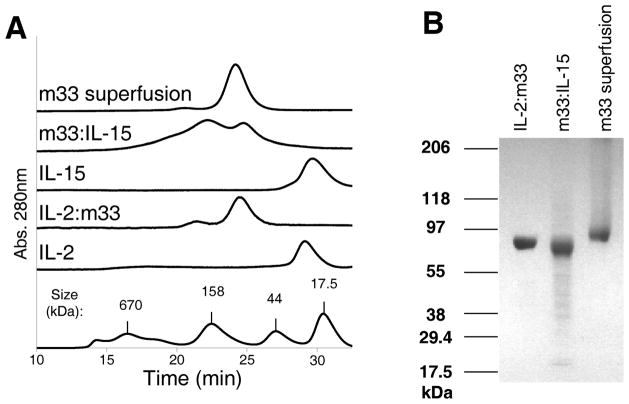
The secreted, soluble fusions were isolated from supernatants by binding to nickel beads, and further purified by size exclusion chromatography. The profiles obtained by size exclusion chromatography showed that the fusions eluted at a larger size than the free cytokines alone (Fig. 3A). Two peaks were observed for the m33:IL-15 fusion, suggesting that this preparation may have contained dimeric or multimeric proteins. However, minimal dimerization or aggregation was observed for IL-2:m33 or the m33 superfusion that included the IL-15Rα sushi domain (Fig. 3A). SDS-PAGE of the reduced and alkylated purified preparations showed that all three proteins migrated as a single band (Fig. 3B). However, all of the fusions ran significantly larger on SDS-PAGE gels than would be predicted by the protein sequences (Fig. 1), suggesting that each may be glycosylated. For example, the m33 superfusion contains 8 potential NX(S/T) sites that could be N-glycosylated (scTCR Vα Asn 74; scTCR Vβ Asn 24 and Asn 73; IL-15Rα sushi domain Asn 18; and IL-15 Asn 56, Asn 59, Asn 71, and Asn 111). The sizes of the proteins were the same from batch to batch, suggesting a consistent pattern of glycosylation. We verified that the proteins migrated at the expected sizes after treatment with N-Glycosidase F (PNGase F, data not shown). Previous results showed that differential glycosylation of an IL-15:scFv fusion could occur depending on the relative orientation of the domains; in their construct, the more highly glycosylated orientation (IL-15 N-terminal relative to scFv) was less active in CTLL-2 proliferation assays.36 Similarly, we noted that the IL-15:m33, which was constructed but not pursued due to low protein yields, was larger by SDS-PAGE than m33:IL-15, likely due to increased glycosylation. However, that construct was equally active in CTLL-2 proliferation assays as m33:IL-15 (data not shown).
Figure 3. Purification of scTv:cytokine fusion proteins.
(A) Size exclusion chromatography (S200) traces for purified fusion proteins and free cytokines. (B) SDS-PAGE gradient gel (4–20% acrylamide) showing purified fusion proteins after boiling, reduction with DTT, and alkylation with iodoacetamide. Gel was stained with Coomasie blue.
Specific binding of m33:cytokine fusions
As m33 scTv binds to SIY/Kb with an affinity similar to many affinity matured antibodies (KD=30nM, 56), we reasoned that we may be able to directly detect binding of the m33 fusion proteins to cells that express SIY/Kb using flow cytometry. T2-Kb cells were loaded with either OVA (null) or SIY (agonist) peptides, and then incubated with the different m33 fusions. To detect bound fusions, we used an antibody to the corresponding linked cytokine or the IL-15Rα. Significant specific binding was observed only to cells that expressed the cognate pepMHC complex, SIY/Kb, using all three fusions: m33:IL-15 (Fig. 4A), IL-2:m33 (Fig. 4B), and the m33 superfusion (Fig. 4C). The superfusion could also be detected with the anti-IL-15 antibody (data not shown), but the signal was weaker, similar to the results seen in the IL-15 ELISA (Fig. 2C).
Figure 4. Peptide/MHC-specific binding of scTv:cytokine fusion proteins.
