What does “allosteric” mean?
In the context of receptors, the word allosteric is now widely used. It is, perhaps, not helpful for clarity of thought that different authors often use it to mean somewhat different things (Colquhoun, 1998).
At one extreme, the term “allosteric antagonist” can often be translated as “we have got an antagonist and we are not sure what it does, but it appears not to be competitive.” This means much the same as noncompetitive, a word which pharmacologists had always supposed to mean action at a different site, though with no postulate as to how the effect was mediated. In fact, noncompetitive usually means nothing more than not competitive, and therefore says nothing about mechanisms.
At the other extreme, Monod et al. (1965) gave a sharply delimited definition, which is as follows (slightly paraphrased for brevity): (a) allosteric proteins are oligomers, the protomers of which are associated in such a way that they all occupy equivalent positions; (b) there is one site on each protomer, for each ligand that can combine with it; (c) the conformation of each protomer is constrained by its association with other protomers; (d) two [conformational] states are accessible to allosteric oligomers; (e) as a result, the affinity of one (or several) of the sites toward the corresponding ligand is altered when a transition occurs from one to the other state; (f) when the protein goes from one state to another state, its molecular symmetry is conserved.
The term allosteric (allos = other, different; stereos = solid) was introduced by Monod and Jacob (1961) who said, in a discussion of end-product inhibition, “From the point of view of mechanisms, the most remarkable feature of the (inhibition of the synthesis of a tryptophan precursor by tryptophan) is that the inhibitor is not a steric analogue of the substrate. We therefore propose to designate this mechanism as ‘allosteric inhibition.’”
At this earlier stage, the word allosteric meant little other than what pharmacologists would have referred to as noncompetitive antagonism. Soon afterward, Monod et al. (1963) said, concerning such noncompetitive regulation of enzyme activity, “The effect of these regulatory agents appears to result exclusively from a conformational alteration (allosteric transition) induced in the protein when it binds the agent.” This shifted the emphasis toward the central role of conformation changes, as first postulated by Wyman and Allen (1951). This emphasis culminated in the influential paper by Monod et al. (1965; see also Changeux [1993] for an account of the background).
Probably the nearest thing there is to a consensus at the moment is that allosteric refers to any mechanism in which a protein can exist in two (or more) distinct conformations that differ in their affinity for a ligand. This usage has been endorsed by Wyman and Gill (1990). An allosteric regulator is anything that binds better to one conformation than the other (i.e., almost everything). Although the definition of Monod et al. (1965) explicitly limits the term to oligomeric molecules that show cooperativity, it is now common to use the term for monomeric receptors that do not fall into this category.
In what sense can agonist activation of a channel be described as allosteric?
The original definition of allosteric involved two ligands. In the case of agonist activation of an ion channel, we have only one. But selective binding to one conformation describes just what we want. If the agonist has a higher affinity for the open channel than for the shut channel, then the equilibrium will be shifted toward the open channel. This is just a consequence of the principle of Le Châtelier (1850–1936). To that extent, there is nothing very new about the idea, though its first explicit application to ligand binding by proteins didn’t occur until the prescient paper by Wyman and Allen (1951). They were, as far as we know, the first people to suggest that selective affinity for two different global conformations of a protein (hemoglobin) is what was responsible for the ability of small ligands to switch the conformation of the protein to the form with higher affinity.
Since then, there have been two extreme views of conformation changes in large proteins. At one extreme, the view has been that proteins can exist in a very large number of conformations, with many different paths that can lead from one stable conformation to another. Although this may be strictly true, it precludes any detailed analysis of mechanisms: it amounts to throwing up your hands and saying it’s just too complicated. At the other extreme, the approach has been that of classical chemical kinetics, which postulates that the system can exist in a limited number of discrete conformations, with transitions from one state being essentially instantaneous (on the observable time scale). These discrete states consist of minima in the energy landscape: wells that are sufficiently deep that, when the protein is in one of them, it will stay there for long enough to be detected. Of course, there will be a near-infinite number of microstates, as well as the main energy minima, but as long as these transitions between them are fast enough that the microstates are close to being at equilibrium (on the time scale of experimental measurements), they don’t alter the prediction that both macroscopic and single-channel measurements will be described by exponential components (Colquhoun and Hawkes, 1982).
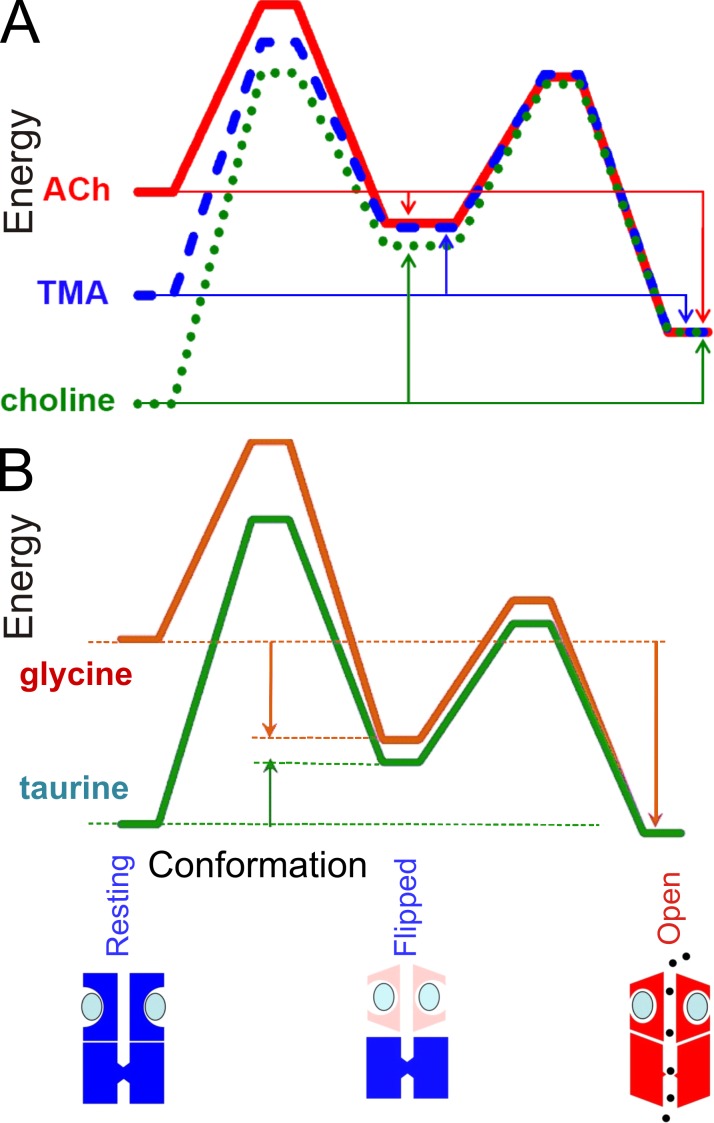
All of the arguments used here can be put in terms of energies rather than in terms of rate constants and equilibrium constants. These two ways of expressing results are exactly equivalent. For some purposes, energies (see Fig. 4) are more convenient (e.g., for comparison with bond energies), but for most purposes we prefer to think in terms of rate constants, which have a simple physical interpretations, e.g., as the number of transitions per second (see section entitled A numerical example).
