Abstract
Parkinson disease entails profound loss of nigrostriatal dopaminergic terminals, decreased vesicular uptake of intra-neuronal catecholamines, and relatively increased putamen tissue concentrations of the toxic dopamine metabolite, 3,4-dihydroxyphenylacetaldehyde (DOPAL). The objective of this study was to test whether vesicular uptake blockade augments endogenous DOPAL production. We also examined whether intracellular DOPAL contributes to apoptosis and, since alpha-synuclein oligomers may be pathogenetic in Parkinson disease, oligomerizes alpha-synuclein. Catechols were assayed in PC12 cells after reserpine to block vesicular uptake, with or without inhibition of enzymes metabolizing DOPAL—daidzein for aldehyde dehydrogenase and AL1576 for aldehyde reductase. Vesicular uptake was quantified by a method based on 6F- or 13C-dopamine incubation; DOPAL toxicity by apoptosis responses to exogenous dopamine, with or without daidzein+AL1576; and DOPAL-induced synuclein oligomerization by synuclein dimer production during DOPA incubation, with or without inhibition of L-aromatic-amino-acid decarboxylase or monoamine oxidase. Reserpine inhibited vesicular uptake by 95–97% and rapidly increased cell DOPAL content (p=0.0008). Daidzein+AL1576 augmented DOPAL responses to reserpine (p=0.004). Intracellular DOPAL contributed to dopamine-evoked apoptosis and DOPA-evoked synuclein dimerization. The findings fit with the “catecholaldehyde hypothesis,” according to which decreased vesicular sequestration of cytosolic catecholamines and impaired catecholaldehyde detoxification contribute to the catecholaminergic denervation that characterizes Parkinson disease.
Key words or phrases: Dihydroxyphenylacetaldehyde, DOPAL, aldehyde dehydrogenase, reserpine, Parkinson disease, monoamine oxidase
Parkinson disease is associated with drastic depletion of dopamine in the corpus striatum (Ehringer & Hornykiewicz 1960) and dopaminergic neuron loss in the substantia nigra (Halliday et al. 1990), as well as decreased norepinephrine contents in several brain areas (Ehringer & Hornykiewicz 1960, Goldstein et al. 2011, Kish et al. 1984). Lewy bodies, a neuropathologic hallmark of Parkinson disease, contain abundant alpha-synuclein, and synuclein oligomers are thought to play a key pathogenetic role in Parkinson disease (Winner et al. 2011, Kazantsev & Kolchinsky 2008).
Mechanisms of loss of catecholaminergic neurons in Parkinson disease and related disorders and relationships between synucleinopathy and endogenous catecholamines remain incompletely understood. The relatively selective involvement of monoaminergic neurons in Parkinson disease suggests that the transmitters themselves might be sources of “autotoxins.” Catecholamines leak continuously from storage vesicles into the cytosol and are recaptured by vesicular uptake (Eisenhofer et al. 2004a, Eisenhofer et al. 2004b). Buildup of cytosolic dopamine is toxic in neurons lacking vesicles (Chen et al. 2008); elevation of cytosolic dopamine levels reduces survival of cultured midbrain neurons (Mosharov et al. 2009); pharmacologic depletion of dopamine in PC12 cells reduces toxicity exerted by subsequent treatment with the complex-1 inhibitor rotenone combined with the sympathomimetic amine methamphetamine (Dukes et al. 2005); and reduced vesicular storage of dopamine augments methamphetamine-induced toxicity (Guillot et al. 2008, Fumagalli et al. 1999).
A well studied mechanism of catecholamine toxicity is spontaneous oxidation to form quinones (Graham 1978, Jana et al. 2011). Dopamine also undergoes enzymatic oxidation to form potentially harmful aldehydes (Blaschko 1952). Monoamine oxidase (EC 1.4.3.4) in the outer mitochondrial membrane catalyzes conversion of cytosolic dopamine to 3,4-dihydroxyphenylacetaldehyde (DOPAL). DOPAL is toxic (Panneton et al. 2010) by at least four mechanisms—protein cross-linking (Rees et al. 2009), oxidation to quinones (Anderson et al. 2011), production of hydroxyl radicals (Li et al. 2001), and oligomerization and precipitation of alpha-synuclein (Burke et al. 2008). Moreover, post-mortem putamen tissue from Parkinson disease patients contains increased DOPAL relative to dopamine (Goldstein et al. 2011). In the setting of reduced vesicular uptake, impaired aldehyde detoxification might increase cytosolic DOPAL levels further, exacerbating the neurodegeneration.
Few studies have measured endogenous DOPAL, and none has addressed whether blockade of vesicular uptake augments DOPAL generation. Addressing this issue was the main objective of the present experiments. We used PC12 cells as a model of dopaminergic neurons, because PC12 cells synthesize, store, and release dopamine; they contain monoamine oxidase of the A type only; they express vesicular and cell membrane catecholamine transporters; and they contain DOPAL (Lamensdorf et al. 2000) and two DOPAL metabolites, dihydroxyphenylacetic acid (DOPAC) and dihydroxyphenylethanol (DOPET). Since PC12 cells express catechol-O-methyltransferase (EC 2.1.1.6), whereas dopaminergic neurons do not, the cells were preincubated for 24 hours in medium containing the catechol-O-methyltransferase inhibitor, tolcapone. This cell line normally expresses little alpha-synuclein. Therefore, to determine whether DOPAL produced intra-cellularly oligomerizes synuclein we used PC12 cells that conditionally over-express alpha-synuclein (Ito et al. 2010).
The present study was designed to test whether (1) reserpine-induced blockade of vesicular uptake and the attendant increase in cytosolic dopamine enhance production of deaminated metabolites of endogenous dopamine, including DOPAL; (2) inhibition of aldehyde dehydrogenase (EC 1.2.1.4) and aldehyde reductase (EC. 1.1.1.21) augments the DOPAL response to vesicular uptake blockade; (3) reserpine at a dose that blocks vesicular uptake does not affect cell trans-membrane uptake of the dopamine analogs, 6-fluorodopamine (Eisenhofer et al. 1989) or 13C-dopamine; (4) inhibition of aldehyde dehydrogenase and aldehyde reductase augments toxicity of elevated intracellular dopamine; and (5) DOPAL mediates DOPA-induced oligomerization of alpha-synuclein.
