Abstract
Objectives
The exact cause(s) of apparent overestimation of β cell mass (BCM) with vesicular monoamine transporter type 2 (VMAT2) PET imaging in type 1 diabetes (T1D) is unknown. The objectives were to estimate in baboons non-displaceable binding of [18F]fluoropropyl (FP)-(+)- dihydrotetrabenazine (DTBZ) with its inactive enantiomer, [18F]FP-(−)-DTBZ, to validate the use of the reference tissue (renal cortex or spleen) in VMAT2 quantification; and also to compare specific pancreatic VMAT2 binding with that of the striatum in the same baboon brains because high specific binding signal for the pancreas would be desirable for its accurate quantification.
Methods
Baboons (Papio Ursinus) had multiple dynamic abdominal and brain PET scans each for 2 h with (+) and (−) enantiomers on separate occasions. Data were analyzed by compartmental models to estimate non-displaceable (VND) and specific (VS) VMAT2 binding in respective organs.
Results
[18F]FP-DTBZ PET showed excellent target tissue signal and specific VMAT2 binding in the pancreas (Vs =41± 11 mL/cm3) at nearly 80% that of the striatum. Directly estimated non-displaceable binding in the pancreas (VND =12 ± 1 mL/cm3) was similar to that of the renal cortex, spleen or cerebellum.
Conclusion
[18F]FP-DTBZ PET shows excellent specific VMAT2 binding in both the pancreas and striatum in baboons. The renal cortex or spleen as the reference tissue in VMAT2 quantification appears supported. However, further studies appear warranted to directly estimate pancreatic non-displaceable binding in humans including T1D patients and also to clarify the cause of the apparent overestimation of BCM in T1D.
Keywords: Vesicular monoamine transporter, β-cell mass, PET, [18F]FP-DTBZ
1. Introduction
The vesicular monoamine transporter type 2 (VMAT2) is found in the dopaminergic terminals of the brain. It has been well established that positron emission tomography (PET) imaging using radiotracers derived from dihydrotetrabenazine (DTBZ) can be used as a biomarkers of striatal dopaminergic innervation. VMAT2 PET imaging using radiolabeled DTBZ and its analogs has been used in the evaluation of central nervous system disorders such as Parkinson’s disease, Lewy body dementia, and Tourette’s syndrome. VMAT2 is also found on the membrane of putative vesicles that store insulin and dopamine within β-cells of the endocrine pancreas. VMAT2 also appears to have the potential to be used as a PET imaging biomarker of β-cell mass (BCM). Accurate noninvasive BCM measurements may ultimately aid in more clearly defining the disease process and management of patients with type 1 diabetes (T1D) and type 2 diabetes (T2D).
We previously evaluated VMAT2 as a potential BCM biomarker by [11C]DTBZ PET imaging in the first proof-of-concept and cross sectional study of healthy human volunteers and patients with long standing T1D [10]. T1D patients were predicted to have little or no BCM based on metabolic measurements. VMAT2 binding potential (BPND) in the pancreas, which is proportional to the VMAT2 density [12], was reduced only modestly in T1D patients [10]. The presence of significant PET VMAT2 binding signal in the T1D population was unexpected because histological studies suggest near complete depletion of BCM in long standing T1D. Recently, studies using an 18F-labeled VMAT2 ligand, [18F]fluoropropyl (FP)-(+)-DTBZ, showed more marked reductions by 40% in pancreatic VMAT2 BPND in long-standing T1D patients compared with healthy controls. However, there was still significant VMAT2 binding signal in the pancreas of T1D patients and the exact cause(s) of this finding is unknown. [18F]FP-(+)-DTBZ (Ki = 0.11 nM) has almost 8-fold higher affinity for VMAT2 binding sites than does [11C]DTBZ (Ki = 0.97 nM). Although β-cells are low in abundance (1–2 % of the total pancreatic tissue) and dispersed throughout the pancreas, [18F]FP-(+)-DTBZ provided exquisitely excellent target tissue (pancreas) signal-to-noise ratios on PET images with a peak pancreatic time activity of over 20 standardized uptake values (SUV) in humans [11]. Together with the advantage of a longer physical half life of 18F (110 min) compared with 11C (20 min), [18F]FP-(+)-DTBZ currently appears to be the tracer of choice for VMAT2 imaging of pancreatic beta cell mass.
