The purpose of the Perspectives in General Physiology is to provide a forum where scientific uncertainties or controversies, or important problems, are discussed in an authoritative, yet open manner. The Perspectives are solicited by the editors—often based on recommendations by members of the editorial advisory board. To frame the issue, two or more experts are invited to present brief points of view on the problem; these are published consecutively in the Journal. One or more experts and the organizer review the contributions, but the comments and opinions expressed in the Perspectives are those of the authors and not necessarily those of the editors or the editorial advisory board. The Perspectives are accompanied by a few editorial paragraphs that introduce the problem and invite the submission of comments, in the form of letters to the editor, which usually are published four months after publication of the Perspectives. After the letters to the editor have been published, further responses are limited to full manuscripts.
In this issue of the Journal, David Colquhoun and Remigijus Lape (University College, London), Sandipan Chowdhury and Baron Chanda (University of Wisconsin-Madison), and Frank T. Horrigan (Baylor College of Medicine) provide three different perspectives on conformational coupling in ligand- and voltage-gated channels.
After seminal contributions by A.V. Hill (1910) and J. Wyman (Wyman and Allen, 1951), the modern era of conformational coupling in proteins (sometimes denoted allosteric coupling) began in the mid-1960s with the work of Monod et al. (1965) and Koshland et al. (1966), who developed theoretical frameworks, the Monod–Wyman–Changeux (MWC) and Koshland–Némethy–Filmer (KNF) models, respectively, for describing the cooperative binding of ligands to oligomeric proteins (see Edsall, 1980, for a historical tracing of the concept of allosteric coupling).
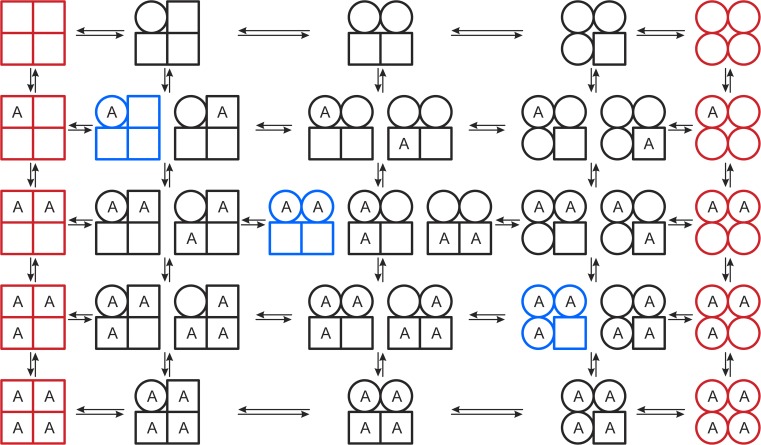
The MWC and KNF models are special cases of a more general model (Eigen, 1968), which is depicted in Fig. 1 for the case of agonist (A) binding to a tetrameric protein. (The general model was examined in detail by Herzfeld and Stanley, 1974.) The key features of the general model are: first, each subunit exists in two conformations (traditionally denoted T and R, which in Fig. 1 are represented by squares and circles, respectively); second, the agonist has higher affinity for the R than for the T conformation, such that agonist binding shifts the T↔R equilibrium toward R; and third, the T↔R equilibrium for a subunit is determined also by the conformational state of its neighbors. The MWC model represents the limit where the T↔R equilibrium is constrained by molecular symmetry, thus precluding the existence of hybrid oligomers having both T and R subunits, which leads to a model represented by concerted quaternary conformational transitions between the red columns at the extreme left and right in Fig. 1. The KNF model represents the limit where the T↔R equilibrium is tightly coupled to agonist association/dissociation, with no quaternary constraints, which leads a linear model represented by the top/left and the bottom/right states plus the blue oligomers on the top/left to bottom/right diagonal in Fig. 1. Both models provide for good fits of experimental data of ligand binding to oligomeric proteins; the number of parameters needed to describe a binding curve, however, will in general be higher for the KNF model than for the MWC model.
Figure 1.
A general scheme for allosteric binding of a ligand (A) to a tetrameric protein, where each subunit can exist in two states: T, represented by squares, and R, represented by circles. This general model encompasses both the MWC model, which is represented by quaternary conformational transitions between the red columns at the extreme left and right in the scheme, and the KNF model, which is represented by the top/left and the bottom/right red states plus the blue oligomers on the top/left to bottom/right diagonal. (The scheme does not include states that differ only in placement of A in the T or R subunits of a state with n T and [4 – n] R subunits [0 ≤ n ≤ 4], meaning that the complete scheme includes 44 states, rather than the 36 depicted here.) Modified after Eigen (1968).
The MWC model soon was adapted to account for the agonist activation of the nicotinic acetylcholine receptors (nAChRs) (Karlin, 1967), and a version of the KNF model, in which the T↔R equilibrium varies as a function of the membrane potential difference, has been extensively used to account for the voltage activation of voltage-dependent channels. The key experimental evidence in favor an MWC-like model for nAChR gating, however, was not obtained until the mid-1980s, when M.H. Jackson (1984, 1986) recorded spontaneous openings of un-liganded nAChRs and quantified the open probability of the un-liganded channel, ∼3 × 10−7. These accomplishments, which were possible only because of the single-molecule resolution provided by the patch clamp, seem to be the first direct evidence for an MWC-type mechanism.