MATERIALS AND METHODS
Materials
PC12 cells and the cell culture media, F12K and RPMI1640, were from the American Type Culture Collection; CellBIND culture dishes from Corning (Lowell, MA); tolcapone (to block catechol-O-methyltransferase) from Orion Pharma (Espoo, Finland); dopamine, reserpine (to block vesicular uptake), daidzein (to inhibit aldehyde dehydrogenase), and hydrogen peroxide (30%) from Sigma; AL1576 (to inhibit aldehyde reductase) from Alcon Laboratories (Fort Worth, TX); epidermal growth factor from R&D Systems (Minneapolis, MS); and Guava Nexin® reagent (for apoptosis assays) from Millipore (Haywood, CA). 6F-Dopamine was obtained in solution (about 1.35 mM) from the NIH PET Department after having passed quality control for i.v. administration to humans. DOPAL standard was synthesized in the laboratory of and provided by Dr. Kenneth L. Kirk (NIDDK). d4-Dopamine internal standard was from CDN Isotopes (Quebec, Canada). 13C-Dopamine (13C at each of the 6 positions on the benzene ring) was obtained from Cambridge Isotope Laboratories (Andover, MA). For Western blotting, detection of protein used mouse anti-alpha-synuclein antibody (Invitrogen, Camarillo, CA) and goat anti-mouse IRDye 800CW secondary antibody (LI-COR, Lincoln, NE, USA). PC12 cells conditionally over-expressing alpha-synuclein in a tetracycline-inducible manner (Ito et al. 2010) were kindly provided by Drs. Ito and Nakaso (Department of Neurology, Institute of Neurological Sciences, Faculty of Medicine, Tottori University, Japan). Human recombinant alpha-synuclein 1–140 (MW 14,460 kDa) was purchased from Calbiochem (La Jolla, CA).
PC12 Cell Culture
PC12 cells were kept frozen in liquid nitrogen until passaged for experiments. Most experiments involved adherent cells; however, these were unavailable for a time, and some experiments involved non-adherent cells. There were no obvious differences in cell contents of catechols between adherent and non-adherent cell cultures. The PC12 cells were not differentiated.
Adherent cells were grown in F12K medium supplemented with 15% heat-inactivated horse serum, 2.5% fetal bovine serum, and 40 ng/mL epidermal growth factor on CellBIND culture dishes. Non-adherent cells were grown in RPMI1640 with 10% heat inactivated horse serum and 5% fetal bovine serum. The cells were incubated at 37 °C in an atmosphere of 5% carbon dioxide. Media were changed several times per week and passaged once per week.
Prior to plating for the experiments, cells were trypsinized, suspended in media, counted, and the suspensions diluted with medium to provide 200,000 cell/mL. One mL of these suspensions was transferred to each well. After 48 hours, the medium was removed and replaced with 1 mL of the same medium but containing 10 μM tolcapone. Experiments began after 24 hours of incubation with tolcapone-containing medium (“baseline”).
Inhibition of Vesicular Uptake
Effects of reserpine on levels of endogenous catechols in cells and medium were examined after 24 hours of exposure to tolcapone, by the addition of 6.0 μL (final concentration 0.6% vol:vol) dimethylsulfoxide vehicle alone or vehicle containing reserpine, to attain a final concentration of 10 μM reserpine in the medium. At 10, 20, 30, 60, 120, 180, or 240 minutes after addition of reserpine or vehicle, the medium was removed and centrifuged. Adherent cells were scraped into 400 μL of a 20:80 mixture of 40 mM phosphoric acid and 200 mM acetic acid. For non-adherent cell cultures, the content of each well was centrifuged, the supernatant removed, and the sedimented cells taken up in 400 μL of the same solution used for adherent cells. The separated medium and the disrupted cell solutions were frozen until assayed for catechol contents, as described below.
Enzyme Inhibition
For experiments about effects of aldehyde dehydrogenase inhibition by daidzein or aldehyde reductase inhibition by AL1576 on catechol levels and catechol responses to reserpine, combinations of daidzein (final concentration 10 μM), AL1576 (final concentration 1 μM), and dimethylsulfoxide vehicle were added to the medium at the same time as tolcapone. The cells were incubated for 24 hours before acute experiments with reserpine. For the acute experiments daidzein, AL1576, or daidzein+AL1576 were continued in the medium along with tolcapone.
6F-Dopamine or 13C-Dopamine Incubation
For experiments with 6F-dopamine, the medium of cells that had been preincubated with tolcapone for 24 hours was replaced with medium containing 1–2 μM of 6F-dopamine and 6.0 μL dimethylsulfoxide vehicle with or without 10 μM reserpine. Cells and medium were separated at 20 or 180 minutes and frozen until assayed for endogenous and 6F-catechols, as described below. Analogous experiments were performed with 13C-dopamine, except that cells were incubated with reserpine for 10 minutes before 13C-dopamine was added to the medium.
Apoptosis
To estimate the contribution of DOPAL to cytotoxicity induced by increasing cytosolic dopamine, we measured dopamine-induced apoptosis, with or without pre-incubation with daidzein+AL1576. We reasoned that if DOPAL contributed to dopamine-induced apoptosis, then pre-treatment with daidzein+AL1576 should exacerbate the apoptosis, and the extent of increase in apoptosis with vs. without daidzein+AL1576 would provide a measure of the contribution of DOPAL. As a positive control we used serum withdrawal for 24 hours, as this is well known to evoke apoptosis.
For measuring apoptosis induced by dopamine, about 2 × 105 cells were plated (12 wells/plate) and incubated for 24 hours in F12 medium containing 15% horse serum and 2.5% bovine serum at 37 °C in 5% CO2. The cells were then treated with dopamine (100 μM) for another 24 hours in the same medium with 10 μM tolcapone added to block catechol-O-methyltransferase. After incubation cells were collected and treated with Guava Nexin® Reagent (Millipore, Hayward, CA) for 20 minutes in the dark. The Guava Nexin® assay detects phosphatidylserine on the external membrane of apoptotic cells. Apoptotic cells were counted by flow cytometry using the Guava device. Results were expressed in terms of apoptotic cells as a function of total cells.
Conditional Over-expression of Alpha-synuclein
To over-express alpha-synuclein, PC12 cells conditionally over-expressing alpha-synuclein in a tetracycline-inducible manner were cultured for 10 days using medium that did not contain doxycycline. For confirmation of alpha-synuclein expression, Western blot analysis was done.
Cells over-expressing alpha-synuclein were exposed to 100 μM L-DOPA and 10 μM copper (II) for 6 hours, with or without 10 μM pargyline to inhibit monoamine oxidase or 10 μM carbidopa to inhibit L-aromatic-amino-acid decarboxylase. The cells were then lysed and cytosolic proteins isolated using a Qproteome cell compartment kit (Qiagen, Valencia, CA). Cytosolic protein (500–600 μg) was further purified by immunoprecipitation (Pierce Classic kit, Thermo Scientific, Rockford, IL). Alpha-synuclein aggregation was analyzed by Western blotting using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). Detection of protein was performed with mouse anti-alpha-synuclein antibody (1:200) and goat anti-mouse IRDye 800CW (1:10,000) secondary antibody. The intensity of bands was quantified by LI-COR Odyssey software. Cell lysates were also assayed for DOPAL and other catechols.
