Abstract
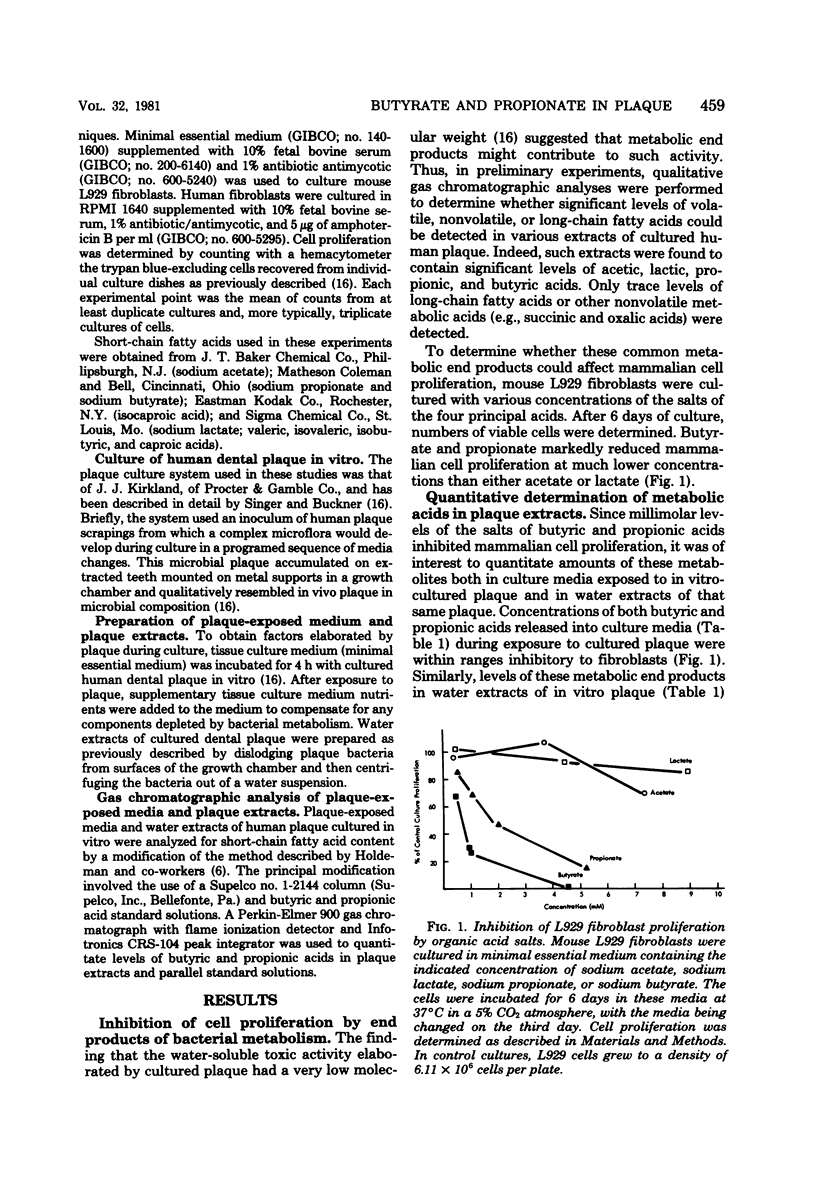
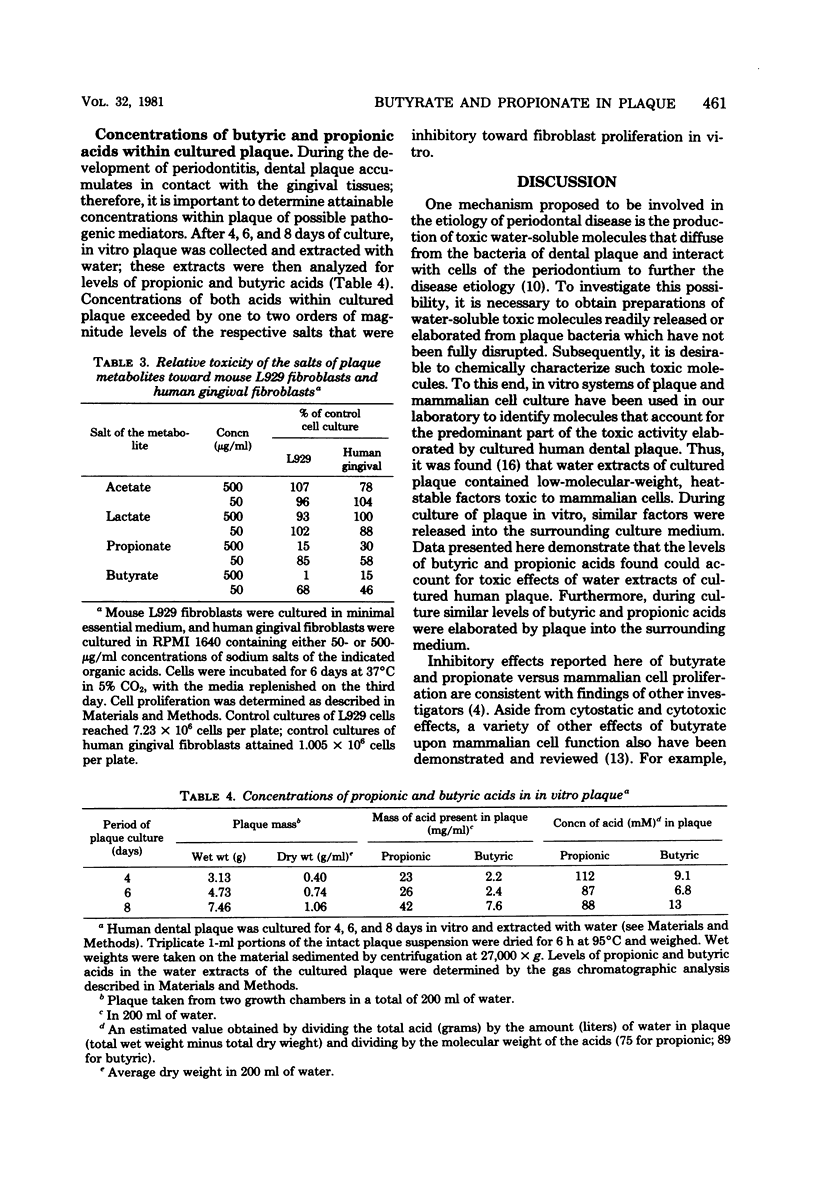
Extracts of in vitro-cultured human dental plaque contain factors toxic to mammalian cells. Previous studies demonstrated that those toxic factors most readily released from cultured plaque had very low molecular weights and were heat stable. Studies reported here demonstrate that metabolic end products including short-chain fatty acids were present in fractions containing the low-molecular-weight, heat-stable factors. The salts of two of these acids, butyrate and propionate, inhibited proliferation of both mouse L929 cells and human gingival fibroblasts. Furthermore, when tested at concentrations present in plaque extracts, the inhibitory effects of butyrate and propionate accounted for essentially all the inhibitory potential of the extracts. These findings, taken together with those of other groups, suggest that butyrate and propionate, end products of dental plaque metabolism, may have an etiological role in periodontal disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Geddes D. A. Acids produced by human dental plaque metabolism in situ. Caries Res. 1975;9(2):98–109. doi: 10.1159/000260149. [DOI] [PubMed] [Google Scholar]
- Ginsburg E., Salomon D., Sreevalsan T., Freese E. Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2457–2461. doi: 10.1073/pnas.70.8.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian H. K., Riggs M. G., Swartz L. A., Ingram V. M. Effect of n-butyrate on DNA synthesis in chick fibroblasts and HeLa cells. Cell. 1977 Nov;12(3):855–860. doi: 10.1016/0092-8674(77)90284-7. [DOI] [PubMed] [Google Scholar]
- Kyner D., Zabos P., Christman J., Acs G. Effect of sodium butyrate on lymphocyte activation. J Exp Med. 1976 Dec 1;144(6):1674–1678. doi: 10.1084/jem.144.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Levine M., Adams R. L., Cowley G. C. Effect of dental plaque extracts on mammalian cells in vitro. J Periodontal Res. 1973;8(5):296–303. doi: 10.1111/j.1600-0765.1973.