SUMMARY
Background and purpose
Ziconotide is a peptide that blocks N-type calcium channels and is anti-hyperalgesic after intrathecal delivery. We here characterize the spinal kinetics of intrathecal bolus and infused ziconotide in dog.
Experimental approach
Male beagle dogs (N = 5) were prepared with chronic intrathecal (IT) lumbar injection and cerebrospinal fluid (LCSF) sampling catheters connected to vest-mounted pumps. Each dog received: i) IT bolus ziconotide (10 µg + 1 µCi 3H-inulin), ii) IT infusion for 48 hr of ziconotide (1 µg/100 µL/hr), iii) IT infusion for 48 hr of ziconotide (5 µg/100 µL/hr), and iv) intravenous injection of ziconotide (0.1 mg/kg). After IT bolus, LCSF ziconotide and inulin showed an initial peak and biphasic (distribtution/elimination) clearance (ziconotide T1/2 α / ß = 0.14 and 1.77 hr, and inulin T1/2 α / ß = 0.16 and 3.88 hr, respectively). The LCSF: plasma ziconotide concentration ratio was 20,000: 1 at 30 min, and 30: 1 at 8 hr. IT infusion of 1 and then 5 µg/hr resulted in LCSF concentrations that peaked by 8 hr and remained stable at 343 and 1380 ng/mL, respectively, to the end of the 48-hr infusions. Terminal elimination T1/2 after termination of continuous infusion was 2.47 hr. Ziconotide LCSF: cisternal CSF: plasma concentration ratios after infusion of 1 µg/hr and 5 µg/hr were 1: 0.017: 0.001 and 1: 0.015: 0.003, respectively. IT infusion of ziconotide at 1 µg/hr inhibited thermal skin twitch by 24 hr, and produced modest trembling, ataxia, and decreased arousal. Effects continued through the 48-hr infusion period, increased in magnitude during the subsequent 5 µg/hr infusion periods, and disappeared after drug clearance.
Conclusions and Implications
After intrathecal bolus or infusion, ziconotide displays linear kinetics that are consistent with a hydrophilic molecule of approximately 2500 Da that is cleared slightly more rapidly than inulin from the LCSF. Behavioral effects were dose dependent and reversible.
Keywords: spinal, kinetics, antinociception, blood pressure, analgesia, PRIALT®, ziconotide, intrathecal, SNX-111
INTRODUCTION
Ziconotide is the synthetic equivalent of a 25 amino acid peptide originally identified in the venom of the cone snail, Conus magus. (1,2) Early work emphasized that ziconotide and related peptides are highly selective antagonists of the N-type voltage-sensitive calcium channel. (3,4,5) N type channels are considered to be important for terminal transmitter release and we have shown that intrathecal delivery of ziconotide will indeed block release of substance P from small nociceptive primary afferents. (6) Subsequent work revealed that block of this channel would exert a potent anti- hyperalgesic and anti-allodynic effect in models of tissue and nerve injury when given intrathecally in the rat. (7,8,9,10) Autoradiographic binding studies showed that the densest localization of the ziconotide binding site in the spinal cord is in the dorsal spinal lamina (substantia gelatinosa) (11), a region known to be pivotal in processing nociceptive information. (12)
Studies in humans have also shown that intrathecal bolus injection or continuous infusion of ziconotide is efficacious in a variety of acute and chronic pain states (13, 14, 15, 16, 17,18) and in ameliorating spasticity secondary to spinal injury. (19) Initial studies reported significant adverse effects with dose incrementation, (15, 20) which may have reflected an incomplete appreciation of the kinetics of redistribution of the intrathecally delivered drug. To address this issue, we undertook pharmacokinetic studies to characterize the spinal kinetics of ziconotide after bolus intrathecal injection, during and after continuous intrathecal infusion, and after intravenous delivery in a well-defined beagle dog model that uses chronically placed lumbar intrathecal catheters. (21,22,23,24)
METHODS
Studies were carried out under protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
Drug/Injectate
Ziconotide and saline diluent were furnished by the Neurex Corporation (now Elan Corporation, South San Francisco, CA). Ziconotide for injection was prepared by aseptic dilution with saline diluent. Solutions were not filtered. Drug dilutions and reconstitutions were made just prior to dosing.
Animals
Male beagle dogs (N = 5) were obtained from Harlan Sprague Dawley, Inc. (Ridgeland Farms, Mt. Horeb, WI). These dogs were between 9 and 18 months old and weighed between 12 and 16.5 kg at the start of the study. All animals had free access to dry dog food, with the exception of the nights prior to scheduled clinical pathology, surgery, and sedation. Tap water was available ad libitum. Animals were individually housed in 4- foot by 7-foot inside runs.
Surgical preparation
After an initial acclimation (3–7 days), dogs underwent surgical implantation of intrathecal injection and sampling catheters to be used for repeated intrathecal dosing and sampling. Catheter placement was performed following strict aseptic precautions. All catheters were fabricated and packaged on site and locally sterilized by e-beam irradiation. Surgery was performed approximately 72 to 96 hr (study day −4 and −3) prior to initiation of dosing. Animals received atropine (0.04 mg/kg, IM) prior to sedation with xylazine (Rompun; 1.5 mg/kg, IM). Tribrissen (15–20 mg/kg, oral, twice daily for 5 days) was given as a prophylactic antibiotic. Anesthesia was mask-induced and the animals were then intubated and maintained under spontaneous ventilation with 1–2% Halothane and 60% N2O and 40%O2. Animals were continuously monitored for oxygen saturation, inspired and end-tidal gas, expired CO2, N2O, O2 and heart and respiratory rates. Surgical areas were shaved and prepped with chlorhexadine scrub and solution. Dexamethasone sodium phosphate (0.25 mg/kg, IM) was given immediately following completion of intrathecal catheter placements.
Using sterile technique, a skin incision was made on the lower back of the neck. The cisterna magna was then exposed by combined blunt and sharp dissection. The dura was then exposed and two small incisions (0.5 mm) were made and the prepared catheters were then individually inserted and passed caudally. The catheters were fabricated of polyethylene PE10 (0.4 mm O.D.), for the infusion catheter, and PE50 (0.97 mm O.D.), for the sampling catheter (Intramedic©, Becton Dickinson). The PE10 catheter was passed approximately 40 cm to the L2–3 level. The PE50 catheter was passed through an adjacent incision approximately 38 cm. The internal segments of the catheters were then connected to 50 cm PE100 external segments and tunneled subcutaneously and caudally to exit on the upper left and right back at the level of the scapula. Confirmation of the appropriate placement of the catheters in the intrathecal space was based on the free outflow of CSF. The incision was closed in layers using 3–0 Vicryl®. With closure of the incision, anesthesia gases were turned off and the animals allowed to recover under observation. Butorphanol tartrate (Torbugesic; 0.04 mg/kg, IM) was administered to relieve post-operative discomfort. A nylon vest (Alice King Chatham Medical Arts, Hawthorne, CA) was placed on each dog following surgical recovery and a Panomat C-10 infusion pump (Disetronics Medical Systems, Plymouth, MN) was placed in the left vest pocket and connected to the PE50 sampling catheter. The pump was set to deliver approximately 100 µL/hr of 0.9% w/v Sodium Chloride for Injection, USP, to maintain sampling catheter patency.
Study Design
Each dog received: i) one intrathecal bolus injection of ziconotide (10 µg in 1 mL), ii) chronic intrathecal infusion over two sequential 48-hr intervals of two concentrations of ziconotide (1 µg/hr IT followed by 5 µg/hr IT, respectively, at 100 µl/hr), and iii) lastly, an intravenous bolus injection of ziconotide (0.1 mg/kg). All intrathecal bolus injections were spiked with 1 µCi 3H-inulin and followed by 0.3 mL 0.9% (w/v) Sodium Chloride for Injection, USP, as catheter flush.
Behavioral Observations
Animals were observed for arousal, motor function, and motor strength on a 4-point scale (22). Morbidity/mortality and clinical observations were noted twice daily (morning and late afternoon). On dosing days, the morning clinical observations were conducted prior to dosing. Appetite, presence of stool and urine, general behavior, overt signs of toxicity, and body temperatures were recorded daily.
Heart rate and blood pressures were measured using a tail cuff manometer (Dinamap 8100, Critikon). Respiratory rates were measured by visualization of chest expansion and contraction. These measurements were made periodically before and after each injection and during infusion intervals.
The thermally evoked nociceptive skin twitch response was measured using a probe with approximately 1 cm surface area maintained at approximately 62.5°C ± 0.5°C with a feedback. (24) The probe was applied to shaven lumbar areas of the back. This stimulus results in a brisk contraction of the local, underlying musculature within 1–3 sec of the probe placement. Upon appearance of this response, the probe was removed and the latency recorded. Failure to respond within 6 sec was cause to remove the probe and assign that value (6 sec) as the latency.
Kinetics
LCSF (approximately 0.3 mL) and blood samples (approximately 1 mL) were collected from each animal before and during dosing intervals. Blood samples were collected on wet ice and prepared as plasma in lavender EDTA-coated test tubes. Cisternal CSF samples (approximately 0.3 mL) were obtained by percutaneous puncture (1.5 inch 22 ga spinal needle) in the lightly anesthetized dog three times: i) just prior to the initiation of 1 µg/hr ziconotide chronic intrathecal infusion, ii) 48 hr later, just prior to the dose change to 5 µg/hr ziconotide infusion, and iii) 48 hr later just prior to termination of chronic intrathecal infusion. All samples were collected onto dry ice and stored frozen at approximately −20°C ± 10°C until assay.
Ziconotide assays were performed using a validated radioimmunoassay (RIA) using 125I-ziconotide and a ziconotide-specific antibody. In this assay, the 125I-ziconotide-antibody complexes are separated from unbound 125I-ziconotide by adsorption to charcoal and the radioactivity in the complexes measured by gamma counting. Ziconotide in a sample inhibits the binding of 125I-ziconotide to the antibody and the amount of ziconotide in the sample is calculated by comparison to a standard curve. The lower limit of quantification of the RIA for dog CSF and plasma is 0.07 ng/ml of ziconotide. Standard curves and blanks were established using dog cisternal cerebrospinal fluid and dog plasma. Additional details and characteristics of this assay are presented elsewhere. (25)
Sacrifice
After completion of the final dosing interval and sample collection, animals were deeply anesthetized with sodium pentobarbital and intubated, with ventilation being manually maintained. The chest was then opened, the aorta catheterized, and the blood cleared by perfusion at 80 to 160 mmHg of pressure, with approximately 4 L of 0.9% saline. The spinal column was then exposed by laminectomy. Methylene blue dye was injected through both intrathecal catheters to establish their patency and to visualize their position relative to each other.