Effects of Hydrogen Peroxide vs. DOPAL on Alpha-Synuclein Dimerization
Alpha-synuclein (1.6 μM) dissolved in 100 mM Tris-HCl buffer (pH 7.4) was incubated with DOPAL (1–30 μM) or hydrogen peroxide (10–300 μM) at 37 °C for 60 min. Dimers of alpha-synuclein were quantified by Western blotting and the LI-COR system.
Catechol Assays
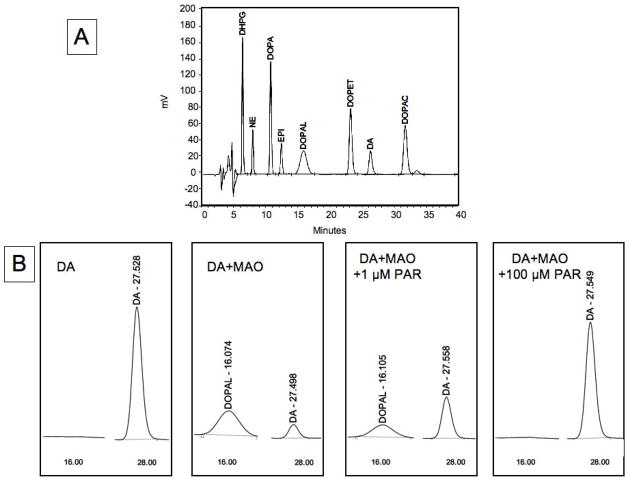
Endogenous and 6F-labeled catechols in cells and medium were assayed in our laboratory by HPLC with electrochemical detection after batch alumina extraction (Holmes et al. 1994). Identity of the DOPAL standard was confirmed by mass spectrometry, nuclear magnetic resonance, and liquid chromatography with time-of-flight mass spectrometry. Injection of DOPAL into the HPLC-electrochemical apparatus resulted in an unusual, characteristically wide chromatographic peak (Figure 1). When dopamine was incubated with monoamine oxidase-A, the combination generated the DOPAL peak. Incubation of dopamine with monoamine oxidase-A and pargyline to inhibit monoamine oxidase abolished production of the DOPAL peak, demonstrating its derivation from oxidative deamination of dopamine. We synthesized 6F-DOPAL from 6F-dopamine by the same approach, to provide a standard by which to identify 6F-DOPAL in chromatographs.
Figure 1.
Chromatographs of catechol standards. (A) Typical chromatograph of catechol standards; (B) Chromatographic segments after incubation of dopamine (DA) with monoamine oxidase (MAO, A type) with or without pargyline to block MAO. Other abbreviations: DHPG=dihydroxyphenylglycol; NE=norepinephrine; EPI=epinephrine. Note conversion of DA to DOPAL via MAO.
Cell catechol contents were assayed from 100–200 μL aliquots of the 400 μL of disrupted cells. For samples of medium, 500 μL was assayed. Catechol concentrations in cell lysates were expressed in units of pmoles per well. Cell counts were measured using a Cellometer device (Nexcelom Bioscience, Lawrence, MA).
For assaying cell concentrations of endogenous and 13C-dopamine, we used ultra-high performance liquid chromatography with tandem quadrupole mass spectrometry. d4-Dopamine (2 ng) was added to each sample as an internal standard. For elution of catechols from alumina, 0.1% formic acid was used. The samples were injected into a Waters Premier XE system under isocratic conditions.
Since our methodology for simultaneous measurement of dopamine, 13C-dopamine, and d4-dopamine has not yet been published, we provide more details as follows.
The system consisted of a Waters Acquity UPLC Core System containing a Binary Solvent Manager and Sample Manager with Column Heater Module and a Quattro Premier XE tandem quadrupole mass spectrometer. Carrier gas was generated by a nitrogen generator (Peak Scientific, Billerica, MA). The collision gas was high purity (99.9%) argon (Roberts Oxygen). Vacuum was generated by an Edwards XDS dry vacuum scroll pump (Edwards, Sanborn, NY). The sample manager was fitted with a 50 μl loop and a 250 μl syringe. The injection mode was partial loop with needle overfill. Column temperature was 30 °C, sample compartment 10 °C. source temperature 140 °C, and desolvation temperature 450 °C. Gas flow was 1000 L/hour and cone gas flow 50 L/hour. Cone voltage was 34 V, capillary voltage 1.0 kV, collision voltage 17 V, and collision energy 17.
Results were acquired in multiple reaction monitoring mode with positive ionization. Parent to daughter transitions for dopamine were 136.95 to 90.80 m/z, d4-dopamine 140.95 to 94.80 m/z, and 13C-dopamine 142.95 to 96.80 m/z. Inter-channel delay was 0.005 s, inter-scan delay 0.005 s, and dwell time 0.05 s.
Peak integration was performed using Waters QuanLynx Method Editor V4.1 software, using the following integration parameters: Smoothing enabled, Method Mean, Iterations 2, Width 2; Apex track parameters: Peak to peak baseline noise 10, Peak width at 5% height 30, baseline start at threshold 0.0, baseline end at threshold 0.5, detect shoulder peaks: No.; Standard peak detect ion parameters: Peak to peak noise amplitude 5440, balance 30, splitting 90, detect shoulder peak threshold 30, reduce tail 50, reduce height 10; View threshold parameters: Threshold relative height 1.5, threshold absolute height 0, threshold relative area 2.0, threshold absolute area 0.
Dopamine concentrations (in units of pg/mL) were calculated from peak areas (PAs) using the following formula.
“Extracted” refers to compounds after batch alumina extraction.
Data Analysis and Statistics
Neurochemical data were displayed using KaleidaGraph 4.01 (Synergy Software, Reading, PA). Fractional changes at 20 minutes from baseline in the same experiments were compared by two-tailed, dependent-means t-tests. Differences between drug treatments across experiments were compared by two-tailed, independent-means t tests. For testing hypotheses involving 3 or more treatments, factorial analyses of variance were used with Fisher’s PLSD post-hoc test. A p value less than 0.05 defined statistical significance.
