Abstract
Objective
Our goal was to determine whether behavioral economic constructs—including impulsivity (i.e., steep discounting of delayed food and monetary rewards), the relative reinforcing value of food (RRVfood), and environmental enrichment (i.e., the presence of alternatives to unhealthy foods in the home and neighborhood environments)—are significant pretreatment predictors of overweight children’s weight loss within family-based treatment.
Method
Overweight children (N = 241; ages 7–12 years; 63% female; 65% non-Hispanic White) enrolled in a 16-week family-based obesity treatment with at least one parent. At baseline, children completed a task to assess RRVfood and delay discounting measures of snack foods and money to assess impulsivity. Parents completed questionnaires to assess environmental enrichment.
Results
Children who found food highly reinforcing and steeply discounted future food rewards at baseline showed a blunted response to treatment compared with children without this combination of risk factors. High environmental enrichment was associated with treatment success only among children who did not find food highly reinforcing. Monetary discounting rate predicted weight loss, regardless of children’s level of RRVfood.
Conclusions
Investigation is warranted into novel approaches to obesity treatment that target underlying impulsivity and RRVfood. Enriching the environment with alternatives to unhealthy eating may facilitate weight loss, especially for children with low RRVfood.
Keywords: pediatric obesity treatment, impulsivity, reinforcing value of food, environmental enrichment
Tailoring pediatric obesity treatments requires an understanding of the individual factors that explain why some children show successful weight loss during treatment, whereas others do not. Only a few studies have examined these individual factors—referred to as pretreatment predictors—among children provided pediatric weight loss treatment (Braet & Beyers, 2009). To date, the most frequent psychological factors examined have been aspects of eating pathology, but it is difficult to draw conclusions about their role in children’s weight loss due to a limited number of studies and differences in treatment characteristics and predictors examined. For example, one study found that children reporting binge eating (i.e., overeating with a sense of loss of control) lost less weight during outpatient family-based treatment (Wildes et al., 2010), whereas others have found no relation between binge eating and children’s weight loss in either an outpatient family-based treatment (Levine, Ringham, Kalarchian, Wisniewski, & Marcus, 2006) or an inpatient treatment (Goossens, Braet, Van Vlierberghe, & Mels, 2009). Still others have examined eating pathology and other pretreatment predictors of longer term weight loss several months or years following treatment cessation, rather than the predictors of the immediate weight loss during treatment (Braet, 2006; Braet & Beyers, 2009; Moens, Braet, & Van Winckel, 2010). In these studies, it is unclear whether the identified predictors are important to treatment success and/or to weight maintenance between treatment and follow-up assessment.
Behavioral economics offers a robust theoretical framework to identify predictors of weight loss but has yet to be applied in this manner. Behavioral economics involves the study of the psychological and economic factors that drive choice, including food and activity choice (Epstein, Leddy, Temple, & Faith, 2007; Epstein, Salvy, Carr, Dearing, & Bickel, 2010). Weight loss requires negative energy balance, in which energy expenditure exceeds energy intake; negative energy balance involves a series of choices that people make regarding their eating and activity. Within a behavioral economic framework, people’s choices to obtain commodities (e.g., food) or engage in activities are influenced by the constraints on those choices as well as the availability of substitutes for those commodities or activities (Bickel et al., 2007). Importantly, people differ in how strongly these constraints and substitutes impact their choices; these individual differences may lead to variation in energy consumption and expenditure, thus impacting weight loss (Epstein, Leddy, et al., 2007; Epstein, Salvy, et al., 2010). These individual differences should be especially important in outpatient behavioral weight-loss treatment, which teaches behavioral skills and guides participants in making healthier choices but does little to modify or restrict the commodities available in the participants’ environment.
Choice is driven in part by the effort needed to obtain one commodity versus another. Commodities for which people will exert considerable effort are identified as powerful reinforcers. In general, food is a powerful reinforcer because it satisfies a critical physiological need (energy consumption); however, its ability to reinforce behavior varies across individuals, contexts, and foods, leading to both adaptive and maladaptive eating behavior (Epstein, Leddy, et al., 2007). For example, individuals who are highly reinforced by foods, especially palatable, high-energy dense (HED) foods, may consume more food than is needed to meet energy demands and may consume food even when satiated, causing positive energy balance. To simulate real-world choice scenarios, in which one choice is made relative to alternative choices, research often examines the relative reinforcing value of food (RRVfood); that is, how reinforcing a particular food—typically HED foods like pizza or candy bars—is relative to other reinforcers (e.g., money or time spent with friends). Research suggests that overweight children and adults have higher RRVfood than their leaner peers (Epstein, Temple, et al., 2007; Saelens & Epstein, 1996; Temple, Legierski, Giacomelli, Salvy, & Epstein, 2008). Moreover, high RRVfood has been linked to future weight gain in children (Hill, Saxton, Webber, Blundell, & Wardle, 2009).
Knowledge of an individual’s level of RRVfood may be critical for understanding how the environment may influence children’s ability to make changes to eating and activity behavior necessary for weight loss. In the present study, environmental enrichment refers to the availability of alternatives to unhealthy eating in the environment and includes healthy foods, recreational equipment and facilities, and facilities and items to promote cognitive stimulation. Intuitively, a highly enriched environment should promote healthy behavior by providing alternatives to unhealthy foods and therefore facilitate weight loss, yet empirical support is lacking. Epstein and his colleagues (Epstein, Roemmich, Stein, Paluch, & Kilanowski, 2005) did not find that increasing alternatives to eating improved weight-loss outcomes or reduced energy intake among overweight children. It is possible that the intervention was not successful because too narrow a range of the available alternatives was used, and these alternatives were not viable substitutes for eating. Alternatively, RRVfood may moderate the relation between environmental enrichment and weight loss. For children with high RRVfood, alternatives to unhealthy eating may provide an insufficient substitute for preferred HED food, whereas for children with low RRVfood, alternatives may successfully compete with HED food, resulting in increased time spent engaged in that healthy alternative and improved weight-loss outcomes.