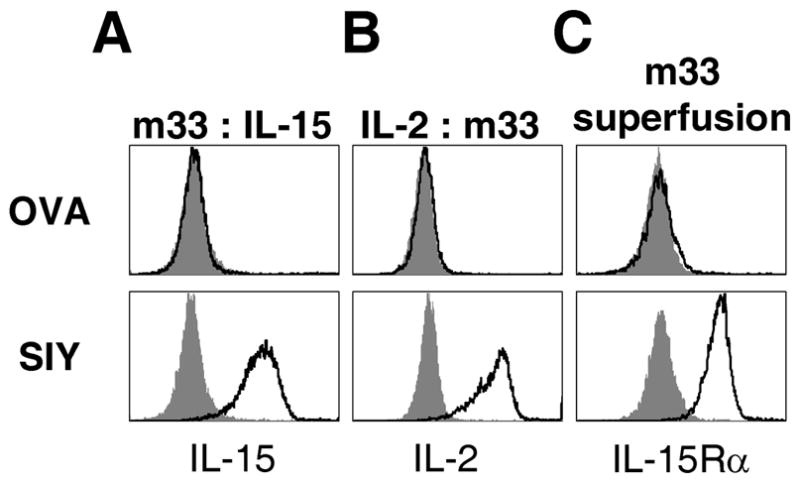
(A–C) T2-Kb cells were loaded with either null (OVA) or specific (SIY) peptide and then incubated with the indicated scTv:cytokine fusions. After washing, cells were incubated with antibodies to the fused cytokines: (A) biotinylated anti-IL-15 antibody, followed by streptavidin-Alexa 488, (B) biotinylated rat anti-IL-2 antibody, followed by with streptavidin-PE and (C) polyclonal goat anti-IL-15Ra antibody, detected with biotinylated polyclonal rabbit anti-goat antibody followed by streptavidin-Alexa 647. In each panel, the solid line trace represents staining by the primary antibody and secondary reagent and the filled, gray histogram represents staining by the secondary reagent only.
While it was clear that ligand-bound fusions were recognized by polyclonal anti-cytokine or anti-cytokine receptor antibodies, it was possible that the epitopes that bind to the cytokine receptors could still be occluded. To determine if the cytokine domain of the fusion molecules could bind to their respective receptors while the fusion was bound to the SIY/Kb, we performed an alternative flow cytometry-based experiment. First, we screened various cell lines for expression levels of IL-2Rα and IL-15Rα (see Supplementary Fig. 1). Based on these results, the CTLL-2 cell line was shown to express both receptors, and the MC57 fibrosarcoma cell line was shown to express IL-15Rα. These cell lines were incubated with the soluble m33:cytokine fusions. After washing, the bound fusion was incubated with SIY/Kb streptavidin tetramer in order to directly examine if the m33 scTv was properly folded and capable of binding to its cognate ligand. CTLL-2 cells were bound by both m33:IL-15 and IL-2:m33 (Fig. 5A), consistent with this cell line containing the receptors for both IL-15 and IL-2. In contrast, MC57 cells bound considerably better to the m33:IL-15 fusion than to the IL-2:m33 fusion (Fig. 5B), consistent with this cell line expressing high levels of the IL-15 receptor, but no IL-2Rα above the detection limit of flow cytometry (see Supplementary Fig. 1). As detection was performed with the SIY/Kb tetramer, these results also show that both the scTv domains and the cytokine domains of the purified fusion proteins are capable of the expected, specific binding.
Figure 5. Cytokine-receptor binding of scTv:cytokine fusion proteins.
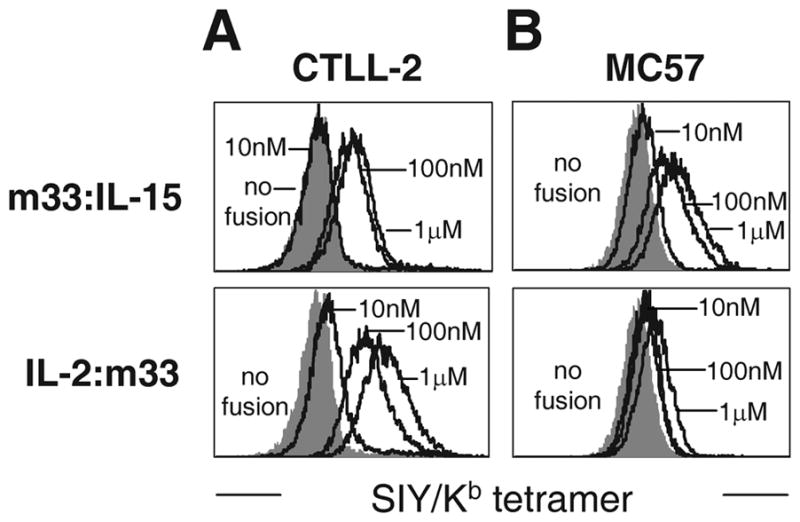
Cytokine receptor-expressing cells (see Supplemental Figure) (A) CTLL-2 or (B) MC57 were incubated with the indicated concentration of scTv:cytokine fusion, then washed and stained with SIY/Kb streptavidin tetramer.