RESULTS
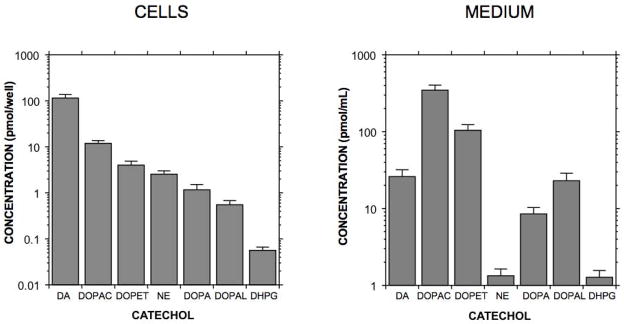
Dopamine was the main catechol detected in PC12 cells. In descending order of cell lysate concentrations, the other detected catechols were DOPAC > DOPET > norepinephrine > dihydroxyphenylalanine > DOPAL > dihydroxyphenylglycol (Figure 2A). No epinephrine was detected. DOPAL corresponded to 0.35% of dopamine. The pattern of catechol levels in the medium at baseline (after 24 hours pre-incubation in medium containing tolcapone) differed substantially from that in cell lysates (Figure 2B), with concentrations of the deaminated metabolites DOPAC and DOPET exceeding and with the DOPAL concentration about the same as that of dopamine.
Figure 2.
Mean (± SEM) concentrations of endogenous catechols in PC12 cells. Same abbreviations as in Figures 1 and 2. (Left) Cell lysate concentrations, in pmol/well; (Right) incubation medium concentrations, in pmol/mL. Note that DA is the main endogenous catechol in PC12 cells, with DOPAL constituting less than 1% of DA content. In contrast, deaminated metabolites of DA are the main endogenous catechols in incubation medium.
Reserpine depletes endogenous dopamine
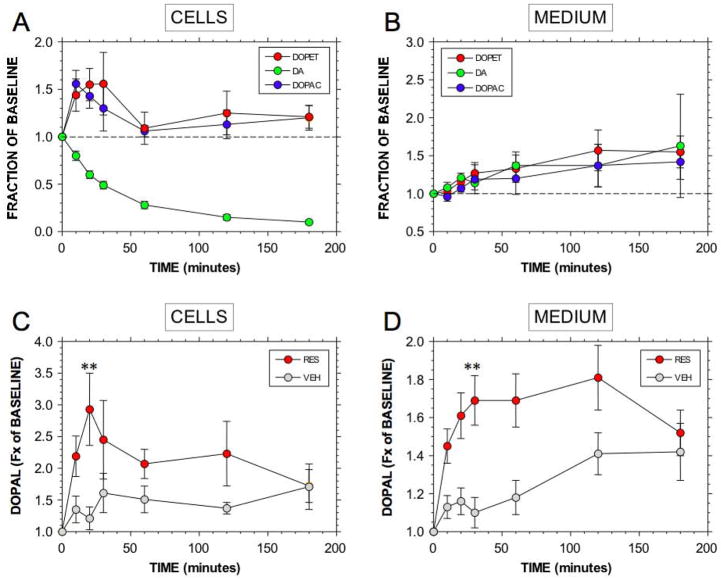
Reserpine rapidly and markedly depleted cellular dopamine, by about 1/2 at 20 minutes (p<0.0001 vs. baseline) and at least 90% at 180 minutes (p<0.0001, N=7; Figure 3A). Dopamine in the medium increased slightly but not significantly (Figure 3B).
Figure 3.
Mean (± SEM) fractions of baseline for cell and medium contents of dopamine (DA) and its deaminated metabolites as a function of time after reserpine addition. (A) Cell DA, DOPET, and DOPAC; (B) Medium DA, DOPET, and DOPAC; (C) Cell DOPAL; (D) Medium DOPAL. Other abbreviations: VEH=dimethylsulfoxide vehicle. Note rapid generation of deaminated metabolites including DOPAL, while cell DA content concurrently declines. (**) DOPAL concentration at 20 minutes greater for RES than for VEH, p<0.01.
Reserpine increases formation of endogenous deaminated metabolites of dopamine, including DOPAL
Reserpine rapidly increased cellular contents of the deaminated metabolites DOPAL, DOPET, and DOPAC; Figure 3A, C). Peak increases in DOPET (p<0.0001), DOPAC (p<0.0001), and DOPAL (p=0.0008) were attained at 20 minutes, when DOPAL averaged 2.7 times baseline. Thereafter, concentrations of the deaminated metabolites declined. Relative increases from baseline were similar for DOPAL, DOPET, & DOPAC. Reserpine also rapidly increased DOPAL concentrations in the medium (p=0.0003 at 20 minutes; Figure 3D). At both 20 minutes and 3 hours of incubation with 6F-dopamine, a compound co-chromatographing with 6FDOPAL was noted, with or without reserpine treatment (data not shown).
Inhibition of aldehyde dehydrogenase and aldehyde reductase increases endogenous DOPAL production
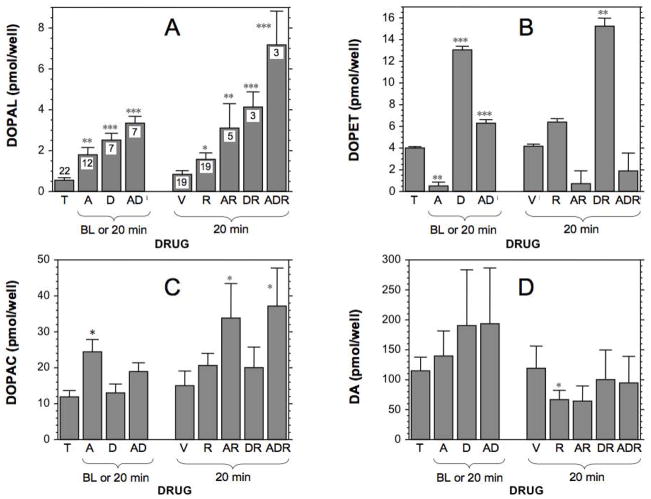
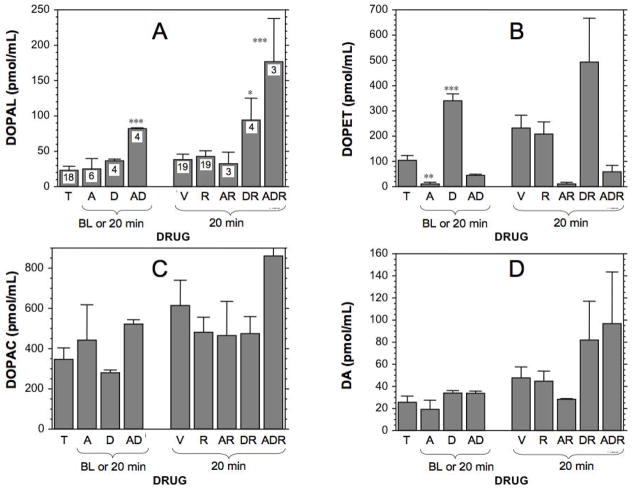
Both aldehyde dehydrogenase inhibition by daidzein and aldehyde reductase inhibition by AL1576 increased DOPAL levels in cells (Figure 4) and medium (Figure 4). Daidzein increased and AL1576 markedly decreased cell lysate and medium contents of DOPET (Figure 4B, 5B). Neither drug affected lysate or medium DOPAC (Figure 4C, 5C). Combined pre-incubation with daidzein+AL1576 produced larger increases in DOPAL than did either drug alone, in both cells and medium. Pre-incubation with daidzein or AL1576 augmented DOPAL responses to reserpine. The largest endogenous DOPAL responses observed in both cells and medium were with daidzein+AL1576+reserpine.
