Abstract
Objectives
Meningitis and bacteremia due to Neisseria meningitidis are rare but potentially deadly diseases that can be prevented with immunization. Beginning in 2008, Arizona school immunization requirements were amended to include immunization of children aged 11 years or older with meningococcal vaccine before entering the sixth grade. We describe patterns in meningococcal vaccine uptake surrounding these school-entry requirement changes in Arizona.
Methods
We used immunization records from the Arizona State Immunization Information System (ASIIS) to compare immunization rates in 11- and 12-year-olds. We used principal component analysis and hierarchical cluster analysis to identify and analyze demographic variables reported by the 2010 U.S. Census.
Results
Adolescent meningococcal immunization rates in Arizona increased after implementation of statewide school-entry immunization requirements. The increase in meningococcal vaccination rates among 11- and 12-year-olds from 2007 to 2008 was statistically significant (p<0.0001). All demographic groups had significantly higher odds of on-schedule vaccination after the school-entry requirement change (odds ratio range = 5.57 to 12.81, p<0.0001). County demographic factors that were associated with lower odds of on-schedule vaccination included higher poverty, more children younger than 18 years of age, fewer high school graduates, and a higher proportion of Native Americans.
Conclusions
This analysis suggests that implementation of school immunization requirements resulted in increased meningococcal vaccination rates in Arizona, with degree of response varying by demographic profile. ASIIS was useful for assessing changes in immunization rates over time. Further study is required to identify methods to control for population overestimates in registry data.
Neisseria meningitidis (N. meningitidis) is a bacterium that causes meningitis and bacteremia, which often result in brain damage, amputations, and death. Meningococcal disease has an estimated case fatality rate of 10%–14%.1 N. meningitidis can be transmitted through contact with large-droplet respiratory secretions of infected patients or asymptomatic carriers. Meningococcal disease is a nationally notifiable disease in the United States; in 2009, 980 cases of meningococcal disease were reported (all serogroups).2
Three vaccines are currently licensed in the U.S. to prevent meningococcal disease. In May 2005, the Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) recommended one dose of quadrivalent meningococcal conjugate vaccine (MCV4/Menactra®) for all children aged 11–12 years, those entering high school, and others at increased risk, such as people traveling to an infectious area or those with a preexisting medical condition.1 In 2007, ACIP’s recommendations were adjusted to include routine immunization of all 11- to 18-year-olds at the earliest opportunity.3 As a consequence, national uptake of the vaccine appears to be increasing. The National Immunization Survey-Teen (NIS-Teen) estimated that coverage of meningococcal vaccine was 53.6% (95% confidence interval [CI] 52.4, 54.9) in adolescents aged 13–17 years in the U.S. in 20094 and increased to 62.7% (95% CI 61.5, 63.9) in 2010.5 Arizona coverage rates for 13- to 17-year-olds were 69.7% (95% CI 63.4, 75.3) in 20094 and 78.9% (65.3%) in 2010.5
Rates of vaccination against meningococcal disease for children and adolescents in Arizona can also be estimated using the Arizona State Immunization Information System (ASIIS). Since 1998, the Arizona Department of Health Services (ADHS) has used ASIIS to facilitate reporting and storage of immunization data for Arizona children.6 Under Arizona Revised Statute §36-135, providers are required to report all immunizations administered to children younger than 18 years of age to ADHS using ASIIS.7
In 2008, the Arizona school-entry requirements were changed to include new meningococcal vaccine specifications for children entering sixth grade.7,8 The requirements indicated that unless exempt, children who were 11 years of age or older entering sixth grade who had not yet received a vaccine to prevent meningococcal disease had to be vaccinated by September 1, 2008. The requirements also included grades seven through 12, but these grades were phased in incrementally, taking effect each year on September 1.