Choice is also driven by the time frame for receiving a valued commodity. Delay discounting (DD) is the concept that people generally discount the value of a commodity or reward that is not received immediately as a function of the delay to its receipt (Bickel et al., 2007). Consistently choosing smaller, immediate rewards over larger, delayed rewards is described as steep discounting of delayed rewards (Green & Myerson, 2004) and is a behavioral operationalization of impulsivity (Ainslie, 1975). More impulsive individuals may succumb more often to immediate temptations rather than adhere to long-term positive health goals, leading to poorer food choices and heightened obesity risk. Indeed, impulsivity has been shown to be a predictor of excess weight in children and adults, both cross-sectionally (Johnson, Parry, & Drabman, 1978; Weller, Cook, Avsar, & Cox, 2008) and prospectively (Francis & Susman, 2009; Sutin, Ferrucci, Zonderman, & Terracciano, 2011). Two studies have examined the influence of children’s impulsivity on their weight-loss success, using inhibitory control tasks rather than the DD paradigm, and show conflicting results. In one study (Nederkoorn, Jansen, Mulkens, & Jansen, 2007), more impulsive children were less successful in an 8-week outpatient treatment. In contrast, Pauli-Pott, Albayrak, Hebebrand, and Pott (2010) found that impulsivity predicted greater weight loss during a 1-year weight-loss and maintenance outpatient treatment among 10- to 12-year-olds, but not among 7- to 9-year-olds.
The significant differences in treatment characteristics and duration within these prior studies prohibit firm conclusions regarding the role of impulsivity in children’s weight loss. Additionally, RRVfood may moderate the effects of impulsivity on children’s success during treatment, which is consistent with neurobiological models of eating behavior (Alonso-Alonso & Pascual-Leone, 2007; Appelhans, 2009; van den Bos & de Ridder, 2006). Mesolimbic brain regions motivate reward-seeking behavior and underlie RRVfood, whereas regions of the prefrontal cortex underlie executive control over cognition and behavior and mediate impulsivity. Executive control may inhibit mesolimbic-based motivation to consume HED food unless executive control is deficient, in which case high RRVfood may drive behavior, resulting in unhealthy overeating. Alternatively, if RRVfood is low, then the need to override the drive to eat may be minimized. Thus, it follows that RRVfood may moderate the effects of impulsivity on eating behavior. A recent behavioral study supports this notion, which showed a significant interaction between women’s DD and RRVfood on food consumption (Rollins, Dearing, & Epstein, 2010). Specifically, differences among low- and high-impulsive women in laboratory calorie consumption were evident only among women with high—but not low—RRVfood. This moderating effect of RRVfood suggests that high impulsivity alone would not necessarily inhibit weight-loss success; thus, the conflicting results in previous research on the role of impulsivity in children’s weight loss may have partly resulted from not accounting for children’s RRVfood.
In the present study, we tested RRVfood, impulsivity, and environmental enrichment as pretreatment predictors of weight loss among overweight children undergoing a family-based weight-loss treatment (FBT). We predicted that high RRVfood and impulsivity independently would predict poorer weight loss and that high RRVfood would moderate the effects of impulsivity on children’s weight loss, such that impulsivity would be a stronger predictor of weight loss among children with high RRVfood than among children with low RRVfood. We also predicted that greater environmental enrichment at the start of treatment would predict greater weight loss and that this effect would be moderated by RRVfood, such that the effects of environmental enrichment would be strongest among children with low RRVfood.
Method
The present study was part of a larger randomized controlled trial investigating the efficacy of family-based weight maintenance interventions following a standard FBT program. Participants were recruited and assessed at two clinical sites in two large metropolitan areas within the United States. Recruitment occurred between the fall of 2009 and fall of 2010. Research approval was obtained at both sites.
Participants
Participants were 241 overweight children (body mass index [BMI] ≥ 85th percentile based on Centers for Disease Control 2000 norms [Kuczmarski et al., 2000]; age 7–12 years), who had at least one overweight parent (BMI ≥ 25 kg/m2). Each child participated with at least one parent, but only one parent provided data. Families were recruited through local media outlets (television, newspaper, Internet, radio), schools and organizations, referrals from pediatrician offices, and clinics treating weight problems. Families were excluded if there was any contravening psychological, emotional, or diagnosed eating disorder; drug or alcohol dependence; low English comprehension; physical disability or illness that precluded moderate to vigorous physical activity or required severe dietary restriction; or new medication regimen that affected the individual’s weight in either the child or the participating parent. Consent was obtained from all participating parents and assent from children. See Table 1 for baseline sample characteristics.
Table 1.
Baseline Sample Characteristics
| Variable | Total sample (n = 241) | Low RRVfood (n = 128) | High RRVfood (n = 112) | Comparison of low and high RRVfood |
|---|---|---|---|---|
| Age, years | 9.9 (1.3) | 10.1 (1.3) | 9.8 (1.4) | t(238) = 1.8, p = .08 |
| Sex, % female | 62.7% (n = 151) | 64.8% (n = 83) | 60.7% (n = 68) | χ2(1, n = 240) = 0.4, p = .51 |
| Race/ethnicity, % | χ2(3, n = 240) = 0.5, p = .92 | |||
| White (non-Hispanic) | 64.7% (n = 156) | 64.8% (n = 83) | 64.3% (n = 72) | |
| Black | 15.4% (n = 37) | 14.1% (n = 18) | 17.0% (n = 19) | |
| Hispanic | 10.4% (n = 25) | 10.9% (n = 14) | 9.8% (n = 11) | |
| Other | 9.5% (n = 23) | 10.2% (n = 13) | 8.9% (n = 10) | |
| Site | χ2(1, n = 240) = 5.4, p = .02 | |||
| Seattle, % | 42.3% (n = 102) | 44.6% (n = 45) | 55.4% (n = 56) | |
| St. Louis, % | 57.7% (n = 139) | 59.7% (n = 83) | 40.3% (n = 56) | |
| Socioeconomic status | 43.1 (10.4) | 44.1 (10.5) | 41.9 (10.1) | t(230) = 1.6, p = .11 |
| Child percent overweight | 66.0 (26.1) | 64.3 (25.3) | 67.9 (27.0) | t(238) = −1.1, p = .29 |
| Parent BMI | 38.2 (9.3) | 38.1 (9.7) | 38.4 (8.9) | t(238) = −0.2, p = .83 |
| Environmental enrichment | 71.0 (15.6) | 70.9 (15.3) | 71.1 (16.0) | t(232) = −0.1, p = .91 |
| Money discounting | 0.07 (0.08) | 0.07 (0.08) | 0.07 (0.08) | t(237) = −0.1, p = .90 |
| HED food discounting | 4.4 (3.1) | 4.5 (3.1) | 4.3 (3.1) | t(238) = 0.5, p = .51 |
Note. Means (and standard deviations) are reported unless otherwise indicated. RRVfood = relative reinforcing value of food; BMI = body mass index; HED = high-energy dense foods.