Figure 4.
Mean (± SEM) cell lysate concentrations of endogenous catechols after exposure to various drug combinations. (A) DOPAL; (B) DOPET; (C) DOPAC; (D) dopamine. Cells were incubated for 24 hours with tolcapone (T) and combinations of the aldehyde reductase inhibitor AL1576 (A) or aldehyde dehydrogenase inhibitor daidzein (D) and then incubated acutely for 20 minutes with reserpine (R) or dimethylsulfoxide vehicle (V). Asterisks for baseline (BL) or 20 minutes are for comparisons with T at 0 minutes. Asterisks for 20 minutes are for comparisons with VEH at 20 minutes. (*) p<0.05; (**) p<0.01; (***) p<0.001) by post-hoc testing after ANOVA. Numbers represent the number of observations. AD=AL1576+daidzein; AR=AL1576+reserpine; DR=daidzein+reserpine. The highest DOPAL concentrations in cell lysates were noted when cells were incubated with AL1576, daidzein, and reserpine (ADR).
Figure 5.
Mean (± SEM) medium concentrations of endogenous catechols after exposure to various drug combinations. Same abbreviations and meanings of asterisks and numbers as for Figure 4. The highest DOPAL concentrations observed in medium were noted when cells were incubated with AL1576+daidzein+reserpine (ADR).
Reserpine blocks vesicular uptake without affecting cellular uptake
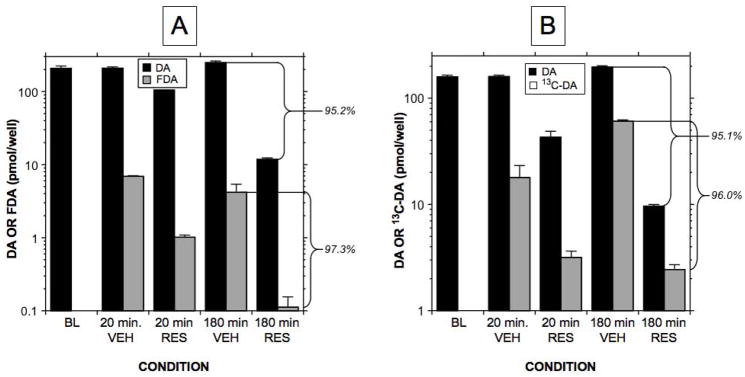
Reserpine treatment decreased 6F-dopamine content by 85.4% at 20 minutes (p<0.0001) and 97.3% at 3 hours (p<0.0001; Figure 6A and Table 1). In contrast, 6FDOPAC was increased slightly by 3.2% at 20 minutes and 11.0% at 3 hours. 6F-norepinephrine was detected at 3 hours, indicating vesicular uptake and slow intravesicular conversion of 6F-dopamine to 6F-norepinephrine. In cells co-incubated with 6F-dopamine and reserpine no 6F-norepinephrine was detected. Between 20 minutes and 3 hours of incubation with 6F-dopamine, 6F-dopamine in the medium often decreased to undetectable levels, while 6FDOPAC increased substantially. Reserpine treatment produced similar drastic effects on cell contents of endogenous and 13C-dopamine by 3 hours (compare with Figures 2A and 6B). Vehicle treatment did not affect concentrations of 6F-dopamine, 6FDOPAC, or 13C-dopamine at either time point.
Figure 6.
Effects of reserpine (RES) treatment on cell concentrations of endogenous and tracer-labeled dopamine. (A) Responses of endogenous and 6-fluorodopamine (6FDA) after co-incubation with 6FDA and dimethylsulfoxide vehicle or RES; (B) Responses of endogenous and 13C-dopamine after co-incubation with 13C-dopamine and dimethylsulfoxide vehicle or RES. 6FDA was assayed by liquid chromatography with electrochemical detection and 13C-dopamine by ultra-high performance liquid chromatography with mass spectrometry. Percents indicate differences between RES and vehicle. RES treatment was associated with profound decreases in cell concentrations of both endogenous and tracer-labeled dopamine.
Table 1.
Medium Mean (± SEM) Concentrations (pmol/mL) of Endogenous and 6F-Catechols. Data averaged for 3–5 experiments.
| Treatment | Time | DA | DOPAC | FDA | FDOPAC |
|---|---|---|---|---|---|
| VEH+6FDA | 20 min | 40±16 | 1423±90 | 352±108 | 34±9 |
| RES+6FDA | 20 min | 36±15 | 1449±86 | 346±108 | 33±8 |
| VEH+6FDA | 3 hours | 64±9 | 1470±152 | 4±3 | 547±63 |
| RES+6FDA | 3 hours | 44±2 | 1650±112 | 2±2 | 586±70 |
DOPAL contributes to DA-induced apoptosis
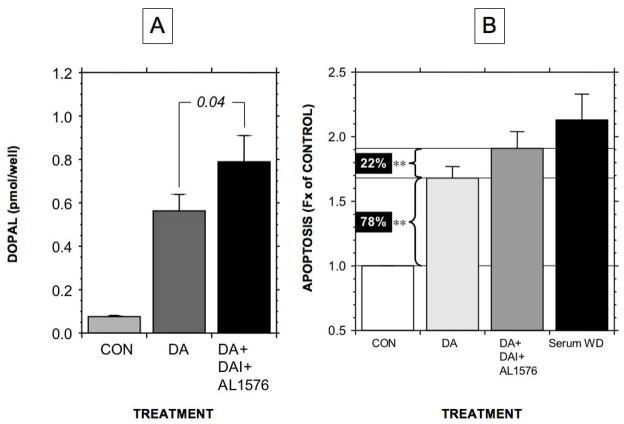
Incubation of PC12 cells with exogenous dopamine increased cellular DOPAL content (Figure 7A) and evoked apoptosis that was about 80% that produced by serum withdrawal (Figure 7B). When cells were incubated with dopamine and daidzein+AL1576, the amount of apoptosis increased to 90% of that produced by serum withdrawal. Incubation with daidzein+AL1576 augmented the cellular DOPAL response to exogenous dopamine (Figure 7A). As indicated by the lines and brackets in Figure 7B, co-incubation with dopamine and daidzein+AL1576 augmented dopamine-induced apoptosis by 22% (p<0.01).
