Abstract
Lateral hypothalamic (LH) orexin neurons are essential for the expression of a cocaine place preference. However, afferents that regulate activity of these orexin neurons during reward behaviors are not completely understood. Using tract tracing combined with Fos staining, we examined LH afferents for Fos induction during cocaine preference in rats. We found that ventral bed nucleus of the stria terminalis (vBNST) was a major input to the LH orexin cell field that was significantly Fos-activated during cocaine conditioned place preference (CPP). Inactivation of vBNST with baclofen plus muscimol blocked expression of cocaine CPP. Surprisingly, such inactivation of vBNST also increased Fos induction in LH orexin neurons; as activity in these cells is normally associated with increased preference, this result indicates that a vBNST-orexin connection is unlikely to be responsible for CPP that is dependent on vBNST activity. Because previous studies have revealed that vBNST regulates dopamine cells in ventral tegmental area (VTA), known to be involved in CPP and other reward functions, we tested whether vBNST afferents to VTA are necessary for cocaine CPP. We found that disconnection of vBNST and VTA (using local microinjections of baclofen plus muscimol unilaterally into vBNST and contralateral VTA) significantly attenuated expression of cocaine preference. However, blocking ionotropic glutamatergic afferents to VTA from vBNST did not significantly reduce cocaine preference. These results indicate that a non-glutamatergic vBNST-VTA projection is involved in expression of cocaine preference.
Keywords: rat, orexin, hypocretin, bed nucleus of the stria terminalis, lateral hypothalamus, ventral tegmental area, cocaine, conditioned place preference
Introduction
Orexins (also named hypocretins) have been shown to play a role in mediating an array of behaviors related to reward and motivation. In particular, inhibition of the orexin 1 receptor (OX1R) attenuates seeking behaviors for alcohol (Lawrence et al., 2006), cocaine (Smith et al., 2009a; Smith et al., 2009b), nicotine (Hollander et al., 2008), amphetamine (Hutcheson et al., 2011), and high fat (Borgland et al., 2009). However, the neural circuitry underlying orexin’s role in reward-seeking and related behaviors is poorly understood.
Orexin neurons, particularly in LH, potentially have ideal neuronal connections to regulate reward processing. For example, they project to areas involved in motivation and reward such as the nucleus accumbens (NAc) and ventral tegmental area (VTA) (Peyron et al., 1998; Fadel and Deutch, 2002; Baldo et al., 2003), and these areas express high levels of orexin receptors (Trivedi et al., 1998; Marcus et al., 2001; Korotkova et al., 2003). Several studies have shown that orexin input to VTA is important in reward-related behaviors (Narita et al., 2006; Harris et al., 2007; Espana et al., 2011; James et al., 2011; Mahler et al., 2012), and recent evidence indicates that orexin projections to other brain areas are also involved in drug seeking (Schneider et al., 2007; Hollander et al., 2008).
Although orexin projections to reward-related areas appear to play a significant role in reward and motivation, until recently very little was known about inputs to orexin neurons that regulate their activity during drug-seeking. LH orexin neurons receive afferents from several reward-related brain regions such as nucleus accumbens shell (NAcS), BNST, central amygdala (CeA), lateral septum (LS), and prelimbic and infralimbic cortices (Sakurai et al., 2005; Yoshida et al., 2006; Marchant et al., 2009). LH orexin inputs from the LS and NAcS have been implicated in cocaine CPP (Sartor and Aston-Jones, 2012), beer seeking (Millan et al., 2010) and food intake (Baldo et al., 2004). However, it remains to be determined if other afferents are also important in regulating LH orexin activity during reward-related behaviors.
In the current study a combination of tract-tracing, cocaine CPP, and staining for the immediate early gene product, Fos, was used to identify brain regions that may be important in activating LH orexin neurons and associated cocaine preference. We found that inputs to LH from vBNST show Fos activation during cocaine CPP in proportion to the preference expressed, and inactivation of vBNST blocked cocaine preference. Surprisingly, however, vBNST inactivation increased Fos expression in orexin neurons in LH, indicating that activation of these cells was unlikely to mediate the role of vBNST in cocaine preference.
Several studies have revealed a strong functional and anatomical projection from vBNST to VTA (Georges and Aston-Jones, 2001, 2002; Massi et al., 2008; Briand et al., 2010). Therefore, in the second group of experiments here, we determined if vBNST afferents to VTA are necessary for expression of cocaine place preference. Using a vBNST-VTA bilateral disconnection approach, we found that vBNST afferents to VTA are critical for cocaine preference.
Methods
Animals
Male Sprague Dawley rats (initial weight approximately 300-325g, Charles River, Raleigh, NC) were pair-housed under a reversed 12-h light/dark cycle and had ad libitum access to food and water. Animals were housed in a temperature and humidity-controlled animal facility at the Medical University of South Carolina (AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at MUSC and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals. A total of 110 rats were used in these experiments.
Stereotaxic surgery
In Experiment 1, microinjections of cholera toxin b subunit (CTb) were made into LH using methods similar to those described in previous publications (Delfs et al., 1998; Chen et al., 1999; Sartor and Aston-Jones, 2012). In brief, rats were anesthetized with ketamine/xylazine (56.5/8.7 ml/kg, ip) and placed in a stereotaxic apparatus. A glass pipette (10 μm tip diameter) was lowered into the LH orexin field (A/P −2.8, M/L 1.7, DV −8.8 from skull surface), and 30 nl of CTb (0.5% dissolved in 0.1 M phosphate buffer, Sigma) was unilaterally delivered via pressure injection. The pipette was left in place for 15 minutes after the injection to allow for CTb diffusion and to minimize backflow up the pipette tract.