Statistical and Kinetic Analyses
Behavioral scores (arousal, muscle tone, and coordination), physiological parameters (blood pressures and heart and respiratory rates) and skin twitch response latencies are presented as mean and standard error of the mean (S.E.M.). The pharmacokinetic analyses were done using PK Solutions software (PK Solutions, Version 2.0 SUMMIT Research Services 1374 Hillcrest Drive, Ashland, OH 44805, Copyright 1999). Area under the concentration-time curves (AUC) for plasma and CSF were estimated by the trapezoidal rule up to the last observed concentration (Clast). For concentrations below the limit of detection, a value of one-half the detection limit was used. If the last concentration was detectable, then AUC0-∞ was estimated as AUC up to the last observed concentration (AUC0-τ) + Clast/λz, where λz is the elimination rate constant estimated by linear regression of the terminal portion of the concentration-time curve. Clearance (CL) was calculated as F*D/AUC, where F=bioavailability and D = dose administered. Apparent volume of distribution (Vz) was estimated by CL*λz, and relates the amount of drug in the body to the sampled matrix concentration during the terminal phase. Volume of distribution at steady-state (Vss) was estimated as (D)(AUMC0-∞)/(AUC0-∞), where AUMC is the total area under the first moment curve, and relates the amount of drug in the body to the sampled matrix concentration at steady-state. The analysis used curve stripping methods, where drug concentration data in blood or CSF are resolved into a series of rate constant terms corresponding to the absorption, distribution, and elimination phases. Half-lives are then estimated as 0.693/k, where k is the corresponding rate constant for that phase. Two rate constants were estimated, and correspond to either: distribution and elimination (when the concentrations are being measured in the matrix where the dose was administered), or absorption/distribution and elimination (when the concentrations are being measured in a different compartment from where the dose was administered). The following parameters were determined:
-
1)
Bolus intrathecal injection: CSF and plasma levels of ziconotide and 3H-inulin versus time. For LCSF and plasma: T½ of distribution (T½-α) (LCSF) or absorption/distribution (plasma), T½ of elimination (T½-β), peak concentration (Cmax), time to peak concentration (Tmax), area under the concentration-time curve (AUC0-∞), ratio of LCSF ziconotide (µg/mL) to LCSF inulin
-
2)
(DPM) versus time, and ratio of LCSF ziconotide to plasma ziconotide versus time. A general (rough) estimate of the absolute bioavailability of ziconotide in plasma (Fplasma), following bolus IT ziconotide administration, can be determined using the following equation: , where CLIV = plasma CL after an IV dose and CLIT = plasma CL/F after the intrathecal dose.
-
2)
Continuous intrathecal infusion: CSF and plasma levels of ziconotide versus time to steady state, steady state concentration (mean of values measured from time of steady state to end of infusion), clearance at steady-state, calculated as intrathecal infusion rate divided by steady-state concentration, ratio of LCSF ziconotide to plasma ziconotide versus time, and ratio of LCSF to cisternal CSF to plasma ziconotide at steady state.
-
3)
Termination of continuous intrathecal infusion:CSF and plasma levels of ziconotide and 3H-inulin versus time. For LCSF and plasma: T½ of elimination (T½-β), and ratio of LCSF ziconotide to plasma ziconotide versus time.
-
2)
-
4)
Bolus intravenous injection: Peak concentration (Cmax) for LCSF and plasma ziconotide, time to peak concentration (Tmax), and area under the time-concentration curve (AUC0-∞). Similar to section 1 above, a general (rough) estimate of the absolute bioavailability of ziconotide in CSF (FCSF), following bolus IV ziconotide administration, can be determined using the following equation: , where CLIV = CSF CL/F after IV administration and CLIT = CSF CL after intrathecal administration.
RESULTS
All animals survived surgery without mishap and completed the intended protocol. At the time of sacrifice, all catheters were patent and were located in the targeted lumbar spinal region with the tip of the sampling catheter located approximately 2 cm rostral to the tip of the dosing catheter. Mean body weight at the time of these studies was 14.5 ± 0.9 kg. Plasma samples were obtained for assay for all animals. Lumbar CSF samples were reliably obtained from 4 of the 5 animals. These samples were employed for the subsequent analysis of ziconotide concentrations.
Kinetics
Intrathecal Bolus
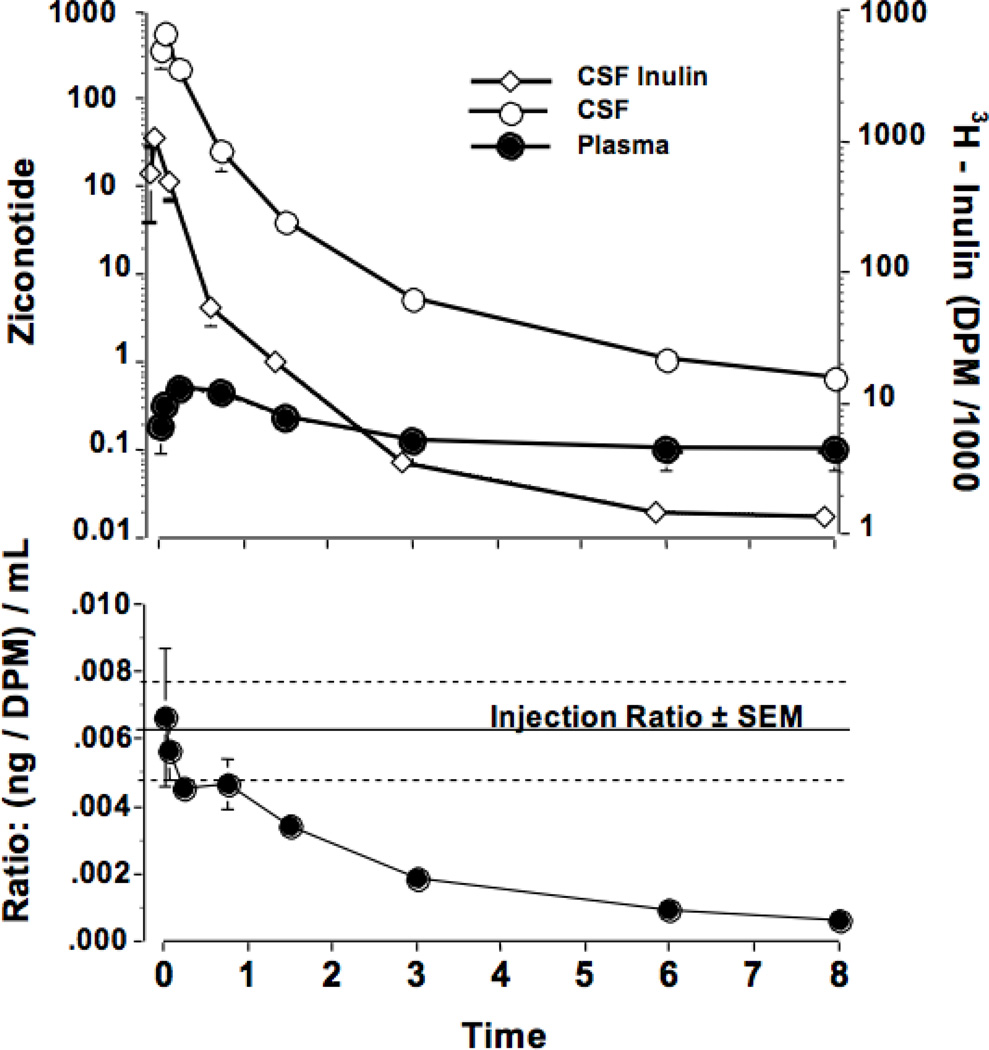
Intrathecal (IT) bolus delivery of ziconotide (10 µg) resulted in peak concentrations in LCSF by the first sampling interval (i.e., 3 min) and with a biphasic (distribution/elimination) clearance (T1/2 α / ß = 0.14 and 1.68 hr, respectively). (See Figure 1 and Table 1.) Examination of ziconotide concentrations in plasma revealed a peak at 20 min. At this time, the CSF : plasma ratio was approximately 20,000: 1 and declined over the ensuing 8 hr to approximately 30: 1. Based on median plasma CLIV dose and plasma CLIT dose values, Fplasma = 53.5%. Note: this general estimate assumes linear PK, which may not apply over the wide plasma ziconotide concentration range evaluated.
Figure 1.
The concentrations of ziconotide (ng/mL) and inulin (DPM/mL) in the lumbar CSF (top) as a function of time after the bolus intrathecal injection of ziconotide (10µg) and inulin (1µCi) in 1 mL. The bottom panel presents the ratio of ziconotide to inulin as a function of time after intrathecal delivery. Each point represents the mean and SEM of data from 5 dogs. Calculated kinetics are presented in Table 1.
Table 1.