Figure 7.
Contributions of DOPAL produced intracellularly on apoptosis in PC12 cells. (A) Mean (± SEM) DOPAL concentration in control cells (CON), cells incubated with dopamine (DA), and cells incubated with DA and AL1576+daidzein to inhibit metabolic breakdown of DOPAL. (B) Mean values for proportion of apoptotic treated cells relative to untreated control cells, expressed as fractions (Fx) of control, in cells exposed to exogenous DA, with or without AL1576+daidzein. Dopamine produced about 80% of the amount of apoptosis evoked by serum withdrawal (WD). It was estimated that at least 22% of apoptosis during dopamine exposure was attributable to DOPAL. (**) p<0.01 for comparison of dopamine+daidzein+ AL1576 compared to dopamine alone.
DOPAL contributes to DA-induced synuclein oligomerization
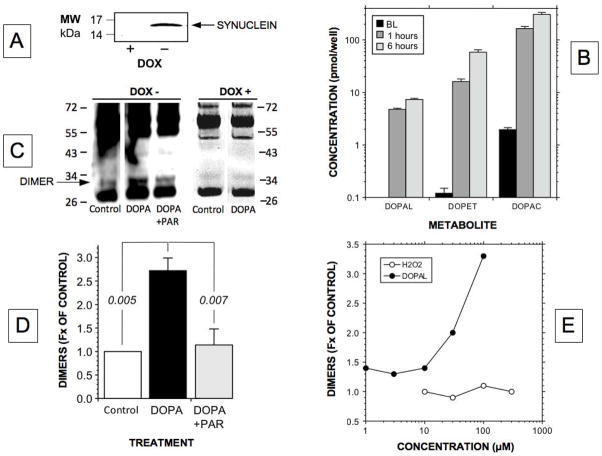
As expected, PC12 cells conditionally over-expressing synuclein had increased synuclein content, based on Western blotting (Figure 8A). DOPA incubation time-dependently increased cell contents of DOPAL, DOPET, and DOPAC by about 100-fold each (Figure 8B shows averaged results for 3 experiments). The peak cell DOPAL concentration attained during incubation with DOPA was about the same as that attained during incubation with reserpine+daidzein+AL1576.
Figure 8.
Contributions of DOPAL produced intracellularly on alpha-synuclein oligomerization in PC12 cells. (A) Western blotting demonstration of alpha-synuclein in PC12 cells expressing alpha-synuclein conditionally in a doxycycline- (DX)-dependent manner. Arrow indicates band at the molecular weight (MW) of alpha-synuclein; (B) Cell mean (± SEM) concentrations of DOPAL, DOPET, and DOPAC, without and with incubation for 1 or 6 hours with exogenous DOPA, in cells conditionally over-expressing alpha-synuclein. Note that attained DOPAL concentrations (about 7 pmol/well) were similar to those in PC12 cells not over-expressing alpha-synuclein and not exposed to exogenous DOPA but treated with reserpine, daidzein, and AL1576 (see Figure 5A). (C) Western blot showing increase in alpha-synuclein dimers when alpha-synuclein was incubated with DOPA and no increase when alpha-synuclein was incubated with DOPA in the setting of the monoamine oxidase inhibitor pargyline (PAR). For comparison a western blot from a different experiment shows absence of alpha-synuclein dimers, with or without DOPA (there is a vague band in the Control and DOPA lanes that upon close inspection corresponds to more than 34 kD and therefore differs from the synuclein dimer); (D) Cell mean (± SEM) concentrations of alpha-synuclein dimers, expressed as fractions of control, after incubation with exogenous DOPA, with or without PAR. DOPA treatment was associated with augmented synuclein dimerization; PAR abolished this augmentation; (E) alpha-synuclein dimer concentration relative to control upon alpha-synuclein incubation with hydrogen peroxide (H2O2) or DOPAL. Note that DOPAL evoked alpha-synuclein dimerization, whereas hydrogen peroxide did not.
Incubation of PC12 cells over-expressing alpha-synuclein with DOPA resulted in augmented formation of alpha-synuclein dimers (Figure 8C shows a representative Western blot and Figure 8D averaged results for 4 experiments). Pargyline (N=3, Figure 8D) or carbidopa (N=2) treatment prevented DOPA-induced dimerization of alpha-synuclein. Neither pargyline nor carbidopa alone affected alpha-synuclein dimerization (data not shown).
Since enzymatic deamination of dopamine produces DOPAL and hydrogen peroxide in a 1:1 molar ratio, DOPAL was compared with hydrogen peroxide across a range of concentrations in the ability to oligomerize alpha-synuclein. As shown in Figure 8E, DOPAL potently evoked alpha-synuclein dimerization, whereas hydrogen peroxide did not.
DISCUSSION
Here we report that in PC12 cells, which normally contain dopamine, the catecholaldehyde DOPAL, and the DOPAL metabolites DOPAC and DOPET, pharmacologic blockade of vesicular uptake increases cell and medium contents of endogenous DOPAL; and inhibition of enzymes that metabolize DOPAL to DOPAC and DOPET augments DOPAL responses to vesicular uptake blockade. We also obtained evidence that intracellular buildup of DOPAL contributes to both apoptosis and to alpha-synuclein oligomerization.
Catecholaminergic cells and neurons are characterized by ongoing passive leakage of vesicular catecholamines into the cytosol (Eisenhofer et al. 2004a), with active reuptake mediated by the vesicular monoamine transporter. Reserpine-induced blockade of vesicular uptake therefore would be expected to result in net translocation of stored dopamine into the cytosol, where dopamine is susceptible to oxidative deamination catalyzed by mitochondrial monoamine oxidase. Consistent with such translocation, reserpine treatment rapidly depleted cellular dopamine content, by about 1/2 at 20 minutes and at least 90% at 3 hours, associated with simultaneous rapid increases in cell and medium contents of DOPAL. These results agree with previously reported findings in PC12 cells exposed to the complex I inhibitor rotenone (Lamensdorf et al. 2000), which can produce an animal model of Parkinson disease (Sherer et al. 2003), redistributes dopamine from vesicles into the cytosol (Watabe & Nakaki 2008), and down-regulates the type 2 vesicular monoamine transporter (Sai et al. 2008); however, whether rotenone inhibits vesicular uptake of intra-neuronal catecholamines, such as by following the fate of tracer-labeled dopamine, has not yet been demonstrated.