In Experiments 2, 3 and 4, guide cannulae (22 gauge, Plastics One Inc.) were implanted unilaterally or bilaterally 2 mm above vBNST (±3.5 M/L, −0.1 A/P, −6.1 D/V, 15° angle lateral to medial). The guide cannulae were fastened to the skull using acrylic cement and obturators were inserted to prevent blockage. Five minutes prior to the preference test, injector cannulae (28 gauge, Plastics One, Inc.) were lowered through and 2 mm below the guide cannulae, and one of the following was infused: artificial cerebrospinal fluid (aCSF), a cocktail of the GABA receptor agonists baclofen and muscimol (B-M, 0.3/0.03nmol respectively), or the OX1R antagonist SB-334867 (1 mM SB; 1-(2-methylbenzoxazol- 6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride, Tocris). In Experiment 3, unilateral B-M injections outside of vBNST (n=5) and aCSF injection into vBNST (n=4) were combined and referred to as control injections because there were no differences in CPP scores or Fos expression between these two groups (t-values < 1.2, p > 0.05).
In the bilateral disconnection studies (Experiments 5 and 6), guide cannulae were implanted unilaterally above vBNST and contralateral VTA (±2.1 M/L, −5.2 A/P, −6.9 D/V, 10° angle M/L). Five minutes before the preference test injector cannulae (28 gauge Plastics One Inc.) were lowered through and 2 mm below the guide cannulae, and one of the following was infused: aCSF, the GABA receptor agonist cocktail baclofen plus muscimol (B-M, 0.3/0.03nmol respectively), or a cocktail of the glutamate receptor antagonists, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 0.7 mM) and (2R)-amino-5-phosphonovaleric acid (AP5, 1.6 mM). All drugs were dissolved in aCSF and injection volumes were 0.3 μl. The injector cannulae were kept in place for 1 minute to allow for diffusion of drug. Animals with B-M or CNQX-AP5 injections outside of vBNST or VTA (missed injections) were used for control data.
Conditioned place preference
CPP experiments utilized methods previously employed by our laboratory (Harris and Aston-Jones, 2003; Harris et al., 2004; Harris et al., 2005; Sartor and Aston-Jones, 2012). Briefly, the CPP apparatus consisted of 2 distinct compartments that were separated by a removable partition. In a pre-test acclimation session, animals were allowed free access to both sides of the chamber for 15 minutes via a doorway in the partition. The time spent on each side of the chamber was recorded automatically. None of the animals had an initial bias on the pre-test (<100 s time difference). Two days later, animals were conditioned for 3 consecutive days. During conditioning the animals were injected with cocaine (10 mg/kg, NIDA Research Triangle Park, NC) and confined to one side of the chamber by a solid partition for 30 minutes, or injected with saline and confined to the other side of the chamber for 30 minutes. Additional animals received saline injections on both sides of the chamber and were used as controls in Experiment 1 (non-conditioned, n=6). As in our previous publication (Sartor and Aston-Jones 2012), cocaine conditioned rats that did not show a preference on test day (<75 sec more time on the cocaine side) were also included in the non-conditioned group in Experiment 1 (n=7). Conditioning occurred in morning and afternoon sessions (at least 4 hr apart). Two days after the last conditioning day, animals were tested for cocaine preference in a 15-minute session with free access to both sides of the chamber via a doorway in the partition. The time spent on both sides of the chamber was automatically measured via photobeam breaks and custom software. Animals that were saline conditioned or cocaine conditioned and did not show preference on test day were combined because there was no difference in preference scores (t(11) = 0.46, p = 0.7) or CTb/Fos labeling in each brain region (t-values < 2.0, p > 0.05) between these groups. Where mentioned, a counter-balanced within-subject design was used to test for cocaine preference in some groups.
Immunohistochemistry
Fos was visualized in orexin- and CTb-positive neurons using a double labeling immunohistochemical procedure. For this, rats were deeply anesthetized with ketamine and xylazine (100 and 20 mg/kg, i.p., respectively) 90 minutes after the CPP test and then perfused transcardially with cold 0.9% saline followed by 4% paraformaldehyde. Brains were cut in 40um-thick tissue sections and then processed for visualization of Fos and orexin. Visualization of Fos was performed by incubating the sections in rabbit anti-Fos (1:5,000, Santa Cruz) overnight, and by incubation in biotinylated donkey anti-rabbit secondary antibody (1:500, Jackson) for two hours, and finally by incubation in avidin-biotin complex (ABC, 1:500) for 1.5 hours. Detection of Fos was performed with 3,3′-diaminobenzidine (DAB, Sigma) followed by nickel ammonium sulfate, producing a purple-black reaction product in the nucleus. Subsequently, in the same sections orexin was visualized by incubation in goat anti-orexin overnight (1:1,000 Santa Cruz), biotinylated donkey anti-goat secondary antibody (1:500) for two hours, and avidin-biotin complex (1:500) for 1.5 hours. Finally sections were incubated in DAB to stain the cytoplasm brown in orexin neurons.
For visualization of Fos in CTb neurons, Fos was stained as described above followed by incubating the same tissue sections in goat anti-CTb (1:20,000 List Labs) overnight, and then biotinylated donkey anti-goat secondary (1:500, Jackson), followed by ABC (1:500). Finally, sections were incubated in DAB to yield a brown reaction product in the cytoplasm.
Histology
Following the CPP test, each rat was deeply anesthetized with ketamine-xylazine, and pontamine sky blue (2% in 0.5 M Na acetate, 300 nl) was injected through the cannulae before the rat was perfused transcardially as described above. Brains were cut in 40μm-thick coronal sections through the vBNST and VTA, which were mounted directly on slides and counterstained with neutral red to confirm cannulae placements.