Figure 4.
Energy diagrams. Energy diagrams that show diagrammatically the changes in free energy that accompany transitions between the three states in which fully liganded receptors can exist (resting, flipped, and open) for muscle nicotinic (A) and glycine (B) receptors. Diagrams are shown for the full agonists (ACh in A, glycine in B), and for partial agonists. They are aligned vertically on the energy of the open state. The nearly exact superimposition of the results for the gating step shows this step is much the same for all agonists. The differences between agonists lie largely in the transition from resting to flipped states. The vertical arrows represent the energy changes (equilibrium constants) for transitions between the three conformations. For full agonists (acetylcholine, glycine) the transition from resting to flipped conformation is downhill, whereas for less efficacious agonists (TMA, choline, taurine) it is uphill.
From this point forth in the article, we shall assume that discrete states provide a description of the activation of ligand-gated ion channels that is sufficiently accurate to be useful in understanding what’s happening. With that assumption, the tasks to be tackled become the following: (a) determine how many open and shut states exist, and how they are connected, and (b) make estimates of the transition rates between each pair of states.
If these aims can be achieved it becomes possible to predict the response to agonist under any conditions. For example, if the mechanism and values for rate constants are obtained from single-ion channel recordings in the steady state, it is possible to calculate the macroscopic response to any applied agonist concentration (the calculation is simplest for step changes in agonist concentration but it can be done for any time course, so synaptic currents can be predicted; Burzomato et al., 2004). Just about anything else can be predicted too. For example, the noise spectrum can be predicted, as can the single-channel behavior in other types of experiment, e.g., single channels after a concentration jump (Colquhoun et al., 1997).
Development of kinetic mechanisms
Ligand-gated ion channels have proved to be easier to analyze in detail than voltage-gated channels. There are two reasons for this. First, concentration has a real zero (the electrochemical equivalent of zero concentration is a membrane potential of minus infinity). Second, we know a priori the nature of some of the shut states (those with 0, 1, 2,… ligands bound).
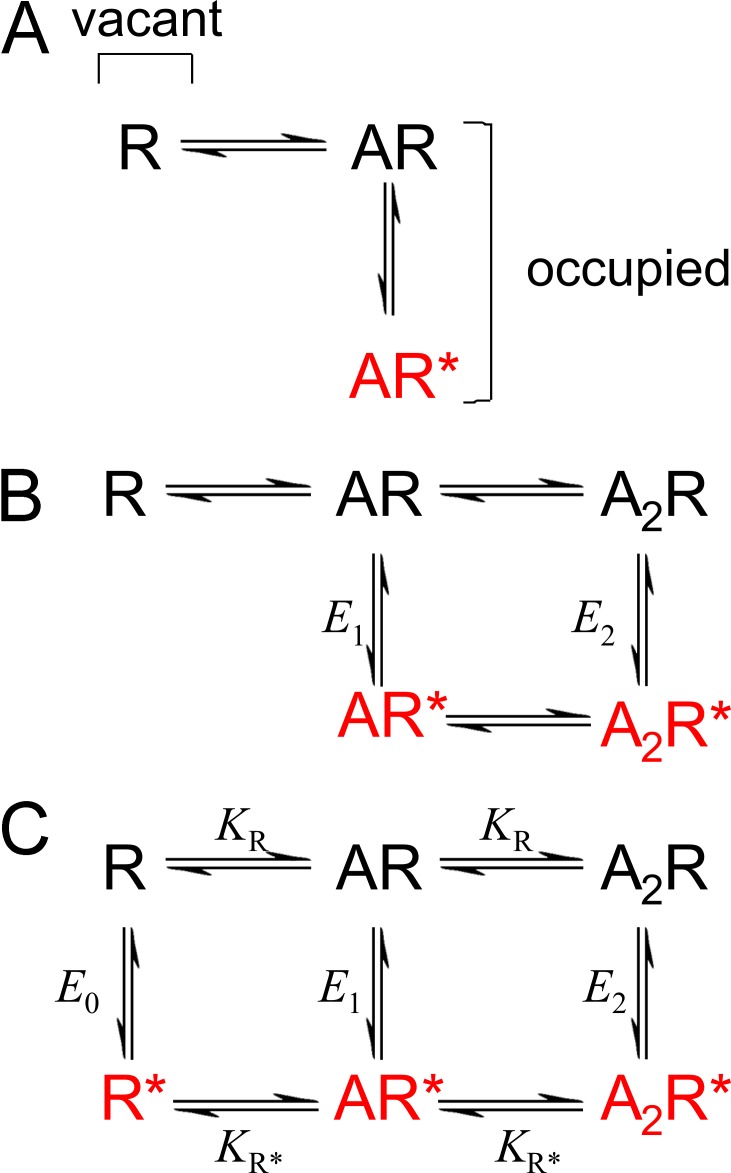
The first applications of discrete-state kinetics to drug receptors can be traced back to A.V. Hill and A.J. Clark in the 1920s and 1930s (Colquhoun, 2006). But it was Del Castillo and Katz (1957) who can lay claim to the first workable mechanism for an agonist-activated channel. Their explanation for the existence of partial agonists lay in the first explicit distinction between the binding of agonist and the subsequent conformation change (Fig. 1 A). In this mechanism, the affinity of the agonist for the open conformation was supposed to be essentially infinite.
Figure 1.
Classical mechanisms. (A) The mechanism proposed by Del Castillo and Katz (1957): the first explicit separation of binding and gating. (B) A typical example of the type of mechanism used to describe the nicotinic receptor, with two subunits, and monoliganded openings as well diliganded. (C) The MWC mechanism. All three models postulate the existence of only two conformations of the channel protein, shut (black) and open (red).
As information about the nicotinic receptor accumulated, the Del Castillo-Katz mechanism was generalized to allow for the fact that the nicotinic receptor has two binding sites. When single-channel methods were developed, it became possible to incorporate into the mechanism two binding affinities (not necessarily equal) and to resolve mono-liganded openings (Colquhoun and Sakmann, 1981). A typical mechanism of this sort is shown in Fig. 1 B.
Colquhoun and Sakmann (1985) used single-ion channel recording to make estimates of all the rate constants for this scheme. Despite the fact that maximum likelihood fitting methods didn’t exist in 1985, the results have held up well. Until 2004, variants of this mechanism were used universally to describe the results obtained with the nicotinic acetylcholine receptor and the glycine receptor.
Almost everything we know now about the energetics of gating in ligand-activated channels is based on kinetic analysis of single channels. The ability to discriminate between rival mechanisms, and the number of rate constants that can be estimated from data, are far greater than with other methods.