Procedure
Children’s response to FBT in the present study was examined prior to randomization into one of three weight-loss maintenance conditions. Therefore, all children in the present study received identical assessments and interventions. Baseline assessments were completed over two visits, typically within the 2 months prior to beginning FBT, and were administered by trained research assistants with at least bachelor’s-level degrees. Following baseline assessment, families participated in a 16-week FBT, which has demonstrated success at reducing child relative body weight (Wilfley et al., 2007). The treatment targeted (a) diet modification (reduce energy intake and improve dietary quality) and (b) physical activity promotion (increase physical activity to 90 min per day for children and 60 min per day for parents, at least 5 days per week), through behavioral modification strategies (promote stimulus control, self-regulatory, and self-monitoring strategies). Treatment sessions were held weekly for 16 weeks and included a 30-min individual family meeting and separate 45-min child and parent groups.
Measures
Anthropometrics
Child and parent heights were measured with shoes removed using a stadiometer, to the nearest 0.1 cm. Child and parent weights were also measured with shoes removed and in light clothing to the nearest 0.1 kg, on a calibrated electronic scale. Height and weight data were used to calculate relative body weight at baseline, Week 9 of FBT, and immediately following FBT (16 weeks). For children, percent overweight was calculated (the percentage the child’s BMI is above the median BMI for the child’s age and sex) (Kuczmarski et al., 2000). For parents, BMI (kg/m2) was calculated.
Demographics
Parents completed a brief demographics questionnaire (i.e., child and parent race/ethnicity, age, and sex). Parents’ occupation and level of education were used to calculate the Barratt Simplified Measure of Social Status—a measure adapted from Hollingshead (1975), designed to be a proxy for socioeconomic status (SES), with higher values indicating higher SES.
Impulsivity
We assessed DD of both primary (HED food) and secondary (money) reinforcers using self-report measures. The first measure asks children to make hypothetical choices between immediate monetary rewards and larger delayed rewards (e.g., “Would you prefer $50 today or $100 in 6 months?”). It contains 27 items across three larger delayed reward magnitudes (small: $25–$35; medium: $50–$60; large: $75–$85). The hyperbolic discounting parameter (k) is computed separately for each magnitude, with larger k values representing steeper discounting of delayed monetary rewards. The k value was positively correlated across small, medium, and large magnitude rewards (r = .54–.73, p < .001), suggesting acceptable internal reliability, and was thus averaged into a single k value. The validity of this measure was established in adults, in which it was shown that heroin addicts discount delayed monetary rewards more steeply than nonaddicts (Kirby, Petry, & Bickel, 1999). It has also been used successfully in children to predict future weight gain (Duckworth, Tsukayama, & Geier, 2010) and has acceptable convergent validity with other measures of impulsivity in children, especially informant reports (Duckworth & Kern, 2011).
DD of HED food rewards was assessed using an adaptation of a measure developed by Mischel and Metzner (1962). It contains nine questions, which ask children whether they would like one portion of a preferred HED food today or two portions of that HED food at different future time points, ranging from 1 day to 1 year. Discounting is determined by calculating the ratio between the number of immediate rewards chosen and the number of delayed rewards chosen, with higher discounting scores representing steeper discounting of delayed HED food rewards. This task has been shown to be sensitive to delay length in similarly aged children, such that preference for delayed rewards inversely correlates with delay length (Mischel & Metzner, 1962). In the present sample, HED food DD scores correlated significantly with monetary DD (r = .25, p < .01), suggesting some degree of convergent validity between the two measures.
RRVfood
The RRVfood task (Goldfield, Epstein, Davidson, & Saad, 2005) asks children to indicate their preference for completing hypothetical work (i.e., pressing a clicker a certain number of times) to obtain a preferred HED food (same food as used in the impulsivity assessment) or a monetary reward ($0.25). The task begins with an equal amount of hypothetical work required to obtain either reward (i.e., 20 presses). With each subsequent item, the number of hypothetical clicker presses required to obtain the HED food increases on a fixed ratio progressive schedule of 20 presses per question (up to 240 presses on the final item), whereas the hypothetical work required to obtain the monetary reward is held constant (20 presses). The point just before the child switches from choosing HED food to choosing money on two consecutive trials is referred to as the switch point. The validity of this measure was established in an adult study, in which it correlated significantly with a laboratory RRVfood task (r = .49) and was sensitive to food deprivation (Goldfield et al., 2005). Finally, it has been successfully used in children as a predictor of future weight gain (Hill et al., 2009).
Environmental enrichment
Parents completed questionnaires assessing the availability of alternatives to HED food in the home and neighborhood environment. Items from the HOME measure (Strauss & Knight, 1999) and a scale developed by Rosenberg and colleagues (2010) assessed the availability of fruits and vegetables, electronics, physical activity equipment, and cognitively stimulating items (e.g., books, musical instruments) in the home. The Cognitive Enrichment subscale of the HOME measure is inversely rated to children’s amount of television watching and their risk of obesity (Strauss & Knight, 1999), and the scale developed by Rosenberg et al. (2010) has demonstrated acceptable test–retest reliability (ICC = .54–.92) and construct validity, including associations between activity equipment and sedentary and physical activity. The Land Use Mix–Diversity subscale from the Neighborhood Environment Walkability Scale (NEWS; Cerin, Saelens, Sallis, & Frank, 2006) assessed the proximity of local community facilities (e.g., parks, playgrounds, recreation centers, and libraries) to the child’s home. The NEWS has acceptable test–retest reliability (ICC = .58–.80) and is sensitive to objective differences in the built environment (Saelens, Sallis, Black, & Chen, 2003). These subscales were combined to create a global index, with higher scores indicating greater environmental enrichment.