Data Analysis
Preference scores were calculated as the time spent in the cocaine-paired side minus the time spent in the saline-paired side on the CPP test. The resulting preference scores were compared within or between groups using Student’s t-test or one-way analysis of variance (ANOVA). When a significant F-value was obtained pairwise comparisons were carried out with a Newman-Keuls test. GraphPad Prism v5 was used for statistical analysis. The risk of Type 1 error (α) was set at p < 0.05.
The percentages of CTb or orexin neurons with Fos (doubly labeled neurons) were averaged across two sections per animal and compared between groups using Student’s t-test. Correlations between percentages of CTb neurons that were Fos-positive and preference scores were performed using Pearson’s correlation coefficient test. An observer blind to the experimental conditions manually counted cells with a point-counter tool using Openlab image processing software (Improvision, Ltd.).
Results
Experiment 1
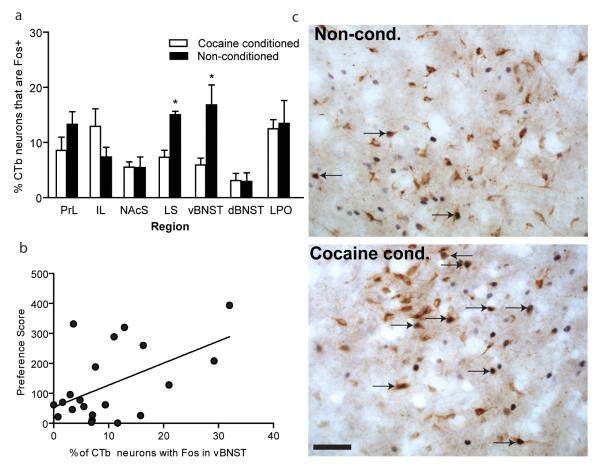
A combination of tract tracing, cocaine CPP, and staining for Fos was used to identify LH afferents activated during cocaine preference. Consistent with previously published data (Sakurai et al., 2005; Yoshida et al., 2006), we identified several brain areas that contained numerous retrogradely labeled (CTb+) neurons after injection of CTb into LH (Table 1). Many retrogradely labeled neurons in LS were Fos-activated during cocaine preference, as recently described (Sartor and Aston-Jones 2012). We also found that vBNST inputs to LH were significantly Fos-activated by cocaine CPP expression: 17% of CTb+ (LH-projecting) neurons in vBNST expressed Fos during cocaine preference (Cocaine-conditioned, n=8) compared to 7% in control, non-conditioned animals (n=13) (t(19) = 3.4, p = 0.003) (Figure 1a,c). The PrL, IL, NAcS, and LPO all contained ≥ 5% CTb+ neurons that were Fos-positive during the cocaine CPP; however, none of these areas showed a significant increase in Fos expression in CTb+ neurons after the CPP test (p > 0.05 each). The insula cortex, central and medial amygdala and ventral tegmental area contained < 1% CTb neurons with Fos after the CPP test (data not shown), indicating that these areas may not be involved in regulating LH orexin neurons during cocaine preference.
Table 1.
Mean (±SEM) number of CTb+ and CTb+/Fos+ neurons after CTb injections in LH in non-conditioned and cocaine conditioned animals.
| Number of CTb+ neurons |
Number of CTb+/Fos+ neurons |
|||
|---|---|---|---|---|
| Non-cond. | Coc cond. | Non-cond. | Coc cond. | |
| PrL | 14.8 (2.0) | 22.4 (3.7) | 1.6 (0.5) | 3.1 (0.8) |
| IL | 19.8 (2.1) | 26.3 (3.3) | 2.6 (0.6) | 1.9 (0.5) |
| NAcS | 29.9 (2.4) | 35.0 (6.5) | 1.7 (0.3) | 1.7 (0.7) |
| vBNST | 30.4 (4.1) | 28.3 (4.3) | 1.6 (0.3) | 5.4 (1.7)* |
| dBNST | 32.7 (3.4) | 41.1 (8.1) | 0.8 (0.3) | 1.1 (0.4) |
| LPO | 29.5 (1.7) | 23.4 (3.2) | 3.3 (0.5) | 3.6 (1.4) |
vBNST Coc cond. > vBNST Non-cond. in # of CTb+/Fos+ neurons, p<0.05.
Figure 1. LH afferents activated during cocaine place preference.
(a) LH inputs from several brain regions are activated during cocaine preference. Note that the percentages of Fos-activated neurons in vBNST that project to LH orexin area were significantly activated during cocaine preference (cocaine conditioned) compared to non-conditioned animals (non-conditioned: saline conditioned or cocaine conditioned animals without a preference) (Non-cond. n=13, Cocaine cond. n=8, *p<0.05 indicates significant difference from non-cond. by t-test). The data shown here for Fos activation of LS afferents to the LH orexin area are from our recent report (Sartor and Aston-Jones 2012). (b) Significant positive correlations between percentages of CTb neurons with Fos in vBNST and preference scores (Pearson’s correlation, r=0.52, *p<0.05). (c) Representative immunohistochemical staining for CTb and Fos in vBNST in cocaine conditioned and non-conditioned animals. Note the substantial increase in the number of Fos-activated neurons in vBNST that project to LH (CTb/Fos doubly labeled neurons) during cocaine preference compared to non-conditioned control animals. Arrows indicate CTb (brown neurons) and Fos (black nuclei) doubly labeled neurons. Scale bar= 100μm.
Further analysis revealed a significant correlation between the percentage of LH-projecting neurons in vBNST that were Fos+ and the corresponding preference score for each animal (r = 0.52, p = 0.02) (Figure 1b). Together, these results indicated that vBNST, but not several other afferents to LH, are activated in proportion to the degree of preference animals exhibit for cocaine and may play a role in driving this preference. Thus, vBNST inputs to LH were studied in greater detail below.
