Abstract
The intracellular location of ADP-glucose pyrophosphorylase (AGP) in developing pericarp of tomato (Lycopersicon esculentum Mill) has been investigated by immunolocalization. With the use of a highly specific anti-tomato fruit AGP antibody, the enzyme was localized in cytoplasm as well as plastids at both the light and electron microscope levels. The immunogold particles in plastids were localized in the stroma and at the surface of the starch granule, whereas those in the cytoplasm occurred in cluster-like patterns. Contrary to the fruit, the labeling in tomato leaf cells occurred exclusively in the chloroplasts. These data demonstrate that AGP is localized to both the cytoplasm and plastids in developing pericarp cells of tomato.
AGP converts Glc-1-P and ATP to ADP-Glc and PPi. The product of this reaction, ADP-Glc, is the major substrate for starch synthases (Preiss, 1991). Substantial evidence from the analysis of the starch-deficient mutants (Tsai and Nelson, 1966; Lin et al., 1988; Hylton and Smith, 1992), transgenic plants (Müller-Röber et al., 1992; Stark et al., 1992), control analysis of photosynthate partitioning (Neuhaus and Stitt, 1990), and kinetic models (Pettersson and Ryde-Pettersson, 1989) firmly establish that AGP catalyzes an essential step for starch biosynthesis in both photosynthetic and nonphotosynthetic tissues.
AGP in higher plants is a heterotetramer composed of two small and two large subunits. Recently, multiple forms of both the small and the large subunits have been found in several plants. Several isoforms of the large subunit were observed when the purified potato (Solanum tuberosum L.) tuber AGP was subjected to high resolution 2-D PAGE (Okita et al., 1990). The identification of three AGP large subunit cDNAs from potato tuber suggests that multiple polypeptides are not the result of proteolytic degradation or posttranslational modification (Cognata et al., 1995). Similarly, multiple AGP polypeptides have been detected in pea (Pisum sativum L.) and rice endosperm (Hylton and Smith, 1992; Nakamura and Kawaguchi, 1992). Multiple cDNA clones for AGP have also been isolated from barley (Hordeum vulgare L.; Villand et al., 1992), Arabidopsis (Villand et al., 1993), maize (Zea mays L.; Giroux and Hannah, 1994), broad bean (Weber et al., 1995), pea (Burgess et al., 1997), and sweet potato (Bae and Liu, 1997).
Whereas the presence of isoforms seems to be a common feature of plant AGPs, the significance of their occurrence is not presently known. One possible explanation is that individual isoforms could have different intracellular locations. It was proposed that AGP of cereal endosperm exists in the cytoplasm as well as in the amyloplasts (Hannah et al., 1993; Villand and Kleczkowski, 1994). Recently, substantial immunological evidence demonstrated that a plastidial form and a major cytoplasmic form of the enzyme exist in barley and maize endosperm (Denyer et al., 1996; Thorbjørnsen, et al., 1996). The occurrence of this phenomenon in plants other than cereals is unknown.
Purification and characterization of AGP from tomato (Lycopersicon esculentum L.) fruit revealed the existence of two small subunit isoforms and three large subunit isoforms (Chen and Janes, 1997). To determine the subcellular location of AGP isoforms in developing tomato fruit pericarp, we used immunocytochemical techniques at the light and electron microscope levels using a highly specific anti-tomato fruit AGP antibody. This study demonstrates that AGP is also localized to both the cytoplasm and plastids in developing pericarp cells of tomato.
MATERIALS AND METHODS
Tomato (Lycopersicon esculentum Mill. var Laura) plants were grown in the greenhouse under a 16-h light/8-h dark cycle. Fruit were collected 2 weeks postanthesis (fresh weight about 30 g). The inner pericarp tissue of the fruit and mature fourth leaves were utilized in this study and processed immediately as described below.