Immediately following these changes to the school-entry requirements, ADHS launched an education and awareness campaign. The campaign aimed to raise awareness of vaccination requirements and educate the public on the role of vaccines in preventing diseases caused by pathogens such as N. meningitidis. The campaign, which was designed by ADHS staff and CDC’s “It’s Their Turn” campaign partners, included print and electronic materials as well as media coverage. The 2008 summer and fall campaign targeted adolescents, their parents, school personnel, health-care providers, and community organizations. Results indicated that the campaign was successful and had a high degree of parental acceptance, but reported that a small yet significant proportion of the population chose not to vaccinate.9
Although meningococcal vaccine coverage appears to be increasing in Arizona and nationally, questions remain about the level of vaccine uptake in Arizona subpopulations and how changes to school immunization requirements affected uptake across the state. The purpose of this study was to use ASIIS data to determine the coverage rates for meningococcal vaccines in Arizona in 11- and 12-year-old children and determine the influence that statewide school vaccination requirements have on the odds of an on-schedule meningococcal vaccination. This study also aimed to enumerate demographic characteristics associated with differences in geographic response to vaccination requirements for school entry.
METHODS
Data and inclusion criteria
We extracted de-identified individual records for meningococcal vaccinations administered from January 2006 to January 2011, for children born between January 1, 1993, and January 1, 2000, from ASIIS. The dataset included records for children in the specified age range regardless of whether the child’s record contained a meningococcal immunization. Records containing ambiguous birth dates were excluded. We used the following variables in the analysis: patient ZIP code, patient date of birth, date of meningococcal vaccination, provider identification, and provider type (e.g., private or public). Patient ZIP codes were matched to corresponding Arizona counties, and records with ZIP codes indicating addresses outside of Arizona were omitted from county and regional analyses.
Analysis of vaccination data from the immunization registry
We calculated proportions of children vaccinated at 11 and 12 years of age for each school year (SY) for SY 2006–2007 through SY 2009–2010. Because ASIIS does not record a child’s grade level, and most children start sixth grade at either 11 or 12 years of age, we assumed that the school-entry requirement applied to all children who were 11 or 12 years of age (as of September 1 of the given school year). Children who received the meningococcal vaccine during their 11th or 12th years were considered on schedule.
We calculated overall vaccination rates for children aged 11 and 12 years, both prior to and after implementation of the school requirement (SYs 2006–2010). We calculated coverage rates using the number of children who were either 11 or 12 years of age with meningococcal vaccination, divided by the total number of children in ASIIS who were 11 or 12 years of age during that school year. For 2010, we also calculated immunization coverage rates using population estimates from the 2010 U.S. Census. Using this external source of population data helps provide perspective on limitations of ASIIS population estimates. To determine if the meningococcal vaccination requirement had a significant impact on vaccination uptake among eligible children in Arizona, we performed Pearson’s corrected Chi-square analysis for vaccination in 2007 vs. 2008.
Grouping county demographics followed by regression modeling
In addition to the changing school requirements for meningococcal vaccine, additional demographic factors (e.g., race/ethnicity, education level, and income) may influence vaccination rates. Many of these statistics are available at the county level from the 2010 U.S. Census. To determine which demographic variables may explain differences among Arizona counties and provide insight into how to cluster counties into groupings, we performed a principal components analysis (PCA). The PCA is a useful technique in exploratory data analysis for finding patterns in complex datasets with many dimensions (e.g., many potentially inter-correlated variables).10,11 Its goal is to extract the important information from the dataset, represent it as a set of new orthogonal variables called “principal components,” and display the pattern of similarity of the observations and the variables as points on a map.11 We used the PCA method because when a multitude of variables are available, such as in the case of U.S. Census data, variables are more likely to be correlated with each other. A major benefit of PCA is that it functions as an exploratory analysis that identifies the most important variables—that is, those variables that are responsible for the most variation in the response.
Variables for the PCA were taken from county--specific information reported in the 2010 U.S. Census. A total of 13 variables were included in the PCA, including the percentage of the population that is younger than 18 years of age; the percentage that is white, Hispanic, or Native American; the percentage of high school graduates; the percentage of home owners; the median home value, household income, and people per household; the percentage living in poverty; the number of people per square mile; federal dollars spent per capita; and the percentage of 2005–2009 U.S. veterans.