Apparent overestimation of BCM with PET VMAT2 binding measurements in these studies [10, 11] may be due to the limitations in PET quantification using reversible receptor (transporter) binding radiotracers such as [18F]FP-(+)-DTBZ. PET signal from the pancreas after a bolus injection of [18F]FP-(+)-DTBZ consists of two major components, non-displaceable (free + nonspecific binding) and displaceable (specific) VMAT2 binding. Specific binding can be estimated from kinetic model-based PET data analysis if the non-displaceable component can also be estimated. VMAT2 PET imaging of the brain allows for the estimation of the non-displaceable binding portion of the striatum using the cerebellar cortex, which is devoid of VMAT2 (called the reference tissue). However, there is no comparable reference tissue within the pancreas because β cells are scattered throughout the pancreas. Instead, the renal cortex has been used as the reference tissue to estimate non-displaceable binding in the pancreas because it is known to be devoid of VMAT2. However, if non-displaceable binding in the pancreas is significantly greater than in the renal cortex, PET measured BCM using the renal cortex as the reference tissue would be overestimated.
One approach to estimate this non-displaceable binding is to conduct a separate PET experiment in which all VMAT2 sites are blocked by pre-administering a large pharmacologically-active dose of non-radioactive VMAT2 antagonists such as DTBZ. In this VMAT2 blocked condition, all pancreatic [18F]FP-DTBZ signal is due to non-displaceable binding. However, this approach is not desirable at least in humans due to the potential pharmacological effects of blocking all VMAT2 sites including those in the brain. Alternatively, to directly estimate this nondisplaceable binding within the pancreas, we can perform a second PET experiment using a stereoisomer of [18F]FP-(+)-DTBZ, namely [18F]FP FP-(−)-DTBZ. Binding of DTBZ to VMAT2 is stereospecific. The (−)-enantiomer has practically no affinity for VMAT2 (Ki > 3,000 nM vs. Ki = 0.11 nM for the (+)-enantiomer) [14, 15, 17].
In this study, we estimated non-displaceable binding of [18F]FP-(+)-DTBZ directly within the pancreas using PET imaging of baboons with [18F]FP-(+)-DTBZ and its enantiomer, [18F]P-(−)-DTBZ and compared this pancreatic non-displaceable binding with that of the renal cortex and spleen estimated with [18F]FP-(+)-DTBZ, the spleen being another potential reference tissue. In addition, PET imaging of the same baboon’s brains was also performed so that a direct comparison of the magnitude of specific pancreatic VMAT2 binding with the specific striatal VMAT2 binding could be made. We found that the specific binding signals were of similar magnitude suggesting that accurate quantification of VMAT2 binding in the pancreas can be made as in the striatum.
2. Materials and methods
2.1. Animals
A total of five adult male baboons (Papio Ursinus, 22 ± 6 kg) had multiple PET scans. Four baboons had both [18F]FP-(+)-DTBZ and [18F]FP-(−)-DTBZ abdominal scans; three had an additional [18F]FP-(+)-DTBZ brain scan and one had only a [18F]FP-(+)-DTBZ brain scan. Multiple scans in the same animal were separated by at least 2 weeks between scans. Study procedures were approved by the Institutional Animal Care and Use Committees of the Columbia University.
2.2. Radiochemistry
Both [18F]FP-(+)-DTBZ and its inactive enantiomer, [18F]FP-(−)-DTBZ were prepared from their respective enantio-pure desmethyl precursors obtained from Monomerchem, Inc (Research Triangle Park, NC, USA) as per previously described methods. The radiochemical purity of either radiotracer was greater than 98%, and the specific radioactivity was 37–74 TBq/mmol (1000–2200 Ci/mmol) at synthesis completion.