Tissue Preparation
Tomato inner pericarp tissue was cut into small blocks (about 2 mm3) and then immediately fixed in 100 mm phosphate buffer (pH 7.2) with 3% (w/v) paraformaldehyde and 1.25% (v/v) glutaraldehyde for 3 to 4 h at room temperature. After the tissue blocks were washed with 100 mm phosphate buffer (pH 7.2), they were dehydrated through a graded ethanol series (10–100%) and infiltrated with London Resin White (Electron Microscopy Sciences, Fort Washington, PA) according to the manufacturer's protocol. Polymerization was conducted at 40°C for 24 h, at 50°C for 24 h, and then at 60°C for 36 h. For carbohydrate-specific staining the inner pericarp tissue was fixed and embedded in wax as described previously (Wang and Lou, 1994).
Immunolabeling and Observation
For light microscope observation, thin sections (180–200 nm) cut by a LKB ultramicrotome were mounted onto gelatin-coated glass slides (Superfrost/plus, Fisher Scientific). The sections were first incubated with TBST buffer (20 mm Tris, pH 7.4, 500 mm NaCl, and 0.1% Tween 20) containing 2% (w/v) BSA at room temperature for 1 h and then incubated in either preimmune serum or antiserum (both diluted 1:2000 in TBST buffer containing 0.1% BSA) raised against tomato fruit AGP (Chen and Janes, 1997) for 3 h. Following washes in antibody diluent, sections were incubated for 1 h in goat anti-rabbit IgG antibody conjugated with 20 nm gold (Electron Microscopy Sciences) diluted 1:50 as above, and then rinsed consecutively in antibody diluent, TBST buffer containing 2% (w/v) BSA and distilled water. Immunogold particles were enlarged by incubation with silver-enhancement solution (ICN) following the manufacturer's recommendations. The sections were counterstained with 0.05% safranin solution and viewed using a light microscope.
For electron microscope observation, ultrathin sections (70–80 nm) were collected on nickel grids and processed for immunogold labeling as described above (without the silver enhancement step). The ultrathin sections were double stained with uranyl acetate-lead citrate (Wang, 1994) and examined with a JEO100CX electron microscope.
RESULTS AND DISCUSSION
To establish the intracellular location of the AGP isoforms revealed by 2-D PAGE analysis of the purified tomato fruit enzyme, a polyclonal antibody raised against the purified enzyme preparation was used. The antiserum reacts with each of the AGP isoforms. Moreover, this reaction was also highly specific, i.e. no other proteins in the tomato fruit crude extract cross-reacted with the antiserum (Chen and Janes, 1997). The material used for AGP purification, the inner pericarp at 2 weeks postanthesis, was also used for immunolocalization in the present study to ensure that each of the AGP isoforms observed by 2-D PAGE is present.
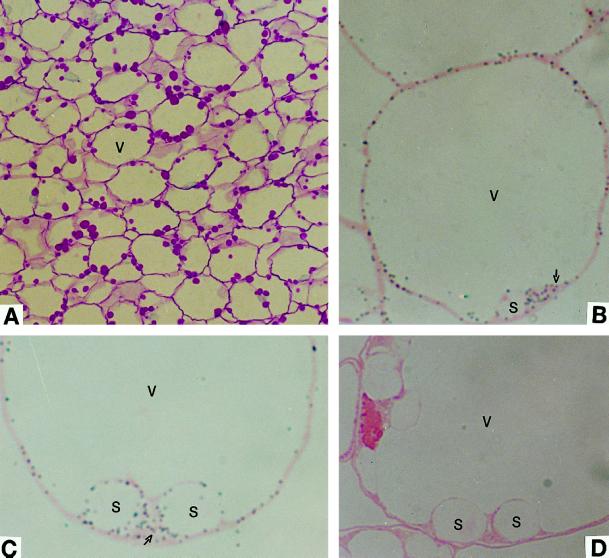
Figure 1A shows carbohydrate-specific staining of the inner pericarp. The cells at this sampling stage contain a large central vacuole and numerous amyloplasts that contain starch grains, which stain red by the periodic acid-Schiff reagent.