The PCA provides insight into which of the 13 variables are most important to drive demographic heterogeneity in meningococcal vaccine coverage among Arizona counties. Those variables considered most important were then selected to perform a hierarchical cluster analysis using Ward’s linkage to assign counties into groups with similar demographic compositions. We performed multivariate logistic regression to determine the odds of vaccination by end of age 12 years for each demographic group following the meningococcal immunization requirement in 2008.
RESULTS
The final dataset comprised 954,953 ASIIS records that met the aforementioned inclusion criteria. Annual immunization coverage rates for 2006–2010 were calculated as the number of children who received meningococcal vaccination by end of age 11 years according to ASIIS, divided by the total number of 11-year-old children in ASIIS. We repeated the calculation for 12-year-olds, and again for all children aged 6–18 years. Across all years, 506,375 children (59.3% of the ASIIS population) received meningococcal vaccinations between 6 and 18 years of age. Of the 506,375 vaccinated children, the majority (n=339,801, 67.1%) were vaccinated by 12 years of age, and 96.4% of those children were vaccinated at either 11 or 12 years of age (12,368 were vaccinated before 11 years of age). This finding suggests that the majority of Arizona children who received the immunization were vaccinated before entering sixth grade.
We also reported immunization coverage for 2010 using the U.S. Census for children aged 11 and 12 years and compared ASIIS-derived immunization rates with U.S. Census-derived rates. We found that in 2010, there were 89,797 11-year-olds in Arizona according to the U.S. Census, whereas ASIIS reported 139,747 11-year-olds for the same year. The lower population estimate from Census data resulted in a higher immunization rate of 74.9% compared with the ASIIS-only coverage rate of 48.8% (Table).
Table.
Percentage of Arizona children with at least one dose of meningococcal vaccine, by school year and age:a ASIISb vs. 2010 U.S. Census-derivedc rates
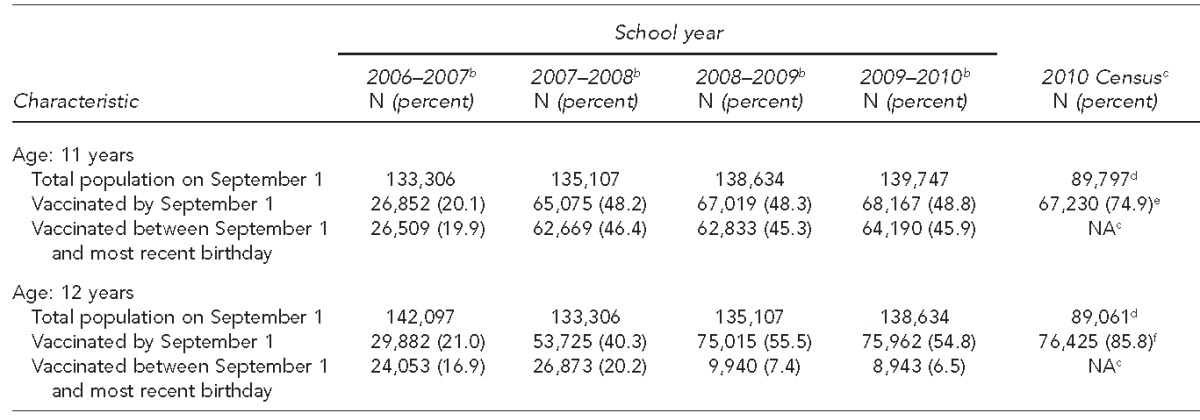
aAge as of September 1 of the specified school year
bAccording to records in ASIIS
cThe U.S. Census Bureau measures decennial Census data, thereby limiting U.S. Census-derived immunization rate comparison with 2010.