Immunostimulatory function of m33:cytokine fusions
To verify that the fusion proteins could stimulate proper effector functions, we used the cytokine-dependent cell line, CTLL-2 in two different assay formats. First, CTLL-2 cells were incubated with various concentrations of each cytokine or the fusions to determine if they could support the proliferation of CTLL-2 cells. Both IL-2 and the IL-2:m33 fusion showed similar EC50 levels (effective dose at 50% maximum; 0.15 and 0.43 nM, respectively) and maximum levels of proliferation (Fig. 6A). However, CTLL-2 cells cultured with various concentrations of IL-15, m33:IL-15, or m33 superfusion showed distinct properties for each (Fig. 6B). While the EC50 for m33:IL-15 was similar to IL-15 (0.54 vs. 4 nM), the maximal proliferation induced was lower, indicating that this reagent would be less effective at stimulating a localized anti-tumor response. In contrast, the m33 superfusion induced levels of proliferation that were even higher than IL-15 alone, with a low EC50 value of 0.53 nM. The precise values of EC50 fit from these data should not be over-interpreted, especially in the case of samples where saturation is not reached in the concentration range tested, such as IL-15. The low levels of stimulation by the m33:IL-15 fusion as compared to IL-15 alone were somewhat surprising. This may result from a reduced fraction of properly folded or accessible IL-15 in the fusion mixture, as aggregation of this construct was consistently seen, while free IL-15 was observed to be a soluble monomer (see Fig. 3A). These results suggest that the m33 superfusion, containing the IL-15Rα domain, may be the most effective candidate for recruitment and stimulation of immune cells.
Figure 6. Cytokine-specific functional activity of scTv:cytokine fusion proteins.
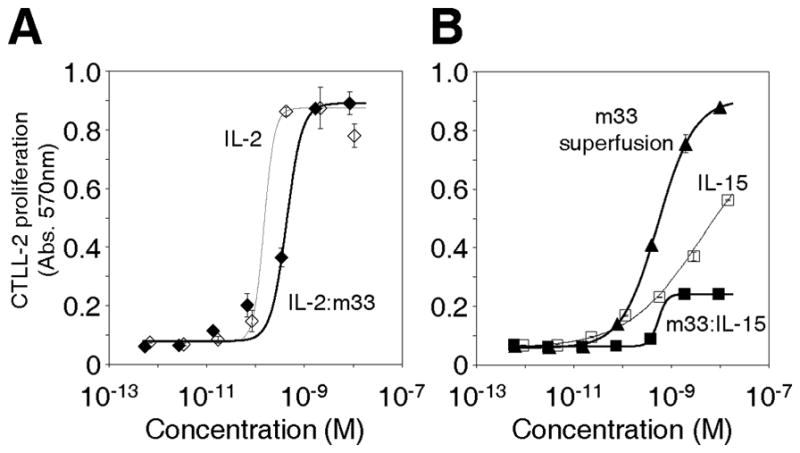
(A,B) Proliferation of CTLL-2 cytokine-dependent cells when cultured with (A) IL-2 or IL-2:m33, or (B) IL-15, m33:IL-15, or m33 superfusion.