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Levine M., Adams R. L., Cowley G. C., Mason D. K. Some characteristics of the cytotoxic material in human dental plaque extracts. Arch Oral Biol. 1974 Dec;19(12):1145–1152. doi: 10.1016/0003-9969(74)90243-x. [DOI] [PubMed] [Google Scholar]
- Mackler B. F. Plaque dialysate effects on human lymphocyte blastogenesis and inflammatory responses. Arch Oral Biol. 1975 Jul;20(7):423–428. doi: 10.1016/0003-9969(75)90228-9. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K. Effect of sodium butyrate on mammalian cells in culture: a review. In Vitro. 1976 Feb;12(2):125–132. doi: 10.1007/BF02796360. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simmons J. L., Fishman P. H., Freese E., Brady R. O. Morphological alterations and ganglioside sialyltransferase activity induced by small fatty acids in HeLa cells. J Cell Biol. 1975 Aug;66(2):414–424. doi: 10.1083/jcb.66.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. E., Buckner B. A. Characterization of toxic extracts of in vitro cultured human plaque. J Periodontal Res. 1980 Nov;15(6):603–614. doi: 10.1111/j.1600-0765.1980.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Möenbo D., Langebaek J., Frandsen A. Microbiota of gingivitis in man. Scand J Dent Res. 1978 May;86(3):174–181. doi: 10.1111/j.1600-0722.1978.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Smith C. C., Henneberry R. C. Induction of functional beta-adrenergic receptors in HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):873–877. doi: 10.1073/pnas.74.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Theilade J. Role of plaque in the etiology of periodontal disease and caries. Oral Sci Rev. 1976;9:23–63. [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Wright J. A. Morphology and growth rate changes in Chinese hamster cells cultured in presence of sodium butyrate. Exp Cell Res. 1973 Apr;78(2):456–460. doi: 10.1016/0014-4827(73)90091-8. [DOI] [PubMed] [Google Scholar]