Figure 1.
Light micrographs showing immunolocalization of AGP in developing pericarp of tomato fruit. A, Carbohydrate-specific staining. Starch grains stained red. B and C, Anti-tomato fruit AGP serum detected a signal (gray dots) in both plastids and cytoplasm (arrows). D, The preimmune control serum detected no signal. S, Starch granule; and V, vacuole. A, ×351; B, ×1560; and C and D, ×1950.
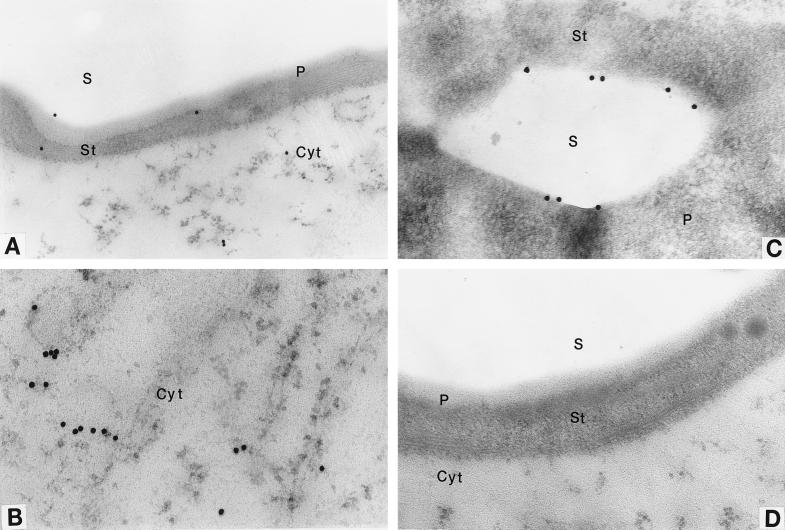
The intracellular localization of AGP was first studied at the light microscope level. Because the immunogold particles (20 nm in diameter) are beyond the resolution limit of the light microscope, they were enlarged by the silver-enhancement technique, which facilitates visualization of the dark-gray dots. Gray deposits were observed in both the plastids and cytoplasm of tomato pericarp cells (Fig. 1, B and C). The signal was not evenly distributed in the cytoplasm, with denser particles clustered near plastids (Fig. 1C). The preimmune serum control showed no gray particles (Fig. 1D), indicating that the antibody is specific. The intracellular distribution of AGP was further examined by an immunoelectron microscope. Consistent with the light microscope findings, immunogold particles were observed in both the plastids (Fig. 2, A and C) and cytoplasm (Fig. 2, A and B). The labeling in the plastids was not uniformly distributed. No immunogold particles were observed inside the starch granule, contrary to what was found in the maize (Zea mays L.) endosperm (Miller and Chourey, 1995). Some of the particles occurred at or near the surface of the starch grains (Fig. 2C), whereas others were localized mostly in the stroma (data not shown). Similar to the plastids, labeling in the cytoplasm was not uniform, and most was localized in cluster-like patterns. Overall, the signal was not as strong as that observed in amyloplasts of potato (Solanum tuberosum L.) tuber (Kim et al., 1989) and maize endosperm (Miller and Chourey, 1995). This is consistent with the very low specific activity of AGP in tomato fruit crude extract (Chen and Janes, 1997). No immunogold signal was detected with preimmune control serum (Fig. 2D).
Figure 2.
Electron micrographs showing immunolocalization of AGP in developing pericarp of tomato fruit. Anti-tomato fruit AGP serum was used for immunolocalization. A, Immunogold particles reside in both plastids and cytoplasm. B, Immunogold particles reside in the cytoplasm. C, Immunogold particles reside at or near the starch granule boundary. D, Preimmune serum control. P, Plastid; S, starch granule; St, stroma; and Cyt, cytoplasm. A, ×24,180;B, ×31,200;C, ×30,420; and D, ×28,860.