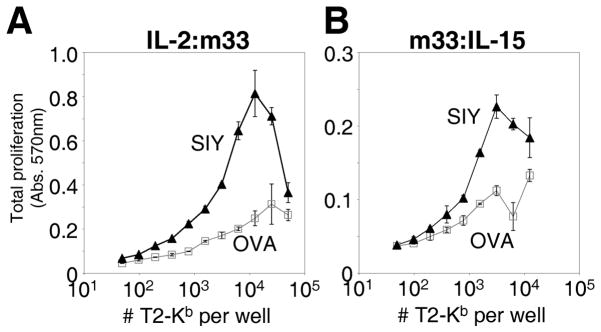
In the second approach, we wished to determine if the cytokines within the fusions could be presented, possibly in “trans,” by antigen presenting cells with the specific pepMHC to the cytokine dependent CTLL-2 line. In this experiment, the T2-Kb cell line was incubated with either the cognate peptide SIY or the control peptide OVA, washed, and incubated with 1 μM of the IL-2:m33 or m33:IL-15 fusions. After washing, the loaded cells were incubated with the CTLL-2 line for 48 hours, and proliferation was monitored (note that some proliferation of the APC is also detected in this assay). In the case of both fusions, a significant level of SIY/Kb-specific proliferation was observed, above that seen with the OVA/Kb control (Fig. 7A,B). At high concentrations of cells and cytokine fusion, the level of viable cells decreases, especially for the more potent IL-2:m33 fusion (Fig. 7A). This is likely due to rapid proliferation, crowding, and death in the assay prior to detection of live cells at the end of the incubation period. Thus, these fusion proteins have the ability to stimulate cytokine-dependent proliferation of immune cells in an antigen-specific format. While we can not confirm that this was indeed “trans” presentation (i.e. activation of CTLL-2 proliferation could be at least in part mediated by soluble fusion released into the media during the assay), the binding of m33 to SIY/Kb would still yield enhanced localization of the cytokine at the site of specific antigen such as a tumor.
Figure 7. Cytokine-specific functional activity of pepMHC-bound cytokine fusions.
T2-Kb cells were loaded with either null (OVA) or specific (SIY) peptide, incubated with scTv:cytokine fusions, and washed before co-culture with CTLL-2. Specific proliferation in the presence of the peptide-loaded antigen presenting cells and IL-2:m33 (A) or m33:IL-15 (B) was measured.
In this format, evaluation of the m33 superfusion was complicated by high activation mediated by APCs loaded with null peptide (OVA) as well as SIY peptide (see Supplementary Fig. 2A,B). This may be due to low level expression of cytokine receptors on the APCs (Supplementary Fig. 2C) that may dominate the interaction with this fusion,61 despite low detection of m33 superfusion binding to OVA-pulsed T2-Kb cells by flow cytometry (Fig.4C). It will be interesting to see how potential interactions with cytokine receptors will affect distribution of these fusions in vivo.
Discussion
T cell antigens in the form of peptides bound to products of the MHC represent a powerful, and highly diverse class of targets for both cancer and virus-based therapeutics. Whereas classical antibody-based targeting of viable cells is restricted to antigens displayed as genuine cell surface proteins, using TCRs widens the repertoire of targetable surface molecules vastly because pepMHC antigens are the cell surface representation of the entire array of intracellular molecules derived from aberrantly expressed or mutated proteins. T cell receptors, the antigen recognition molecules for this class of antigens, can thus provide the specificity required for targeting these antigens. When used in soluble form for the delivery of immunostimulatory molecules, the TCR provides an opportunity to enhance the cellular immune response not only at the site of the tumor or viral infection, but importantly in the draining lymph nodes for the lesions, where various immune cells (including T cells) are often first stimulated.41–44
One of the major hurdles in using soluble TCRs for this purpose has been the low affinities associated with wild type TCRs. Most naturally occurring TCRs bind weakly, and require co-receptor binding to direct an efficient cellular response (reviewed in 47). One strategy that was employed to address the issue of low affinity was to use a murine T cell clone against a human pepMHC (p53/HLA-A2), resulting a CD8-independent response.62 This TCR, called 264, was fused as a soluble, three-domain (VαVβCβ) to IL-2. When injected i.v. repeatedly beginning 24 h after injecting cancer cells also i.v., the TCR-fused cytokine was somewhat more successful in reducing the number of lung metastases than the unconjugated IL-2.50 More recently, a study by Wong and colleagues was published that used the same three-domain 264 TCR as a targeting element.27 In their in vitro studies, a bivalent construct was generated by fusing the scTCR individually to either the IL-15 or the IL-15Rα sushi domain, relying on binding between cytokine and receptor fragment (Kd = 0.4–1.5nM, 26) to dimerize the TCR. The resulting TCR bivalency allowed detectable binding to cells presenting pepMHC antigen, but it is unclear that at the typically low antigen densities associated with normally processed antigens, a strategy requiring multivalent binding will be practical. Perhaps to help overcome this issue, the same study explored the use of a TCR/CD8 heterodimer of the OT-1 TCR63 to improve binding to class I MHC. It remains to be seen if this latter approach will result in reduced pepMHC specificity. Another potential issue with non-covalent linkage between IL-15 and the IL-15Rα sushi domain is that the IL-15-containing protein will associate with the normal full-length IL-15Rα (Kd =38pM, 26) widely expressed on non-hematopoietic cells of the host, preventing effective targeting of the pepMHC by the bivalent construct. In our study, the problems associated with weak wild-type TCR binding affinity have been overcome by in vitro TCR engineering to affinities in the same range as therapeutic antibodies.56, 64–66 This allowed us to fuse a scTv to the cytokines or cytokine complexes in a single polypeptide chain, enabling high affinity pepMHC binding.