2.3. Positron Emission Tomography (PET) Procedure
Animals were scanned on an HR+ scanner (Siemens, Knoxville, TN, USA) in a two-(abdomen) or three (brain)-dimensional mode under 2% isoflurane anesthesia for 120 minute with 22 frames of increasing frame length (3 × 15 s, 2 × 60 s, 4 × 120 s, 2 × 300 s and 10 × 600 s) starting with an intravenous injection (over 30 sec) of either [18F]FP-(+)-DTBZ or [18F]FP-(−)-DTBZ (115 ± 49 MBq). Vital signs were monitored (DataScope Corp, Paramus, NJ, USA) and body temperature was maintained at 37° C. A 10 minute transmission scan was acquired before the tracer injection for attenuation correction. PET data were corrected for attenuation, scatter, and randoms and reconstructed using filtered back-projection with a Shepp filter (cutoff frequency = 0.5 cycles). For the input function measurement, multiple arterial samples were concurrently collected via a femoral artery using an automated sampling system during the first 4 minutes (11 samples) and manually thereafter at longer intervals at 5, 6, 8, 12, 16, 20, 30, 50, 70, 90, and 120 min) (a total of 22 samples). Six samples collected at 2, 4, 12, 10, 50 and 70 minutes were analyzed by radio- high-performance liquid chromatography (HPLC) to determine the fraction of un-metabolized parent radioactivity. The data points were fitted with a sum of two exponential functions. Additionally, plasma fractions (fP) of radiotracer unbound to plasma protein were determined using the ultrafiltration method previously described.
2.3. PET Data Analysis
For [18F]FP-(+)-DTBZ abdominal scans, regions of interest (ROIs) were manually defined by visual inspection over the pancreas (≈ 6 cm3) and spleen (≈ 10 cm3) on transverse slices and the renal cortex (right + left ≈ 12 cm3) on coronal slices of summed images over 120 minutes by MI who is a radiologist and nuclear medicine physician experienced in anatomical and functional images of the abdomen. For [18F]FP-(−)-DTBZ abdominal scans, the summed images were co-registered to corresponding [18F]FP-(+)-DTBZ summed images by using the iterative co-registration module in biomedical image data analysis software, PMOD (PMOD Technologies Ltd., Zurich, Switzerland); the same ROI defined on the latter were used with minor modifications when needed as assessed by visual inspection. For [18F]-FP-(+)-DTBZ brain scans, T1 weighted 1.5 T magnetic resonance (MR) scans obtained previously were co-registered to the summed images in PMOD. These images were manually reoriented along the anterior-posterior commissural line. ROIs were manually drawn over the striatum (right + left ≈ 1.8 cm3) and cerebellum (≈ 6 cm3) on the fused PET/MR images. ROI time activity curve (TAC) data were decay corrected and expressed as SUV, which is the tissue radioactivity normalized by injected tracer dose and body weight. TAC data were fitted with standard compartment models for reversibly binding radiotracers to receptors (transporters) using metabolite-corrected plasma TAC data as input functions to estimate the total distribution volume, VT (mL/cm3) using kinetic analysis modules implemented in PMOD.
For [18F]FP-(+)-DTBZ, target tissue VT containing VMAT2 (pancreas or striatum) was defined by the sum of the non-displaceable (free + nonspecific binding) (VND) and specific binding (VS) compartment distribution volumes. VS is proportional to VMAT2 density in tissue (Bmax) and is defined by where KD is the dissociation constant and fP is the free radiotracer fraction in plasma. VT in the reference tissue (renal cortex, spleen or cerebellum), containing no appreciable amounts of VMAT2 was equal to VND in that organ. For the negative enantiomer, [18F]FP-(−)-DTBZ, however, VT in the pancreas is equal to VND in the pancreas because it has little affinity for VMAT2. These VND values were compared to assess the approximation of reference tissue VND to pancreatic tissue VND. Additionally, the magnitudes of VS values were compared between striatum and pancreas.