Unliganded openings and the Monod-Wyman-Changeux (MWC) mechanism
It’s obvious from the Boltzmann distribution law that unliganded channel openings can occur. But the energetics of wild-type receptors means that they are very rare. Consequently, the full MWC mechanism (Fig. 1 C) can be fitted only to gain-of-function mutant receptors that show more frequent spontaneous opening in the absence of agonist.
There are at least two respects in which the MWC mechanism provides a poor fit. It predicts that there will be only one shut state and one open state at saturating concentrations, and it predicts that there will be only one open state at zero concentration. Neither of these is true, at least for some mutants, though the deviations are more prominent for the glycine receptor than they are for the nicotinic receptor. Grosman and Auerbach (2000) were the first to show that unliganded channel openings in some spontaneously active mutant nicotinic receptors showed complex bursting activity which resembled that seen in fully liganded channels, a result that was very surprising at the time and which cannot be described by MWC. The number of shut states in MWC is clearly not enough to fit all of the observations. In our study, we show various ways in which more shut states can be added to remedy this deficiency.
In the case of the nicotinic receptor, the MWC model has been used as an approximate description of the channel gating (Auerbach, 2012). Insofar as the MWC approximation is adequate, Auerbach and his colleagues were able to estimate the equilibrium constant, E0, for opening of the unliganded wild-type nicotinic receptor. They used two different methods, and the results show impressive agreement. E0 = 7 × 10−7. So an unliganded wild-type nicotinic receptor would have a probability of being open of less than 1 in a million. These estimates depend on three quite strong assumptions: (1) the effect of each separate mutation on E0 was energetically independent of the others; (2) that all the cycles in the mechanism obey microscopic reversibility; and (3) none of the mutants that are used affect the relative binding to shut and open conformations, so the effect of the mutations was attributable entirely to change in E0.
Their results were roughly in accordance with these assumptions, so their conclusion that the main effect of mutations is to change E0 is justified, to a first approximation. This has some plausibility because one would expect that a change in structure produced by a mutation (away from the binding site) would occur in much the same way whether or not an agonist was bound (assumption 3). However, many observations on the nicotinic receptor (including some of Auerbach’s) are not consistent with the basic MWC mechanism (Fig. 1 C), although the inadequacy of MWC is much more obvious with the glycine receptor, which shows more openings with less than full liganding than nicotinics. It seems unlikely that most mutations will leave relative binding affinities unchanged; certainly there is no direct evidence for this. Neither is microscopic reversibility a law of nature (assumption 2; see the section entitled A numerical example).
Intermediate shut states: Flip and primed mechanisms
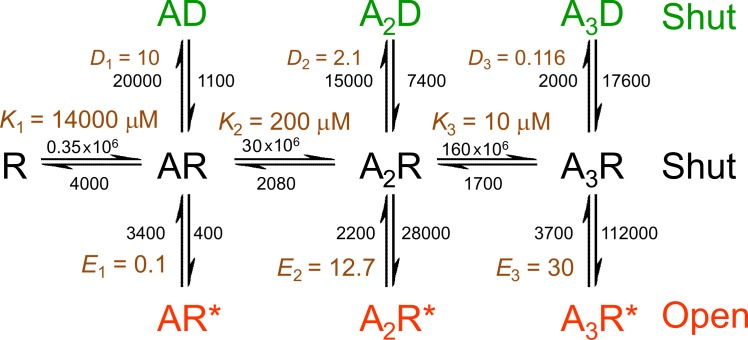
Although mechanisms such as those in Fig. 1 B provided a reasonably good description of the nicotinic receptor, quite often the fit was improved by introduction of another shut state. This was commonly added as a short-lived shut state that followed the open state, like a short-lived desensitized state (Salamone et al., 1999; Hatton et al., 2003). There was no independent reason to believe in the physical existence of such a state: it was introduced in an arbitrary way to improve the fit. This is unsatisfactory because kinetic mechanisms are of value only insofar as the postulated states have real physical existence. In the case of the glycine receptor, the inadequacy of mechanisms of the sort shown in Fig. 1 B was much more obvious than for the nicotinic receptor. A good fit could be obtained only by introducing three extra shut states, as in Fig. 2.
Figure 2.
A mechanism that can fit results with heteromeric glycine receptors. A Jones-Westbrook type of mechanism adapted for the heteromeric glycine receptor, with three binding sites. Three extra shut states are postulated, distal to open states (red). Although they are denoted as the D conformation (green), they bear no obvious relationship to macroscopic desensitization in this case. Rate constants are marked in black for each transition, and equilibrium constants are marked in brown. The values are means of fits to three sets of data, taken from Table 4 of Burzomato et al. (2004). Note that equilibrium constants for each set were averaged, rather than calculated from the averaged rate constants. Therefore, the ratio of the mean rate constants will not generally be the same as the mean equilibrium constant, especially when the scatter between sets is large (e.g., for D1).
The mechanism in Fig. 2 gave a good fit to observation with the heteromeric glycine receptor, but it has two problems. One is that the extra shut states are totally arbitrary: there is no independent reason to believe they exist. The second objection is that the fit with mechanism of Fig. 2 suggested that the three sequential bindings to the resting conformation showed strong cooperativity: the first binding appeared to be low affinity (K1 = 14 mM), when one site is already occupied the second binding appeared tighter (K2 = 0.2 mM) and the third even tighter (K1 = 0.01 mM). Although not impossible, this seems quite unlikely because the binding sites are quite a long distance from one another and the mechanism postulates no global conformation change while still in the resting conformation, so one would not expect a binding site to be able to sense when other sites are occupied.
Both of these objections can be overcome if the three extra shut states are supposed to lie between the resting conformation and the open state, as shown in Fig. 3 (Burzomato et al., 2004). The transitions are labeled with the estimated rate constants, and the corresponding equilibrium constants.
Figure 3.
The flip mechanism for a glycine receptor with three binding sites. Three extra shut states are postulated, as in Fig. 2, but they are now located between the resting conformation (black) and the open conformation (red), so they represent an intermediate conformation (the flipped conformation, green) that must be adopted before the channel can open. The values are means of fits to three sets of data, taken from Table 5 of Burzomato et al. (2004; see legend of Fig. 2 for description of averaging). The unliganded flipped and open conformations are shown for completeness, but they are grayed out because the observations on wild-type receptors contained no information about these states.
The mechanism in Fig. 3, dubbed the flip mechanism, has, like Fig. 2, three extra shut states. But unlike Fig. 2, the extra shut states are postulated to lie between the resting conformation and the open conformation. The intermediate flipped conformation therefore represents a state which, while still shut, is such that opening can occur. Flipping must occur before opening is possible. It is an appealing feature of this mechanism that a good fit to the data can be obtained without having to postulate any cooperativity in the binding to an individual conformation. The microscopic equilibrium constant is postulated to be the same for the first, second, and third binding to the resting conformation. Likewise the equilibrium constant for binding to a vacant site on the intermediate (“flipped”) conformation is assumed to be the same regardless of how many other sites are occupied.