Statistical Analyses
Preliminary t tests and chi-square tests revealed differences in the characteristics of children who completed FBT (n = 185) versus those who did not (n = 56). Bivariate correlations and t tests revealed the relations among DD, RRVfood, environmental enrichment, and baseline relative weight. Next, bivariate correlations and chi-square tests revealed the relations between potential covariates and weight loss in order to identify covariates to include in the primary analyses. The primary research aims were tested using two sets of mixed-model hierarchical regression analyses, using all available data after baseline, in which time (i.e., 9- and 16-week time point) was entered as a repeated measure, and covariates and predictors of interest were entered as time-invariant fixed effects. The restricted maximum likelihood method estimated a first-order autoregressive covariance matrix, which accounts for correlations among within-subjects’ error residuals across time points. In the first set of primary analyses, we tested whether RRVfood moderated the effects of DD on changes in relative weight across FBT (i.e., from baseline to 9 weeks to 16 weeks). The base model included relevant covariates, as well as the repeated measure of time. The DD × Time interaction was added in Step 2; the RRVfood and RRVfood × Time interaction were added in Step 3. The DD × RRVfood and DD × RRVfood × Time interactions were added in the final step. These analyses were conducted separately for HED food and monetary DD. Second, we tested whether RRVfood moderated the effects of environmental enrichment on changes in relative weight across FBT. Four nested models were tested, identical to the four described above, with the exception that environmental enrichment replaced DD in all steps. In all sets of analyses, chi-square tests assessed changes in the −2 restricted log-likelihood to determine whether the step improved fit over the nested model. If a step improved model fit (p < .05), the predictors added in that step were examined, and the coefficient estimates, their 95% confidence intervals, and their standard errors are reported for significant predictors. Simple slopes were computed to interpret significant interactions, and standardized beta coefficients (β) were computed to assess the effect size. Mixed-model regression analyses were run using the SPSS 19.0 MIXED procedure. In a final set of analyses, we determined how the main findings translated into actual weight loss by calculating the actual (not predicted) weight loss and percentage of change in weight for children with the various combinations of RRVfood and DD, and RRVfood and environmental enrichment. In these analyses, we used a median split to categorize children into low and high categories of DD and environmental enrichment and used the dichotomization strategy previously described for RRVfood. We calculated Cohen’s d to determine the standardized mean difference in weight loss between categories of children.
Results
Preliminary Analyses
Of the 241 children who entered FBT, 185 (76.8%) completed the 16-week treatment. No differences were observed between those children who completed FBT versus those who did not for child age, sex, minority status, SES, DD (monetary or HED food), RRVfood, environmental enrichment, child baseline percent overweight, or parent baseline BMI (all ps > .10). RRVfood data were missing for one child due to assessor error, monetary DD data were considered invalid for one child due to highly inconsistent responding across the three reward magnitudes, and environmental enrichment data were missing for six children due to questionnaire data inadvertently not being collected at baseline. Three children’s change in percent overweight at either 9 or 16 weeks fell outside of three standard deviations of the mean. These outliers did not alter the pattern of findings and were included in the results reported below. We log-transformed monetary and HED food discounting scores to correct for positive skew, and we standardized discounting and environmental enrichment scores before analysis (M = 0, SD = 1). RRVfood scores were also highly skewed, such that 53.3% of the sample did not respond to food or responded that they would work equally hard for food and money (i.e., switch point < 2). A log-transformation could not normalize the data (Shapiro–Wilks W [240] = 0.81, p < .01), and therefore, we used a dichotomization strategy to categorize children with low RRVfood (n = 128, switch point < 2) or high RRVfood (n = 112, switch point ≥ 2).
Baseline relative weight did not correlate with any of the continuous predictors (ps > .49) or differ between children with high versus low RRVfood (p = .90). HED food DD correlated positively with monetary DD (r = .25, p < .01) and correlated negatively with environmental enrichment (r = −.18, p < .01). RRVfood was unrelated to either DD measure or to environmental enrichment (all ps > .70). Environmental enrichment also correlated positively with SES (r = .22, p < .01), suggesting that they are related (e.g., SES may impact a family’s ability to enrich their homes with nonfood alternatives, such as recreational equipment); however, the modest correlation also suggests that environmental enrichment is not a strong proxy for SES. Child racial/ethnic minority status and sex were related to weight loss (ps < .01) and were included in the primary analyses, whereas SES, baseline percent overweight, parent baseline BMI, and child age were unrelated to weight loss (ps > .20) and were excluded from the main analyses. We also examined clinical site because initial analyses revealed that RRVfood scores varied across the two clinical sites (see Table 1); however, site was unrelated to weight loss and did not interact with any of the primary predictors of interest (ps > .20); therefore, we excluded it from the main analyses.
Main Analyses of Behavioral Economic Predictors
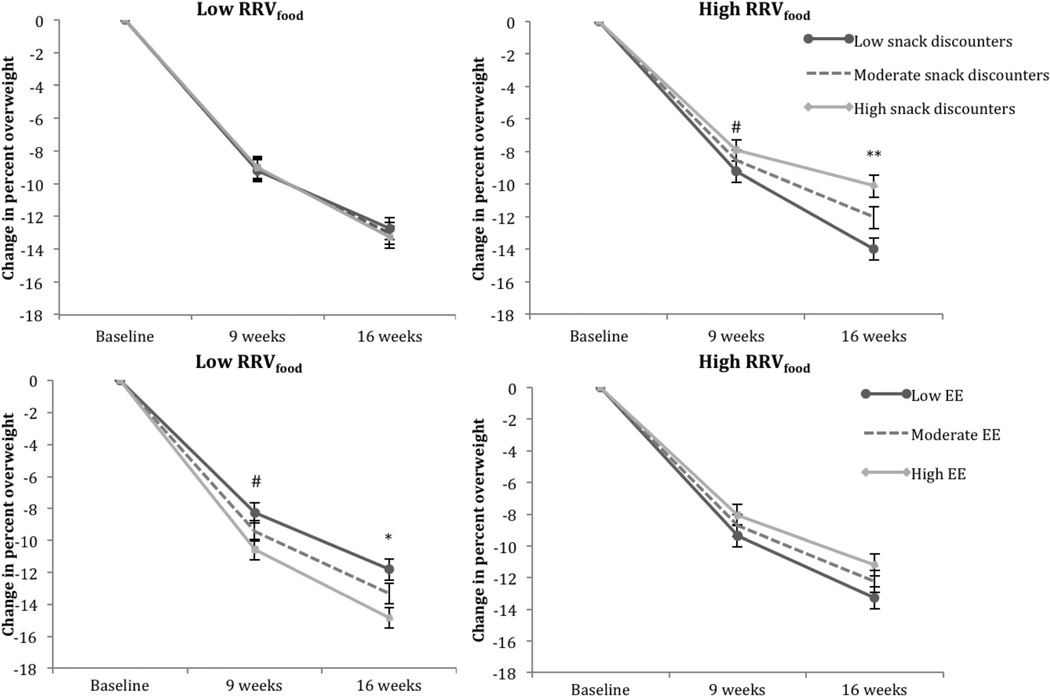
The effects of DD and RRVfood on change in percent overweight across treatment are displayed in Table 2. In examining the independent and interactive effects of money DD and RRVfood, only Step 2 improved model fit (p = .025). In this step, the main effect of money DD was significant (p = .008) but did not interact with time (p > .10), suggesting a similar effect at Weeks 9 and 16. We computed simple slopes to examine these findings, which showed that children who more steeply discounted monetary rewards lost less weight by the 9-week time point (B = 1.01, CI [0.06, 1.96], p = .037, β = .19) and by the 16-week time point (B = 1.51, CI [0.54, 2.47], p = .002, β = .18). In the model including the independent and interactive effects of HED food DD and RRVfood, only Step 4 improved model fit (p = .002). In this final step, HED food DD interacted with RRVfood (p = .011) and with RRVfood × Time (p = .009). Simple slopes analyses suggested that for children with high RRVfood, steeper HED food discounting predicted less weight loss by 16 weeks (B = 2.19, CI [0.83, 3.55], p = .002, β = .40) and marginally less weight loss by 9 weeks (B = 1.20, CI [−0.14, 2.54], p = .077, β = .22). For children with low RRVfood, HED food discounting did not predict weight loss at either 9 weeks (p = .78) or 16 weeks (p = .51). The top panel of Figure 1 displays this three-way interaction.
