Abstract
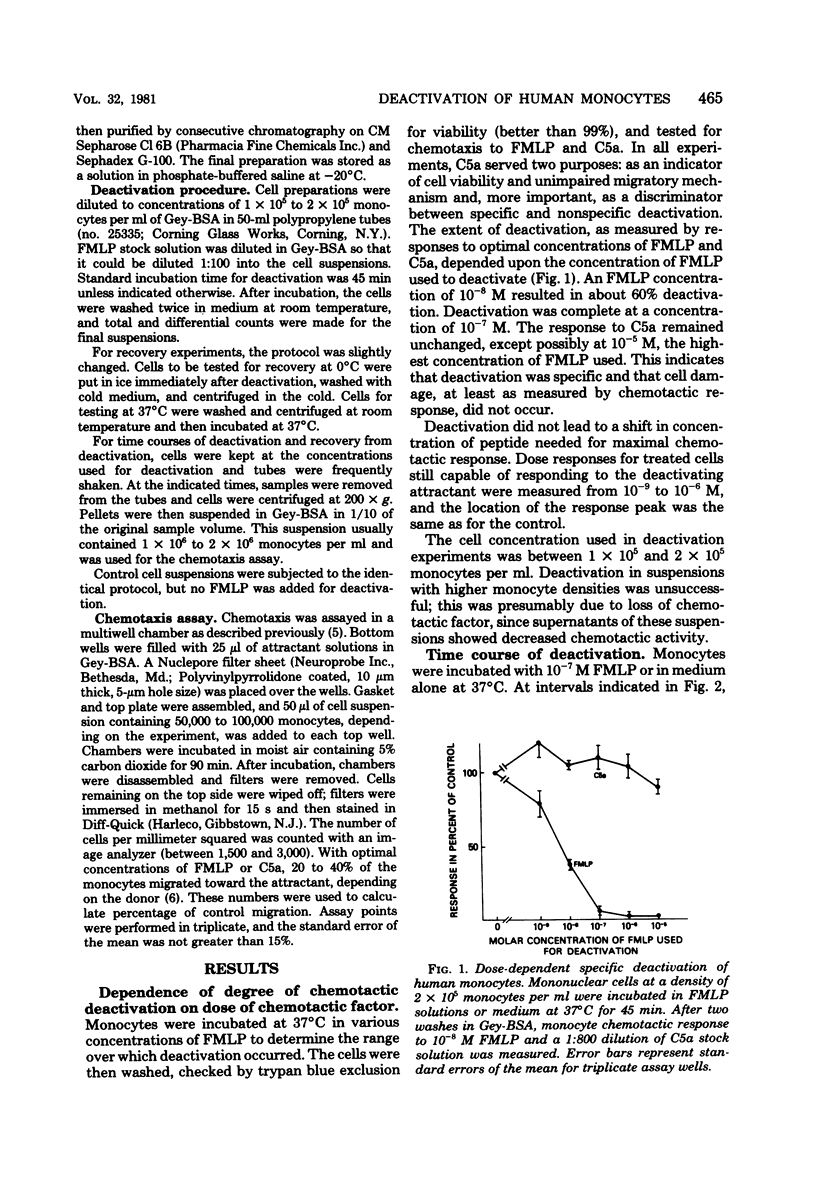
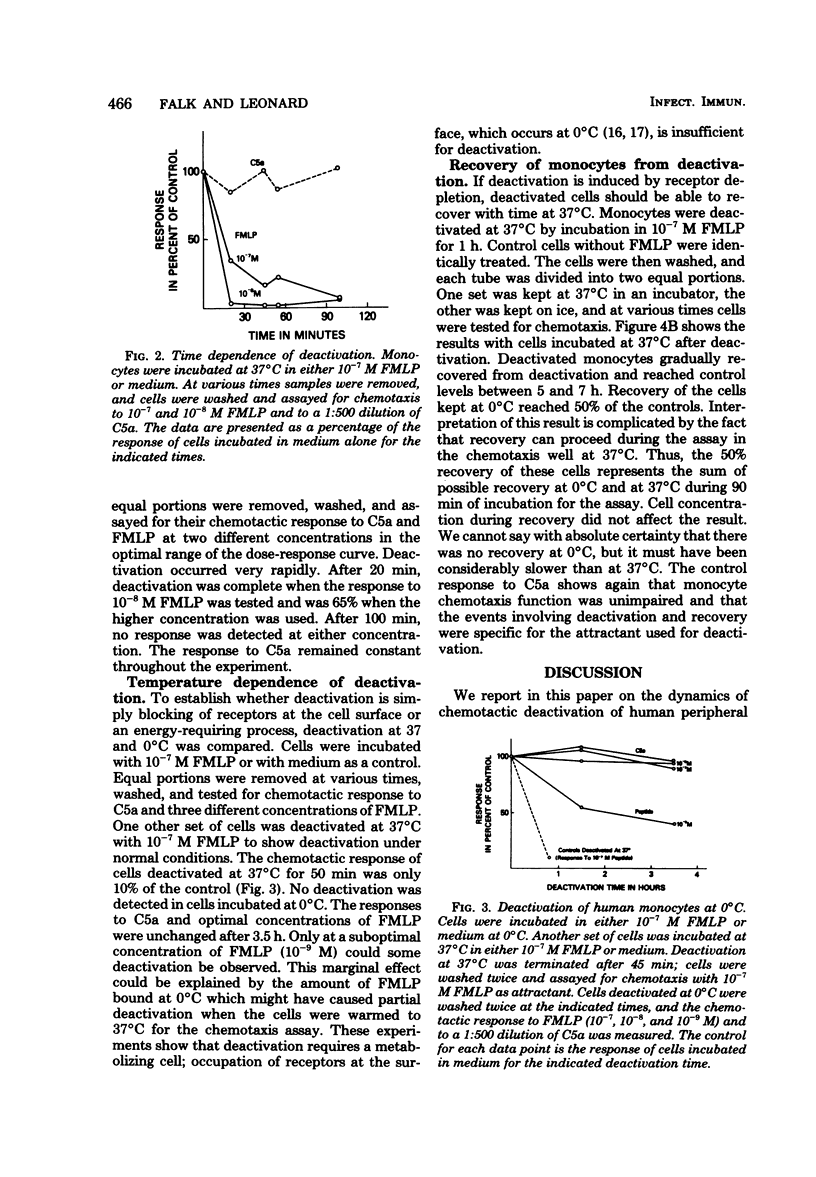
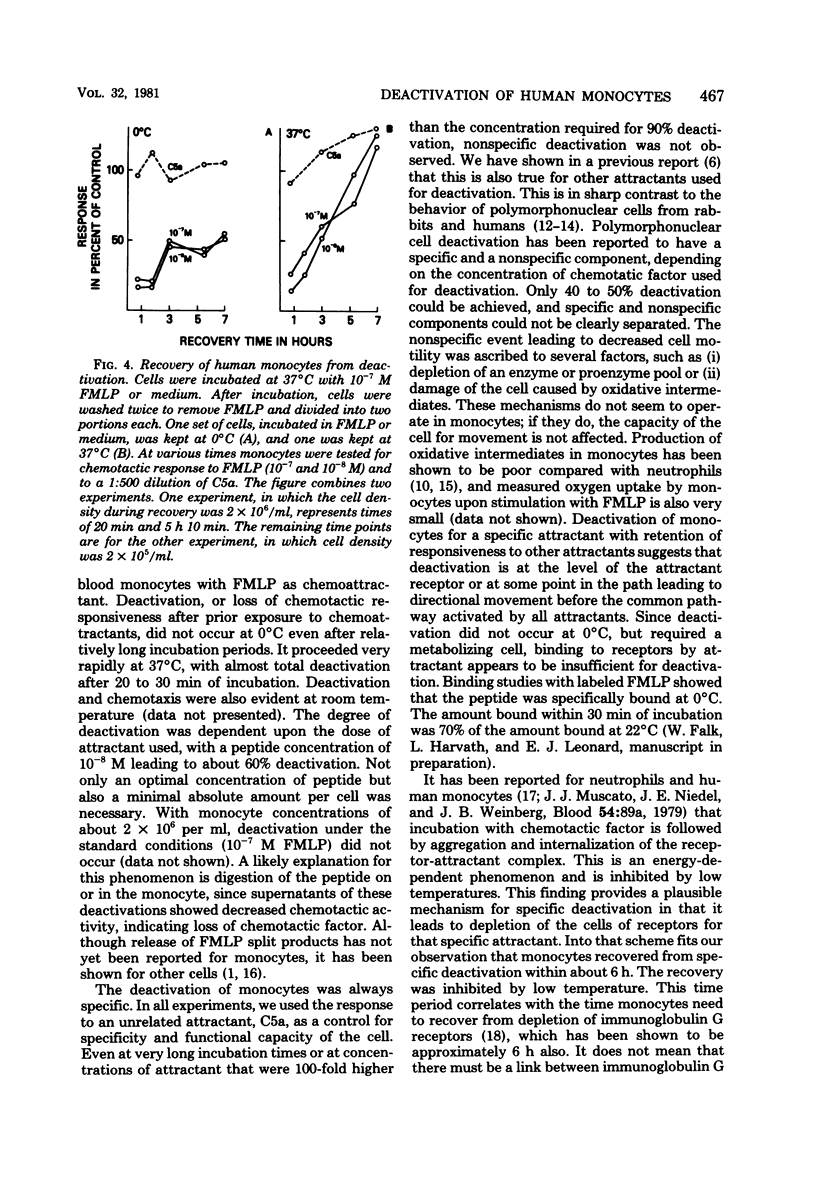
The chemotactic deactivation of human monocytes was studied to provide insight into the mechanism of chemotaxis. Deactivation was dependent on the dose of chemoattractant and time of incubation. A concentration in the cell suspension of 10(-8) M N-formylmethionylleucyl phenylalanine (FMLP) for 45 min at 37 degrees C led to 60% suppression of the subsequent specific chemotactic response. Higher concentrations of FMLP led to almost 100% specific suppression. Deactivation was specific under all conditions used. The response to a nonrelated chemoattractant, human serum-derived C5a, was unaffected by incubation in FMLP. Deactivation was also transient. If cells were deactivated at 37 degrees C with FMLP, they recovered within 6 h at 37 degrees C from this deactivation. Both phenomena, deactivation and recovery from deactivation, were temperature dependent. Monocytes could not be deactivated at 0 degrees C, and they did not