Summary of Ziconotide (10 µ g) and [3H]Inulin (approx. mean dose: 1.77×106 dpm) PK following Bolus Intrathecal Administration.
| PK Parameters |
Statistics | Ziconotide | [3H]Inulin | |
|---|---|---|---|---|
| Plasma | CSF | CSF | ||
| N | 4 | 4 | 5 | |
| C0 (ng/mL or dpm/mL) |
||||
| Median | NA | 8781 | 1348776 | |
| Range | NA | 6007 – 11187 | 707500 – 2551985 | |
| Cmax (ng/mL or dpm/mL) |
||||
| Median | 0.52 | 6698 | 1189820 | |
| Range | 0.3 1– 0.72 | 5470 – 6840 | 649480 – 1566460 | |
| Tmax (h) |
||||
| Median | 0.25 | 0.06 | 0.08 | |
| Range | 0.25 – 0.75 | 0.03 – 0.08 | 0.03 – 0.08 | |
| T½-α (hrs) | ||||
| Median | 0.61 | 0.14 | 0.14 | |
| Range | 0.2–2.1 | 0.06–0.23 | 0.06–0.32 | |
| T½-β (h) | ||||
| Median | 1.25 | 1.68 | 3.04 | |
| Range | 0.79 – 4.08 | 1.59 – 2.14 | 2.49 – 7.78 | |
| AUC∞ (ng.h/mL or dpm.h/mL) |
||||
| Median | 0.85 | 1879 | 487562 | |
| Range | 0.61 – 3.91 | 1488 – 2936 | 258576 – 593643 | |
| CLa or CL/Fb (mL/h) |
||||
| Median | 11740b | 5.53a | 4.66a | |
| Range | 2559 – 16425b | 3.41 – 6.72a | 1.79 – 5.79a | |
| Vza or Vz/Fb (mL) |
||||
| Median | 17160b | 12.9a | 18.8a | |
| Range | 12932 – 33328b | 8.5 – 20.8a | 6.4 – 65.0a | |
| Vss (mL) |
||||
| Median | NA | 1.32 | 2.34 | |
| Range | NA | 0.96 – 1.66 | 0.69 – 8.49 | |
Note 1: C0 = initial concentration (back-extrapolated to dose time; NA = not applicable.
Note 2: Vss is much smaller than Vz, indicating that appreciable elimination of drug occurs before distribution equilibrium is achiePved.
3H-Inulin, injected intrathecally concurrently with ziconotide, displayed a clearance profile similar to that of ziconotide with a similar T1/2α but a longer T1/2 ß (T1/2 α and ß = 0.16 and 3..04 hr, respectively).The slower inulin clearance is confirmed by inspection of the ratio of ziconotide: inulin concentrations determined concurrently in LCSF of each sample over time. This ratio fell from approximately 0.006 ng/DPM (essentially equivalent to that which was injected) to approximately 0.0005 by 8 hr. (See Figure 1)
Intravenous Bolus
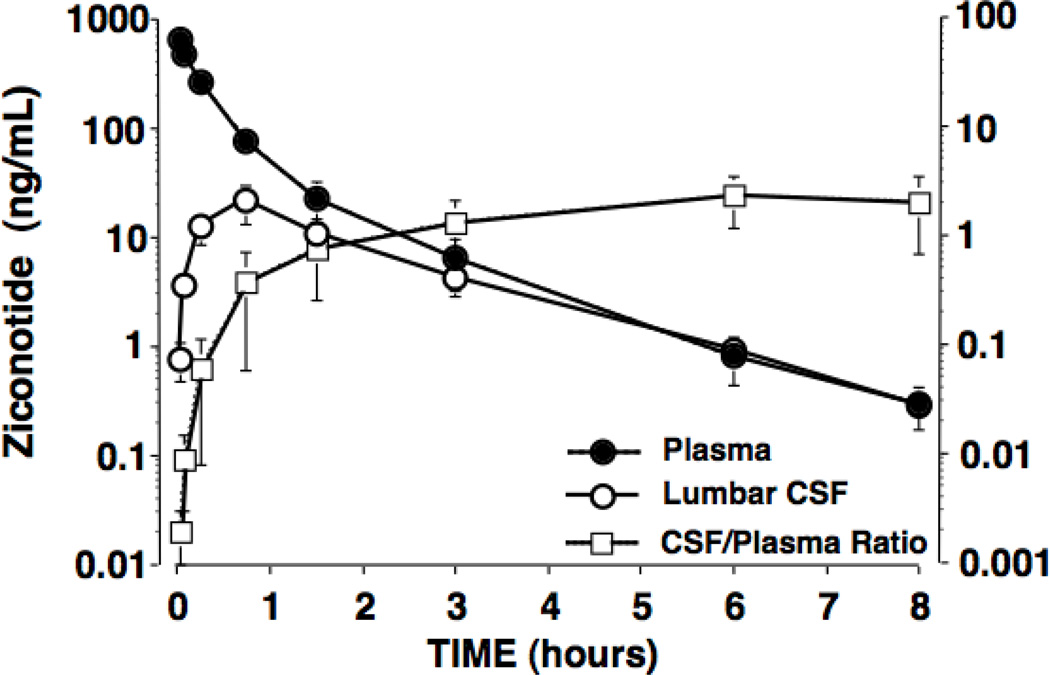
IV bolus delivery of 0.1mg/kg ziconotide resulted in an immediate peak plasma concentration (approximately 900 ng/mL), a terminal elimination half-live of 1.3 hrs. (See Figure 2 and Table 2.) The LCSF Cmax after IV delivery was 21 ng/mL observed at 45 min. At plasma Cmax, the plasma: LCSF ratio was 850: 1, declining to 3: 1 at 45 min, and to 1: 1 at 8 hr. Based on median CSF CLIV dose and CSF CLIT dose values, FCSF = 0.013%. Note: this general estimate also assumes linear PK, which may or may not apply over the wide CSF ziconotide concentration range evaluated.
Figure 2.
The concentrations of ziconotide (ng/mL) in the plasma and lumbar CSF, and the ratio of CSF: plasma levels after the bolus intravenous delivery of ziconotide (0.1 mg/kg). Results presented represent the mean and SEM of data from 5 dogs. Calculated kinetics are presented in Table 2.
Table 2.
Summary of Ziconotide PK following Bolus Intravenous Administration (0.1 mg/kg)
| PK Paramete |
Statistics | Ziconoti | |
|---|---|---|---|
| Plas | CS | ||
| N | 5 | 4 | |
| C0 (ng/mL) |
Median | 9 | N |
| Range | 419 – 1138 | N | |
| Cmax (ng/mL) |
Median | 6 | 20 |
| Range | 428 – | 6.6 – | |
| Tmax (h) |
Median | 0. | 0. |
| Range | 0.03 – 0.08 | 0. | |
| T½-β (h) | Median | 1. | 1. |
| Range | 1.06 – 1.43 | 0.95 – | |
| AUC∞ (ng·h/mL) |
Median | 2 | 38 |
| Range | 185 – | 15.9 – | |
| CLa or CL/Fb (mL/h) |
Median | 628 | 4256 |
| Range | 3027 – 8613a | 22184 – 91574b | |
| V a or V /Fb z z (mL) |
Median | 1264 | 102615 |
| Range | 4618 – 17786a | 30436 – 243703b | |
| Vss (mL) |
Median | 23 | N |
| Range | 1979 – 7840 | N | |
NA = not applicable.
Intrathecal Infusion
After the initiation of the 1 µg/hr intrathecal infusion, ziconotide concentration in LCSF peaked by 8 hr and remained essentially stable through the 8–48 hr infusion (median CSF concentration for 8–48 hr = 286 ng/mL). (See Table 3.) Plasma levels of ziconotide peaked by 8 hr and remained essentially stable through the remaining 8–48 hr infusion (median plasma concentration for 8–48 hr was at the limits of detection for 3 animals)
Table 3.
Summary of Ziconotide Concentrations in Plasma and Lumbar CSF with Continuous Intrathecal Infusion for 48 hrs with 1µg/hr followed by 48 hrs of 5µg / h and then Elimination from Lumbar CSF after Termination of 5 µg/h infusion
| PK Parameters |
Statistics | Ziconotide | |||
|---|---|---|---|---|---|
| Plasma | CSF | ||||
| 1 µg/h | 5 µg/h | 1 µg/h | 5 µg/h | ||
| Cmax (ng/mL) |
N | 5 | 5 | 5 | 4 |
| Median | 0.00 | 1.26 | 449 | 2475 | |
| Range | 0.00 – 0.30 | 0.96 – 1.54 | 181 – 630 | 1579 – 3705 | |
| Css (8 – 48 h) (ng/mL) |
N | 5 | 5 | 5 | 4 |
| Median | 0.00 | 1.06 | 286 | 1620 | |
| Range | 0.00 – 0.25 | 0.92 – 1.36 | 122 – 576 | 990 – 2429 | |
| Tmax (h) |
N | 2 | 5 | 5 | 4 |
| Median | 28.0 | 24.0 | 24.0 | 16.0 | |
| Range | 8.0 – 48.0 | 8.0 – 48.6 | 8.0 – 24.0 | 8.0 – 48.6 | |
| CLa or CL/Fb (mL/h) |
N | 2 | 5 | 5 | 4 |
| Median | 8029b | 4702b | 3.50a | 3.25a | |
| Range | 4054 – 12005b | 3668 – 5454b | 1.73 – 8.18a | 2.06 – 5.05a | |
| T½-β (h) | N | ND | 5 | ND | 4 |
| Median | ND | 0.94 | ND | 2.35 | |
| Range | ND | 0.84 – 1.31 | ND | 1.99 – 3.18 | |
Note 1: Calculated Css values are the average of concentrations 8 – 48 hours after the start of IT infusion.
Note 2: CSF concentration values during IT infusion appeared quite variable; hence, the PK estimates above should be interpreted with some caution. Further, several plasma concentration values for the 1 µg/h infusion regimen were BLQ, which precluded or limited the assessment of PK estimates.