In other words, the shut states in Fig. 3 are exactly as postulated in the Monod et al. (1965) mechanism. The appearance of cooperativity arises from the fact that the flipped conformation has a higher affinity for the agonist than the resting conformation, rather than from interaction between binding sites. The only difference in Fig. 3 is that the unoccupied flip state has been omitted for the purposes of fitting results with wild-type receptors (hence it is showed grayed out). Such a state must exist: it is omitted from the fitted mechanism only because unliganded openings are not detectable in the wild-type glycine receptor so the recordings contain no information about it.
For mutant receptors that show openings in the absence of agonist, it would obviously be necessary to include the missing states in Fig. 3 (unliganded flipped and open states). More importantly, the flip mechanism in Fig. 3 assumes that all subunits flip simultaneously—a concerted transition—even if only one glycine molecule is bound. This is the least plausible part of the mechanism, though if flipping of other subunits is rapid once the first has flipped then flipping might appear to be concerted.
Notice that the flipped conformation is supposed to be shut, so the time spent (on average about 8 µs for the wild-type glycine receptor) is not included in the rise-time of the channel-opening transition, as observed in the single-channel recording. This is still too fast to resolve.
Application of the flip model to partial agonists (Lape et al., 2008) provided a novel interpretation of partial agonism. Contrary to what had been assumed in the 50 years since Del Castillo and Katz (1957), the rate constants for the opening and shutting of the channel appeared to be much the same for both full and partial agonists. The reason that partial agonists are ineffective lay in the preopening flip step. This makes sense, because the change in conformation that occurs on flipping is likely to be close to the agonist-binding site, and therefore likely to be sensitive to the structure of the agonist. The results are shown as energy diagrams in Fig. 4, for both the nicotinic receptor (top) and the glycine receptor (bottom). The scaling of such diagrams is arbitrary (Andersen, 1999). They have been shifted vertically to align the energy of the open state. The near-perfect superimposition of the graphs for the flipped to open transition for full and partial agonists illustrates their very similar open and shutting rates. In contrast, the flipping reaction is downhill for full agonists but uphill for partial agonists.
A more general variant of the flip mechanism was proposed by Mukhtasimova et al. (2009). They were writing about the nicotinic receptor, which has two binding sites. They proposed that individual subunits flip independently, so, rather than there being only one flipped state for each degree of liganding, there are two (if the subunits are assumed to be equivalent). Either one or both of the ligand-binding subunits can be flipped. This makes sense: the least plausible aspect of the flip model is the postulate of concerted flipping. They referred to the change in conformation to an intermediate shut state as priming rather than flipping, but the idea is much the same.
Corringer et al. (2012) describe this mechanism as improbable, but they do so on the basis of principles, not data, and they have produced no alternative explanation of our observations, or those of Mukhtasimova et al. (2009).
Fig. 5 illustrates the fully primed mechanism for a receptor with two binding sites (it was first proposed for the nicotinic receptor). It has been known since 1981 (Colquhoun and Sakmann, 1981) that the nicotinic receptor, with low concentrations of acetylcholine, produces short openings, usually isolated, and bursts of longer openings. This has commonly been explained by attributing the short openings to monoliganded receptors and the long ones to diliganded receptors, though it has been known from the start that there tend to be more short openings at higher concentrations than are predicted by this hypothesis (Colquhoun and Sakmann, 1981, 1985).
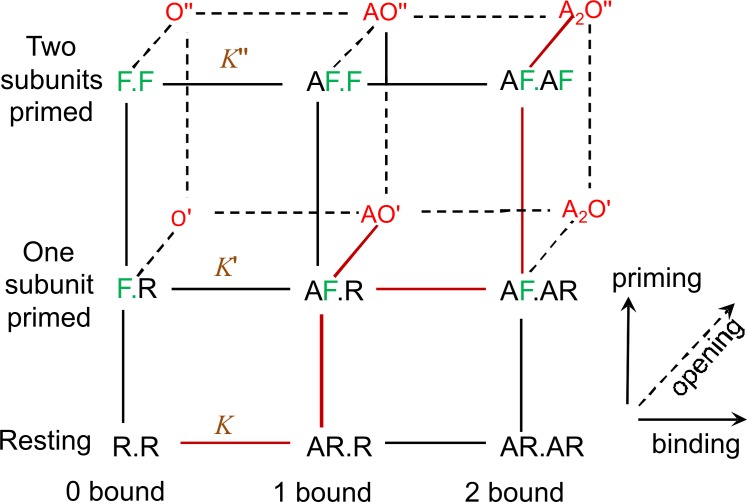
Figure 5.
The full primed mechanism. It is shown for a receptor with two binding sites, such as the nicotinic receptor (Mukhtasimova et al., 2009). The agonist is denoted A. The two binding subunits are shown as R.R when both are in the resting conformation, as R.F when one is primed/flipped (green) and as F.F when both are primed/flipped. Open states (red) are denoted O′ if one subunit is flipped/primed and O′′ if both are. Equilibrium constants for binding are shown in brown, with primes to indicate the number of primed subunits.
This view was upset by unexpected findings with mutant receptors which are active in the absence of any agonist. It was found that these too could produce both short isolated opens and bursts of longer opening, very like those elicited by agonists (Grosman and Auerbach, 2000 ; Grosman, 2003; Mukhtasimova et al., 2009; Purohit and Auerbach, 2009). All this makes sense if, as proposed by Mukhtasimova et al. (2009), the short openings result from single-primed channels rather than single-liganded channels, and long openings result from double-primed channels.
Linear free energy relationships
Support for flip/primed states come from pioneering work of Grosman et al. (2000), reviewed by Auerbach (2007). By use of linear free energy relationships, they postulate a series of intermediate states between the resting conformation and the open conformation. By making many hundreds of mutants, they identified domains of the nicotinic receptor in which all amino acids had similar Φ values. This suggested that each of these domains moves as a block during the opening process. Furthermore, there was a very plausible gradient in Φ values which they interpreted as meaning that part of the molecule close to the binding site moved earlier in the opening process than parts that are close to the channel gate. It seems that there was a “conformational wave” (Grosman et al., 2000) that started at the binding site and culminated with channel opening. The opening itself must occur very rapidly because channel openings have an irresolvably fast rise time: the actual opening of a channel is indistinguishable from an instantaneous conformation change.
The passage through the postulated intermediate states was too fast to allow anything to be inferred about rates of transitions between them. However the analysis methods used in Auerbach’s laboratory, particularly SKM idealization, do not allow estimation of fast rates directly from data. So it remained possible that one or more of the intermediate states might last for sufficiently long to be detected in experimental measurements. That was done first for the glycine receptor where it is more obvious (Burzomato et al., 2004). But the nicotinic receptor seems to also have a detectable intermediate (Lape et al., 2008; Mukhtasimova et al. 2009), which provides a far more plausible explanation of the slight imperfections of fits with mechanisms like Fig. 1 B than postulation of an arbitrary extra states.
What is the structural basis for flipping or priming?
Fitting mechanisms is useful only insofar as the postulated states have physical reality. Burzomato et al. (2004) suggested that the flipping reaction, a change of shape that precedes channel opening, might correspond to the “domain closure” seen in structural studies (Gouaux, 2004; Lester et al., 2004).