dAs of 2010
eVaccinated by 11 years of age
fVaccinated by 12 years of age
ASIIS = Arizona State Immunization Information System
NA = not applicable
Increases in on-schedule vaccination rates following state requirement change
During SY 2006–2007, only 20.1% of 11-year-olds and 21.0% of 12-year-olds in the registry received the meningococcal vaccine. This proportion increased during SY 2007–2008 to 48.2% of 11-year-olds and 40.3% of 12-year-olds. Vaccination coverage for 11-year-olds remained constant, and the proportion of children vaccinated by the end of age 12 years continued to rise in SY 2008–-2009 (Table). The increase in on-schedule vaccination rates between 2007 and 2008 was statistically significant (Pearson’s corrected Chi-square value = 2,426.07, degree of freedom = 1, p<0.0001) at 95% CI. We observed a large difference in observed immunization rates when data from the 2010 U.S. Census were used as denominators for coverage rate calculations.
Variability in vaccination uptake associated with demographics
Variability in county demographic characteristics can be reasonably described by the components from the PCA. The first three components described 75.74% of the total variability among Arizona counties. Component 1 accounted for 41.88%, Component 2 accounted for 20.70%, and Component 3 accounted for 13.16% of the variability.
Component 1 was driven by a high Native American population, fewer high school graduates, lower income, and higher poverty. Component 2 was driven by a high Hispanic population, more children younger than 18 years of age, fewer veterans, and higher income. Component 3 was driven by a high Native American population, fewer Hispanic people, more high school graduates, and higher home values.
We identified seven key variables from the first two components of the PCA that were responsible for the majority of demographic diversity among Arizona counties. We performed hierarchical cluster analysis with Ward’s linkage to assign counties to groups with similar demographic profiles based on the following key variables: percentage of the population younger than 18 years of age, percentage living in poverty, percentage Native American, percentage Hispanic, percentage white, percentage high school graduates, and percentage home owners (Figure 1).
Figure 1.
Eight demographic profiles of Arizona counties based upon similarities in household, education, income, and race/ethnicity, along with their associated odds of an on-schedule vaccination by end of age 12 years, following the 2008 statewide rule change requiring meningococcal vaccination
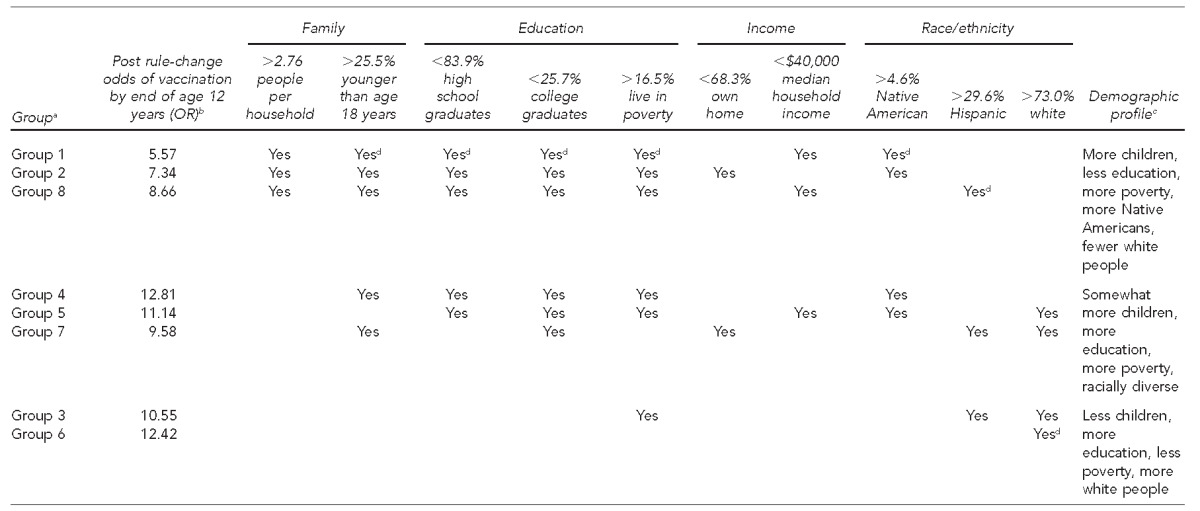
aGroups are listed in the order of their perceived sociodemographic risk profile.