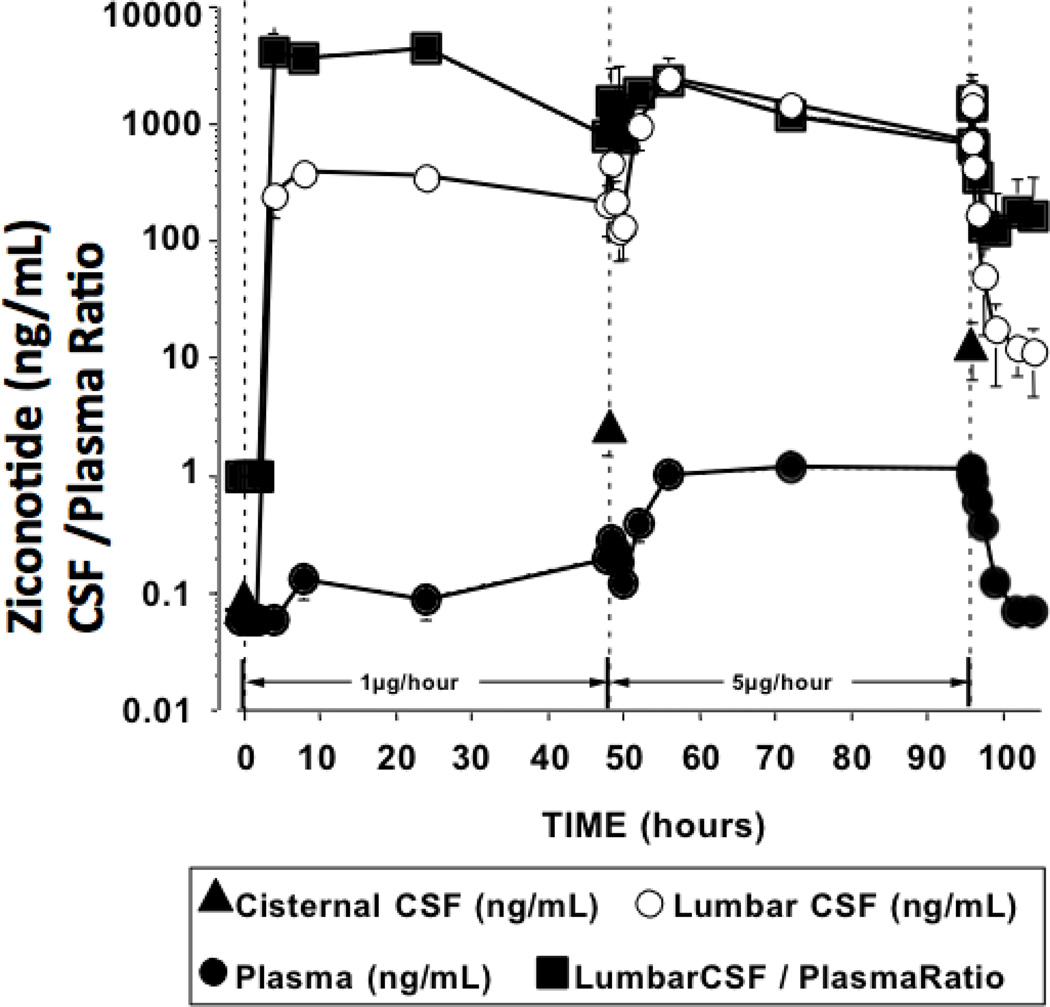
After initiating a 5-fold increase in infusion dose (5 µg/hr), time to peak CSF concentrations was also approximately 8 hr and remained essentially stable through the 8–48 hr infusion (median CSF concentration for 8–48 hr = 1620 ng/mL). (See Table 3.) With 5 µg/hr, a similar concentration- time profile was observed in plasma, with the 8–48 hr group mean concentration being 1.1 ng/mL. (See Figure 3 and Table 3.) The ratio of ziconotide in LCSF versus plasma was approximately 2400: 1 at 8 hr, 1200: 1 at 24 hr and 600: 1 at 48 hr.
Figure 3.
The concentrations of ziconotide (ng/ml) in the plasma, lumbar CSF, and the cisternal CSF, and the ratio of CSF: plasma levels at intervals before, during, and after continuous infusion of ziconotide. From 0 to 48 hr, ziconotide was infused at 1µg/hr. From 48 to 96 hr ziconotide was delivered at the rate of 5µg/hr. Cisternal CSF was sampled immediately prior to the initiation of infusion just before changing the infusion rate to 5 µg/hr and just before terminating the infusion of 5µg/hr. Results presented represent the mean and SEM of data from 5 dogs. Calculated kinetics for the two infusion rates and for the clearance of ziconotide after termination of 5 µg/hr infusion in Table 3.
Following termination of the 5 µg/hr infusion, LCSF ziconotide concentrations fell, showing a monophasic clearance with a mean terminal elimination T1/2 of 2.35 hr (See Figure 3/Table 3.)
Cisternal CSF ziconotide concentrations, prior to initiating infusion of 1 µg/hr, were below detection. After 48 hr of the 1 µg/hr infusion, and then at 48 hr after the initiation of the 5 µg/hr infusion, the mean cisternal CSF concentrations of ziconotide were 2.3 ng/mL and 13.5 ng/mL, respectively. (See Figure 3.) Calculation of the LCSF: cisternal CSF: plasma ratio using ziconotide concentrations measured concurrently in those spaces at 48 hr after the infusion of 1 µg/hr, and then at 48 hr after 5 µg/hr, showed comparable values: 1: 0.017: 0.001 and 1: 0.015: 0.003, respectively (where 1 is taken as the concentration of ziconotide in LCSF 48 hr after the initiation of 1 or 5 µg/hr infusions and all other values are expressed as a ratio of that value). (See Table 4.)
Table 4.
Ratio of Lumbar:Cisternal:Plasma Ziconotide Concentrations Measured Concurrently at 48 Hours After Intrathecal Infusion of 1µg and 5µg Ziconotide
| Lumbar | Cisternal | Plasma | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Post 1µg/hr | 1 ± 0 | 0.0171 ± 0.0114 | 0.0007 ± 0.0004 |
| Post 5µg/hr | 1 ± 0 | 0.0153 ± 0.0082 | 0.0026 ± 0.0021 |
Pharmacodynamics
General Behavior
After intrathecal bolus injection of ziconotide, 3 of the 5 dogs exhibited whole body trembling. Panting was observed in 2 of the 5 dogs at approximately 1 hr post injection. All 5 dogs showed decreased arousal and activity but remained responsive 8 hr post injection. Dogs returned to normal baseline behavior 24 hr after injection. Almost immediately following the intravenous bolus injection of ziconotide, 4 of the 5 dogs vomited and 2 of the 5 had scleral vasodilation and facial erythema. At approximately 5 min post injection, whole body trembling, laryngeal spasms, decreased muscle tone and slight sedation were observed in 3 of the 5 dogs. All observed effects were absent 24 hr post injection. At approximately 4–8 hr after initiation of 1 µg/hr continuous intrathecal infusion of ziconotide, all 5 dogs exhibited whole body trembling, ataxia and decreased arousal and activity levels. The observed whole body trembling and ataxia continued through the 1 µg/hr 48-hr infusion period. There was a tendency for this to increase in severity after the first 24 hr of the 5 µg/hr 48-hr infusion period, which immediately followed the first infusion period. At 24 hr post termination of the intrathecal infusion, 3 of the 5 dogs had diarrhea and 1 had hematuria.
Antinociception
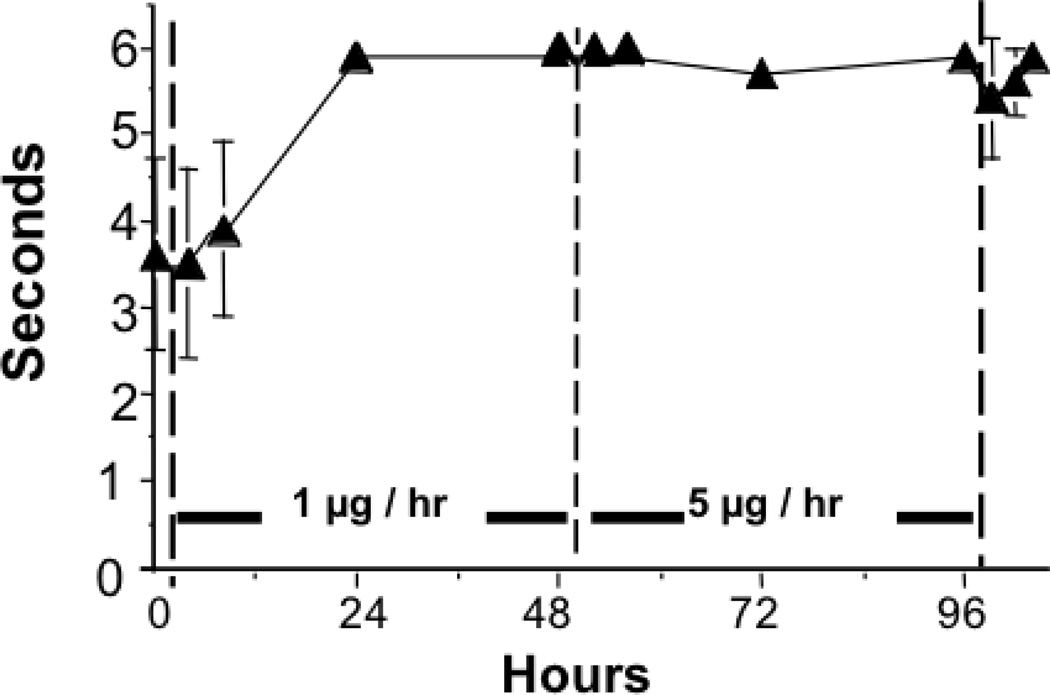
Neither bolus intravenous nor intrathecal injection of ziconotide had an effect upon skin twitch response (data not shown). During intrathecal infusion, all dogs showed no significant anti-nociceptive behavior at 8 hr, but displayed a complete block of the skin twitch response by 24 hr after initiation. Animals remained maximally blocked through the remainder of the 1 µg/hr infusion and the subsequent 5 µg/hr infusion. (See Figure 4).
Figure 4.
The skin twitch response latency during the continuous intrathecal infusion of ziconotide. From 0 to 48 hr, ziconotide was infused at 1 µg/hr. From 48 to 96 hr, ziconotide was delivered at the rate of 5 µg/hr. Results presented represent the mean and SEM of data from 5 dogs.