2.4. Statistical Analysis
Data are expressed as mean ± SD. Student’s t testing (paired or unpaired) was used to compare group mean data values. A p value of < 0.05 was considered statistically significant.
3. Results
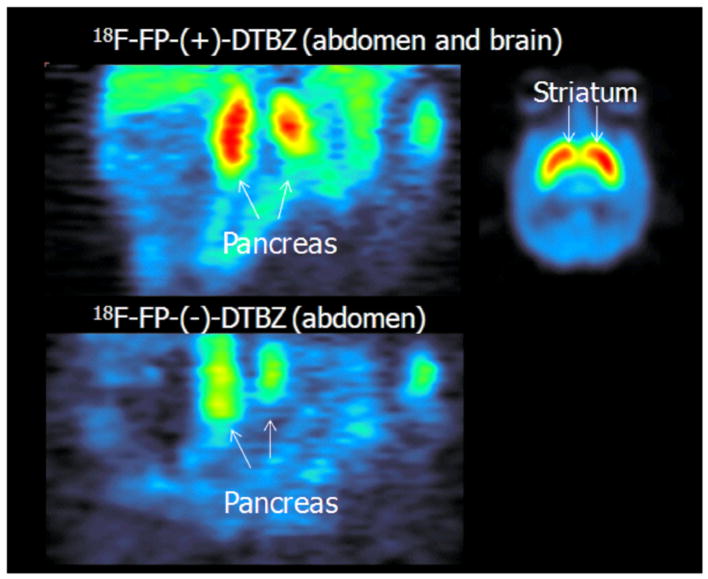
[18F]FP-(+)-DTBZ abdominal scans showed excellent tracer uptake in the pancreas (peak uptake SUV =8.8 ± 2.0 at around ≈ 20 min) with relatively slow washout thereafter. There was also excellent tracer uptake in the renal cortex and spleen with very early peak uptake, within minutes, and a very quick washout thereafter (Fig. 1A). [18F]FP-(+)-DTBZ brain scans also showed excellent tracer uptake in the striatum; the striatal time activity course was similar to that of the pancreas while that of the cerebellum was similar to the renal cortex or spleen of abdominal scans (Fig. 1C). [18F]FP-(−)-DTBZ abdominal scans, on the other hand, showed a quick peak (6.9 ± 1.7 SUV at ≈ 6 min) and a fast washout with a time course similar to those of the renal cortex and spleen (Fig. 1B). Coronal images of the pancreas summed over 120 minutes using both [18F]FP-(+)-DTBZ (top left) and [18F]FP-(−)-DTBZ (bottom) and are a summed [18F]FP-(+)-DTBZ transverse image through the mid striatum (top right) are shown in Fig. 2.
Fig. 1.
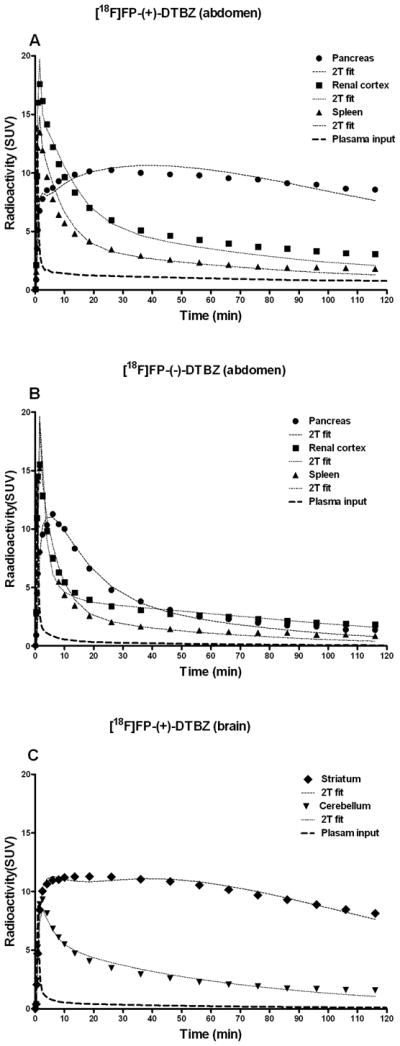
Regional and metabolite corrected plasma (input) time activity data obtained in the same one baboon with [18F]FP-(+)-DTBZ (A) and [18F]FP-(−)-DTBZ (B) for the pancreas, spleen and kidney; and [18F]FP-(+)-DTBZ for the brain (C). The two tissue compartment model (2T) fitted curves are shown by thin dotted curves.