bA 2008 statewide rule change required meningococcal vaccination for school entry at sixth grade.
cStatistics within each characteristic were assigned risk thresholds according to state-reported averages in the 2010 Census. Groups falling outside these thresholds are noted with a “yes” for that statistic and contributes to an overall group demographic profile.
dDenotes that the group has the lowest or highest value for that statistic
OR = odds ratio
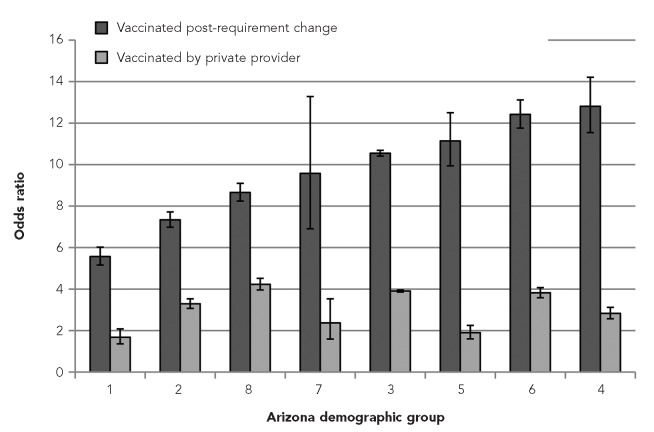
Based on logistic regression modeling in all groups, the odds of an on-schedule vaccination (vaccination at 11 or 12 years of age) were significantly higher after the Arizona rule change. The odds ratios (ORs) for vaccination by age 12 years following the Arizona rule change ranged from 5.57 to 12.81, demonstrating that substantial variability in odds exists among the eight demographic groups (Group 1 OR = 5.57, Group 2 OR = 7.34, Group 3 OR = 10.55, Group 4 OR = 12.81, Group = OR = 11.14, Group 6 OR = 12.42, Group 7 OR = 9.58, and Group 8 OR = 8.66) (Figure 1). For example, Group 1, which had the highest poverty rates, most children per family, fewer white people, and more Native Americans, had significantly lower odds of vaccination by end of age 12 years following the rule change (OR55.57, 95% CI 5.16, 6.02) than that of Group 4 (OR512.81, 95% CI 11.54, 14.21) (Figure 2). These findings suggest that additional demographic factors may be contributing to the observed differences in ORs.
Figure 2.
Logistic regression model ORsa for meningococcal vaccination of children by end of age 12 years, by Arizona demographic groups based on household, income, education, and racial profiles
aThis figure illustrates the relationship between the lower ORs in demographic groups 1, 2, and 8 as opposed to ORs in the other demographic groups. These three groups represent the demographic groups with overall highest poverty rates, more children per family, lowest educational attainment, and highest proportion of Native Americans with fewer white people. The ORs are for either the odds of an on-schedule (by end of age 12 years) meningococcal vaccination following the Arizona statewide rule change in 2008 requiring vaccination (dark gray bars), or the odds of an on-schedule vaccination (by end of age 12 years) given by a private provider vs. a public provider (light gray bars).
OR = odds ratio
Logistic regression using patient-level variables (e.g., age at vaccination, vaccination date, and provider type) found that provider type explained some of the variation in vaccination uptake rates; i.e., a patient’s odds of an on-schedule vaccination by a private provider were 1.5 to 4.5 times that of public providers (data not shown).