At 3 hours of co-incubation with 6F-dopamine or 13C-dopamine, reserpine markedly decreased cell 6F-dopamine content by 97% and 13C-dopamine content by 96%, confirming extremely efficient blockade of vesicular uptake by reserpine in PC12 cells. Since reserpine did not affect concentrations of either 6FDA or 6FDOPAC in the medium, reserpine did not impede cellular catecholamine uptake.
DOPAL is converted to DOPAC by aldehyde dehydrogenase and to DOPET by aldehyde reductase. Normally the former route is favored, and the main endogenous metabolites of dopamine are acids (Kopin 1985). Thus, in the present study, cellular content of the acid DOPAC exceeded that of the alcohol DOPET. Since inhibition of aldehyde dehydrogenase by daidzein increased and inhibition of aldehyde reductase by AL1576 decreased DOPET levels, the two enzymes are alternatively available to act on cytosolic DOPAL. Inhibition of either enzyme increased cell DOPAL, inhibition of both increased DOPAL further, and reserpine exposure in the setting of AL1576+daidzein was associated with the highest cell and medium concentrations of endogenous DOPAL seen in the study, several times those in untreated cells.
These findings may help explain inconsistent literature about toxicity of DOPAL in nigrostriatal dopaminergic neurons. In human neuroblastoma SH-SY5Y cells, incubation with exogenous dopamine in the setting of disulfiram to block aldehyde dehydrogenase was found to increase DOPAL levels and to evoke substantial toxicity (Legros et al. 2004a). In rats treated with semi-chronically with levodopa and benserazide to increase dopamine production in the brain, however, disulfiram treatment did not alter striatal dopamine content (Legros et al. 2004b). Based on the present results, perhaps levodopa treatment with aldehyde dehydrogenase inhibition would have exerted cytotoxicity had the study included inhibition of vesicular uptake or of aldehyde reductase.
Mice with genetically determined very low activity of the type 2 vesicular monoamine transporter or with double knockout of the genes encoding aldehyde dehydrogenase 1A1 and 2 have aging-related behavioral and neuropathologic findings mimicking those in Parkinson disease (Caudle et al. 2007, Wey et al. 2012). The current findings lead straightforwardly to the hypotheses that pharmacologic inhibition of aldehyde dehydrogenase and aldehyde reductase should exacerbate neurodegeneration in the former animal model, while pharmacologic inhibition of vesicular uptake should exacerbate neurodegeneration in the latter model. If so, this would provide much needed gene-environment interaction models for the pathogenesis of Parkinson disease (Bronstein et al. 2009).
Incubation of PC12 cells with dopamine evoked apoptosis, with severity corresponding to about 80% that seen with serum withdrawal, in line with previous reports (Shinkai et al. 1997, Jana et al. 2011). To examine the contribution of DOPAL produced within the cells to dopamine-induced apoptosis, we incubated PC12 cells with dopamine, with or without daidzein+AL1576 to inhibit enzymatic breakdown of DOPAL. From the increase in apoptosis in the setting of daidzein+AL1576 we estimated that at least 22% of dopamine-evoked apoptosis was attributable to DOPAL. Lewy bodies, cytosolic inclusions in monoaminergic neurons, are a pathologic hallmark of sporadic Parkinson disease.
Lewy bodies contain abundant alpha-synuclein (Spillantini et al. 1997), rare patients with familial Parkinson disease have mutations or replication of the gene encoding alpha-synuclein abnormalities of (Polymeropoulos et al. 1997, Singleton et al. 2003), and genome-wide association studies have consistently reported statistical associations between Parkinson disease and genotypic variants of the alpha-synuclein gene (Edwards et al. 2010, Satake et al. 2009). According to the “catecholaldehyde hypothesis,” reduced vesicular uptake and impaired aldehyde detoxification contribute to the catecholaminergic denervation and synucleinopathy that characterize Parkinson disease.
For the catecholaldehyde hypothesis to hold credence one would expect a link between DOPAL and alpha-synucleinopathy. This link has indeed been found: exogenous DOPAL oligomerizes and precipitates alpha-synuclein (Burke et al. 2008). In the present study, the findings of DOPA-induced dimerization of alpha-synuclein and prevention of this effect by either carbidopa to block conversion of DOPA to dopamine or pargyline to block conversion of dopamine to DOPAL indicate that DOPAL formed intracellularly from cytosolic dopamine contributes to alpha-synuclein oligomerization. Considering that alpha-synucleinopathy may directly or indirectly interfere with vesicular sequestration of intra-neuronal catecholamines (Guo et al. 2008, Park et al. 2007, Mosharov et al. 2009, Adamczyk et al. 2006) and with enzymatic detoxification of catecholaldehydes (Nasstrom et al. 2011, Jinsmaa et al. 2009, Lee et al. 2001), induction of multiple positive feedback loops could lead to rapidly progressive neurodegeneration. The present results do not imply, however, that increased oligomerization of alpha-synuclein accounts for DOPAL-induced apoptosis. One may reasonably doubt this causal sequence, because PC12 cells normally contain relatively little alpha-synuclein.
DOPAL production from DOPA necessarily entails hydrogen peroxide production, in a 1:1 molar ratio, upon enzymatic deamination of dopamine. To evaluate the relative contributions of DOPAL and hydrogen peroxide to synuclein oligomerization after DOPA incubation, we compared DOPAL with hydrogen peroxide in the ability to dimerize alpha-synuclein. Whereas hydrogen peroxide up to a concentration of 300 μM did not dimerize alpha-synuclein, DOPAL did so at concentrations above about 30 μM. Prevention by pargyline of DOPA-induced alpha-synuclein oligomerization therefore is much more likely to be from decreased DOPAL generation than from decreased hydrogen peroxide generation.
Whether levodopa treatment for Parkinson disease accelerates the neurodegenerative process has been a persistently controversial and provocative topic in clinical neurology. Based on the findings that DOPA oligomerizes alpha-synuclein, that the oligomerization occurs via increased DOPAL production, and that the attained intracellular DOPAL concentration upon exposure to exogenous DOPA is within a physiologic range, perhaps better understanding about determinants of production and metabolism of DOPAL and about DOPAL-induced synuclein oligomerization in individual patients will help resolve this important matter.