Fig. 2.
Selected coronal slices summed over 120 min showing the pancreas with [18F]FP-(+)-DTBZ (top left) and [18F] FP-(−)-DTBZ (bottom); and a selected transverse slice summed over 120 min showing the striatum with [18F]FP-(+)-DTBZ from the same baboon shown in Fig. 1.
Plasma time activity data for both enantiomers showed very similar patterns of metabolite production with parent fractions, approximately 85%, 25% and 12% at 2 min, 20 min and 70 min, respectively; they both showed one metabolite peak at the retention time of 2 min on HPLC chromatogram. The free plasma fraction, fP, was excellent for both enantiomers: [18F]FP-(+)-DTBZ (0.31 ± 0.5) was slightly lower than [18F]FP-(−)-DTBZ (0.41 ± 0.05) (p < 0.05).
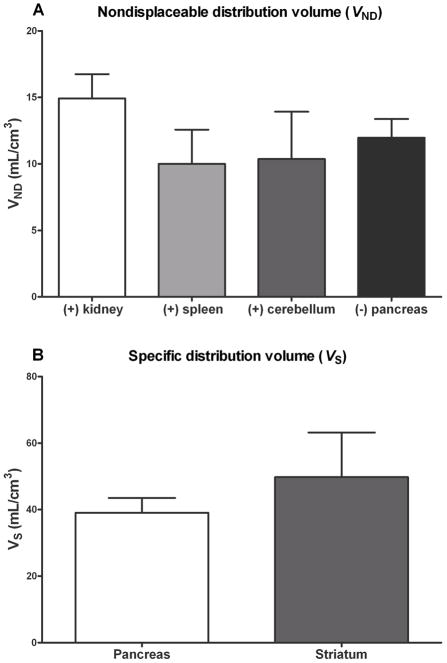
The two tissue compartment model (2T) fitting of TAC data for the both enantiomers was significantly superior to that of the one tissue model (1T) fitting for all regions. Therefore, the 2T fitting results are presented here (Fig. 1). VT was well identified by 2T fitting in all regions (the standard error of the estimates expressed as percentages being 10% ± 3%). The mean VT (mL/cm3) values of the pancreas and striatum were 53 ± 10 and 61 ± 13, respectively. The mean VND (mL/cm3) values for the renal cortex, spleen, cerebellum and the pancreas (the last being estimated by [18F]FP-(−)-DTBZ) were 15 ± 4, 10 ± 3, 10 ± 4 and 12 ± 1, respectively. Thus, the mean VND of the renal cortex, spleen and cerebellum (reference tissues) were similar (p ≈ 0.3) although not identical to the mean VND values directly estimated in the pancreas by [18F]FP-(−)-DTBZ (Fig. 3). The mean pancreas VS (41± 11 mL/cm3) values, which represented approximately 75% of VT were slightly lower (38 ± 9) and higher (43 ± 8) using the renal cortex and spleen as the reference tissue, respectively. Pancreatic VS was excellent in magnitude representing nearly 80% that of the striatum (Fig. 3).
Fig. 3.
Nondisplaceable distribution volumes (VND) of the renal cortex, spleen, cerebellum and pancreas (A), where (+) and (−) indicate [18F]FP-(+)-DTBZ and [18F]FP-(+)-DTBZ, respectively, and specific distribution volumes of the pancreas and the striatum (B). Error bars indicate positive standard deviation (SD).