Temperature
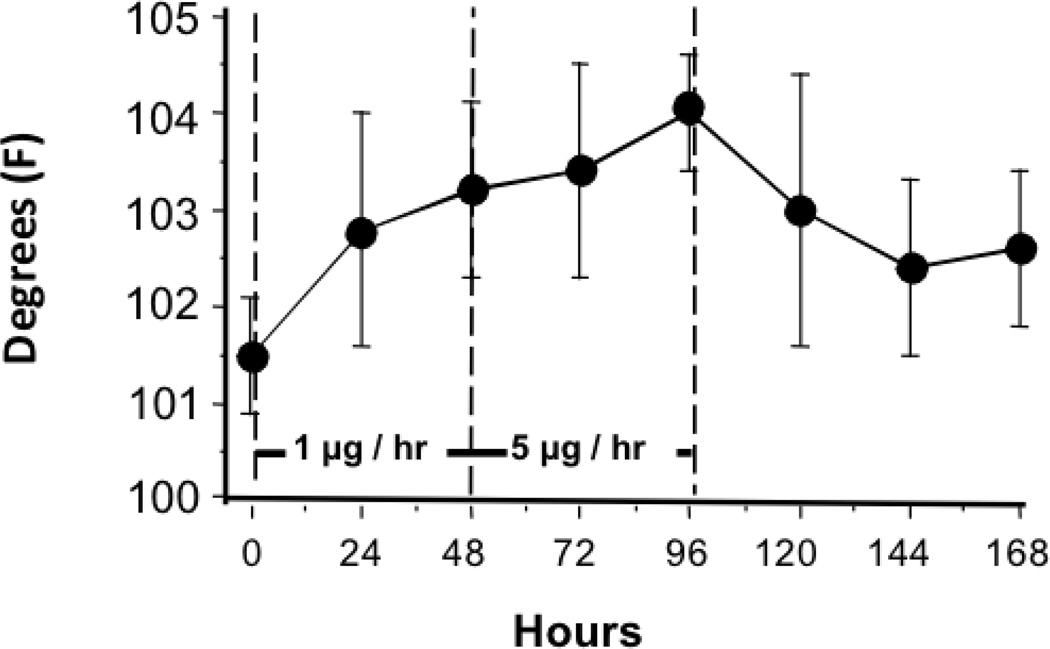
At 24 hr after bolus intrathecal and intravenous ziconotide, body temperature was not different from pretreatment baseline measurements (data not shown). With continuous intrathecal infusion of 1 µg/hr, temperature increased modestly by approximately 1.3°C and 1.7°C at 24 and 48 hr, respectively. With initiation of the 5 µg/hr infusion, temperature displayed a continued additional rise of approximately 0.2°C and 0.8°C at 24 and 48 hr, respectively. All elevated temperatures returned to baseline values 24–72 hr after termination of the 5 µg/hr infusion. (See Figure 5.)
Figure 5.
The rectal temperature assessed during the continuous intrathecal infusion of ziconotide. From 0 to 48 hr, ziconotide was infused at 1µg/hr. From 48 to 96 hr, ziconotide was delivered at the rate of 5µg/hr. Results presented represent the mean and SEM of data from 5 dogs.
Blood Pressure and Heart Rate
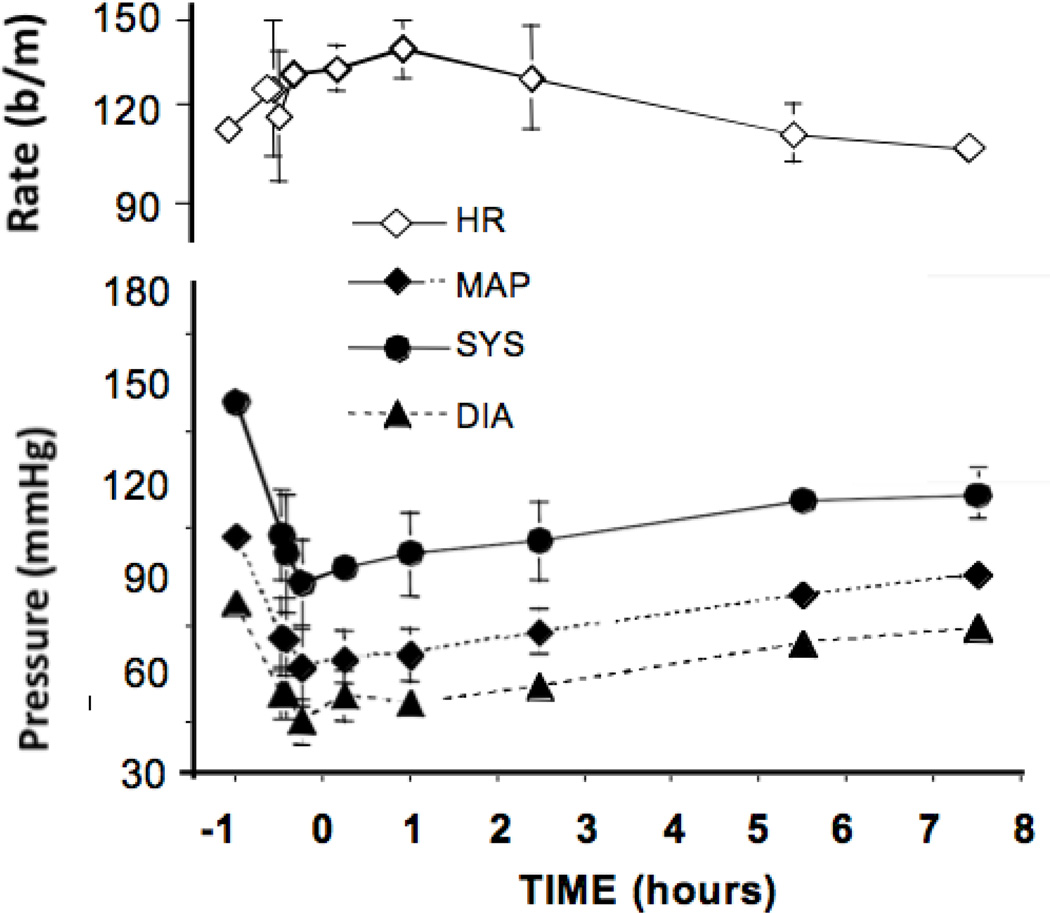
Bolus intrathecal ziconotide (10 µg) was associated with a modest decline in mean, diastolic, and systolic pressures and a mild tachycardia. Bolus intravenous injection of ziconotide (0.1 mg/kg), however, resulted in a profound (approximately 40 mmHg) and long lasting (>8 hours) fall in mean, diastolic, and systolic pressures and this was accompanied by a corresponding tachycardia. (See Figure 6).
Figure 6.
The blood pressure and heart rate responses observed after the intravenous delivery of a bolus of ziconotide (0.1 mg/kg) at t = 0. Results presented represent the mean and SEM of data from 5 dogs.
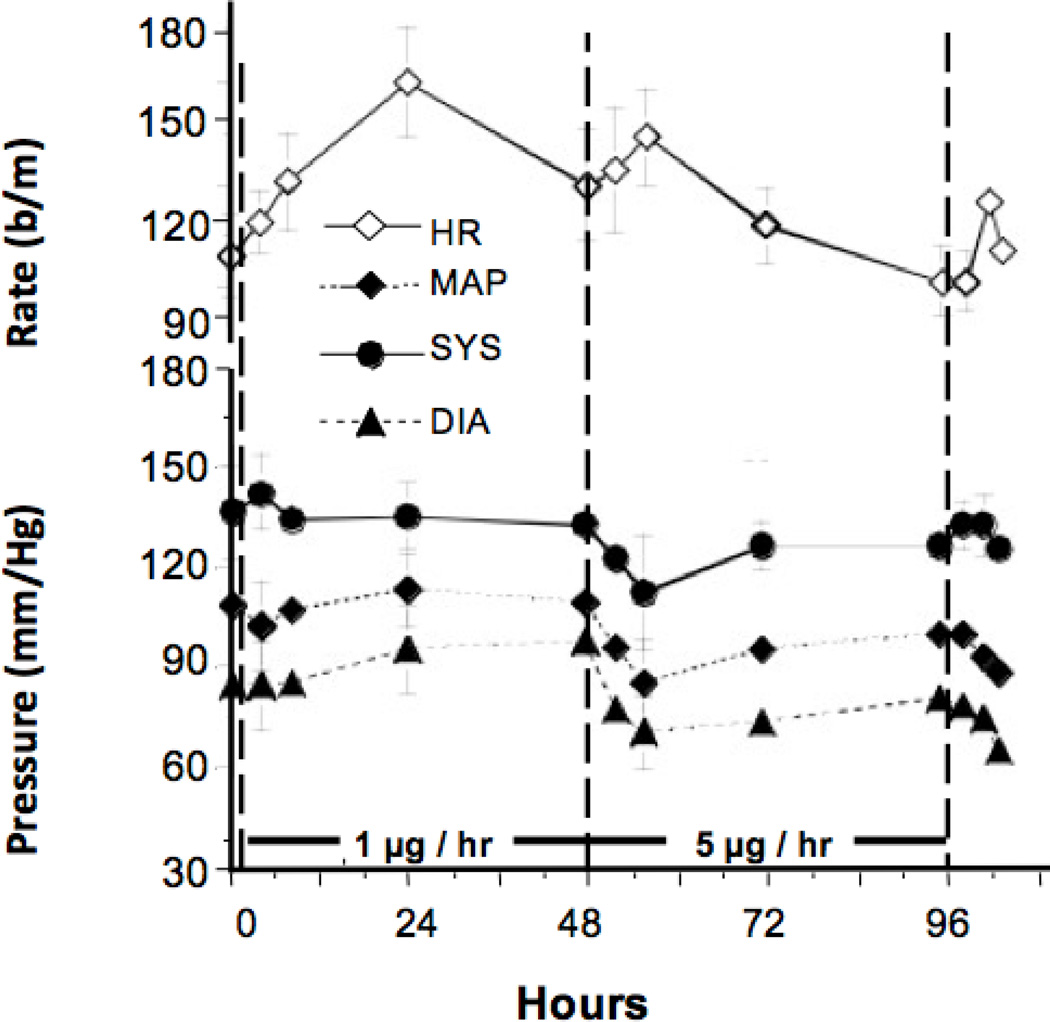
Continuous intrathecal infusion of 1 µg/hr had no effect upon blood pressure but did result in tachycardia by 8–24 hours, peaking at around 24 hr. After initiation of the 5 µg/hr dose, mean, diastolic, and systolic blood pressures showed a prominent reduction that persisted for the remainder of the infusion. This was accompanied by a tachycardia and a general reduction in heart rate to baseline levels by 72–96 hr. (See Figure 7.)
Figure 7.
The blood pressure and heart rate responses observed during the continuous intrathecal infusion of ziconotide. From 0 to 48 hr, ziconotide was infused at 1 µg/hr. From 48 to 96 hr, ziconotide was delivered at the rate of 5 µg/hr. Results presented represent the mean and SEM of data from 5 dogs.