Experiment 2
To determine if vBNST is essential for the expression of cocaine preference, additional rats were conditioned with cocaine CPP and then given bilateral injections of the GABAA/B receptor cocktail B-M or aCSF into vBNST immediately prior to the CPP preference test using a within-subjects, counterbalanced design (n=6). Figure 2 shows that bilateral microinjections of B-M into vBNST significantly attenuated cocaine preference compared to aCSF injections in vBNST or injections of B-M nearby but outside of vBNST (missed B-M, n=6) (F2, 15 = 15.06, p = 0.0003). Injections of B-M outside of vBNST (missed B-M preference scores: 160 ±10 and 215 ±95 for dBNST and LPO, respectively) did not significantly affect expression of cocaine preference compared to aCSF injections in the vBNST (t(10 ) = 1.8, p = 0.1). Microinjections of B-M had no significant effect on general motor activity during the CPP test, as measured by the number of crosses between the two chambers (F2,15 = 0.3, p = 0.9). Interestingly, a significant and non-significant trend in Fos-induction was found in non-orexin (aCSF = 70±11.5, B-M = 117±12.3; t(4) = 2.7, p = 0.04) and orexin neurons in LH (aCSF = 12 ±2.1, B-M = 19.8 ±3.3, p > 0.05), respectively, following this bilateral vBNST inactivation.
Figure 2. Bilateral inactivation of vBNST attenuated cocaine preference.
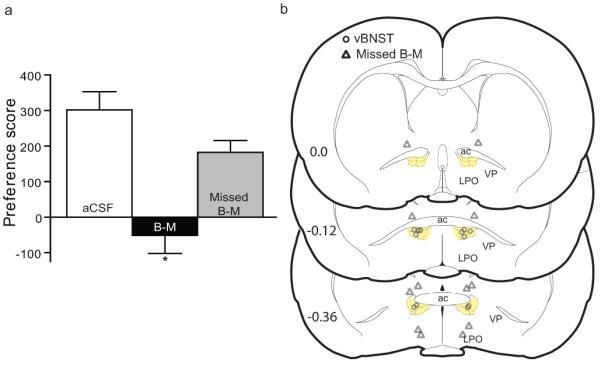
(a) Bilateral microinjections of B-M into vBNST (B-M), but not outside of vBNST (Missed B-M) or vehicle injections in vBNST (aCSF), significantly attenuated cocaine preference (n=6, *p<0.01 indicates significant difference by Newman-Keuls post-hoc test). (b) Cannulae placements in vBNST (open circles) and B-M injections outside of vBNST (Missed B-M, triangles). The vBNST is highlighted in yellow. ac= anterior commissure, VP= ventral pallidum, LPO= lateral preoptic nucleus.
Experiment 3
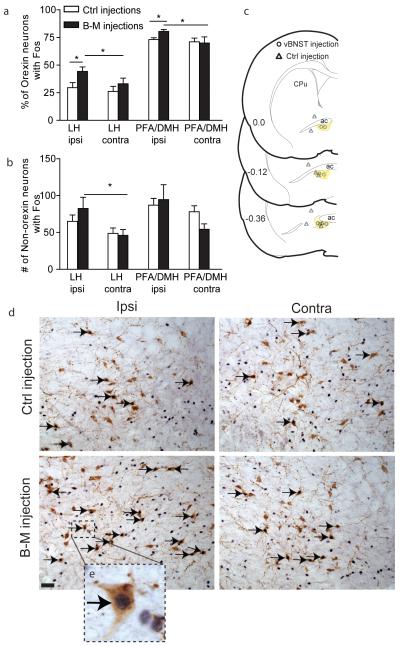
To determine if vBNST connections regulate LH orexin activity during expression of cocaine CPP (as indexed by Fos induction), additional animals received a unilateral microinjection of B-M or vehicle in vBNST immediately prior to the preference test. The unilateral approach was utilized because bilateral inactivation of vBNST decreases preference, and such altered behavior could affect Fos in orexin neurons, thereby confounding interpretation of Fos changes. Because virtually all vBNST neurons that project to the LH are ipsilateral, inactivating vBNST unilaterally should reveal effects in ipsilateral orexin neurons without affecting contralateral orexin neurons or CPP behavior. As shown in Figure 3, we found that unilateral microinjections of B-M in vBNST (n=7) significantly increased Fos expression in orexin and non-orexin neurons in the ipsilateral LH and PFA/DMH compared to injections of B-M outside of vBNST, to aCSF injections in vBNST (Control injections, n=9), or to the contralateral hemisphere (t values >2.1, p < 0.05) (Fig. 3a-c). Unilateral microinjections of B-M into vBNST, however, did not alter preference scores (Ctrl preference score = 167.3 ±28.8 sec vs. B-M preference score = 152.4 ±47.6 sec, t(14)=0.3, p=0.8). These manipulations did not alter general exploration of the chamber as measured by the number of crosses between chambers (t(14) = 0.04, p = 0.9).
Figure 3. Unilateral inactivation of vBNST and orexin/Fos staining in LH and PFA/DMH.
(a,b) Percentages of orexin neurons with Fos and numbers of non-orexin neurons with Fos following unilateral B-M microinjection in vBNST. Ipsilateral (ipsi) and contralateral (contra) are in reference to the unilateral B-M injection in vBNST. Significant increases in the percentage of Fos-activated orexin neurons, and number of Fos+ non-orexin neurons, were found following vBNST B-M injection (*p<0.05 indicates significant difference by t-test). (c) Unilateral B-M cannulae placements in vBNST (vBNST injections, open circles, n=7) and outside of vBNST or aCSF microinjection placements in vBNST (Ctrl injections, triangles, n=9). (d) Representative staining for orexin (brown cytoplasm) and Fos (black nuclei) in LH following unilateral vBNST inactivation. Note the increased number of orexin neurons with Fos (double labeled) and non-orexin neurons with Fos (Fos only) in LH, ipsilateral to the B-M injection in vBNST. Double-labeled orexin/Fos neurons are indicated with black arrows. Scale bar= 100μm. CPu= caudate putamen, ac= anterior commissure.