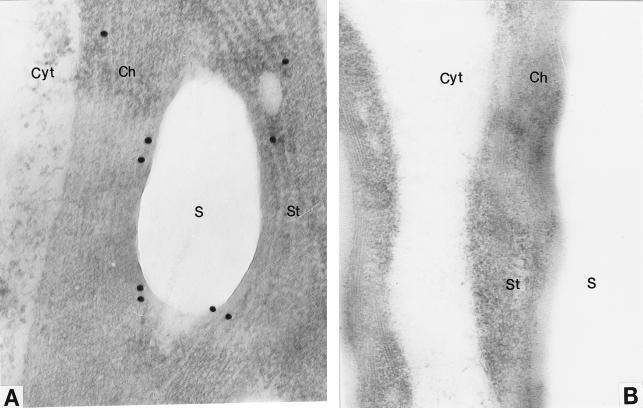
Reliability of immunolocalization results depends on the following factors: (a) specificity of the antiserum, (b) absence of artifacts caused by antigen mobility during sample preparation and labeling, and (c) absence of nonspecific binding of antiserum. The specificity of the antiserum is high, as suggested by western-blot analysis (Chen and Janes, 1997). To minimize artifacts caused by antigen movement, immunolocalization was done at both the light and electron microscope levels and the results were consistent with each other. Furthermore, as mentioned above, the particles were not randomly distributed. No labeling was observed in the central vacuole or inside the starch grains. To test the specificity of the cytoplasmic labeling, tomato leaf tissue served as a control. It is well documented that the AGP protein is located exclusively in the chloroplasts of leaf tissue (Echeverria and Boyer, 1986; Robinson and Preiss, 1987). We used the same immunolocalization procedure as for tomato pericarp, and labeling occurred exclusively in the chloroplasts of leaf cells (Fig. 3A). This provides evidence that the cytoplasmic labeling of developing pericarp cells is fruit specific and that they are not likely to be artifacts of sample preparation.
Figure 3.
Electron micrographs showing immunolocalization of AGP in tomato leaves. Anti-tomato fruit AGP serum was used for immunolocalization. A, Immunogold particles reside in the stroma of chloroplasts. B, Preimmune serum control. Labels are as in Figure 2. A, ×54,600; B, ×42,900.
This immunolocalization study clearly establishes that AGP is both plastidial and cytoplasmic in developing pericarp cells of tomato fruit, thereby extending the existence of cytoplasmic AGP to plant tissues other than cereal endosperm. However, its occurrence appears to be both species and tissue dependent. Potato tuber immunocytological studies (Kim et al., 1989) and transgenic plant experiments (Stark et al., 1992) indicate that AGP is located exclusively within the amyloplast. In contrast to barley (Hordeum vulgare L.) and maize endosperm, the majority of tomato pericarp AGP appears to reside within the plastid. We observed a greater degree of labeling in the plastids than in the cytoplasm.
Whether the plastidial and cytoplasmic AGP in tomato pericarp represent two distinct isoforms is not presently known. In barley and maize endosperm these two forms are distinct, as revealed by differences in the size of the small subunit (Denyer et al., 1996; Thorbjørnsen et al., 1996). Tomato pericarp contains two isoforms of the small subunit and three isoforms of the large subunit (Chen and Janes, 1997). The data presented here establish that AGP isoforms exist in both plastids and cytoplasm. However, the localization of each specific isoform within the plastid or cytoplasm remains unknown. It is also possible that the cytoplasmic AGP we observed is simply untransported precursors of AGP subunits resulting from inefficiency of the protein-translocation machinery on the plastid membranes. Isoform-specific antibodies are needed to answer these questions. Recently, we isolated four cDNAs coding for AGP in tomato fruit (B.-Y. Chen and H. W. Janes, unpublished data), which will facilitate the production of these antibodies.