Mukhtasimova et al. (2009) took this further by producing direct evidence that priming step results from “transition of a C-loop from the uncapped to the capped conformation.” They used a spontaneously active mutant receptor, with serine in place of the conserved leucine in the middle of the pore. They substituted a cysteine residue at the tip of the C-loop of each binding site of the mutant receptor and inserted another cysteine in each of the two juxtaposed subunits. They then looked at spontaneous channel opening, in the absence of agonist, before and after applying an oxidizing reagent. Before oxidation, single receptor channels activate in episodes of predominantly brief openings. After oxidation, however, receptor channels activate in episodes of long openings in quick succession. This suggests that the disulphide bridge locks the C-loops in the capped conformation, thereby producing a doubly primed state that generates long-lived channel openings.
Estimation of rate constants and the problem of over-parameterization
Realistic mechanisms for agonist activation of ion channels, such as those in Fig. 1 B and Figs. 2–4, have 5–10 discrete states and many free rate constants (14 in Fig. 3). Estimation of 14 rate constants is impossible by macroscopic methods, though use of fast concentration jumps may come close in favorable cases (Franke et al., 1991; Milescu et al., 2005). In contrast, single-channel recordings can estimate up to 18 (mechanism in Fig. 2) free rate constants in favorable cases (Burzomato et al., 2004). This can be achieved by using time-course idealization, followed by maximum likelihood fitting with exact missed event correction with the HJCFIT program (Colquhoun and Sigworth, 1995; Colquhoun et al., 2003).
It’s clear that it is possible to identify rate constants only for transitions that occur sufficiently frequently that the observations contain information about them. That’s why the unliganded flipped and open states have to be omitted from the mechanisms fitted to wild-type nicotinic and glycine receptors, despite the fact that these states must exist.
The resolving power of single-ion channel records is such that we could obtain reasonable estimates of all 14 free parameters in the flip model, because all the transitions occur sufficiently frequently in the wild-type α1β glycine receptor. But the number of rate constants is larger for primed models, and so far it hasn’t been possible to estimate all the rate constants.
The flip model (Fig. 3) has 12 states when unliganded branch is included. The equivalent primed model for a glycine receptor has 28 states, with 53 connections and 106 rate constants. If all cycles obey microscopic reversibility, then the number of connections set by microscopic reversibility is 53 – 28 + 1 = 26 (Colquhoun et al., 2004), so the number not so set is 53 – 26 = 27. Each connection set by microscopic reversibility reduces the number of free rate constants by one, so that the number of free rate constants is 106 – 26 = 80. This is far more that anyone has succeeded in fitting so far. That is why Mukhtasimova et al. (2009) fitted only a subset of states (marked as brown connections in Fig. 5).
More recently, we have found that the flip mechanism also provides an adequate description of the action of choline on the nicotinic receptor (Lape et al., 2009) and also for the α2 homomeric glycine receptor (Krashia et al., 2011). However we found that the mutant glycine receptor, α1K276E, could not be fitted satisfactorily by the flip model, but could be fitted with the primed mechanism (Lape et al., 2012). But, like Mukhtasimova et al. (2009), we have been able to fit only a subset of the primed mechanism with parameters that are well-defined by the data.
Although it is easy to see that unliganded flipped and open states must be omitted if no spontaneous openings can be seen, that is a special case. In general, it is to be expected that every receptor, and every mutant of the same receptor, will visit some states more often than others (see Fig. 8). Thus, even if the same basic reaction mechanism holds for all of them, some states will be visited so rarely that they can’t be identified from observations. Lape et al. (2012) tried omitting states that had low occupancies and/or states that were visited infrequently (as in Eq. 3 and Fig. 8), as methods to decide which states to omit from the primed mechanism when fitting results with the mutant glycine receptor, α1K276E. But these values had to be calculated on the basis of an over-parameterized fit and so may well not be accurate. In general, the optimum choice of which states to omit is an unsolved statistical problem.
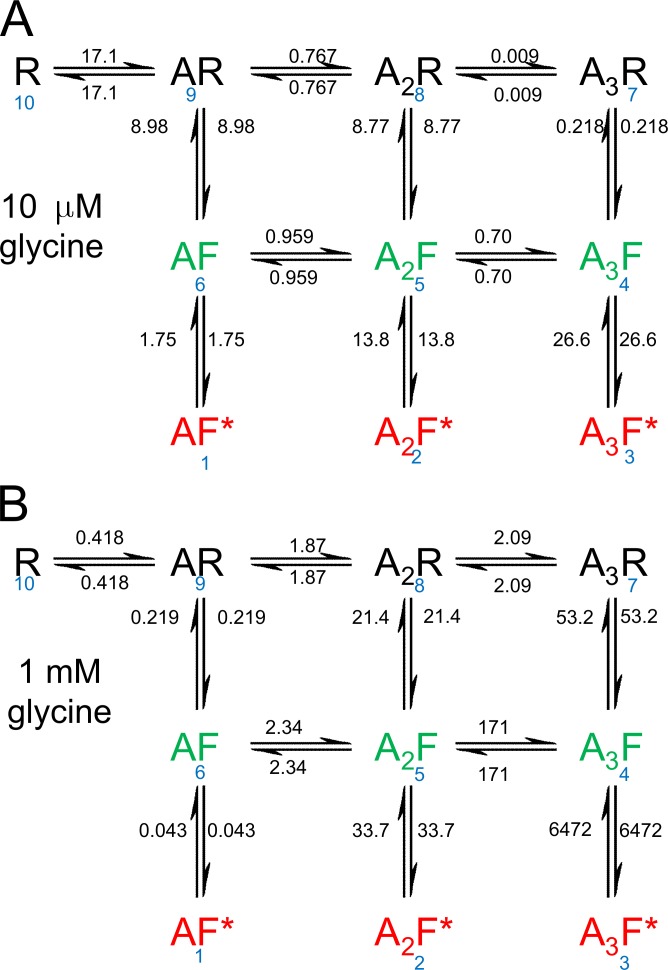
Figure 8.
Transition frequencies at equilibrium. The number of transitions per second was calculated from Eq. 3 and marked on each transition arrow. They are shown for 10 µM glycine (A) and 1 mM glycine (B) at equilibrium. The frequencies are calculated from the same rate constants as used for Fig. 7. The state numbering is shown in blue.
Errors that result from omitting intermediate shut states
It may be asked, what are the likely errors if intermediate states are omitted from the model that is used to fit the data? The simplest case arises if the flipping reaction is very fast. For example, when A3R and A3F in Fig. 3 are treated as a single-shut state, [A3R, A3F], the apparent dissociation rate constant from [A3R, A3F] will be the true dissociation rate constant multiplied by the factor 1/(1 + F3). This factor is the equilibrium fraction of [A3R, A3F] that is in A3R. This error can be quite large for a high efficacy agonist (large F3). Thus, omission of the intermediate state from the model will result in underestimation of the true dissociation rate of agonist from the fully liganded resting conformation, and so overestimation of the resting affinity. It is possible that an error of this sort in the estimation of the affinity for the resting conformation might contribute to the correlation between efficacy and apparent affinity that was observed in Fig. 4 of the article by Jadey and Auerbach (2012).