Respiratory Rate
After bolus intrathecal ziconotide (10 µg), 3 of the 5 dogs displayed panting 20 min post injection, but respiratory rates remained otherwise unaffected through 6 hr. (Data not shown.) Bolus intravenous injection of ziconotide (0.1 mg/kg) resulted in a moderate decrease in respiratory rates for approximately 3 hr post injection. This decrease in respiratory rate corresponds with the observed slight sedation previously mentioned. (Data not shown.) Continuous intrathecal infusion had no effect on respiratory rates. (Data not shown.)
DISCUSSION
In the present study, we sought to characterize the spinal kinetics of ziconotide, a relatively large hydrophilic peptide (25 amino acids; approximate molecular weight 2500 Da), in the chronically spinal catheterized beagle dog.
Pharmacokinetic Model
The canine model has been widely employed in defining the physiological effects of a variety of intrathecal antinociceptive agents, (26) including several opiates, (23, 24), alpha2 adrenergic agonists, (27, 28), neostigmine (22); NSAIDs (ketorolac) (29), and growth factors (21) given as a bolus injection and/or infusion. The use of separate chronically placed sampling and infusion catheters permits ongoing drug delivery and sampling without contamination of the sampling route with the infusate. The use of the cisternal approach reduces the likelihood of changes in local drug distribution due to local leakage at the dural catheterization site. An important limitation of this and any sampling model is that removal of the lumbar CSF (LCSF) serves to diminish the pool of available drug and likely causes an overestimation of the rate of clearance. The impact of this intervention is reduced by limiting the number and volume of samples. Importantly, given the effect of sampling, we believe it is necessary to have concurrent comparisons with diffusion standards, such as inulin, to permit the reliable determination of the pharmacokinetic behavior of the target drug. The inert water-soluble agent, inulin, is widely used as a marker for the extravascular / extracellular space. (See, for example (30) Its large size (approximately 5000 Da) reduces its ability to diffuse though extracellular channels and its clearance from CSF is believed to reflect the CSF clearance through rostral bulk flow and absorption in the arachnoid granules and subsequently into the venous drainage. (30,31,32) Agents cleared as inulin is are, accordingly, believed to be primarily removed by a similar route.
Intrathecal injection
The lumbar CSF space is a poorly stirred volume with limited local fluid movement. (33,34, 35, 36) Accordingly, upon delivery into the intrathecal space, the injectate undergoes an initial local redistribution that depends upon the volume and rate of delivery. Once delivered, the injectate shows an initial fall in concentration that reflects: i) a progressive dilution into the LCSF volume driven by oscillatory pressure waves. (34) A second elimination phase is typically noted which reflects: i) ongoing local dilution in the CSF, ii) movement into the adjacent spinal parenchyma, and iii) movement into and through the adjacent meninges into the epidural space. (35,37). Such biphasic clearances have been observed after bolus delivery in all drugs studies in this model (see below)
Previous work has shown that the principal variables governing the second phase of CSF clearance of an intrathecally delivered agent are its physicochemical properties. Low molecular weight, moderately lipid soluble molecules (e.g., alfentanil) show a rapid movement from the lumbar CSF into tissue and through the meninges and into the vasculature.(35). In contrast, larger, hydrophilic molecules such as ziconotide or inulin show a more delayed clearance. (21) At the extreme, the clearance of a hydrophilic molecule that diffuses poorly into tissue will display a half-life that approaches that of CSF. In the case of the dog, this has been calculated to be on the order of approximately 2 hr. (32) While systematic data are limited, it appears that hydrophilic molecules over several hundred Da display increasingly similar clearance properties with increasing molecular weight. In the present studies, ziconotide (approximately 2500 Da) displayed an elimination half-life after bolus delivery on the order 3 hr. This is longer than the T1/2 calculated for smaller hydrophilic molecules in this model (e.g. 0.9 hr for ketorolac (29); and 1.2 hr for morphine; Allen and Yaksh, unpublished data). This clearance is slightly faster than that for inulin (3.9 h), and may some reflect binding in parenchyma and/or meninges. Interestingly, IT BDNF, a large growth factor (27 KDa) showed a surprisingly rapid clearance from the CSF (0.7 hrs_(21). Such difference may reflect on other routes of clearance of free agent including binding to meninges. In contrast, comparison of the initial distribution phase for ziconotide and inulin (as well as the other hydrophilic molecules) indicates that they are essentially identical, emphasizing that the initial dilution after bolus delivery represents a local dilution in the CSF. Calculation of the ziconotide: inulin ratios, after bolus delivery, revealed that, consistent with the comparable calculated half-lives, both molecules showed a similar acute redistribution. On the other hand, consistent with the shorter T1/2ß, ziconotide LCSF concentrations fell more rapidly than those of inulin, such that the ziconotide: inulin ratio fell by a factor of approximately 3 and 12 at 3 hr and 8 hr, respectively. This difference in the decline of lumbar ziconotide concentrations may reflect: i) a slightly more rapid clearance of ziconotide into tissue, ii) adherence of the drug to local sites (i.e., specific and non-specific binding), and/or iii) metabolism of ziconotide. Specific binding has been shown for ziconotide in spinal tissues (11) and local absorption in the meninges is not unlikely. Protein binding in CSF is not likely a factor, as protein concentrations are exceedingly low compared to plasma. As regards metabolism, ziconotide is a peptide and can undergo peptidic hydrolysis in the blood (38), though it appears relatively stable in in vivo studies. (25)
Intravenous injection
Assessment of elimination after bolus IV delivery revealed a half life of 1.6 hrs, faster than previously reported in rats and dogs after steady state infusion.(25) The elimination T1/2 of ziconotide in CSF following IV injection was longer than that in plasma (1.64 hr vs 0.75 hr), but was comparable to the elimination T1/2 in CSF when ziconotide was given by IT bolus (1.2 hr). This suggests that transfer of ziconotide out of the CSF is the rate-limiting for its spinal action.
Intrathecal infusion
Dogs
Several observations emphasize the linearity of IT ziconotide pharmacokinetics. 1) LCSF ziconotide concentrations remained stable upon reaching steady state between 8 and 48 hr of infusion of ziconotide at 1 and 5 µg/hr. 2) LCSF ziconotide concentrations at these infusion doses corresponds to a ratio of approximately 1: 4, a value similar to the 5-fold incremental dosing ratio. 3) Examination of the LCSF concentrations of ziconotide after termination of intrathecal infusion revealed a T1/2 of 2.35 hr which is longer than the T1/2 calculated with bolus delivery (1.68 hr). This raises the likelihood that the longer T1/2 observed after continuous infusion reflect a clearance of ziconotide from spools such as the tissue and meninges filled with continued exposure. Examination of plasma levels during continuous infusion revealed a progressive rise relative to the LCSF concentration over the 48-hr interval, from 20,000: 1 at 8 hr to 600: 1 at 48 hr, reflecting the approach to time-dependent equilibration of steady state CSF levels with plasma levels.
Based on the estimated ziconotide CL in CSF from bolus dosing (i.e., median value = 5.53 mL/h), and assuming linear PK, predicted steady-state CSF concentrations for a given IT infusion regimen can be determined from the following equation:
Hence, for IT infusion rates of 1000 and 5000 ng/h, predicted CSF Css values would be 181 and 904 ng/mL, respectively. Measured lumbar CSF levels after these infusions were 419 and 2559, ng/mL respectively (Table 3). Similarly, predicted steady-state plasma Css values for these IT infusion rates would be 0.09 and 0.43 ng/mL. Measured plasma levels after IT infusion were found to be 0.11 and 1.26 pg.mL, respectively. (Table 3)
Cisternal CSF levels of ziconotide at steady state (with continuous infusion) in the dog are proportional to the LCSF levels at steady state. The appearance of drug in the cisternal CSF with lumbar delivery reflects i) movement from the site of injection to the cisterna by bulk CSF redistribution and ii) movement from the site of injection to the blood and thence to the brain. The concentrations in the cisternal CSF with LCSF delivery are determined by i) the LCSF dose delivered, ii) the clearance of drug from the CSF, and iii) the effective dilution volume of the spinal and cisternal CSF into which the injected drug is delivered. Drugs that diffuse readily into tissue, or bind locally, will be accordingly cleared as they diffuse rostrally. Hence, the cisternal: lumbar ratio of such a drug will, on average, be less than that of a drug that does not diffuse into tissue and/or bind locally. Ziconotide LCSF: cisternal ratios at steady state were on the order of 1: 0.02. Similar LCSF: cisternal ratios were observed after IT delivery of a large growth factor (BDNF). (21) Importantly, the respective ziconotide ratios were comparable when assessed after a low and high dose infusion (e.g. 0.011 at 1 µg/hr and 0.019 at 5 µg/hr) suggesting that rostral distribution is proportional to the IT dose of ziconotide. The plasma concentrations measured concurrently are approximately 0.1 times those observed in the cisterna. Given the poor blood-to-CSF redistribution observed in the intravenous study, the presence of ziconotide in the cisternal CSF is consistent with a rostral movement of the molecule from the lumbar intrathecal space (as opposed to a spinal - vascular-cisternal route). A caveat to the above commentary is that the concentrations of ziconotide in the cisternal CSF were assessed at a single time point, 48 hrs after the initiation of the infusion. While lumbar CSF concentrations appeared to be at steady state, at this time, we cannot be certain that there would not be higher levels of cisternal ziconotide at longer intervals.