Another hurdle with targeting by soluble TCRs has been the ability to express adequate amounts of the soluble TCR, especially in the format analogous to scFv fragments of antibodies. We have previously engineered several mouse scTv not only with high-affinity against model pepMHC antigens,56, 64 but also for enhanced stability and better expression in recombinant systems.59 The targeted cytokine therapeutic molecules studied here contained one of these TCRs, the high-affinity scTv m33, which binds to its ligand SIY/Kb with an affinity of ~30 nM.55, 56 This receptor was derived from the well-studied 2C TCR via directed evolution by yeast surface display, resulting in high affinity as well as a stabilized scTv scaffold that can be produced in large amounts. We have recently described a strategy for generating stabilized human scTv fragments against human pepMHC.54 Use of the scTv format for soluble TCR targeting will allow direct comparison with scFv targeting strategies that have been well-studied for specificity, biodistribution, and efficacy.
IL-15 has been recently cited as one of the most promising molecules for tumor treatment.10 The localization of IL-15 to both tumor cells and stromal cells is important in anti-tumor responses. Studies of IL-15-secreting tumors in mice showed that NK cell-mediated rejection depended on presentation of the IL-15Rα-bound cytokine on the surface of the tumor cells, but that complete eradication (prevention of recurrence) also depended on IL-15Rα on the stromal cells, as well.22 Furthermore, it has been suggested that IL-15 may be able to overcome the tolerance exhibited by some cancer-specific T cells,12 and to facilitate the generation of memory T cells against the cancer.5 Accordingly, it is important to direct the cytokine not only to the site of the tumor, where some anergic T cells may reside, but also to antigens cross-presented on the tumor stroma,43 as well as to the site of draining lymph nodes where T cells are typically thought to undergo activation. Targeting the stroma will be particularly valuable in the case of antigen-loss variants, which would normally complicate treatments with conventional antibody-based therapeutics, since destruction of tumor stroma can eliminate even those cells within a tumor that have individually lost expression of the target antigen.45, 46
Conclusions
In an effort to use a high-affinity TCR to deliver immunomodulatory cytokines, we have characterized here the first in this class of stimulatory scTv:cytokine fusions. We were able to show that both the IL-2 and IL-15 fusions were expressed, folded, and functional for binding to appropriate cytokine receptors on cell surfaces, and were able to drive cytokine-dependent proliferation. Specific, high affinity binding of the m33 scTv domain was maintained to the model tumor antigen SIY/Kb. These fusions were functional when added directly to cells and when bound to cells with target pepMHC, presenting the cytokine moiety in trans.
The inclusion of covalently-linked IL-15Rα sushi domain in the m33 superfusion construct led to high yield production of very stable protein that was more effective in CTLL-2 proliferation assays than IL-15 itself, either alone or fused to the m33 scTv. The addition of the targeting TCR as shown here has the potential to effectively deliver this complex with increased local concentration to tumors, tumor stroma, and tumor draining lymph nodes.
While we recognize that these TCR-based fusion proteins will require further study for pharmacokinetic properties and in vivo tumor targeting, the design and characterization of these protein fusions described here provides a foundation for additional work, and shows the feasibility of using stabilized, high-affinity engineered scTv fragments to target to tumors and sites of infections.
Supplementary Material
Supplementary Figure 1 characterizing the expression of IL-2Rα and IL-15Rα on various immortalized cell lines is included.