Likewise, the effective opening rate constant estimated when fitting the model without intermediate will differ from its true value by a factor of F3 /(1 + F3), so the error will be greatest for partial agonists. When the flipping step is not much faster than other steps, as suggested by the estimates of these rates (Burzomato et al., 2004; Lape et al., 2008), then simulations suggest that errors of roughly this size occur in the dissociation rate constant and the opening rate constant, but also substantial errors can occur in the shutting rate constant and the association rate constant too.
The true affinity for the resting conformation of the receptor has been elusive. Substantial errors in its values are likely to occur if intermediate shut states are omitted while fitting data.
The interpretation of the affinity change that results from flipping
The flip and primed mechanisms work because the flipped conformation has a higher affinity for the agonist than the resting conformation. It was a striking feature of the results for the wild-type glycine receptor that this increase in affinity resulted not from a decrease in the dissociation rate, but from an increase in the association rate constant (Burzomato et al., 2004). For binding of glycine to the resting conformation, the association rate constant was unusually low, 0.58 × 106 M−1 s−1, but for binding to the flipped conformation it was 1.5 × 108 M−1 s−1. The dissociation rate constant was actually faster for the high affinity form, not slower. The structural interpretation of this observation is not obvious. The words “domain closure” or “capping” suggest a physical obstruction to dissociation that could increase affinity by decreasing the dissociation rate, but this is not what’s seen. In any case, such a physical obstruction might be expected to hinder association as much as it obstructed dissociation, and if that were the case there would be little change in affinity.
The observation of an increased association rate constant suggests that the flipped conformation provides much easier access to the binding site and, to a lesser, extent easier egress, too. The structural basis for these changes remains to be elucidated.
For the nicotinic receptor, this effect is not detectable, but the increase in affinity that results from flipping is much smaller than for the glycine receptor, only about twofold (Lape et al., 2008), compared with 65-fold for glycine. That is probably why the intermediate states eluded detection for so long.
A numerical example
It is often discussed whether agonist binds first to unliganded channels that are already in the high-affinity conformation, or whether they bind first to the low-affinity resting conformation which subsequently isomerizes. Once we have estimates of all the rate constants, it becomes easy to answer questions such as these as shown in the following example.
As an example, we can take the flip model for the glycine receptor, using the rate constants that were estimated by Burzomato et al. (2004). The numbers used are those that were estimated for the set of recordings that were used for the figures in that paper. The values for the rate constants (averaged over three datasets) are shown in Fig. 3. There are several ways to look at what’s happening when an agonist binds.
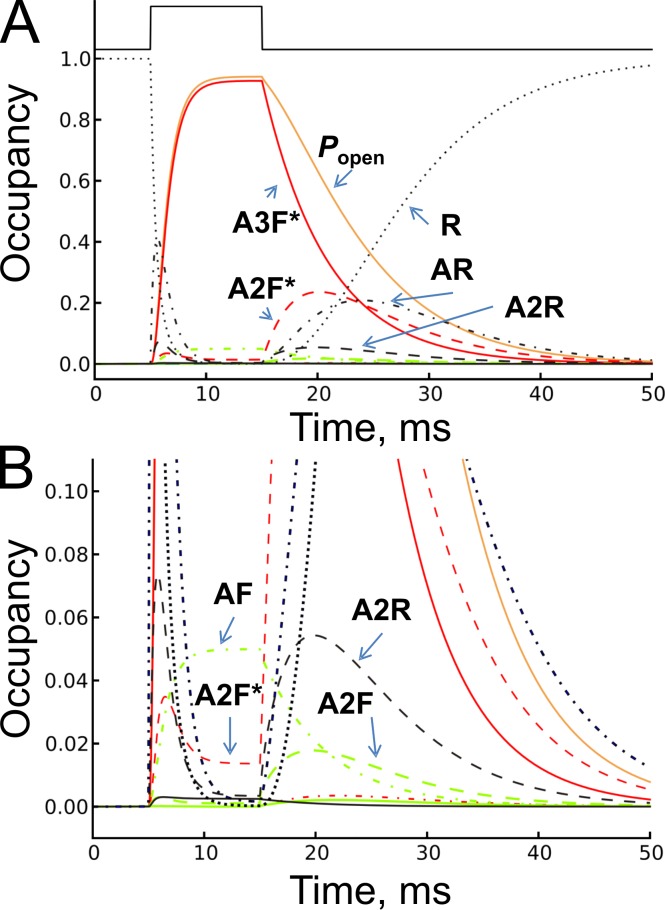
Fig. 6 shows the time course of the occupancies of all 10 states after a jump in glycine concentration from 0 to 1 mM and then back to 0. The receptors are initially all in the unoccupied resting state (R, state 10). They eventually reach the equilibrium occupancies, which are shown (as percentages) in Table 1. At 1 mM, most receptors (93%) are in the triple-liganded open state at equilibrium. In contrast, at 10 µM glycine, most receptors (93%) are still unoccupied at equilibrium, and there are more diliganded open channels (0.56%) than triliganded open channels (0.38%).
Figure 6.
The time course of occupancies. The time course of glycine receptor state occupancies is shown over the full range, 0–1 in A, and as an enlarged view of occupancies below 0.1 in B. The occupancies were calculated from Eq. 1 and plotted for all 10 states in the flip mechanism (Fig. 3) after a step in glycine concentration from 0 to 1 mM, and, 10 ms later, a step from 1 mM to zero (the concentration profile is shown on top of panel A as black solid line). Occupancies of the resting conformation are plotted in black, flipped conformation is plotted in green, and open conformation is plotted in red. Solid lines represent triliganded states, dashed lines those of diliganded states, and dashed-dot lines show monoliganded states. Occupancy of the unliganded resting state R (state 10) is plotted as a dotted black line and the total open probability as a solid orange line.
Table 1.
Equilibrium occupancies of each state at equilibrium (as a percentage), for two concentrations of glycine
| State | 1AF* | 2A2F* | 3A3F* | 4A3F | 5A2F | 6AF | 7A3R | 8A2R | 9AR | 10R |
| 10 µM | 0.047 | 0.559 | 0.381 | 0.0205 | 0.042 | 0.0288 | 0.001 | 0.139 | 6.21 | 92.57 |
| 1 mM | 0.0015 | 1.36 | 92.77 | 4.99 | 0.103 | 0.0007 | 0.253 | 0.339 | 0.151 | 0.023 |
The concentration pulse is assumed to be rectangular so the occupancies can be calculated from
| (1) |
where p(t) is a 1 × 10 row vector that contains the 10 occupancies, pi(t), i = 1,…,10, and Q is the matrix that contains the rate constant for transition from state i to state j in the off-diagonal elements (Colquhoun and Hawkes, 1977, Eq. 23; Colquhoun and Hawkes, 1995a).