4. Discussion
Human VMAT2 binding PET signal in patients with long-standing T1D was higher than expected in trials using [11C]DTBZ. Additionally, our preliminary [18F]FP-(+) DTBZ PET evaluation of human subjects with long-standing T1D has also shown more VMAT2 signal than expected. Therefore, we conducted this imaging study to investigate if pancreatic non-displaceable binding of [18F]FP-(+)-DTBZ as estimated by that of renal cortex or spleen is appropriate by using both the positive and negative enantiomers, the latter inactive enantiomer for VMAT2 directly estimating pancreatic nondisplaceable binding in male adult baboons. [18F]FP-(+)-DTBZ PET showed excellent target tissue (pancreas) signal of very high image quality (high signal-to-noise ratios). [18F]FP-(+)-DTBZ that binds reversibly to VMAT2 allowed excellent quantification of specific VMAT2 binding parameters that directly correlate with average VMAT2 density per unit tissue volume (Bmax) [12] (Figs. 1 and 2). Although β-cells are scattered throughout the pancreas accounting for 1–2% of the total volume of the pancreas, the pancreatic specific VMAT2 binding signal was almost comparable to that of the striatum (Fig. 3). The dopaminergic terminals containing VMAT2 are also scattered in the striatum with their trivial volume contribution similar to the β-cell counterpart in the pancreas [20]. Yet PET with high affinity radioligand, [18F]FP-(+)-DTBZ, for these sites allow detection of excellent binding signals and kinetic model based quantification allows for the estimation of specific VMAT2 binding (VS) that directly correlates with the average VMAT2 density in the striatum and pancreas. In other words, while the mass of beta cells is only 1–2% of the pancreas, the β cells themselves richly express VMAT2 such that even when diluted by the exocrine tissue, the relative concentration of VMAT2 approaches that found in striatal tissue.
The results of this study showed that the non-displaceable distribution volume, VND, of the pancreas can be estimated by the VND of the renal cortex or spleen, which accounted for approximately 25% of the total pancreatic VT (i.e., the specific distribution volume, Vs, was three-fold larger at 75% of VT). If our findings can be translated to human PET data analysis, this study confirms the apparent overestimation of BCM by [18F]FP-(+)-DTBZ PET is not due to the underestimation of VND by using the renal cortex as the reference tissue. However, it is possible that VND of the pancreas in T1D might be significantly larger than that in the renal cortex or spleen because of the persistent inflammatory changes (i.e. diffuse pancreatitis and adipose infiltration) commonly observed in the long term T1D pancreas. To explore the possibility that non-displaceable binding in the pancreas might be increased in T1D, we examined in another different in vitro study of specific and non-specific binding of [18F]FP-(+)-DTBZ in preparations of membranes obtained from human fresh frozen cadaveric pancreata obtained from healthy individuals and diabetic patients. In membrane preparations of equal protein content, non-displaceable binding of the [18F]FP-(+)-DTBZ was significantly increased approximately twofold in samples obtained from diabetic individuals relative to samples obtained from healthy individuals (data not shown), although membranous preparation represents only one component of the pancreatic tissue. Additionally, parenchyma fat compositions as determined by 1H-MRS in healthy controls and individuals with T2D are different (10% versus 20% respectively). This preliminary observation support the hypothesis that non-specific binding or it’s in vivo correlate, VND, of [18F]FP-(+)-DTBZ might be different in healthy versus diseased human pancreata. To better address this possibility, we are undertaking to image T1D and healthy human subjects using both (+) and (−) enantiomers to directly estimate VND in the pancreas.
In addition to the issue of nondisplaceable binding in the pancreas in VMAT2 quantification, however, there may be two other factors that could contribute to the apparent overestimation of BCM with [18F]FP-(+)-DTBZ VMAT2 imaging. First, VMAT2 is distinctly absent in the exocrine tissue. However, VMAT2 expression is not completely restricted to β-cells because small percentages of pancreatic polypeptide producing cells (PP) cells as well as the pancreatic innervation are VMAT2 positive [13, 22], although the latter seems to be not significant accounting for only ~1% of total VMAT2 sites [13]. Although the potential contribution of VMAT2 positive PP cells, which are thought not destroyed in T1D, to the apparent overestimation of BCM is also likely to be minor [22], further investigations appear warranted.