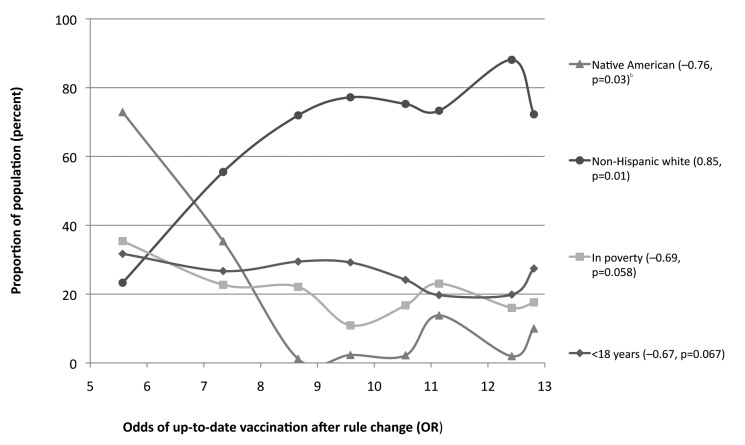
To explore which socioeconomic factors were associated with the lower odds of up-to-date vaccination following the rule change, we compared each group’s OR against several demographic characteristics. We found that groups with a higher percentage of Native Americans, lower percentage of white people, more children younger than 18 years of age, and higher percentage of people living in poverty were associated with a lower OR. Figure 3 shows that as the proportion of non-Hispanic white people increases, the odds of having an up-to-date vaccination following the rule change also increases.
Figure 3.
Associations between demographic profiles and odds ratios for meningococcal vaccination of children by end of age 12 years, following the rule changea in Arizona
aA 2008 statewide rule change required meningococcal vaccination for school entry at sixth grade.
bPearson’s correlation coefficient p-value at 95% confidence interval
OR = odds ratio
DISCUSSION
Meningococcal vaccination rates increased during the study period, suggesting that 2008 adolescent school immunization requirements were successful in improving vaccine coverage. Similar results were found in a study of North Dakota adolescent immunization rates12 and in a study about hepatitis A vaccination uptake in Arizona.13 Concurrent activities—an educational campaign launched after the rule change,9 increasing provider focus on adolescent immunizations, and communication improvements surrounding meningococcal vaccine—may have also influenced trends in immunization rates.
We observed differences in vaccination coverage when using registry vs. Census-derived populations. In 2010, the U.S. Census Bureau reported 89,797 total 11-year-old children and 89,061 total 12-year-old children in Arizona.14 Closely corresponding year and birth date criteria (as of September 1, 2009) showed that ASIIS contained 139,747 records of 11-year-olds and 138,634 total 12-year-olds. Registry population overestimates have been observed in other states12 and may be explained by children who have left the state but remain active in the registry, an issue identified by the American Immunization Registry Association.15 Vaccine coverage estimates are higher when using the Census population because it is 60% of the registry’s population estimate. This discrepancy is a significant issue regarding the use of registry data vs. Census data. Because the Census Bureau lacks detailed data (by year, county, and age in a given year) for non-decennial Census years, we used registry information for vaccination coverage calculations and logistic regression analysis.
Our analysis revealed that patients were more likely to receive a meningococcal vaccination by end of age 12 years from private rather than public providers following the vaccination requirement change. Patients served by public providers may be less likely to have a medical home and have less adequate insurance coverage, resulting in lower odds of on-schedule immunization. However, to suggest that there is a reliance on private providers would be an oversimplification of very complex interactions among factors including socioeconomic status, insurance coverage, and availability of health care.
Communities with a greater proportion of individuals having a high school education, higher median household incomes, and a larger proportion of non-Hispanic white residents had the best odds of on-schedule vaccination following the requirement change. That is, the requirement change had less impact on vaccination rates in poorer communities with higher Native American populations and fewer high school graduates. Lower income, lower family education level, and nonwhite race have all been identified as risk factors for underimmunization in other national studies,16 and our findings suggest that these communities might also exhibit less reaction to state vaccination requirement mandates.
The observed difference in responses to requirement changes may be due to a stronger initial response to vaccine recommendations as opposed to requirements. In fact, demographic groups with lower observed response to the requirement change actually had higher immunization coverage prior to the requirement change. This finding suggests that there was a response by these communities to the initial ACIP recommendation in 2005,1 which leads to additional questions about the proportion of public providers who responded to the initial recommendation and differences between responses of public and private providers to recommendations vs. requirements. These questions merit further exploration, as they could impact future targeting of educational campaigns aimed at providers in Arizona.