Table 2.
The Independent and Interactive Effects of Delay Discounting and RRVFood on Children’s Change in Percent Overweight
| Money discounting model | HED food discounting model | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Parameter estimate |
95% CI | p | Step fit (−2LL)c | Parameter estimate |
95% CI | p | Step fit (−2LL)c |
| Step 1 | 2244.50 | 2254.71 | ||||||
| Minority statusa | 2.65 | [0.79, 4.49] | .005 | 2.64 | [0.80, 4.49] | .005 | ||
| Sexb | 2.18 | [0.34, 4.02] | .021 | 2.18 | [0.34, 4.01] | .020 | ||
| Time | −3.77 | [−4.38, −3.16] | <.001 | −3.77 | [−4.38, −3.16] | <.001 | ||
| Step 2 | 2237.16 | 2253.02 | ||||||
| DD | 1.28 | [0.34, 2.23] | .008 | 0.67 | [−0.29, 1.63] | .170 | ||
| DD × Time | 0.50 | [−0.12, 1.11] | .113 | 0.15 | [−0.47, 0.77] | .627 | ||
| Step 3 | 2231.54 | 2247.58 | ||||||
| RRVfood | 1.65 | [−0.22, 3.51] | .084 | 1.55 | [−0.32, 3.42] | .105 | ||
| RRVfood × Time | 0.56 | [−0.66, 1.79] | .366 | 0.52 | [−0.70, 1.74] | .404 | ||
| Step 4 | 2228.52 | 2235.30 | ||||||
| DD × RRVfood | −0.64 | [−2.56, 1.28] | .510 | 2.45 | [0.60, 4.34] | .011 | ||
| DD × RRVfood × Time | −0.24 | [−1.47, 1.00] | .705 | 1.64 | [0.42, 2.86] | .009 | ||
| Step comparison | ||||||||
| Step 2 vs. 1 | χ2(2) = 7.34, p = .025 | χ2(2) = 1.69, p = .430 | ||||||
| Step 3 vs. 2 | χ2(2) = 5.62, p = .060 | χ2(2) = 5.24, p = .073 | ||||||
| Step 4 vs. 3 | χ2(2) = 3.02, p = .221 | χ2(2) = 12.66, p = .002 | ||||||
Note. RRVfood = relative reinforcing value of food; HED = high-energy dense foods; CI = confidence interval; LL = log-likelihood; DD = delay discounting.
Racial/ethnic minority status: 0 = nonminority, 1 = minority.
Sex: 0 = male, 1 = female.
Smaller values represent better fit.
Figure 1.
RRVfood moderated the effects of HED food discounting (top panel) and the effects of environmental enrichment (bottom panel) on change in children’s percent overweight over treatment. Error bars are standard errors of the slope. RRVfood = relative reinforcing value of food; Low = −1 SD below the mean; EE = environmental enrichment; Moderate = mean score; High = +1 SD above the mean. # p ≤ .10. * p < .05. ** p < .01.
The effects of environmental enrichment and RRVfood on change in percent overweight are shown in Table 3. Only Step 4 improved model fit (p = .019). In this final step, environmental enrichment interacted with RRVfood (p = .021), independent of time (p = .29). Simple slopes revealed that for children with low RRVfood, greater environmental enrichment predicted greater weight loss by 16 weeks (B = −1.35, CI [−2.67, −0.03], p = .044, β = −.25) and marginally greater weight loss by 9 weeks (B = −1.08, CI [−2.38, 0.22], p = .10, β = −.20). See the bottom panel of Figure 1.
Table 3.
The Independent and Interactive Effects of Environmental Enrichment and RRVFood on Children’s Change in Percent Overweight
| Predictor | Parameter estimate | 95% CI | p | Step fit (−2LL)c |
|---|---|---|---|---|
| Step 1 | 2225.55 | |||
| Minority statusa | 2.74 | [0.89, 4.59] | .004 | |
| Sexb | 2.27 | [0.42, 4.12] | .016 | |
| Time | −3.79 | [−4.40, 3.17] | <.001 | |
| Step 2 | 2225.55 | |||
| EE | −0.21 | [−1.15, 0.74] | .666 | |
| EE × Time | 0.04 | [−0.57, 0.64] | .900 | |
| Step 3 | 2220.92 | |||
| RRVfood | 1.37 | [−0.53, 3.27] | .158 | |
| RRVfood × Time | 0.47 | [−0.77, 1.71] | .456 | |
| Step 4 | 2212.96 | |||
| EE × RRVfood | 2.20 | [0.34, 4.05] | .021 | |
| EE × RRVfood × Time | 0.65 | [−0.57, 1.86] | .294 | |
| Step comparison | ||||
| Step 2 vs. 1 | χ2(2) = 0.00, p = .999 | |||
| Step 3 vs. 2 | χ2(2) = 4.63, p = .099 | |||
| Step 4 vs. 3 | χ2(2) = 7.96, p = .019 | |||
Note. RRVfood = relative reinforcing value of food; CI = confidence interval; LL = log-likelihood; EE = environmental enrichment.
Racial/ethnic minority status: 0 = nonminority, 1 = minority.
Sex: 0 = male, 1 = female.
Smaller values represent better fit.