Human
These results compare favorably to a report on lumbar CSF PK in humans after an acute (1 hr infusion) of ziconotide in humans. (16) In that work, after one-hour intrathecal bolus administration of ziconotide in doses of 1 – 10 µg, an exponential clearance was noted over 24 hr. Due to longer sampling intervals, the nature of the initial distribution could not be estimated. However, a median LCSF half-life of 4.5 hr and a volume of distribution of 99 mL were calculated and the authors concluded that clearance of ziconotide in humans is due to bulk redistribution of CSF. As in the dog, the pharmacokinetics of CSF ziconotide were linear over the range of doses examined, based on the estimated T1/2 and Cmax values. As in the canine model, the systemic bioavailability of the IT ziconotide was low, with plasma concentrations in the human frequently being below the limit of detection of the ziconotide assay. Several differences preclude an exact comparison of the human and dog data. First, the Wermeling study employed a single catheter for delivery and sampling, so the sampling did not begin until 1 hr after termination of infusion. Accordingly, it is not likely that the drug had reached a steady state. Secondly, the use of a combined injection and sampling system served to sample at the injection site. The dog model employed a sampling catheter approximately 2 cm from the injection site. This allowed for an assessment of the local distribution of injectate over a length of spinal cord considered important for influencing the lumbar dermatomes. Accordingly, we were able to detect an initial distribution at the earliest time point. Third, the use of a combined infusion–sampling catheter precluded an initial sampling at injection. Indeed, in the human study, the first sample was not taken until 2 hrs after the start of infusion and this would lead to an overestimation of time to clearance. In spite of these differences, it is evident that both human and dog data indicate that intrathecal ziconotide has linear kinetics and is cleared slowly.
Pharmacodynamics
Antinociception
Previous work has shown that the thermally evoked skin twitch is a polysynaptic, small-afferent-evoked nociceptive reflex. (39) Other work in the dog has shown this to be affected by opioids and alpha2 agonists given as a bolus. (24, 27) The present study indicates that the bolus delivery of 10 µg of ziconotide had little effect upon the skin twitch. In contrast, the continuous delivery of 1 ug/hr ziconotide produced a maximal block of the skin twitch response by 24 hr that persisted throughout the 5 µg/hr infusion period. These results are consistent with the results in acute thermal nociception in rat models using both bolus injection and continuous infusion of intrathecal ziconotide. (8) The reason for the difference in effect with bolus versus continuous infusion is not clear. One possibility is that the large polycationic ziconotide molecule may diffuse into spinal tissue relatively slowly along its concentration gradient. If the clearance of the drug from the CSF is faster than the time required for the drug to penetrate to its molecular target within the spinal parenchyma (i.e., the substantia gelatinosa), then the bolus delivery may not permit the concentration gradient to persist long enough for effective concentrations of the drug to be reached in the parenchyma. Using a microdialysis probe continuously infused with radioiodinated ziconotide, Valentino, and colleagues (40) estimated the diffusion of the peptide in rat brain parenchyma to be less than 1 mm in 2 hrs. This argument is consistent with the observation that the onset of inhibition of the twitch response is delayed relative to the time to steady state LCSF levels. It should be stressed that the skin twitch is a spinally organized reflex and the present study does not define whether the local effects are related to an alteration in the excitability of the reflex arc as well as an alteration in the ascending sensory message. Nevertheless, the block of such a response in the absence of significant changes in motor function (see below) argues against a general block of segmental motor outflow.
Behavioral Effects
Arousal, muscle tone, and coordination were not affected by intrathecal bolus injection, but were transiently altered by chronic intrathecal infusion of ziconotide. These results emphasize that the doses that were employed were without general effect upon somatomotor function. The modest reduction in arousal after intravenous delivery may reflect an effect secondary to the hypotension that was induced by this agent given intravenously. A dose-related profile of side effects was noted in human studies after spinal delivery. Thus, in humans, intrathecal ziconotide resulted in adverse effects, including mild gait disturbance, nausea, dizziness, confusion, increased body temperature, and somnolence. Modest hypotension has also been reported. (14, 15)
Physiological Effects
Ziconotide given intravenously resulted in hypotension. This likely reflects the direct inhibition of the release of catecholamines from the peripheral terminals by blockade of N-type calcium channels (40, 41) and is consistent with previous reports. (42, 43, 44; 45). After continuous intrathecal infusion at 1 µg/hr, there was little effect upon blood pressure, but at the 5 µg/hr dose, a fall in mean and diastolic pressures, and a lesser fall in systolic pressure, were noted within several hours of altering the infusion rate. Hypothetically, this sympatholytic effect may be mediated by a direct effect upon the excitatory input into preganglionic sympathetic neurons. Spinal alpha2 adrenoceptor agonists induce a direct inhibitory effect upon the excitability of spinal preganglionic sympathetic neurons. (46) However, the 1.12 ng/mL ziconotide observed at steady state (Table 3) is in the range of the IC50 for inhibition of sympathetic function by ziconotide. Thus, we cannot completely exclude the likelihood that at least some component of this effect is peripheral.
Clinical significance of intrathecal ziconotide kinetics
From the above reported observations and analyses, it can be concluded that intrathecally delivered ziconotide is distributed in the lumbar and cisternal CSF as a large molecule in a manner suggesting bulk redistribution in the spinal intrathecal space. The molecule displays linear kinetics that are not altered by continuous infusion, coming to a steady state in LCSF with continuous infusion within an 8-hr interval in this model -- a value consistent with the half-life determined from clearance following bolus delivery or clearance from steady state. Of particular interest in both the humans and dog study was the apparent delay in the onset of the behavioral effects. In the dog study, skin twitch was not blocked at 8 hrs, although the CSF steady state was apparently maximal at this time. In the human work, it was evident that a lag time exists between the onset of analgesia in these pain patients and the LCSF-PK. The source of this delay likely reflects upon the time required for the distribution from the site of delivery and the spinal site of action. In the case of the N-type calcium channel blocker this action lies within the superficial layer of the spinal dorsal horn. (5) Accordingly, after IT delivery, the drug must reach the spinal levels mediating that pain state (e.g., lumbar spinal cord, not roots). After bolus delivery, based on this dog study, this initial redistribution may be relatively brief. However, with the use of infusion, it is likely that the interval will be longer. At any given level, the agent must then penetrate to the site of spinal action. While there are few systematic data, previous work has emphasized that the time to onset of inhibition of dorsal horn neurons firing correlates with the time of onset of analgesia. Agents that are lipid soluble will penetrate rapidly and show a correspondingly more rapid onset than that observed with polar molecules. (47, 48) Moreover, consistent with spinal cord size, the time of onset of effect after a particular IT drug such as morphine is shortest in small species (e.g., mouse) and longest in larger species (e.g., dog and human). Accordingly, it is not surprising that there is a definable hysteresis between LCSF steady state and onset of action of large, relatively poorly diffusible molecules such as ziconotide. These comments reflect upon the likely need to increment the dosing of intrathecal ziconotide to achieve optimal dosing. Based on CSF levels in the dog we would estimate that CSF steady state will require around 8 hrs. One would then consider that time to diffuse into tissue would require an additional interval. It is not possible from these studies to estimate the additional interval, but given the time to analgesia observed in the dog infusion studies, this interval would occur inside of 24 hrs. From a clinical perspective, dose incrementation should therefore not occur at intervals of less than 24 hrs. As non-spinal side effects increase as a function of dose, rapid incrementation would tend to overshoot the minimum therapeutic levels and increase the likelihood of untoward effects. In humans, confusion and other mental changes, as well as autonomic effects, have been reported to occur at roughly 24–48 hr (20, 49) or 48–72 hr (15) intervals after incrementation of intrathecal ziconotide and probably reflect a supraspinal action. The present studies indicate that increasing plasma versus LCSF levels continue to rise through 48 hr. Though systemic ziconotide displays limited systemic bioavailability, the delayed onset of side effects may reflect a role for systemic redistribution. We did not examine cisternal ziconotide concentrations at shorter time intervals than 48 hrs, but it seems likely that supraspinal steady state levels will occur after the LCSF Cmax levels that were observed between 8 and 24 hr. Importantly, the stability and linearity of the kinetics after either bolus or continuous infusion observed in these studies provides assurance that drug effects may be predictably titrated by slower incrementation than used in initial clinical studies of ziconotide. (15, 20, 17, 50)
Acknowledgment
This work was supported by funds from the Elan Corporation and from NINDS-15353. We would like to thank Michael Rathbun and Jean C. Provencher for their expert technical assistance.
The work was performed at the University of California, San Diego, Anesthesiology Research Laboratory.
Footnotes
Author responsibilities
All authors have read and approved the manuscript
Tony L. Yaksh, Ph.D.: Senior author and is responsible for all aspects of the manuscript.
Annelies de Kater Ph.D. : Responsible for the PK analysis of the investigation.
Robin Dean, Ph.D.: Responsible for drug analysis.
Brookie M. Best, Pharm.D., M.A.S. : Responsible for data reduction and analysis.
George P. Miljanich, Ph.D.: Responsible for drafting and revising the manuscript
Conflict of Interest: Dr. Miljanich is co-inventor on several US and ex-US patents covering methods of use of ziconotide as an analgesic drug. Ziconotide is a subject of this contribution. Dr. Miljanich assigned all his patent rights related to ziconotide to Elan Corp. and realizes no financial gain from the sale of ziconotide under these rights.
The other authors reported no conflicts of interest.
A summary of the work was presented at the Annual Meeting of the Society of Toxicology, 1999.