Strengths and limitations
This study had several strengths and limitations. ASIIS offers a large dataset from a well-established surveillance system; in 2009, at least 95% of 19- to 35-month-olds in Arizona had two or more immunizations recorded in ASIIS.17 Data extracted from ASIIS were invaluable in completing this study, although systematic biases are present within this passive surveillance system. One limitation was the population overestimate observed in the immunization registry.9 Another limitation was that missing or incomplete records may have been present. Arizona does not currently conduct statewide reminder recalls, which may help in identifying children who no longer live in a certain jurisdiction. Still, the exceptional statewide coverage of ASIIS,17 the presence of individual-level rather than aggregated data, and data availability make it a sound choice for immunization coverage research. Further exploration of the issue of population overestimates will lead to analytically sound ways to address this challenge.
The NIS-Teen surveyed nationally a total of 2,947 adolescents aged 13–18 years in 2007, 17,835 in 2008, 20,066 in 2009, and 19,257 in 2010.4,5,18,19 In contrast, the 2011 ASIIS dataset used in our study contains a total of 816,980 Arizonans aged 13–18 years (born 1993–1999). The sheer number of patients within ASIIS demonstrates the power of using state immunization registries to investigate trends in vaccine uptake and coverage.
The use of ASIIS data allows for a population-level assessment and flexibility in the analyses that cannot be achieved using other vaccination data sources. Our study employed the use of PCA and OR methods that are more robust than simple rates, even with the inflated denominators in the ASIIS data. Our novel approach was useful for identifying population-level factors associated with changes in vaccination coverage estimates for jurisdictions smaller than the state level and between communities with very different demographic compositions.
We assigned ASIIS records to counties based on patient ZIP code, acknowledging several limitations. Some ZIP codes span several counties; in these cases, the record was assigned to the most populous county, potentially leading to inaccuracies in coverage estimates. Future analyses examining provider or school location may identify additional geographic patterns in vaccine uptake.
More extensive analyses should examine additional factors such as the year the child entered sixth grade, provider demographics, the child’s school, and differences in school practices regarding immunization requirements and exemptions. Because of imperfect data, we used several proxies in our analysis, including age, to estimate entry into the sixth grade. We also used the provider identified as “owner” as the provider of record for the child. This proxy cannot account for instances when a child has moved from one provider/owner to another between the date of meningococcal vaccine administration and when the data were extracted from ASIIS.
More detailed information from additional datasets could bolster future analyses. For example, we could account for children exempt from the immunization requirement, and although most sixth-grade exemptions in 2010 in Arizona were religious/philosophical rather than medical (3,026 of 3,428),20 examining these data will add valuable information for future public health initiatives. School district-level data and detailed demographic data on providers will facilitate exploration into other important areas that may influence immunization coverage. In addition, an exploration of the factors responsible for artificial denominator inflation observed in ASIIS might suggest ways to better account for that inflation in analyses.
CONCLUSIONS
This study offers a unique presentation of important population-level information about changes in vaccine coverage in Arizona in response to a new statewide meningococcal vaccination mandate. Our study made use of ASIIS, a rich and valuable data source, and used novel methods that allowed for flexible analyses of changes to coverage estimates. We also identified demographic characteristics of populations that may be less likely to respond to state mandates for vaccinations. The methods we used may be useful to other immunization programs in which similar initiatives and rules may be under consideration, or where such programs have been implemented but whose results have not yet been measured.
Immunization data from Arizona’s registry, ASIIS, were useful in conducting a descriptive analysis of vaccine coverage for meningococcal vaccine in Arizona and in examining the impact of statewide policy changes in Arizona on the odds of on-schedule vaccination. Meningococcal vaccine coverage increased after ACIP recommendations were released, and again after school-entry requirements were changed in all Arizona demographic populations, but in varying magnitude associated with demographic compositions. The observed differences in response to the school requirement due to demographic factors are important and can help immunization programs effectively target educational messages and/or resources to support adherence to requirements. Issues regarding registry overestimates of the total population may lead to systematic errors in estimating vaccination coverage in a population; estimates should be reserved for understanding trends over time as opposed to an absolute measure of coverage. More work is necessary to determine the most appropriate methods for handling this bias inherent in immunization data sources.