Fig. 6 B shows an enlargement of the bottom part of Fig. 6 A, to more clearly show the occupancy of sites that have small occupancies throughout. Most open channels are fully liganded throughout. Diliganded openings (A2F*, red dashed line) rise to a peak of around 3.5% during the pulse, and then decline as a third ligand is bound. During offset, diliganded openings rise to a peak of over 20% and in the later stages of decay there are more diliganded than triliganded channels. The occupancy of shut flipped states (green lines) is low at all times because of their short mean lifetime, despite the fact that the triliganded flipped state (A3F, state 4) is visited frequently, as shown in Fig. 8.
To understand better what is happening, it’s interesting to look at preferred routes through the 10 states. Except for mutant receptors that show spontaneous activity, the equilibrium favors the low-affinity resting conformation so strongly in the absence of agonist that it will be rare for agonist to bind with unliganded flip conformation, of which little is present. In the present example, the high-affinity unliganded flipped and open states are not even included in the mechanism because they are present in such small amounts that the data contain little or no information about them.
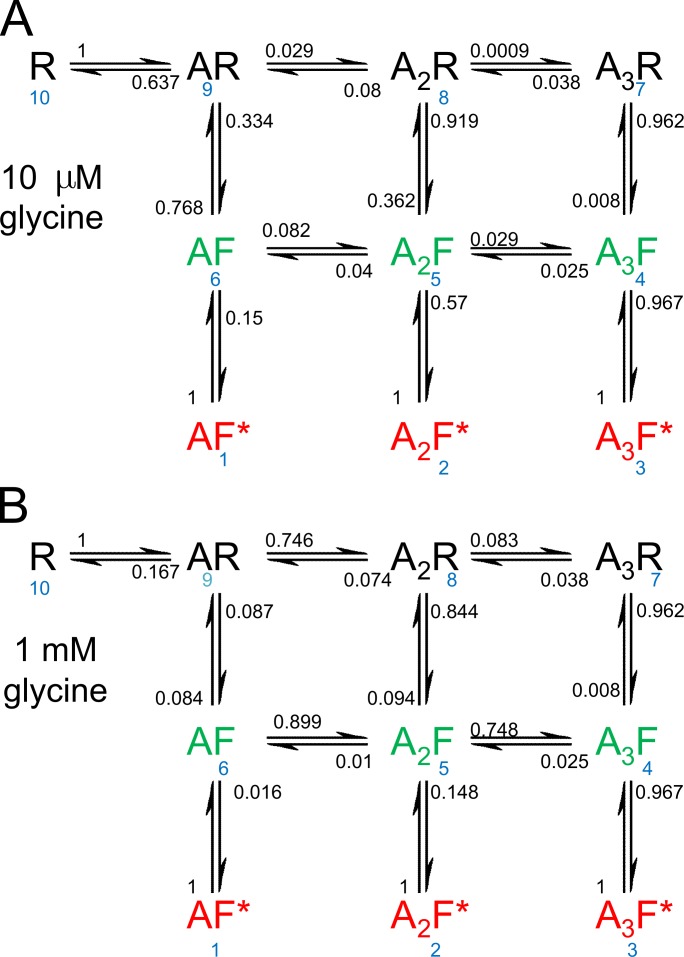
In Fig. 7, the arrows connecting the states are marked with the probabilities that a transition out of a specified state will lead to each of the other states to which it is connected. Thus, the sum of the probabilities on all arrows leading out of a state add to 1. For the transition out of state i into state j this probability is given by
| (2) |
Figure 7.
Transition probabilities. The transition arrows leading out of each state are marked with the probability that the next transition, whenever it occurs, will be in the indicated direction. The sum of the probabilities leading out of each state is 1. The transition probabilities are calculated from Eq. 2, using the rate constants from one of the three sets of observations from Burzomato et al. (2004; the set that was used for the illustrations in that paper). These probabilities are independent of time, but depend on concentration and are shown for 10 µM glycine (A) and 1 mM glycine (B). The state numbering is shown in blue.
(Colquhoun and Hawkes, 1982, Eq. 1.5). These probabilities are shown in Fig. 7. They are independent of time, but depend on concentration. They are the same whether or not the system is at equilibrium. Fig. 7 A shows values at 10 µM glycine and Fig. 7 B shows values at 1 mM glycine.
Another way to look at the pathways through the various states is to look at the number of transitions that occur on each pathway. This depends on the rate constant of the transition in question and it depends also on the occupancy of the state being left. There will not be many transitions from a state that has a low occupancy. The number of transitions per second from state i to state j is
| (3) |
(Colquhoun and Hawkes, 1995b). The frequencies depend on the occupancy of each state at time t, pi(t). The evolution of these occupancies after a jump in glycine from 0 to 1 mM and back was shown in Fig. 5. The transition frequencies at equilibrium are shown in Fig. 8, for 10 µM glycine (Fig. 8 A) and at 1 mM glycine (Fig. 8 B).
At 1 mM glycine, a near-saturating concentration, by far the most frequent transition at equilibrium is between the fully liganded flipped state and the open state, f43 = f34 = 6,472 transitions per second. Notice that transitions have equal frequencies in both directions at equilibrium, because of the assumption of microscopic reversibility. In contrast, the first occupancy of the resting state, f10,9 is rare, only 0.418 per second, because there are few free receptors (R) to be occupied at equilibrium, p10(∞) = 0.023% (Table 1). With 1,000 channels, 418 would associate per second, and 418 would dissociate.
However, at the start of a concentration jump from 0 to 1 mM, the receptor is entirely in the vacant resting conformation, p10(0) = 1, so the frequency of initial associations is much higher: it is p10(0) q10,9 where the association rate is 3k+1 c, and c is the concentration, 1 mM. The initial frequency of bindings is thus 1,850 bindings per second at t = 0, and it falls to 0.418 per second at equilibrium.
It is perhaps a bit misleading to say that the initial rate of binding is 1,850 per second. Each receptor, once it has become occupied is out of play until the agonist dissociates, either directly or after wandering though other states. The mean lifetime of the resting state is, from Eq. 24 in Colquhoun and Hawkes (1995b), 1/q10,9 = −1/q10,10 = 0.54 ms. After 1 s, equilibrium would have been achieved. When the occupancies are time dependent in Eq. 3, it makes sense only if the transition frequency is expressed over a time interval that is short enough that occupancy changes little. It is clear from Fig. 6 that the occupancy of the unliganded resting state (R, state 10) falls rapidly after a concentration jump from 0 to 1 mM, so rather than saying that the initial binding frequency is 1,850 per second, it makes more sense to say it is 1.85 per millisecond, or better still, 0.00185 per microsecond. None of this matters at equilibrium, when occupancies aren’t changing with time.
The transition frequencies (Fig. 8) can be used to identify which transitions predominate. Of course, there will be often oscillations during the transition between one state and another, but if we look only at the direct route from unliganded receptor (R, state 10), to the fully ligand open state (A3F*, state 3) at equilibrium, inspection of the frequencies at 1 mM suggests that, at equilibrium, the predominant route is likely to involve flipping while diliganded, to reach the diliganded flip state (state 5), 10→9→8→5→4→3.