Additional Analyses
To determine whether the covariates unduly influenced the main results described above, we analyzed the same models excluding the covariates (i.e., racial/ethnic minority status and sex). The pattern of results held with only minor changes to the size of the parameter estimates. The main effect of monetary DD of weight loss was slightly stronger (estimate = 1.51, CI [0.55, 2.47], p = .002). The HED Food DD × RRVfood × Time interaction was unchanged (estimate = 1.64, CI [0.41, 2.86], p = .009). Finally, the Environmental Enrichment × RRVfood interaction was slightly weaker (estimate = 1.89, CI [−0.02, 3.81], p = .052). Next, because environmental enrichment correlated with SES, we tested a model including SES as a covariate. In this model, the Environmental Enrichment × RRVfood interaction was stronger (estimate = 2.00, CI [0.08, 3.93], p = .04) compared with the previous model with no covariates. We also tested the models using change in zBMI as the outcome measure and obtained an identical pattern of findings as described above. Finally, we tested the models using children’s relative weight at each time point (rather than their change in relative weight from baseline) and controlled for children’s baseline relative weight. These analyses yielded an identical pattern of results to those described above.
Table 4 shows how the main results translate into actual weight loss and percentage of change in weight from baseline to the end of FBT. The most notable result is the large difference (d = 0.75) in weight reduction between children with low- and high-HED food DD, who also had high RRVfood. Whereas children with high RRVfood but low-HED food DD lost, on average, 3.6 kg (8.0 pounds; 5.9% reduction in weight), children with high RRVfood and high-HED food DD lost only 1.5 kg (3.4 pounds; 2.8% reduction in weight) on average. However, children with low RRVfood lost around 3 kg (6.6 pounds; 5.4% reduction in weight), regardless of their discounting rate. There was also a small difference (d = 0.29) in weight reduction between children with low and high environmental enrichment, who also had low RRVfood. Finally, there was a small-to-medium difference (d = 0.39) in weight loss between children with low and high monetary discounting, irrespective of their RRVfood.
Table 4.
Weight Loss Over Treatment Based on Baseline Levels of Delay Discounting, RRVFood, and Environmental Enrichment
| Low RRVfood | High RRVfood | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | Change in weighta (SD) | % change in weight | Cohen’s d | n | Change in weight (SD) |
% change in weight | Cohen’s d |
| Low-food DD | 48 | −2.9 kg (3.0) | −5.3% | 0.05 | 42 | −3.6 kg (3.2) | −5.9% | 0.75 |
| High-food DD | 48 | −3.1 kg (3.4) | −5.4% | 44 | −1.5 kg (2.4) | −2.8% | ||
| Low EE | 52 | −2.6 kg (3.4) | −4.5% | 0.29 | 44 | −2.5 kg (3.2) | −4.3% | 0.09 |
| High EE | 44 | −3.5 kg (2.9) | −6.4% | 39 | −2.7 kg (3.0) | −4.6% | ||
| Irrespective of children’s RRVfood | ||||||||
| n | Change in weighta(SD) | % change in weight | Cohen’s d | |||||
| Low-money | 85 | −3.5 kg (3.1) | −5.9% | 0.39 | ||||
| High-money | 97 | −2.3 kg (3.0) | −4.2% | |||||
Note. RRVfood = relative reinforcing value of food; DD = delay discounting; EE = environmental enrichment.
Weight in kilograms at the end of treatment minus weight in kilograms at baseline.
Discussion
In the present study, we used a behavioral economic framework to identify internal and external factors that may influence children’s ability to lose weight during treatment. Past research has established the utility of behavioral economics to understand individual differences in food consumption, weight status, and future weight gain (Epstein, Salvy, et al., 2010). The present study extends its utility to explain individual differences in success during a comprehensive FBT. In particular, the results show that impulsivity and environmental enrichment predicted children’s weight loss, but these effects largely depended on the child’s initial level of RRVfood. Children who consistently made impulsive decisions regarding immediate versus larger delayed HED food rewards lost less weight if they had high RRVfood. Moreover, children who lived in an environment enriched with alternatives to unhealthy eating lost more weight if they had low RRVfood. Only monetary discounting exerted a main effect independent of RRVfood, such that children who steeply discounted the value of future monetary rewards lost less weight than did children who discounted less steeply.
The strongest predictor was children’s discounting of delayed HED food rewards, but only among children who were more reinforced by food than by money. Specifically, the results suggest that high RRVfood coupled with steep discounting of delayed HED foods may be a significant impediment to modifying food and activity choices necessary to lose excess weight during family-based treatment. Over time, those individuals with the highest levels of impulsivity and RRVfood may develop reinforcement pathology (Bickel et al., 2012; Carr, Daniel, & Epstein, 2011; Epstein, Salvy, et al., 2010). Reinforcement pathology is thought to develop as HED food reinforcers gain strength and successfully compete with other potential reinforcers to become a central motivating factor underlying food choice. This increase in RRVfood is accompanied by a narrowing in perception for alternatives and a greater discounting of future rewards (Epstein, Salvy, et al., 2010). Although a focus on immediate rewards and a strong motivation to obtain HED foods may have been adaptive in an earlier era in which HED foods were scarce, it is likely maladaptive in today’s obesogenic environment, in which HED foods are abundant and cheap.
Importantly, these behavioral economic constructs were at least marginally significant predictors of weight loss occurring during the first 9 weeks of treatment but were stronger predictors of weight loss during the entire 16-week treatment. This is especially evident in the HED Food DD × RRVfood × Time interaction, in which the negative effects of steep discounting of HED food rewards coupled with high RRVfood increased in strength as treatment continued (see Figure 1, top panel). It is possible that motivational and novelty factors partially outweighed these negative effects early on, but over time, the positive effects of motivation and treatment novelty diminished, leading to even poorer weight loss during the second half of treatment for those children with this combination of reinforcement pathology risk factors. To address the negative effects of impulsivity coupled with high RRVfood, the next generation of weight-loss treatments should incorporate techniques targeted to children who have these factors. One possibility is to train the neurocognitive processes that underlie choice behavior. Previous research has shown that the executive control processes that mediate impulsivity are sensitive to various forms of training in children (see Diamond & Lee, 2011, for a review). In particular, working memory training may be effective, which has been shown to reduce impulsive discounting of delayed rewards in adults with stimulant additions (Bickel, Yi, Landes, Hill, & Baxter, 2011) and to reduce alcohol consumption in problem drinkers (Houben, Wiers, & Jansen, 2011). Working memory training appears to be a domain-general tool to increase the capacity and updating ability of working memory, which in turn improves future-oriented and controlled cognition and behavior (Bryck & Fisher, 2012). Given the domain-general effects, the content of the training may not need to be specific to modifying eating and activity behavior, and its effects may extend to various scenarios in which cognitive control is needed. It is worth testing whether working memory training, in conjunction with FBT, improves weight-loss outcomes beyond FBT alone for children with impulsive decision making and high RRVfood.