Second, although [18F]FP-(+)-DTBZ is highly selective for VMAT2, this radioligand also has a weak affinity for sigma-1 and 2 receptors (Ki = 95 and 110 nM, respectively) [23]. Tsao et al. [23] recently showed in their in vitro binding assays of rat pancreatic and brain striatal tissues that [18F]FP-(+)-DTBZ binds significantly to sigma receptors with low affinity but with high capacity in both rat pancreatic exocrine homogenates as well as in rat islet cell homogenates but not in rat striatum homogenates. They suggested that this low affinity and high capacity sigma receptor binding might contribute to the apparent overestimation of BCM with VMAT2 PET imaging. Of note is that these investigators did not perform binding assays using the negative enatiomer, [18F]FP-(−)-DTBZ, because this inactive enatiomer for VMAT2 is active for sigma receptors with similar binding affinity to that of active [18F]FP-(+)-DTBZ [23]. Thus, the pancreatic non-displaceable distribution volume, VND, directly estimated by [18F]FP-(−)-DTBZ includes this sigma receptor binding contribution, which was only 25% of total VT (the rest of 75% being specific for VMAT2) and was similar to VND estimated with [18F]FP-(+)-DTBZ for the renal cortex, spleen and cerebellum. Therefore, despite the presence of low affinity and high binding capacity sigma receptors, the magnitude of specific VMAT2 binding signal in the pancreas is quite large at least in baboons and would not be responsible for the overestimation of BCM. In fact, additional use of the negative enatiomer in humans to directly estimate the pancreatic non-displaceable binding may turn out to be of great value. Despite these potential limitations, however, [18F]FP-(+)-DTBZ pancreas imaging appears very promising with exquisitely intense target tissue (pancreas) signal of very high PET image quality ( in human with peak SUV > 20). VMAT2 should be an excellent biomarker of BCM, for example, tracking BCM changes over time.
5. Conclusion
[18F]FP-DTBZ PET shows excellent specific VMAT2 binding in both the pancreas and striatum in baboons. The renal cortex or spleen as the reference tissue in VMAT2 quantification appears supported. However, further studies appear warranted to directly estimate pancreatic non-displaceable binding in humans including T1D patients and also to clarify the cause of the apparent overestimation of BCM in T1D.
Acknowledgments
This work was supported by the PHS, NIH, NIDDK (5 R01 DK063567). The authors wish to acknowledge the help of Dr. Mark Slifstein and his team in the baboon imaging experiments and the efforts of Dr. B. Easwaramoorthy in preparation of [18F]-FP-(+ or −)-DTBZ used in certain experiments. This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vander Borght TM, Sima AA, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA. [3H]methoxytetrabenazine: a high specific activity ligand for estimating monoaminergic neuronal integrity. Neuroscience. 1995;68:955–62. doi: 10.1016/0306-4522(95)00167-h. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Mann S, Sossi V, Ruth TJ, Stoessl AJ, Schulzer M, et al. [11C]DTBZ-PET correlates of levodopa responses in asymmetric Parkinson’s disease. Brain. 2003;126:2648–55. doi: 10.1093/brain/awg270. [DOI] [PubMed] [Google Scholar]
- 3.Koeppe RA, Gilman S, Junck L, Wernette K, Frey KA. Differentiating Alzheimer’s disease from dementia with Lewy bodies and Parkinson’s disease with (+)-[11C]dihydrotetrabenazine positron emission tomography. Alzheimer’s & Dementia. 2008;4:S67–76. doi: 10.1016/j.jalz.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Koeppe RA, Gilman S, Joshi A, Liu S, Little R, Junck L, et al. 11C-DTBZ and 18F-FDG PET measures in differentiating dementias. J Nucl Med. 2005;46:936–44. [PubMed] [Google Scholar]