recover from deactivation when kept at 0 degrees C. Thus, specific deactivation appears to require cellular metabolism, involving loss of receptors or blocking of a step between receptor occupancy and response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Jacob H. S. Complement-mediated granulocyte dysfunction in paroxysmal nocturnal hemoglobinuria. Blood. 1976 Jun;47(6):931–939. [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Ho P. P., Young A. L., Southard G. L. Methyl ester of N-formylmethionyl-leucyl-phenylalanine: chemotactic responses of human blood monocytes and inhibition of gold compounds. Arthritis Rheum. 1978 Jan-Feb;21(1):133–136. doi: 10.1002/art.1780210121. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Biggar W. D. Influence of serum-derived chemotactic factors and bacterial products on human neutrophil chemotaxis. Infect Immun. 1977 Jan;15(1):212–220. doi: 10.1128/iai.15.1.212-220.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. E. Chemotaxis and random mobility. Clinical and biologic differentiation. Antibiot Chemother (1971) 1974;19:338–349. [PubMed] [Google Scholar]
- Nelson R. D., Fiegel V. D., Herron M. J., Simmons R. L. Chemotactic deactivation of human neutrophils: relationship to loss of cytotaxin receptor function and temporal nature of the phenomenon. J Reticuloendothel Soc. 1980 Sep;28(3):285–294. [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Herron M., Simmons R. L., Quie P. G. Chemotactic deactivation of human neutrophils: possible relationship to stimulation of oxidative metabolism. Infect Immun. 1979 Feb;23(2):282–286. doi: 10.1128/iai.23.2.282-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. Mechanisms of the inhibition of chemotaxis by phosphonate esters. J Exp Med. 1967 Jun 1;125(6):1001–1020. doi: 10.1084/jem.125.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. I., Whitmer D., Geotzl E. J., Austen K. F. Chemotactic deactivation of human eosinophils by the eosinophil chemotactic factor of anaphylaxis (38527). Proc Soc Exp Biol Med. 1975 Jan;148(1):301–306. doi: 10.3181/00379727-148-38527. [DOI] [PubMed] [Google Scholar]