Altering RRVfood may be more difficult. Behavioral economic theory suggests that enriching the environment should provide children with competing choices, resulting in healthy behavior substituting for energy consumption (Epstein, Salvy, et al., 2010). The present study suggests that this approach may be most effective for children who enter treatment with low RRVfood. Children with high RRVfood may have difficulty using alternatives or may be unaware of them due to a narrowing of perception related to reinforcement pathology (Epstein, Salvy, et al., 2010). Previous research using the approach of only increasing a narrow set of behaviors incompatible with eating did not result in a decrease in energy intake or improve children’s FBT response (Epstein et al., 2005). Thus, it will likely be important to modify the children’s home and neighborhood environment to increase alternatives to HED food (Wilfley, Vannucci, & White, 2010), but first it may be necessary to identify the alternatives that successfully compete with food intake among children with high RRVfood. One promising strategy is to increase social interaction, which has been shown to substitute for eating in overweight children (Salvy, Coelho, Kieffer, & Epstein, 2007).
It is interesting that over 50% of children fell into the low-RRVfood category, despite all children being overweight or obese at the time of baseline assessment. This may seem contradictory to previous research implicating high RRVfood in obesity (e.g., Temple et al., 2008). Given that these were treatment-seeking children, social desirability may have led to diminished responding to HED food, which may not have occurred in previous nontreatment-seeking samples (e.g., Hill et al., 2009; Temple et al., 2008). This possible discrepancy warrants direct comparison of treatment-seeking to nontreatment-seeking overweight children to determine whether they differ in RRVfood. Also, it is important to remember the phenotypic complexity of obesity (Ziauddeen, Farooqi, & Fletcher, 2012). Thus, even though overweight children tend to have higher RRVfood than their leaner peers, there is still substantial variation in RRVfood among both overweight and nonoverweight children, and there are other risk factors for obesity, including genetic, environmental, and other psychological factors.
The interaction between RRVfood and impulsivity only occurred for discounting of HED foods and not for discounting of money. A previous study showed that monetary discounting interacted with RRVfood to predict energy consumption (Rollins et al., 2010), but research with drug abusers has shown discounting of their drug of choice to be greater than that of monetary reinforcers (Bickel et al., 2007). Obese people may be generally more impulsive than nonobese people (e.g., Weller et al., 2008), but may be especially impulsive in the context of food choice. Moreover, previous research shows that directly consumable rewards are discounted differently in adults than is money (Estle, Green, Myerson, & Holt, 2007), and it seems this also would be true in children, who likely have much more direct experience with (and therefore a better understanding of) food than money. Of note, the questionnaire approach we used to measure DD and RRVfood correlates with laboratory-based behavioral measures of these constructs (Epstein, Dearing, & Roba, 2010; Epstein et al., 2003), but they may not be as strong predictors as laboratory-based behavioral measures. Furthermore, the two DD questionnaires had different formats and different ways of calculating discounting, which could explain the discrepant pattern of results. Despite these limitations, the present study supports these measures’ predictive validity with regard to children’s response to FBT.
Another limitation is that eating pathology was not examined in this study. As noted in the introduction, there is some indication that eating pathology is an important predictor of children’s weight loss (Braet & Beyers, 2009). Aspects of eating pathology, including binge eating, have been associated with higher RRVfood and DD in adult women (Goldfield & Lumb, 2008; Manwaring, Green, Myerson, Strube, & Wilfley, 2011), but it is unknown whether these associations occur in children. It is possible that children in this sample with high RRVfood and/or DD were more likely to have eating pathology, which raises the possibility that eating pathology may be an unexamined confounder. That stated, behavioral economic measures provide insight into the underling mechanisms that drive unhealthy food choice, which traditional eating pathology measures may not provide.
A final limitation is that we examined pretreatment predictors in the context of children’s short-term treatment success but not long-term weight maintenance. Thus, it is unclear whether these predictors can be used to determine which children will garner sustained health benefits from FBT and which children will not. That stated, because the present study involves the immediate success during treatment, the findings provide insight into the factors that influence weight loss per se, which may differ from correlates of sustained weight maintenance. It should be noted that these behavioral economic predictors might not be important in every type of weight-loss treatment for children. For example, they may not be meaningful in an inpatient treatment setting, in which children’s environment is significantly altered and their choices are highly restricted. Thus, the present findings require replication and extension in order to firmly establish the utility of behavioral economics in understanding overweight children’s weight loss.
Overall, the present study’s findings suggest that the psychological processes that govern food choice also influence children’s weight-loss success during a family-based behavioral weight-loss treatment. The results highlight the importance of considering external environmental factors in conjunction with these internal processes. Tailoring treatment to individuals’ needs—for example, by targeting impulsivity and high RRVfood along with specific environmental modifications—may maximize children’s weight losses within this type of treatment.
Acknowledgments
This work was supported by National Institues of Health (NIH) Grants 5-RO1HD036904 (National Institue of Child Health and Human Development), 5K24MH070446 (National Institue of Mental Health), and 5T32HL007456 (National Heart, Lung, and Blood Institute [NHLBI]) awarded to Denise E. Wilfley. John R. Best, Kelly R. Theim, and Dana M. Gredysa were supported by NIH Grant 5T32HL007456 (NHLBI), and Richard I. Stein was supported by NIH Grant KL2RR024994 (National Center for Research Resources). This work was also made possible by NIH Grant UL1 RR024992 (National Center for Research Resources), which supports the Washington University Pediatric and Adolescent Ambulatory Research Consortium (WU PAARC) and the WU Institute of Clinical and Translational Sciences, and by the St. Louis Children’s Hospital Foundation, which supports WU PAARC. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. We are grateful to the families who participated in this study.