- 5.Albin RL, Koeppe RA, Wernette K, Zhuang W, Nichols T, Kilbourn MR, et al. Striatal [11C]dihydrotetrabenazine and [11C]methylphenidate binding in Tourette syndrome. Neurology. 2009;72:1390–6. doi: 10.1212/WNL.0b013e3181a187dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer P, Bohnen NI, Minoshima S, Koeppe RA, Wernette K, Kilbourn MR, et al. Striatal presynaptic monoaminergic vesicles are not increased in Tourette’s syndrome. Neurology. 1999;53:371–4. doi: 10.1212/wnl.53.2.371. [DOI] [PubMed] [Google Scholar]
- 7.Anlauf M, Eissele R, Schafer MK, Eiden LE, Arnold R, Pauser U, et al. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem. 2003;51:1027–40. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- 8.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, et al. Longitudinal noninvasive PET-based beta cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–13. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal T, Ding YS, Weinzimmer D, Normandin MD, Labaree D, Ropchan J, et al. Pancreatic beta cell mass PET imaging and quantification with [11C]DTBZ and [18F]FP-(+)-DTBZ in rodent models of diabetes. Mol Imaging Biol. 2011;13:973–84. doi: 10.1007/s11307-010-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, et al. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009;50:382–9. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Normandin MD, Petersen KF, Ding YS, Lin SF, Naik S, Fowles K, et al. In vivo imaging of endogenous pancreatic beta-cell mass in healthy and type 1 diabetic subjects uusing 18F-fluoropropyl-dihydrotetrabenazine and PET. J Nucl Med. 2012;53:908–16. doi: 10.2967/jnumed.111.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Met. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 13.Saisho Y, Harris PE, Butler AE, Galasso R, Gurlo T, Rizza RA, et al. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol. 2008;39:543–51. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami R, Ponde DE, Kung MP, Hou C, Kilbourn MR, Kung HF. Fluoroalkyl derivatives of dihydrotetrabenazine as positron emission tomography imaging agents targeting vesicular monoamine transporters. Nuclear medicine and biology. 2006;33:685–94. doi: 10.1016/j.nucmedbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, et al. In vivo imaging of beta-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med. 2008;49:1171–6. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 16.Koeppe RA, Frey KA, Kuhl DE, Kilbourn MR. Assessment of extrastriatal vesicular monoamine transporter binding site density using stereoisomers of [11C]dihydrotetrabenazine. Cerebral Blood Flow and Metabolism. 1999;19:1376–84. doi: 10.1097/00004647-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Kung MP, Hou C, Goswami R, Ponde DE, Kilbourn MR, Kung HF. Characterization of optically resolved 9-fluoropropyl-dihydrotetrabenazine as a potential PET imaging agent targeting vesicular monoamine transporters. Nucl Med Biol. 2007;34:239–46. doi: 10.1016/j.nucmedbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–9. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 19.Freeby M, Golan R, Saxena C, Kringas P, Carpenter A, Skovronsky, Harris P, Ichise M. PET imaging of pancreatic beta cell mass in humans with type 1 and type 2 diabetes mellitus using 18F-FP-DTBZ. J Nucl Med. 2012;53:91. [Google Scholar]
- 20.Ichise M, Harris PE. Beta-cell Imaging: Opportunities and Limitations REPLY. J Nucl Med. 2011;52:493–5. doi: 10.2967/jnumed.110.085530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–21. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- 22.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–71. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 23.Tsao HH, Skovronsky DM, Lin KJ, Yen TC, Wey SP, Kung MP. Sigma receptor binding of tetrabenazine series tracers targeting VMAT2 in rat pancreas. Nucl Med Biol. 2011;38(7):1029–34. doi: 10.1016/j.nucmedbio.2011.03.006. [DOI] [PubMed] [Google Scholar]