Supplementary Figure 2 characterizing peptide non-specific stimulation of CTLL-2proliferation by m33 superfusion presented in trans by peptide-pulsed T2 cells, along with IL- 2Rβ expression on the cells is included.
Acknowledgments
This work was supported by the National Institutes of Health [R01-GM55767 and P01-CA97296 to D.M.K. and P01-CA97296, R01-CA22677 and R01-CA37516 to H.S.], and a Samuel and Ruth Engelberg/Irvington Institute Postdoctoral Fellowship of the Cancer Research Institute [to JDS].
Abbreviations used
- pepMHC
complex between peptide and MHC class I or II molecule
References
- 1.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 2.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20:276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170:85–100. doi: 10.1111/j.1600-065x.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. Jama. 1994;271:907–913. [PubMed] [Google Scholar]
- 7.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 8.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 10.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 11.Munger W, DeJoy SQ, Jeyaseelan R, Sr, Torley LW, Grabstein KH, Eisenmann J, Paxton R, Cox T, Wick MM, Kerwar SS. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 12.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Carrasquillo JA, Paik CH, Waldmann TA, Tagaya Y. Differences of biodistribution, pharmacokinetics, and tumor targeting between interleukins 2 and 15. Cancer Res. 2000;60:3577–3583. [PubMed] [Google Scholar]
- 14.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, Cocco L, Vitale M. NK cells and cancer. J Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 15.Rao BM, Driver I, Lauffenburger DA, Wittrup KD. High-affinity CD25-binding IL-2 mutants potently stimulate persistent T cell growth. Biochemistry. 2005;44:10696–10701. doi: 10.1021/bi050436x. [DOI] [PubMed] [Google Scholar]
- 16.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 17.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 18.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus D, Chavaillaz PA, Romero CA, Rhode PR, Wong HC. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56:804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, Binder DC, Butz E, Krausz T, Rowley DA, Jabri B, Schreiber H. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res. 2012;72:1964–1974. doi: 10.1158/0008-5472.CAN-11-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8:2736–2745. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 24.Bouchaud G, Garrigue-Antar L, Sole V, Quemener A, Boublik Y, Mortier E, Perdreau H, Jacques Y, Plet A. The exon-3-encoded domain of IL-15ralpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Ralpha. J Mol Biol. 2008;382:1–12. doi: 10.1016/j.jmb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 27.Wong RL, Liu B, Zhu X, You L, Kong L, Han KP, Lee HI, Chavaillaz PA, Jin M, Wang Y, Rhode PR, Wong HC. Interleukin-15:Interleukin-15 receptor {alpha} scaffold for creation of multivalent targeted immune molecules. Protein Eng Des Sel. 2011;24:373–383. doi: 10.1093/protein/gzq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen SK, Ota N, Kishishit S, Kukimoto-Niino M, Murayama K, Uchiyama H, Toyama M, Terada T, Shirouzu M, Kanagawa O, Yokoyama S. Crystal Structure of the interleukin-15. interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007;282:37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- 29.Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, Roffler SR, Tao MH. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Hum Gene Ther. 2010;21:611–621. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 30.Ma W, Yu H, Wang Q, Bao J, Yan J, Jin H. In vitro biological activities of transmembrane superantigen staphylococcal enterotoxin A fusion protein. Cancer Immunol Immunother. 2004;53:118–124. doi: 10.1007/s00262-003-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohlman B, Sweetenham J, Macklis RM. Review of clinical radioimmunotherapy. Expert Rev Anticancer Ther. 2006;6:445–461. doi: 10.1586/14737140.6.3.445. [DOI] [PubMed] [Google Scholar]
- 32.Schanzer JM, Baeuerle PA, Dreier T, Kufer P. A human cytokine/single-chain antibody fusion protein for simultaneous delivery of GM-CSF and IL-2 to Ep-CAM overexpressing tumor cells. Cancer Immun. 2006;6:4. [PubMed] [Google Scholar]
- 33.Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, Ferrini S, Kosmehl H, Neri D, Zardi L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99:1659–1665. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 34.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, Gonzalez-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, Lovato V, Kaspar M, Trachsel E, Menssen HD, Neri D, Garbe C. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17:7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 35.Gillies SD. Immunocytokines: A Novel Approach to Cancer Immune Therapy. In: JL, et al., editors. Targeted Cancer Immune Therapy. Springer Science+Business Media, LLC; Waltham, MA: 2009. pp. 241–256. [Google Scholar]