Experiment 4
As described above, bilateral inactivation of vBNST during CPP blocked cocaine preference, but increased Fos-activation in LH and PFA/DMH. Previous results from our lab showed that LH orexin neurons are activated with cocaine preference (Harris et al., 2005). Together, these findings indicate that LH orexin neurons are unlikely to be involved in the ability of vBNST inactivation to decrease cocaine preference. Given that high levels of orexin-1 receptors and orexin fibers are present in vBNST (Trivedi et al., 1998; Cutler et al., 1999; Marcus et al., 2001), it seemed possible that orexin-mediated activation of vBNST may be involved in cocaine preference, and that an influence of elevated orexin activity after vBNST inactivation would be obviated by vBNST inactivation. If that were the case, we expected that OX1R antagonism in vBNST would decrease cocaine preference. To examine this hypothesis, the OX1R antagonist, SB-334867, was bilaterally microinjected into vBNST after CPP conditioning, prior to the CPP preference test (n=6). As shown in Figure 4, blocking OX1Rs in vBNST in this way had no effect on cocaine preference compared to vehicle injections (paired t-test, t(5) = 0.3, p = 0.8).
Figure 4. Orexin-1 receptor antagonist injections in vBNST.
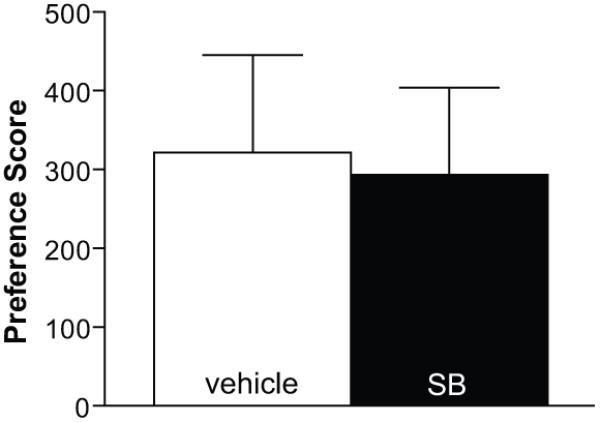
Bilateral injections of the OX1R antagonist SB-334867 (SB) had no effect on cocaine preference compared to vehicle injections (n=6, p>0.05).
Experiment 5
We reasoned that bilateral vBNST inactivation might block cocaine CPP expression but also increase Fos in LH orexin neurons (which is normally associated with increased CPP; Harris et al., 2005) if vBNST projects to a non-hypothalamic region is necessary for cocaine CPP (see Discussion section for a discussion of possible mechanisms involved). Our laboratory previously reported a functional and anatomical projection from vBNST to VTA (Georges and Aston-Jones, 2001, 2002). To determine if afferents to VTA from vBNST are necessary for expression of cocaine preference, we utilized a bilateral disconnection technique in which cocaine conditioned animals received a unilateral B-M microinjection into vBNST and also into contralateral VTA (B-M, n=11) immediately prior to the CPP preference test (Figure 5). Unilateral injections of aCSF in vBNST and contralateral VTA were used as vehicle controls (aCSF, n=8). Figure 6 reveals that bilateral disconnection of vBNST and VTA significantly attenuated cocaine preference compared to aCSF injections in vBNST and VTA or to B-M injections outside of either vBNST and VTA (Missed injections, n=10) (F2,26 = 20.55, p < 0.0001). Moreover, the accurately placed B-M microinjection in vBNST and VTA produced a significant avoidance of the cocaine-paired side compared to aCSF injections (t(17) = 4.3, p = 0.0004). This bilateral disconnection of vBNST and VTA had no significant effect on general motor activity, as measured by the number of crosses between the two CPP chambers (crosses after aCSF = 49.2 ±9.6 vs. after B-M = 34.5 ±6.8, t(17) = 1.5, p = 0.14).
Figure 5. Cannulae placements for vBNST - VTA disconnection studies.
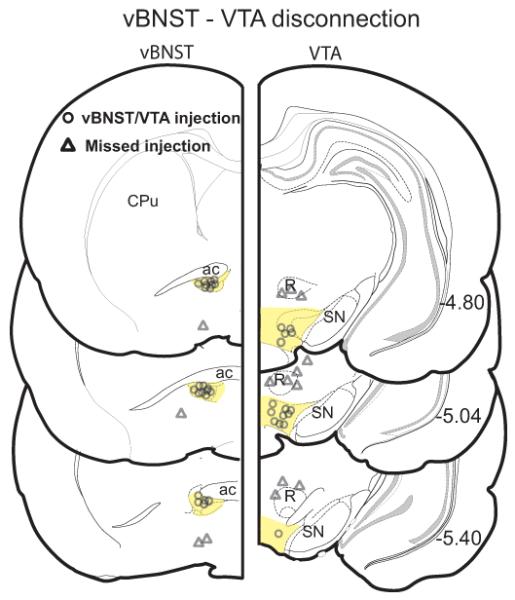
Cannulae placements within a vBNST and contralateral VTA are displayed as open circles (vBNST/VTA injection), and placements outside of vBNST or VTA are represented by open triangles (Missed injection). The vBNST and VTA are highlighted in yellow. CPu= caudate putamen, ac= anterior commissure, R= red nucleus, SN= substantia nigra.