In summary, blockade of vesicular uptake increases endogenous DOPAL production, inhibition of aldehyde dehydrogenase and aldehyde reductase attenuates DOPAL metabolism, the combined manipulations augment DOPAL levels further, and DOPAL produced within cells contributes to apoptosis and alpha-synuclein oligomerization. The results therefore fit with the catecholaldehyde hypothesis. Further studies about potential effects of genetically determined alpha-synucleinopathy on endogenous DOPAL production and about factors influencing DOPAL-induced synuclein oligomerization seem indicated.
Acknowledgments
The research reported here was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
The authors thank Gilberto Carmona, Nelson Cole, Richard Imrich, and Roshanak Mansouri for preliminary work that enabled us to carry out the current experiments.
Abbreviations
- DOPAC
dihydroxyphenylacetic acid
- 6FDOPAC
6F-dihydroxyphenylacetic acid
- DOPAL
dihydroxyphenylacetaldehyde
- DOPET
dihydroxyphenylethanol
- PA
peak area
Footnotes
The authors have no conflicts of interest to disclose.
Authorship Credit:
David S. Goldstein: Conception and design, data analysis, data interpretation, drafting the article, final approval
Patti Sullivan: Data acquisition, data analysis
Adele Cooney: Data acquisition, drafting the article, revising the article, data analysis
Yunden Jinsmaa: Data acquisition, data analysis, conception and design, drafting the article, revising the article
Rachel Sullivan: Data acquisition, data analysis
Daniel J. Gross: Data acquisition, data analysis
Courtney Holmes: Data acquisition, data analysis, drafting the article, revising the article
Irwin J. Kopin: Conception and design, data analysis, drafting the article, revising the article critically for important intellectual content
Yehonatan Sharabi: Conception and design, data analysis, drafting the article, revising the article critically for important intellectual content
References
- Adamczyk A, Kazmierczak A, Strosznajder JB. Alpha-synuclein and its neurotoxic fragment inhibit dopamine uptake into rat striatal synaptosomes. Relationship to nitric oxide. Neurochem Int. 2006;49:407–412. doi: 10.1016/j.neuint.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J Biol Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H. Amine oxidase and amine metabolism. Pharmacol Rev. 1952;4:415–458. [PubMed] [Google Scholar]
- Bronstein J, Carvey P, Chen H, et al. Meeting report: consensus statement-Parkinson’s disease and the environment: collaborative on health and the environment and Parkinson’s Action Network (CHE PAN) conference 26–28 June 2007. Env Health Perspectives. 2009;117:117–121. doi: 10.1289/ehp.11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes AA, Korwek KM, Hastings TG. The effect of endogenous dopamine in rotenone-induced toxicity in PC12 cells. Antioxid Redox Signal. 2005;7:630–638. doi: 10.1089/ars.2005.7.630. [DOI] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74:97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system] Wien Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Hovevey-Sion D, Kopin IJ, Miletich R, Kirk KL, Finn R, Goldstein DS. Neuronal uptake and metabolism of 2- and 6-fluorodopamine: False neurotransmitters for positron emission tomographic imaging of sympathetically innervated tissues. J Pharmacol Exp Ther. 1989;248:419–427. [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004a;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Leaky catecholamine stores: undue waste or a stress response coping mechanism? Ann NY Acad Sci. 2004b;1018:224–230. doi: 10.1196/annals.1296.027. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. Journal of neurochemistry. 2008;106:2205–2217. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JT, Chen AQ, Kong Q, Zhu H, Ma CM, Qin C. Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell Mol Neurobiol. 2008;28:35–47. doi: 10.1007/s10571-007-9227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakaso K, Imamura K, Takeshima T, Nakashima K. Endogenous catecholamine enhances the dysfunction of unfolded protein response and alpha-synuclein oligomerization in PC12 cells overexpressing human alpha-synuclein. Neurosci Res. 2010;66:124–130. doi: 10.1016/j.neures.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochimica et Biophysi Acta. 2011;1812:663–673. doi: 10.1016/j.bbadis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem Res Toxicol. 2009;22:835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Kolchinsky AM. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch Neurol. 2008;65:1577–1581. doi: 10.1001/archneur.65.12.1577. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak KS, Rajput AH, Gilbert JJ, Hornykiewicz O. Cerebellar norepinephrine in patients with Parkinson’s disease and control subjects. Arch Neurol. 1984;41:612–614. doi: 10.1001/archneur.1984.04210080020007. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ. Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J Neurosci Res. 2000;60:552–558. doi: 10.1002/(SICI)1097-4547(20000515)60:4<552::AID-JNR14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neuochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- Legros H, Dingeval MG, Janin F, Costentin J, Bonnet JJ. Toxicity of a treatment associating dopamine and disulfiram for catecholaminergic neuroblastoma SH-SY5Y cells: relationships with 3,4-dihydroxyphenylacetaldehyde formation. Neurotoxicology. 2004a;25:365–375. doi: 10.1016/S0161-813X(03)00148-7. [DOI] [PubMed] [Google Scholar]
- Legros H, Janin F, Dourmap N, Bonnet JJ, Costentin J. Semi-chronic increase in striatal level of 3,4-dihydroxyphenylacetaldehyde does not result in alteration of nigrostriatal dopaminergic neurones. J Neurosci Res. 2004b;75:429–435. doi: 10.1002/jnr.10880. [DOI] [PubMed] [Google Scholar]
- Li SW, Lin TS, Minteer S, Burke WJ. 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res Mol Brain Res. 2001;93:1–7. doi: 10.1016/s0169-328x(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasstrom T, Fagerqvist T, Barbu M, et al. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of alpha-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Rad Biol Med. 2011;50:428–437. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS One. 2010;5:e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SS, Schulz EM, Lee D. Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein. Eur J Neurosci. 2007;26:3104–3112. doi: 10.1111/j.1460-9568.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y, Wu Q, Le W, Ye F, Li Y, Dong Z. Rotenone-induced PC12 cell toxicity is caused by oxidative stress resulting from altered dopamine metabolism. Toxicol In Vitro. 2008;22:1461–1468. doi: 10.1016/j.tiv.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Shinkai T, Zhang L, Mathias SA, Roth GS. Dopamine induces apoptosis in cultured rat striatal neurons; possible mechanism of D2-dopamine receptor neuron loss during aging. J Neurosci Res. 1997;47:393–399. [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Mitochondrial complex I inhibitor rotenone inhibits and redistributes vesicular monoamine transporter 2 via nitration in human dopaminergic SH-SY5Y cells. Mol Pharmacol. 2008;74:933–940. doi: 10.1124/mol.108.048546. [DOI] [PubMed] [Google Scholar]
- Wey M, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: Implications for Parkinson’s disease. PLoS ONE. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that {alpha}-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]