Both the MWC and KNF models describe the equilibrium behavior of the system—the energetic coupling between different states, such as the difference in the free energy of ligand binding to the open- and closed-channel states that is required to change the nAChR open–closed equilibrium from that of the un-liganded channel to 1, ∼9 kcal/mole using Meyer Jackson’s data. These models provide important constraints on how the system operates, but they provide less information about how the two states are coupled and how the binding energy is transmitted to the gate. This question is perhaps best addressed using the general Eigen scheme (Fig. 1), in which ligand binding causes a tertiary conformational change in the subunit, which then propagates to adjacent subunits (or, maybe, domains within a single subunit). That is, the different states are likely to be coupled through the transition landscape that connects the different major states, a situation that is different from the one-step transition between the all-T and all-R oligomers in the MWC model. To survey the fine-structure of the transition landscape, it becomes necessary to examine the gating kinetics. There is indeed evidence for such fine structure. A. Auerbach and colleagues (Grosman et al., 2000; Auerbach, 2010) used rate-energy relations for the closed↔open transitions of amino acid–substituted nAChRs to identify clusters of amino acids that form transition “states,” separating short-lived intermediate states, in the landscape that has to be traversed (the so-called Φ value analysis). Furthermore, D. Colquhoun and colleagues (Burzomato et al., 2004; Lape et al., 2008) have provided for short-lived intermediate states, visible as brief closing transitions, in the gating of glycine receptors. These intermediate “transition” states, whether identified as clusters of amino acids with distinct Φ values or as brief transitions to nonconducting states, are likely to provide the key to how the different states are coupled.
In this series of Perspectives, Colquhoun and Lape focus on the gating of ligand-gated channels, including the intermediate states in the transitions between closed- and open-channel states. Then, Chowdhury and Chanda discuss the gating of voltage-dependent channels, using both MWC-like and KNF-like schemes to dissect the mechanisms by which the voltage sensors may be coupled to the channel pore. Finally, Horrigan considers the situation of channels that are gated by both ligands and voltage, which introduces a new dimension into the description and suggests specific models for the coupling of voltage-sensor movement to the pore gate domain.
Letters to the editor related to these Perspectives should be received no later than Friday, February 1, 2013. The letters may be no longer than two printed pages (approximately six double-spaced pages) and will be subject to editorial review. They may contain no more than one figure, no more than 15 references, and no significant references to unpublished work. Letters should be prepared according to The Journal’s Instructions and can be submitted electronically at http://www.jgp.org.
References
- Auerbach A. 2010. The gating isomerization of neuromuscular acetylcholine receptors. J. Physiol. 588:573–586 10.1113/jphysiol.2009.182774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzomato V., Beato M., Groot-Kormelink P.J., Colquhoun D., Sivilotti L.G. 2004. Single-channel behavior of heteromeric alpha1beta glycine receptors: an attempt to detect a conformational change before the channel opens. J. Neurosci. 24:10924–10940 10.1523/JNEUROSCI.3424-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall J.T. 1980. Hemoglobin and the origins of the concept of allosterism. Fed. Proc. 39:226–235 [PubMed] [Google Scholar]
- Eigen M. 1968. New looks and outlooks on physical enzymology. Q. Rev. Biophys. 1:3–33 10.1017/S0033583500000445 [DOI] [PubMed] [Google Scholar]
- Grosman C., Zhou M., Auerbach A. 2000. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 403:773–776 10.1038/35001586 [DOI] [PubMed] [Google Scholar]
- Herzfeld J., Stanley H.E. 1974. A general approach to co-operativity and its application to the oxygen equilibrium of hemoglobin and its effectors. J. Mol. Biol. 82:231–265 10.1016/0022-2836(74)90343-X [DOI] [PubMed] [Google Scholar]
- Hill A.V. 1910. The possible effects of the aggregation of the molecules of hæmoglobin on its dissociation curves. J. Physiol. 40:4–7 [Google Scholar]
- Jackson M.B. 1984. Spontaneous openings of the acetylcholine receptor channel. Proc. Natl. Acad. Sci. USA. 81:3901–3904 10.1073/pnas.81.12.3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.B. 1986. Kinetics of unliganded acetylcholine receptor channel gating. Biophys. J. 49:663–672 10.1016/S0006-3495(86)83693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. 1967. On the application of “a plausible model” of allosteric proteins to the receptor for acetylcholine. J. Theor. Biol. 16:306–320 10.1016/0022-5193(67)90011-2 [DOI] [PubMed] [Google Scholar]
- Koshland D.E., Jr, Némethy G., Filmer D. 1966. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 5:365–385 10.1021/bi00865a047 [DOI] [PubMed] [Google Scholar]
- Lape R., Colquhoun D., Sivilotti L.G. 2008. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 454:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.P. 1965. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12:88–118 10.1016/S0022-2836(65)80285-6 [DOI] [PubMed] [Google Scholar]
- Wyman J., Allen D.W. 1951. The problem of the heme interactions in hemoglobin and the basis for the Bohr effect. J. Polym. Sci. B Polym. Phys. 7:499–518 [Google Scholar]