Figure 6. Disconnection of vBNST - VTA eliminates cocaine preference.
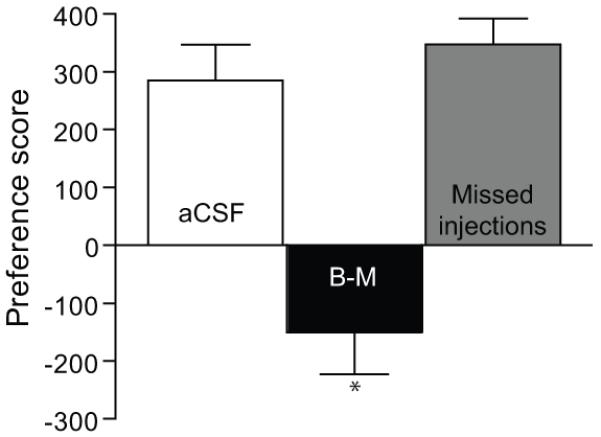
Unilateral B-M injections in vBNST and contralateral VTA (B-M, n=11) significantly attenuated cocaine preference compared to aCSF in vBNST and VTA (aCSF, n=8) or to injections of B-M outside of vBNST and/or VTA (Missed injections, n=10) (*p<0.001 indicates a significant difference using Newman-Keuls post-hoc test). Note that disconnection of vBNST-VTA also caused a significant avoidance of cocaine paired side. Injections of B-M outside of vBNST, VTA, or both had no effect on CPP compared to aCSF microinjections into vBNST and VTA.
Experiment 6
Previous studies indicate that VTA receives glutamatergic inputs from vBNST (Georges and Aston-Jones, 2001, 2002). To determine if glutamatergic afferents to VTA from vBNST are critical for expression of cocaine preference, we again utilized a bilateral disconnection method. In these experiments, conditioned rats received a unilateral B-M microinjection in vBNST and a microinjection of a cocktail of the AMPA/NMDA glutamate receptor antagonists, CNQX/AP5, in contralateral VTA prior to the CPP preference test (B-M CNQX/AP5, n=11). Unilateral microinjections of aCSF in vBNST and contralateral VTA were used as vehicle controls (aCSF, n=8). As shown in Figure 7, unilateral B-M microinjection in vBNST and glutamate receptor antagonists in contralateral VTA did not significantly attenuate cocaine preference (F2,23 = 1.2, p = 0.3), although a noticeable reduction in the average preference score was apparent following this manipulation. These injections in vBNST and VTA had no significant effect on the number of crosses during the CPP test (crosses after aCSF = 57 ±11.3 vs. after B-M/glutamate antagonists = 55.4 ±7.8). In addition, microinjections of B-M or CNQX/AP5 outside of vBNST or VTA (Missed injections, n=7) had no significant effect on preference scores compared to aCSF (t(13) = 0.3, p = 0.8) (Figure 7).
Figure 7. Glutamatergic afferents to VTA from vBNST and cocaine preference.
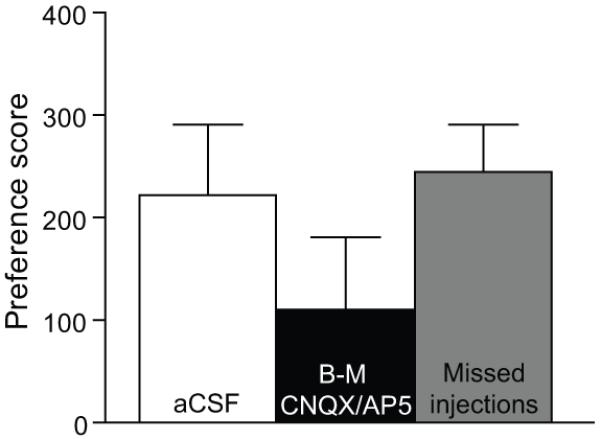
Unilateral inactivation of vBNST with B-M and antagonism of NMDA and non-NMDA receptors in the contralateral VTA with CNQX/AP5 (B-M CNQX/AP5, n=11) did not significantly attenuate cocaine preference compared to aCSF injections in vBNST (aCSF, n=8), or to B-M and CNQX/AP5 injections outside of vBNST or VTA (Missed injections, n=7).
Discussion
Summary of present findings
Although mounting evidence shows a role for orexin in reward processing, little has been known about the circuitry that regulates orexin neurons during reward-related behaviors. Recently, we found that LS afferents to LH orexin neurons are important for expression of cocaine place preference (Sartor and Aston-Jones, 2012). However, orexin neurons in LH receive inputs from many reward-related brain regions (Sakurai et al., 2005; Yoshida et al., 2006; Marchant et al., 2009), and it is unknown if inputs in addition to LS are important in cocaine preference. In the current study, we surveyed LH afferents for Fos activation during cocaine CPP. Consistent with previous results (Sakurai et al., 2005; Yoshida et al., 2006), we found that the LH receives strong inputs from several brain regions, including PrL IL, NAcS, LS, vBNST, and LPO. Despite the many retrogradely labeled afferents to LH, we found that only the LS and vBNST exhibited significant Fos induction with cocaine CPP. As the role of LS afferents to LH in cocaine preference was previously reported (Sartor and Aston-Jones, 2012), here we focused on the vBNST afferents.
The percentages of CTb+ neurons (retrogradely labeled from LH) that were also Fos-activated in vBNST were positively correlated with the amount of preference exhibited on the CPP test day. In addition, using B-M to locally inactivate neurons, we found that vBNST activity is necessary for cocaine CPP. We also unilaterally inactivated vBNST neurons during CPP expression, to evaluate the effect of vBNST projections to LH on Fos expression in orexin cells. Unexpectedly, this manipulation increased Fos expression in ipsilateral LH orexin neurons during cocaine preference.