Contributor Information
John R. Best, Department of Psychiatry, Washington University School of Medicine
Kelly R. Theim, Department of Psychology, Washington University in St. Louis
Dana M. Gredysa, Department of Psychology, Washington University in St. Louis
Richard I. Stein, Department of Psychiatry, Washington University School of Medicine
R. Robinson Welch, Department of Psychiatry, Washington University School of Medicine.
Brian E. Saelens, Seattle Children’s Research Institute, University of Washington
Michael G. Perri, College of Public Health and Health Professions, University of Florida
Kenneth B. Schechtman, Division of Biostatistics, Washington University School of Medicine
Leonard H. Epstein, Department of Pediatrics, University at Buffalo School of Medicine and Biomedical Sciences
Denise E. Wilfley, Department of Psychiatry, Washington University School of Medicine
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA: The Journal of the American Medical Association. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obesity. 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mackillop J, Epstein LH, Carr KA, Mueller ET, Walz T. The behavioral economics of reinforcement pathologies: Novel approaches to addictive disorders. In: Shaffer HJ, editor. Addiction syndrome handbook. Washington, DC: American Psychological Association; 2012. [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90(Suppl. 1):S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity. 2006;14:148–155. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- Braet C, Beyers W. Subtyping children and adolescents who are overweight: Different symptomatology and treatment outcomes. Journal of Consulting and Clinical Psychology. 2009;77:814–824. doi: 10.1037/a0016304. [DOI] [PubMed] [Google Scholar]
- Bryck RL, Fisher PA. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. American Psychologist. 2012;67:87–100. doi: 10.1037/a0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KA, Daniel TO, Epstein LH. Reinforcement pathology and obesity. Current Drug Abuse Reviews. 2011;4:190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerin E, Saelens BE, Sallis JF, Frank LD. Neighborhood Environment Walkability Scale: Validity and development of a short form. Medicine and Science in Sports and Exercise. 2006;38:1682–1691. doi: 10.1249/01.mss.0000227639.83607.4d. [DOI] [PubMed] [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011 Aug 19;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Kern ML. A meta-analysis of the convergent validity of self-control measures. Journal of Research in Personality. 2011;45:259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Tsukayama E, Geier AB. Self-controlled children stay leaner in the transition to adolescence. Appetite. 2010;54:304–308. doi: 10.1016/j.appet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Roba LG. A questionnaire approach to measuring the relative reinforcing efficacy of snack foods. Eating Behaviors. 2010;11:67–73. doi: 10.1016/j.eatbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Experimental and Clinical Psychopharmacology. 2003;11:131–138. doi: 10.1037/1064-1297.11.2.131. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: Clinic and field studies. Annals of Behavioral Medicine. 2005;30:201–209. doi: 10.1207/s15324796abm3003_4. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & Behavior. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D-sub-2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychological Science. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Francis LA, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Archives of Pediatrics and Adolescent Medicine. 2009;163:297–302. doi: 10.1001/archpediatrics.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield GS, Epstein L, Davidson M, Saad F. Validation of a questionnaire measure of the relative reinforcing value of food. Eating Behaviors. 2005;6:283–292. doi: 10.1016/j.eatbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Lumb A. Effects of dietary restraint and body mass index on the relative reinforcing value of snack food. Eating Disorder: The Journal of Treatment and Prevention. 2008;17:46–62. doi: 10.1080/10640260802570106. [DOI] [PubMed] [Google Scholar]
- Goossens L, Braet C, Van Vlierberghe L, Mels S. Weight parameters and pathological eating as predictors of obesity treatment outcome in children and adolescents. Eating Behaviors. 2009;10:71–73. doi: 10.1016/j.eatbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. American Journal of Clinical Nutrition. 2009;90:276–281. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: Training working memory to reduce alcohol abuse. Psychological Science. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Johnson WG, Parry W, Drabman RS. The performance of obese and normal size children on a delay of gratifcation task. Addictive Behaviors. 1978;3:205–208. doi: 10.1016/0306-4603(78)90020-5. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Johnson CL. CDC growth charts: United States. Advance Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Levine MD, Ringham RM, Kalarchian MA, Wisniewski L, Marcus MD. Overeating among seriously overweight children seeking treatment: Results of the children’s eating disorder examination. International Journal of Eating Disorders. 2006;39:135–140. doi: 10.1002/eat.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring JL, Green L, Myerson J, Strube M, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: Evidence for general rather than specific differences. The Psychological Record. 2011;61:561–582. doi: 10.1007/bf03395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Metzner R. Preference for delayed reward as a function of age, intelligence, and length of dealy interval. Journal of Abnormal and Social Psychology. 1962;64:425–431. doi: 10.1037/h0045046. [DOI] [PubMed] [Google Scholar]
- Moens E, Braet C, Van Winckel M. An 8-year follow-up of treated obese children: Children’s, process and parental predictors of successful outcome. Behaviour Research and Therapy. 2010;48:626–633. doi: 10.1016/j.brat.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy. 2007;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Albayrak Ö, Hebebrand J, Pott W. Does inhibitory control capacity in overweight and obese children and adolescents predict success in a weight-reduction program? European Child & Adolescent Psychiatry. 2010;19:135–141. doi: 10.1007/s00787-009-0049-0. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55:420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DE, Sallis JF, Kerr J, Maher J, Norman GJ, Durant N, Saelens BE. Brief scales to assess physical activity and sedentary equipment in the home. International Journal of Behavioral Nutrition and Physical Activity. 2010;7:1–11. doi: 10.1186/1479-5868-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: An environmental scale evaluation. American Journal of Public Health. 2003;93:1552–1558. doi: 10.2105/ajph.93.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvy S-J, Coelho JS, Kieffer E, Epstein LH. Effects of social contexts on overweight and normal-weight children’s food intake. Physiology & Behavior. 2007;92:840–846. doi: 10.1016/j.physbeh.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Knight J. Influence of the home environment on the development of obesity in children. Pediatrics. 1999;103:e85. doi: 10.1542/peds.103.6.e85. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. Journal of Personality and Social Psychology. 2011;101:579–592. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AP, Salvy S, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. The American Journal of Clinical Nutrition. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, de Ridder D. Evolved to satisfy our immediate needs: Self-control and the rewarding properties of food. Appetite. 2006;47:24–29. doi: 10.1016/j.appet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Kalarchian MA, Levine MD, Houck PR, Cheng Y. Self-reported binge eating in severe pediatric obesity: Impact on weight change in a randomized controlled trial of family-based treatment. International Journal of Obesity. 2010;34:1143–1148. doi: 10.1038/ijo.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: A meta-analytic review of randomized controlled trials. Health Psychology. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Vannucci A, White EK. Early intervention of eating- and weight-related problems. Journal of Clinical Psychology in Medical Settings. 2010;17:285–300. doi: 10.1007/s10880-010-9209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: How convincing is the addiction model? Nature Reviews Neuroscience. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]