- 36.Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940–4948. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- 37.Kaspar M, Zardi L, Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118:1331–1339. doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- 38.Sommavilla R, Pasche N, Trachsel E, Giovannoni L, Roesli C, Villa A, Neri D, Kaspar M. Expression, engineering and characterization of the tumor-targeting heterodimeric immunocytokine F8-IL12. Protein Eng Des Sel. 2010;23:653–661. doi: 10.1093/protein/gzq038. [DOI] [PubMed] [Google Scholar]
- 39.Kermer V, Baum V, Hornig N, Kontermann RE, Muller D. An Antibody Fusion Protein for Cancer Immunotherapy Mimicking IL-15 trans-Presentation at the Tumor Site. Mol Cancer Ther. 2012;11:1279–1288. doi: 10.1158/1535-7163.MCT-12-0019. [DOI] [PubMed] [Google Scholar]
- 40.Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207:1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai XF, Gao JX, Liu J, Wen J, Zheng P, Liu Y. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 2001;61:6860–6867. [PubMed] [Google Scholar]
- 42.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, Robinson BW, Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- 43.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 44.Wolkers MC, Stoetter G, Vyth-Dreese FA, Schumacher TN. Redundancy of direct priming and cross-priming in tumor-specific CD8+ T cell responses. J Immunol. 2001;167:3577–3584. doi: 10.4049/jimmunol.167.7.3577. [DOI] [PubMed] [Google Scholar]
- 45.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 46.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178:5727–5734. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. Embo J. 2005;24:2968–2979. doi: 10.1038/sj.emboj.7600771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Card KF, Price-Schiavi SA, Liu B, Thomson E, Nieves E, Belmont H, Builes J, Jiao JA, Hernandez J, Weidanz J, Sherman L, Francis JL, Amirkhosravi A, Wong HC. A soluble single-chain T-cell receptor IL-2 fusion protein retains MHC-restricted peptide specificity and IL-2 bioactivity. Cancer Immunol Immunother. 2004;53:345–357. doi: 10.1007/s00262-003-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merril J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Jones DS, Silverman AP, Cochran JR. Developing therapeutic proteins by engineering ligand-receptor interactions. Trends Biotechnol. 2008;26:498–505. doi: 10.1016/j.tibtech.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Richman SA, Kranz DM. Display, engineering, and applications of antigen-specific T cell receptors. Biomol Eng. 2007;24:361–373. doi: 10.1016/j.bioeng.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Aggen DH, Chervin AS, Insaidoo FK, Piepenbrink KH, Baker BM, Kranz DM. Identification and engineering of human variable regions that allow expression of stable single-chain T cell receptors. Protein Eng Des Sel. 2011;24:361–372. doi: 10.1093/protein/gzq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, Kranz DM. The impact of TCR-binding properties and antigen presentation format on T cell responsiveness. J Immunol. 2009;183:1166–1178. doi: 10.4049/jimmunol.0900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4:55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 57.Jones LL, Colf LA, Bankovich AJ, Stone JD, Gao YG, Chan CM, Huang RH, Garci KC, Kranz DM. Different thermodynamic binding mechanisms and peptide fine specificities associated with a panel of structurally similar high-affinity T cell receptors. Biochemistry. 2008;47:12398–12408. doi: 10.1021/bi801349g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 59.Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18:754–759. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- 60.Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, Reiling N, Bulfone-Paus S. Soluble Interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- 61.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 64.Holler PD, Lim AR, Cho BK, Rund LA, Kranz DM. CD8(−) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J Exp Med. 2001;194:1043–1052. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y, Bennett AD, Zheng Z, Wang QJ, Robbins PF, Yu LY, Li Y, Molloy PE, Dunn SM, Jakobsen BK, Rosenberg SA, Morgan RA. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179:5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 characterizing the expression of IL-2Rα and IL-15Rα on various immortalized cell lines is included.
Supplementary Figure 2 characterizing peptide non-specific stimulation of CTLL-2proliferation by m33 superfusion presented in trans by peptide-pulsed T2 cells, along with IL- 2Rβ expression on the cells is included.