As previous studies showed that Fos activation in LH orexin neurons is positively correlated with increased cocaine preference (Harris et al., 2005), the finding that inactivation of vBNST increased Fos expression in these cells but also attenuated cocaine preference indicated that LH orexin neurons are an unlikely target of vBNST responsible for its role in cocaine preference. There are several possible mechanisms by which inhibition of vBNST reduced preference, but also increased Fos in orexin neurons. First, expression of Fos was elevated in lateral and medial orexin neurons following B-M injections in vBNST. Fos activation in medial orexin neurons has been associated with stress/anxiety/negative attributes (Harris and Aston-Jones 2006). Such elevated negative effects could potentially overcome the positive effects of the LH orexin activation, leading to reduced CPP. Second, it is possible that the LH orexin neurons that are activated by vBNST inhibition are not those that project to the primary mediators of preference (e.g., VTA), and therefore play less of a role in preference overall. Third, Fos induction, a marker often used for neuronal activation, can also be an indication of neuronal inhibition or alterations in signaling cascades without changing neuronal firing (Morgan et al. 1991; Mikkelsen et al 2005). Therefore, it is possible that Fos activation of LH in this study does not reflect increased firing of LH orexin neurons. Finally, it is plausible that orexin is not a primary mediator of preference, but rather is necessary to modulate other areas that are more primary mediators of CPP (e.g., VTA). For example, orexin may serve to modulate responses of VTA DA neurons to glutamate inputs that drive the specific behavioral responses (as we have recently published; Mahler et al., 2012). Thus, LH orexin may be necessary but not sufficient for reward-related behaviors. Therefore, although we demonstrated that vBNST plays a significant role in cocaine CPP, more work is needed to understand how BNST neurons interact with orexin neurons during reward-related behaviors.
Given the high expression of OX1Rs in vBNST (Trivedi et al., 1998; Marcus et al., 2001), one possible explanation for this puzzling situation was that orexin neurotransmission within vBNST may be important for cocaine preference, and that the inactivation of vBNST blocked this pathway. However, OX1R antagonist injections in vBNST did not attenuate cocaine preference, indicating that orexin input to vBNST was not critical for this behavior.
Therefore, we sought targets other than LH orexin neurons where vBNST might act to support cocaine preference. Our laboratory previously showed that the vBNST acts as a potent regulator of DA neurons in VTA (Georges and Aston-Jones, 2002), but it was unknown if this connection is critical during reward-related behaviors. Using a bilateral disconnection technique, we found that afferents to VTA from vBNST are essential for cocaine preference. However, we also found with local microinjections of glutamate antagonists that this projection does not purely depend on activation of iontoropic glutamate receptors in VTA.
The present study utilized a bilateral disconnection technique to determine if vBNST afferents to VTA neurons are necessary for cocaine preference. One caveat of this technique is that it does not differentiate between afferents and efferents. Therefore, one might argue that our results were due to interruption of projections from VTA to vBNST that are important in cocaine preference. However, this alternative explanation is not likely in view of anatomical evidence showing that there are virtually no VTA projections to vBNST (Shin et al., 2008). Dopamine neurons from the VTA do, however, project heavily to dBNST (Risold and Swanson 1997), and this region is strongly interconnected with vBNST (Shin et al,. 2008); other indirect pathways could exist. Therefore, it is possible that indirect connections from VTA to vBNST could be affected by the disconnection technique used in these experiments.
Role of BNST in reward behaviors
Several studies have identified roles for ventral and dorsal BNST in reward-related behaviors (Aston-Jones et al., 1999; Leri et al., 2002; Buffalari and See, 2011) and drug-induced neuroplasticity (Erb et al., 2004; Dumont et al., 2005; Grueter et al., 2006; Colussi-Mas et al., 2007; Dumont et al., 2008; Grueter et al., 2008; Francesconi et al., 2009; Krawczyk et al., 2011; Nobis et al., 2011). We targeted neurons in the ventral BNST because of its strong connections with LH, Fos activation of LH-projecting neurons in this area during cocaine preference, and strong projections from this area to VTA (Georges and Aston-Jones, 2002). In addition, we showed that inactivation of vBNST, but not of dBNST, attenuated cocaine CPP. Therefore, although the ventral and dorsal BNST have been shown to be important in other drug-seeking behaviors (Briand et al., 2010; Buffalari and See, 2011; Wenzel et al., 2011), only the vBNST and its projections to VTA appear to play an important role in cocaine preference.
Relapse to drug-seeking can be evoked by discrete cues, stress or contexts. Inactivation of BNST has been shown to attenuate cue- and stress-induced relapse to cocaine-seeking behaviors (Buffalari and See, 2011). Although recent data has implicated BNST in contextual fear learning (Sullivan et al., 2004), it was unknown if BNST is involved in contextual drug-seeking behaviors. We showed that vBNST is critical for the expression of cocaine place preference, a behavior that reflects context-reward associations (Bardo and Bevins, 2000; Cunningham et al., 2006). In addition, BNST receives strong projections from ventral subiculum (vSUB) (Shin et al., 2008), a region that has been implicated in context-induced drug-seeking behaviors (Lasseter et al., 2010). Therefore, it seems possible that vSUB influences BNST activity and cocaine preference. Consistent with this view, Massi and colleagues reported that vSUB exerts a strong excitatory influence on BNST neurons that project to VTA (Massi et al., 2008). However, further research is needed to determine if a vSUB-BNST, or perhaps a vSUB-BNST-VTA, circuit is involved in context-induced drug-seeking behaviors.