If we assume that the role of the cytoplasmic AGP is for starch biosynthesis, ADP-Glc, the product of the enzyme, must then be transported into the amyloplasts. It was found by in vitro experiments that an adenylate translocator in the amyloplasts can transport ADP-Glc, which is utilized for starch synthesis (Liu et al., 1991; Pozueta-Romero et al., 1991a, 1991b). This adenylate translocator is present in all plastid types (Ardila et al., 1993). In vivo evidence for the presence of the putative ADP-Glc transporter comes from the maize brittle1 (bt1) mutant (Mangelsdorf, 1926; Wentz, 1926). Mutant bt1 kernels have a brittle texture and accumulate about 80% less starch than normal kernels (Tobias et al., 1992). The BT1 gene was cloned (Sullivan et al., 1991) and its encoded proteins were localized in the amyloplast membrane of maize endosperm cells (Cao et al., 1995; Sullivan and Kaneko, 1995). Compared with the normal endosperm, the level of ADP-Glc in the cytoplasm of bt1 endosperm was at least 13-fold higher (Shannon et al., 1996), whereas amyloplasts isolated from bt1 endosperm were less active in ADP-Glc uptake and conversion to starch (Liu et al., 1992). These data suggest that the BT1 protein could function as an ADP-Glc translocator and transport ADP-Glc from the cytoplasm into amyloplasts in vivo. Therefore, this putative ADP-Glc translocator may play a pivotal role in linking the cytoplasmic form of AGP and starch biosynthesis in amyloplasts.
Recently, it was shown that the isolated amyloplasts from potato tuber are able to synthesize starch from ADP-Glc (Naeem et al., 1997). If this is of physiological significance, then results from the transgenic plant experiment (Stark et al., 1992), in which plant expression of an Escherichia coli AGP gene in the amyloplasts increased starch content but expression in the cytoplasm did not, may alternatively indicate that the putative ADP-Glc or adenylate translocator may be rate-limiting. As a result any increase of ADP-Glc in the cytoplasm may have no effect on starch content without corresponding changes in this ADP-Glc or adenylate translocator on the amyloplast membrane. Inconsistent with this argument, BT1 homologs were not detected in potato tubers and other starchy tissues by the BT1 antibody (Cao and Shannon, 1997). Therefore, whether the putative ADP-Glc translocator exists in the amyloplast membrane of tomato pericarp cells may be a key toward understanding the function of the cytoplasmic form of AGP in tomato fruit.
ACKNOWLEDGMENT
We thank Dr. Bruce Wasserman for critical reading of the manuscript.
Abbreviations:
- AGP
ADP-Glc pyrophosphorylase
- 2-D
two-dimensional
Footnotes
This work was supported by the National Aeronautics and Space Administration.