Footnotes
The authors thank Patty Gast at the Arizona Department of Health Services (AZDHS) for her expertise and helpful feedback. The AZDHS determined that this study used de-identified data and was exempt from Institutional Review Board review.
References
- 1.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 2.Hall-Baker PA, Nieves E, Jr, Jajosky RA, Adams DA, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2007 [published erratum appears in MMWR Morb Mortal Wkly Rep 2009;58(34):954] MMWR Morb Mortal Wkly Rep. 2009;56(53):1–94. [Google Scholar]
- 3.Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56(31):794–5. [PubMed] [Google Scholar]
- 4.National state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–23. [PubMed] [Google Scholar]
- 5.National state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–23. [PubMed] [Google Scholar]
- 6.Arizona Department of Health Services, Arizona Immunization Program Office. Arizona State Immunization Information System. [cited 2011 Dec 18]. Available from: URL: http://www.azdhs.gov/phs/asiis.
- 7.Arizona Rev. Stat. §36-135 (1998) [cited 2011 Nov 4]. Available from: URL: http://www.azleg.state.az.us/ars/36/00135.htm.
- 8. Arizona Administrative Code. Tit. 9, Ch. 6, R9-6-702 (2008) [Google Scholar]
- 9.Coronado F, Kenney J, Rigler J, Fredrickson K, Lyons C, Cory J, et al. It’s Their Turn': an educational and awareness initiative to promote adolescent immunization in state and local health departments. Abstract presented at the National Immunization Conference; 2009 Mar 31-Apr 2; Dallas, Texas. [Google Scholar]
- 10.Jolliffe IT. Principal component analysis. 2nd ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 11.Adbi H, Williams LJ. Principal component analysis. WIREs Comp Stat. 2010;2:433–59. [Google Scholar]
- 12.LoMurray K, Sander M. Using the North Dakota Immunization Information System to determine adolescent vaccination rates and uptake. Public Health Rep. 2011;126(Suppl 2):78–86. doi: 10.1177/00333549111260S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst KC, Pogreba-Brown K, Rasmussen L, Erhart LM. The effect of policy changes on hepatitis A vaccine uptake in Arizona children, 1995-2008. Public Health Rep. 2011;126(Suppl 2):87–96. doi: 10.1177/00333549111260S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Census Bureau (US). 2010. census summary file 1, QT-P2—Arizona: single years of age and sex. [cited 2011 Dec 18]. Available from: URL: http://www2.census.gov/census_2010/04-Summary_File_1.
- 15.American Immunization Registry Association. Atlanta: AIRA; 2005. [cited 2011 Dec 18]. Management of moved or gone elsewhere (MOGE) status and other patient designations in immunization information systems: recommendations of the American Immunization Registry Association (AIRA) Modeling of Immunization Registry Operations Workgroup (MIROW) Also available from: URL: http://www.immregistries.org/pubs/mirow.html. [Google Scholar]
- 16.Lowery NE, Belansky ES, Siegel CD, Goodspeed JR, Harman CP, Steiner JF. Rural childhood immunization: rates and demographic characteristics [published erratum appears in J Fam Pract 1999;48:503] J Fam Pract. 1998;47:221–5. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (US) IIS interactive data query tool: immunization information systems annual report data. 2009. [cited 2011 Dec 26]. Available from: URL: http://www2a.cdc.gov/nip/registry/IISAR/IISAR_QUERY.asp.
- 18.Vaccination coverage among adolescents aged 13-17 years— United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(40):1100–3. [PubMed] [Google Scholar]
- 19.National state, and local area vaccination coverage among adolescents aged 13-17 years— United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;58(36):997–1001. [PubMed] [Google Scholar]
- 20.Arizona Department of Health Services. Arizona immunization exemption rates by facility/grade. [cited 2012 Aug 25]. Available from: URL: http://www.azcentral.com/ic/pdf/business/AZ-ImmunizationExemptions-July-2011.pdf.