vBNST projection to VTA: role in cocaine preference
Many studies have demonstrated that glutamatergic afferents to VTA are important in regulating activity of DA neurons (Georges and Aston-Jones, 2001, 2002; Dumont and Williams, 2004; Massi et al., 2008). Furthermore, glutamatergic inputs to VTA DA neurons are involved in neural plasticity that could eventually lead to drug addiction (Geisler and Wise, 2008). Our laboratory reported that activation of NMDA and non-NMDA receptors in the VTA is necessary for the acquisition and expression of cocaine and morphine CPP (Harris and Aston-Jones, 2003; Harris et al., 2004), but the source of this glutamatergic input is unknown. Anatomical and functional data indicate that vBNST is one possible source of the glutamatergic input to VTA (Georges and Aston-Jones, 2002). We found that vBNST afferents to VTA are essential for cocaine preference, but although preference was somewhat decreased it did not appear to be solely mediated by glutamatergic projections. However, it is also possible that our glutamate antagonist microinjections did not effectively cover all of the VTA, so that glutamate plays more of a role in this preference mediated by this projection than indicated by these results. Recently, Geisler and colleagues reported staining for vesicular glutamate transporters (vGLUTs), a marker for glutamatergic neurons, in vBNST neurons that largely did not project to the VTA (Geisler et al., 2007). Therefore, it seems likely that the primary source of input to VTA from vBNST that is involved in cocaine preference involves pathways in addition to glutamatergic projections.
Recent data indicate that BNST is importantly involved in stress and reward (Leri et al., 2002; Briand et al., 2010; Buffalari and See, 2011). In the present study, unilateral inactivation of vBNST and contralateral VTA during a CPP test not only significantly attenuated preference but also caused avoidance of the cocaine-paired side. Cocaine is known to have both rewarding and anxiogenic effects (Yang et al., 1992; Blanchard et al., 1999; Rogers and See, 2007). Therefore, it is possible that inactivating the vBNST-VTA circuit reduces reward-context associations of cocaine, revealing unopposed anxiogenic properties of cocaine associations resulting in an avoidance behavior. Consistent with the idea of a vBNST-VTA circuit involved in anxiety and reward, Briand and colleagues recently reported that vBNST projections to the VTA were activated following exposure to cocaine and stress (Briand et al., 2010). A study by Wenzel and colleagues, however, found that inactivation of the dorsal BNST reduced avoidance of cocaine using a runway self-administration procedure (Wenzel et al., 2011). Although several differences exists between these findings and the current study (inactivation of dBNST vs. vBNST-VTA connection, Pavlovian vs. instrumental behaviors, etc.), these data indicate that subregions of the BNST may be involved in the anxiogenic behavioral effects of cocaine.
In summary, this study extends present understanding of BNST in reward processing by showing that ventral BNST and its connections with VTA are necessary for cocaine place preference. Although strong interconnections between vBNST and LH exist, the current study indicates that vBNST afferents to the LH do not selectively regulate orexin neurons during cocaine preference. Instead, our results show that vBNST projections to VTA are necessary for cocaine preference. Therefore, future studies are needed to determine the significance of vBNST-LH connections and what role orexin plays in this circuit during drug-seeking behaviors.
Acknowledgements
This research was supported by National Institute on Drug Abuse Grants: R01DA017289, R37DA06214 and T32 DA007288. We thank Michael Smith for his excellent technical assistance.
Abbreviations
- ac
anterior commissure aCSF: artificial cerebrospinal fluid
- AP5
(2R)-amino-5-phosphonovaleric acid
- B-M
baclofen plus muscimol
- CeA
central amygdala
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione CPP: conditioned place preference
- CPu
caudate putamen
- CTb
cholera toxin b dBNST: dorsal bed nucleus of the stria terminalis LPO: lateral preoptic area
- LS
lateral septum vBNST: ventral bed nucleus of the stria terminalis VP: ventral pallidum
- VTA
ventral tegmental area
References
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Kaawaloa JN, Hebert MA, Blanchard DC. Cocaine produces panic-like flight responses in mice in the mouse defense test battery. Pharmacol Biochem Behav. 1999;64:523–528. doi: 10.1016/s0091-3057(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang M, Miselis RR, Aston-Jones G. Characterization of transsynaptic tracing with central application of pseudorabies virus. Brain Res. 1999;838:171–183. doi: 10.1016/s0006-8993(99)01680-7. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Geisler S, Zimmer L, Zahm DS, Berod A. Activation of afferents to the ventral tegmental area in response to acute amphetamine: a double-labelling study. Eur J Neurosci. 2007;26:1011–1025. doi: 10.1111/j.1460-9568.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci. 2006;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, Arch JR, Wilson S, Buckingham RE, Evans ML, Leslie RA, Williams G. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nature neuroscience. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Funk D, Borkowski S, Watson SJ, Akil H. Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience. 2004;123:1003–1009. doi: 10.1016/j.neuroscience.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O’Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J Neurosci. 2008;28:9261–9270. doi: 10.1523/JNEUROSCI.2886-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci. 2011;31:8928–8935. doi: 10.1523/JNEUROSCI.0377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British journal of pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2681-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grandes P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J Neurosci. 2008;28:10496–10508. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Soderman A, Kiss A, Mirza N. Effects of benzodiazepines receptor agonists on the hypothalamic-pituitary-adrenocortical axis. Eur J Pharmacol. 2005;519:223–230. doi: 10.1016/j.ejphar.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic Receptors Enhance Excitatory Transmission in the Bed Nucleus of the Stria Terminalis Through a Corticotrophin-Releasing Factor Receptor-Dependent and Cocaine-Regulated Mechanism. Biol Psychiatry. 2011;69:1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Brain Res Rev. 1997;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2009a doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European journal of neuroscience. 2009b;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, Su ZI, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011;217:221–230. doi: 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]