REFERENCES
- 1.Olivera BM, Gray WR, Zeikus R, McIntosh JM, Varga J, Rivier J, de Santos V, Cruz LJ. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- 2.Nadasdi L, Yamashiro D, Chung D, Tarczy-Hornoch K, Adriaenssens P, Ramachandran J. Structure-activity analysis of a Conus peptide blocker of N-type neuronal calcium channels. Biochemistry. 1995;34:8076–8081. doi: 10.1021/bi00025a013. [DOI] [PubMed] [Google Scholar]
- 3.Cruz LJ, Olivera BM. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J. Biol. Chem. 1986;261:6230–6233. [PubMed] [Google Scholar]
- 4.Reynolds IJ, Wagner JA, Snyder SH, Thayer SA, Olivera BM, Miller RJ. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc. Natl. Acad. Sci. U. S. A. 1986;83:8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- 6.Takasusuki T, Yaksh TL. Regulation of spinal substance P release by intrathecal calcium channel blockade. Anesthesiology. 2011;115:153–164. doi: 10.1097/ALN.0b013e31821950c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J. Pharmacol. Exp. Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- 8.Malmberg AB, Yaksh TL. Effect of continuous intrathecal infusion of omega-cnopeptides, N-type calcium-channel blockers, on behavior and antinociception in the formalin and hot-plate tests in rats. Pain. 1995;60:83–90. doi: 10.1016/0304-3959(94)00094-U. [DOI] [PubMed] [Google Scholar]
- 9.Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J. Neurosci. 1994;14:4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowersox SS, Gadbois T, Singh T, Pettus M, Wang YX, Luther RR. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J. Pharmacol. Exp. Ther. 1996;279:1243–1249. [PubMed] [Google Scholar]
- 11.Gohil K, Bell JR, Ramachandran J, Miljanich GP. Neuroanatomical distribution of receptors for a novel voltage-sensitive calcium-channel antagonist, SNX-230 (omega- conopeptide MVIIC) Brain Res. 1994;653:258–266. doi: 10.1016/0006-8993(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 12.Yaksh TL. Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol. Sci. 1999;20:329–337. doi: 10.1016/s0165-6147(99)01370-x. [DOI] [PubMed] [Google Scholar]
- 13.Brose WG, Gutlove DP, Luther RR, Bowersox SS, McGuire D. Use of intrathecal SNX-111, a novel, N-type, voltage-sensitive, calcium channel blocker, in the management of intractable brachial plexus avulsion pain. Clin. J. Pain. 1997;13:256–259. doi: 10.1097/00002508-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Atanassoff PG, Hartmannsgruber MW, Thrasher J, Wermeling D, Longton W, Gaeta R, Singh T, Mayo M, McGuire D, Luther RR. Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Reg. Anesth. Pain Med. 2000;25:274–278. doi: 10.1016/s1098-7339(00)90010-5. [DOI] [PubMed] [Google Scholar]
- 15.Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, Mayo M, McGuire D, Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- 16.Wermeling D, Drass M, Ellis D, Mayo M, McGuire D, O'Connell D, Hale V, Chao S. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J. Clin. Pharmacol. 2003;43:624–636. [PubMed] [Google Scholar]
- 17.Rauck RL, Wallace MS, Leong MS, Minehart M, Webster LR, Charapata SG, Abraham JE, Buffington DE, Ellis D, Kartzinel R Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393–406. doi: 10.1016/j.jpainsymman.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Rauck RL, Wallace MS, Burton AW, Kapural L, North JM. Intrathecal ziconotide for neuropathic pain: a review. Pain Pract. 2009;9:327–337. doi: 10.1111/j.1533-2500.2009.00303.x. [DOI] [PubMed] [Google Scholar]
- 19.Ridgeway B, Wallace M, Gerayli A. Ziconotide for the treatment of severe spasticity after spinal cord injury. Pain. 2000;85:287–289. doi: 10.1016/s0304-3959(99)00255-9. [DOI] [PubMed] [Google Scholar]
- 20.Penn RD, Paice JA. Adverse effects associated with the intrathecal administration of ziconotide. Pain. 2000;85:291–296. doi: 10.1016/s0304-3959(99)00254-7. [DOI] [PubMed] [Google Scholar]
- 21.Yaksh TL, Rathbun ML, Dragani JC, Malkmus S, Bourdeau AR, Richter P, Powell H, Myers RR, Lebel CP. Kinetic and safety studies on intrathecally infused recombinant-methionyl human brain-derived neurotrophic factor in dogs. Fundam. Appl. Toxicol. 1997;38:89–100. doi: 10.1006/faat.1997.2314. [DOI] [PubMed] [Google Scholar]
- 22.Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995;82:412–427. doi: 10.1097/00000542-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR. Chronically Infused Intrathecal morphine in dogs. Anesthesiology. 2003;99:174–187. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Sabbe MB, Grafe MR, Mjanger E, Tiseo PJ, Hill HF, Yaksh TL. Spinal delivery of sufentanil, alfentanil, and morphine in dogs. Physiologic and toxicologic investigations. Anesthesiology. 1994;81:899–920. doi: 10.1097/00000542-199410000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Bowersox S, Mandema J, Tarczy-Hornoch K, Miljanich G, Luther RR. Pharmacokinetics of SNX-111, a selective N-type calcium channel blocker, in rats and cynomolgus monkeys. Dru. Metab. Dispos. 1997;25:379–383. [PubMed] [Google Scholar]
- 26.Yaksh TL, Malkmus SA. In: Animal models of intrathecal and epidural drug delivery, Spinal Drug Delivery. Yaksh TL, editor. Amsterdam: Elsevier Science B.V.; 1999. pp. 317–344. [Google Scholar]
- 27.Sabbe MB, Penning JP, Ozaki GT, Yaksh TL. Spinal and systemic action of the alpha2 receptor agonist dexmedetomidine in dogs. Antinociception and carbon dioxide response. Anesthesiology (Laboratory Investigations) 1994;80:1057–1072. doi: 10.1097/00000542-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Yaksh TL, Rathbun M, Jage J, Mirzai T, Grafe M, Hiles RA. Pharmacology and toxicology of chronically infused epidural clonidine HCl in dogs. Fundam. Appl. Toxicol. 1994;23:319–335. doi: 10.1006/faat.1994.1112. [DOI] [PubMed] [Google Scholar]
- 29.Yaksh TL, Horais KA, Tozier N, Rathbun M, Richter P, Rossi S, Grafe M, Tong C, Meschter C, Cline JM, Eisenach J. Intrathecal ketorolac in and rats dogs. Toxicol. Sci. 2004;80:322–334. doi: 10.1093/toxsci/kfh168. [DOI] [PubMed] [Google Scholar]
- 30.Nara E, Masegi M, Hatono T, Hashida M. Pharmacokinetic analysis of drug absorption from muscle based on a physiological diffusion model: effect of molecular size on absorption. Pharm. Res. 1992;9:161–168. doi: 10.1023/a:1018916802528. [DOI] [PubMed] [Google Scholar]
- 31.Durant PA, Yaksh TL. Distribution in cerebrospinal fluid, blood, and lymph of epidurally injected morphine and inulin in dogs. Anesth. Analg. 1986;65:583–592. [PubMed] [Google Scholar]
- 32.Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kizziar R, Nesbit GM. The quantitative evaluation of cerebral spinal fluid flow. Semin. Ultrasound. CT MR. 2000;21:452–461. doi: 10.1016/s0887-2171(00)90037-4. [DOI] [PubMed] [Google Scholar]
- 34.Loth F, Yardimci MA, Alperin N. Hydrodynamic modeling of cerebrospinal fluid motion within the spinal cavity. J. Biomech. Eng. 2001;123:71–79. doi: 10.1115/1.1336144. [DOI] [PubMed] [Google Scholar]
- 35.Bernards CM. Understanding the physiology and pharmacology of epidural and intrathecal opioids. Best Pract Res Clin Anaesthesiol. 2002;16:489–505. doi: 10.1053/bean.2002.0255. [DOI] [PubMed] [Google Scholar]
- 36.Artru AA. In: Spinal cerebrospinal fluid chemistry and physiology, Spinal Drug Delivery. Yaksh TL, editor. Amsterdam: Elsevier Science B.V.; 1999. pp. 177–237. [Google Scholar]
- 37.Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil [see comments] Anesthesiology. 2000;92:739–753. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Newcomb R, Abbruscato TJ, Singh T, Nadasdi L, Davis TP, Miljanich G. Bioavailability of Ziconotide in brain: influx from blood, stability, and diffusion. Peptides. 2000;21:491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 39.Doucette R, Theriault E, Diamond J. Regionally selective elimination of cutaneous thermal nociception in rats by neonatal capsaicin. J. Comp. Neurol. 1987;261:583–591. doi: 10.1002/cne.902610409. [DOI] [PubMed] [Google Scholar]
- 40.Valentino K, Newcomb R, Gadbois T, Singh T, Bowersox S, Bitner S, Justice A, Yamashiro D, Hoffman BB, Ciaranello R, et al. A selective N-type calcium channel antagonist protects against neuronal loss after global cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7894–7897. doi: 10.1073/pnas.90.16.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehm S, Huck S. Inhibition of N-type calcium channels: the only mechanism by which presynaptic alpha 2-autoreceptors control sympathetic transmitter release. Eur. J. Neurosci. 1996;8:1924–1931. doi: 10.1111/j.1460-9568.1996.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 42.Waterman SA. Role of N-, P- and Q-type voltage-gated calcium channels in transmitter release from sympathetic neurones in the mouse isolated vas deferens. Br. J. Pharmacol. 1997;120:393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuire D, Bowersox S, Fellmann JD, Luther RR. Sympatholysis after neuron-specific, N-type, voltage-sensitive calcium channel blockade: first demonstration of N-channel function in humans. J. Cardiovasc. Pharmacol. 1997;30:400–403. doi: 10.1097/00005344-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Wright CE, Robertson AD, Whorlow SL, Angus JA. Cardiovascular and autonomic effects of omega-conotoxins MVIIA and CVID in conscious rabbits and isolated tissue assays. Br. J. Pharmacol. 2000;131:1325–1336. doi: 10.1038/sj.bjp.0703701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowersox SS, Singh T, Nadasdi L, Zukowska-Grojec Z, Valentino K, Hoffman BB. Cardiovascular effects of omega-conopeptides in conscious rats: mechanisms of action. J. Cardiovasc. Pharmacol. 1992;20:756–764. [PubMed] [Google Scholar]
- 46.Eisenach JC, Tong CY. Site of hemodynamic effects of intrathecal alpha 2-adrenergic agonists [see comments] Anesthesiology. 1991;74:766–771. doi: 10.1097/00000542-199104000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Suzukawa M, Matsumoto M, Collins JG, Kitahata LM, Yuge O. Dose-response suppression of noxiously evoked activity of WDR neurons by spinally administered fentanyl. Anesthesiology. 1983;58:510–513. doi: 10.1097/00000542-198306000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Homma E, Collins JG, Kitah ata LM, Matsumoto M, Kawahara M. Suppression of noxiously evoked WDR dorsal horn neuronal activity by spinally administered morphine. Anesthesiology. 1983;58:232–236. doi: 10.1097/00000542-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Jain KK. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expert Opin Investig Drugs. 2000;9:2403–2410. doi: 10.1517/13543784.9.10.2403. [DOI] [PubMed] [Google Scholar]
- 50.Smith HS, Deer TR. Safety and efficacy of intrathecal ziconotide in the management of severe chronic pain. Ther Clin Risk Manag. 2009;5:521–534. doi: 10.2147/tcrm.s4438. [DOI] [PMC free article] [PubMed] [Google Scholar]