LITERATURE CITED
- Bae JM, Liu JR. Molecular cloning and characterization of two novel isoforms of the small subunit of ADPglucose pyrophosphorylase from sweet potato. Mol Gen Genet. 1997;254:179–185. doi: 10.1007/s004380050406. [DOI] [PubMed] [Google Scholar]
- Burgess D, Penton A, Dunsmuir P, Dooner H. Molecular cloning and characterization of ADP-glucose pyrophosphorylase cDNA clones isolated from pea cotyledons. Plant Mol Biol. 1997;33:431–444. doi: 10.1023/a:1005752311130. [DOI] [PubMed] [Google Scholar]
- Cao H, Shannon JC. BT1, a possible adenylate translocator, is developmentally expressed in maize endosperm but not detected in starchy tissues from several other species. Physiol Plant. 1997;100:400–406. [Google Scholar]
- Cao H, Sullivan TD, Boyer CD, Shannon JC. Bt1, a structural gene for the major 39–44 kDa amyloplast membrane polypeptides. Physiol Plant. 1995;95:176–186. [Google Scholar]
- Chen BY, Janes HW. Multiple forms of ADP-glucose pyrophosphorylase from tomato fruit. Plant Physiol. 1997;113:235–241. doi: 10.1104/pp.113.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognata UL, Willmitzer L, Mulller-Rober B. Molecular cloning and characterization of novel isoforms of potato ADPglucose pyrophosphorylase. Mol Gen Genet. 1995;246:538–548. doi: 10.1007/BF00298960. [DOI] [PubMed] [Google Scholar]
- Denyer K, Dunlap F, Thorbjornsen T, Keeling P, Smith AM. The major form of ADP-glucose pyrophosphorylase in maize (Zea mays L.) endosperm is extra-plastidial. Plant Physiol. 1996;112:779–785. doi: 10.1104/pp.112.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E, Boyer CD. Localization of starch biosynthetic and degradative enzymes in maize leaves. Am J Bot. 1986;73:167–171. [Google Scholar]
- Giroux MJ, Hannah LC. ADPglucose pyrophosphorylase in sh2 and bt2 mutants of maize. Mol Gen Genet. 1994;243:400–408. doi: 10.1007/BF00280470. [DOI] [PubMed] [Google Scholar]
- Hannah LC, Giroux M, Boyer C. Biotechnological modification of carbohydrates for sweet corn and maize improvement. Sci Hortic. 1993;55:177–197. [Google Scholar]
- Hylton C, Smith AM. The rb mutation of peas causes structural and regulatory changes in ADPglucose pyrophosphorylase from developing embryos. Plant Physiol. 1992;99:1626–1634. doi: 10.1104/pp.99.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Franceschi VR, Okita TW, Robinson N, Morell M, Preiss J. Immunocytochemical localization of ADP-glucose pyrophosphorylase in developing potato tuber cells. Plant Physiol. 1989;91:217–220. doi: 10.1104/pp.91.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-P, Caspar T, Somerville CR, Preiss J. A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol. 1988;88:1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K-C, Boyer CD, Shannon JC (1991) Evidence of an adenylate translocator in maize amyloplast membranes. In A Huang, L Taiz, eds, Molecular Approaches to Compartmentation and Metabolic Regulation. American Society of Plant Physiologists, Rockville, MD, pp 236–237
- Liu K-C, Boyer CD, Shannon JC. Carbohydrate transfer into isolated maize (Zea mays L.) amyloplasts (abstract no. 234) Plant Physiol. 1992;99:S-39. [Google Scholar]
- Mangelsdorf PC. The genetics and morphology of some endosperm characters in maize. Conn Agric Exp Stn Bull. 1926;279:509–614. [Google Scholar]
- Miller ME, Chourey PS. Intracellular immunolocalization of adenosine 5′-diphosphoglucose pyrophosphorylase in developing endosperm cells of maize (Zea mays L.) Planta. 1995;197:522–527. [Google Scholar]
- Müller-Röber B, Sonnewald U, Willmitzer L. Antisense inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage proteins. EMBO J. 1992;11:1229–1238. doi: 10.1002/j.1460-2075.1992.tb05167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M, Tetlow IJ, Emes MJ. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997;11:1095–1103. [Google Scholar]
- Nakamura Y, Kawaguchi K. Multiple forms of ADP-glucose pyrophosphorylase of rice endosperm. Physiol Plant. 1992;84:336–342. [Google Scholar]
- Neuhaus HE, Stitt M. Control analysis of photosynthate partitioning. Impact of reduced activity of ADP-glucose pyrophosphorylase or plastid phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana (L.) Heynh. Planta. 1990;182:445–454. doi: 10.1007/BF02411398. [DOI] [PubMed] [Google Scholar]
- Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J. The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol. 1990;93:785–790. doi: 10.1104/pp.93.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson G, Ryde-Pettersson U. Metabolites controlling the rate of starch synthesis in the chloroplast of C3 plants. Eur J Biochem. 1989;179:169–172. doi: 10.1111/j.1432-1033.1989.tb14536.x. [DOI] [PubMed] [Google Scholar]
- Pozueta-Romero J, Ardila F, Akazawa T. ADP-glucose transport by the chloroplast adenylate translocator is linked to starch biosynthesis. Plant Physiol. 1991a;97:1565–1572. doi: 10.1104/pp.97.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta-Romero J, Frehner M, Viale MA, Akazawa T. Direct transport of ADP-glucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc Natl Acad Sci USA. 1991b;88:5769–5773. doi: 10.1073/pnas.88.13.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Biology and molecular biology of starch synthesis and its regulation. Oxf Surv Plant Mol Cell Biol. 1991;7:59–114. [Google Scholar]
- Robinson NL, Preiss J. Localization of carbohydrate metabolizing enzymes in guard cells of Commelina communis. Plant Physiol. 1987;85:360–364. doi: 10.1104/pp.85.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JC, Pien FM, Liu KC. Nucleotides and nucleotide sugars in developing maize endosperm. Synthesis of ADP-glucose in brittle-1. Plant Physiol. 1996;110:835–843. doi: 10.1104/pp.110.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADPglucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Sullivan TD, Kaneko Y. The maize brittle1 gene encodes amyloplast membrane polypeptides. Planta. 1995;196:477–484. doi: 10.1007/BF00203647. [DOI] [PubMed] [Google Scholar]
- Sullivan TD, Strelow LI, Illingworth CA, Phillips RL, Nelson OE., Jr Analysis of the maize Brittle-1 alleles and a defective Suppressor-Mutator-induced mutable allele. Plant Cell. 1991;3:1337–1348. doi: 10.1105/tpc.3.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbjørnsen T, Villand P, Denyer K, Olsen O-A, Smith AM. Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm. Plant J. 1996;10:243–250. [Google Scholar]
- Tobias RB, Boyer CD, Shannon JC. Alteration in carbohydrate intermediates in the endosperm of starch-deficient maize (Zea mays L.) genotypes. Plant Physiol. 1992;99:146–152. doi: 10.1104/pp.99.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C, Nelson OE. Starch-deficient maize mutant lacking adenosine diphosphate pyrophosphorylase activity. Science. 1966;151:341–343. doi: 10.1126/science.151.3708.341. [DOI] [PubMed] [Google Scholar]
- Villand P, Aalen R, Olsen OA, Luthi E, Lonneborg A, Kleczkowski LA. PCR amplification and sequences of cDNA clones for the small and large subunits of ADPglucose pyrophosphorylase from barley tissues. Plant Mol Biol. 1992;19:381–389. doi: 10.1007/BF00023385. [DOI] [PubMed] [Google Scholar]
- Villand P, Olsen OA, Kleczkowski LA. Molecular characterization of multiple cDNA clones for ADPglucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol Biol. 1993;23:1279–1284. doi: 10.1007/BF00042361. [DOI] [PubMed] [Google Scholar]
- Villand P, Kleczkowski LA. Is there an alternative pathway for starch biosynthesis in cereal seeds? Z Naturforsch. 1994;49c:215–219. [Google Scholar]
- Wang Y. Light and electron microscopy study on the endoplasmic reticulum in the microspore of Gossypium hirsutum. Chin J Bot. 1994;6:23–28. [Google Scholar]
- Wang Y, Lou CH. Pollen wall development in Gossypium hirsutum. Acta Agron Sin. 1994;20:277–281. [Google Scholar]
- Weber H, Heim U, Borisjuk L, Wobus U. Cell-type specific, coordinate expression of two ADP-glucose pyrophosphorylase genes in relation to starch biosynthesis during seed development of Vicia faba L. Planta. 1995;195:352–361. doi: 10.1007/BF00202592. [DOI] [PubMed] [Google Scholar]
- Wentz JB. Heritable characters in maize. XXVI. Concave. J Hered. 1926;17:327–329. [Google Scholar]