Another way to look at the route is through the transition probabilities. These are not time dependent, so no assumption of equilibrium is needed. At 1 mM, the probability for this route is . (Note that , because there is only one way to leave state 10). This probability is about 8 times larger than the probability for either of the routes round the outside of the rectangle. The clockwise route, 10→9→ 8→7→4→ 3, gives , whereas the probability for the anticlockwise route is .
At a low concentration of glycine (10 µM), these routes give probabilities of 7.3 × 10−4, 2.8 × 10−5, and 7.7 × 10−4, respectively, so the anticlockwise route and the route via states 8 and 5 are about equally likely, and both are about 30 times more likely than the clockwise route via state 7. The most likely routes at low concentration involve flipping while monoliganded (anticlockwise route) or while diliganded.
The calculation can be made a bit more sophisticated by noting that opening while monoliganded (6→1) or when diliganded (5→2) are not relevant to the calculation. This suggests that π54 should be replaced by the conditional probability of a 5→4 transition, given that the channel does not open (to state 2) from state 5 (probability = 1 − π52). The rules of conditional probability give this as
| (4) |
Likewise the probability of a 6→5 transition, π65, should be replaced by
| (5) |
In this case, the probabilities of opening with one or two ligands bound are sufficiently small that the conclusions about predominant route are not changed by this procedure.
The opening steps
In the flip mechanism, it is assumed that opening can occur only when the intermediate flipped conformation has been reached. The opening step is very fast when fully liganded. More interestingly, the shut–open reaction seems to be much the same regardless of which agonist is used to open. It is just as fast for partial agonists as for full agonists, in both glycine and nicotinic receptors (Lape et al., 2008). The reason that partial agonists can produce only a small maximum response is that they are ineffective in eliciting the flipped conformation, not because the actual opening transition is defective. A picture emerges of a stereotyped change of conformation from shut (flipped) to open, which is much the same whatever agonist elicited it. Another, much older, aspect of the same thing is that it’s been known for a long time that the conductance of a channel is the same whether it is opened by a full or a partial agonist (Colquhoun et al., 1983).
Indeed, the direct observation in experimental records of the sharp transition from shut to open provides the most direct evidence ever found for the existence of discrete conformational states. Although the assumption of discrete states has been made in chemical kinetics for over a century, it was only the observation of single-channel openings that confirmed the validity of this view.
The evidence for the existence of discrete shut states, the transitions between which can’t be observed directly, is inevitably more speculative. If there are three binding sites, then the physical existence for four states (with 0, 1, 2, or 3 ligands bound) is inevitable. The argument for the existence of two forms of fully liganded shut channels (e.g., resting and flipped) is seen most directly by the fact that the shut time distribution for fully saturated channels does not always have a simple exponential form, as predicted by pre-2004 mechanisms, but requires two exponentials (as predicted by flip) or more (as predicted by primed mechanisms).
In the flip model (Fig. 3), it may be noticed that the three open states have no direct connections between them, despite the fact that it must be physically possible for agonist to dissociate from open channels, though possibly too slowly to be detected (Grosman and Auerbach, 2001). In the case of glycine, fitting with routes between the open states gives a worse fit than when these routes are omitted, when the cycles so generated are assumed to obey microscopic reversibility. This assumption forces the ratio of the opening equilibrium constants for mono and diliganded channels to be the same as the ratio for diliganded and triliganded channels. The free fit gives E1 = 1.3, E2 = 13, E3 = 20, so these ratios are far from constant, E2/E1 = 10, but E3/E2 = 1.5. This means that either the model is wrong (Edelstein and Changeux, 2010), or that the opening reaction does not obey microscopic reversibility.
There are at least two examples known of channels that clearly do not obey microscopic reversibility. It was reported by Richard and Miller (1990) in a double-barreled chloride channel, and for one sort of NMDA receptor, NR1-NR2D (Wyllie et al., 1996). In the latter, transitions from the 35-pS level to the 17-pS level are more common than transitions from 17 to 35 pS. A similar asymmetry was found in a mutant NMDA channel (Schneggenburger and Ascher, 1997). That is hardly surprising because any interaction between ion flow (which is not at equilibrium) and gating could cause such an effect (Finkelstein and Peskin, 1984; Läuger, 1985; Rothberg and Magleby, 2001). So it is precisely the opening step that one might expect not to obey microscopic reversibility; the surprising thing is that so many channels appear to obey microscopic reversibility. But the question has not been examined in detail for most channels. The only easy way to detect to detect breaches of microscopic reversibility is by observation of temporal asymmetry of subconductance transitions. However, this method is not useful for muscle nicotinic and glycine receptors because subconductance transitions are rare.
Conclusions
Terms like allosteric and cooperative don’t really refer to mechanisms, and therein lays the limitation of their usefulness. On the other hand, terms like independence, equivalence, and concerted refer to physical phenomena, and those terms are more useful for describing what’s actually happening during the opening of a channel by an agonist.
The shut part of the flip mechanism is the same as that postulated by Monod et al. (1965). The essential features of the flip mechanism are as follows: (a) the binding sites are equivalent (it doesn’t matter which individual site is occupied; so, for example, all three monoliganded states in the resting conformation are indistinguishable). (b) The binding sites are independent. This means that binding to one site is the same regardless of whether or not other sites are occupied. Thus, the equilibrium constant is 520 µM for binding to the resting conformation, the same for all three bindings. And, likewise, the equilibrium constant is 8 µM for all bindings to the flipped conformation. (c) The transition from the resting conformation to the flipped conformation is concerted. This implies that there is only one flipped conformation regardless of the number of ligands bound. This is an explicit physical assumption, though perhaps the least plausible of the assumptions that are made in this mechanism. It is relaxed in the primed model. (d) The finding that glycine has 65-fold higher affinity for the flipped conformation than it has for the resting conformation is what causes flipping and hence opening. This fact, in combination with the concerted conformation, change results in cooperativity of the observed response, despite the fact that the binding steps show no cooperativity. (e) The opening and shutting steps represent a stereotyped conformation change that is much the same regardless of which agonist elicits the openings. The open-shut transitions appear not to obey microscopic reversibility. (f) Questions concerning the preferred routes through the various states in which the receptor can exist can be answered once estimates have been made for the rate constants for each transition.
This Perspectives series includes articles by Andersen, Chowdhury and Chanda, and Horrigan.
Acknowledgments
We are grateful for helpful comments by Professors A.G. Hawkes and L. Sivilotti.
Olaf S. Andersen served as guest editor.
Footnotes
Abbreviation used in this paper:
- MWC
- Monod-Wyman-Changeux
References
- Andersen O.S. 1999. Graphic representation of the results of kinetic analyses. J. Gen. Physiol. 114:589–590 10.1085/jgp.114.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. 2007. How to turn the reaction coordinate into time. J. Gen. Physiol. 130:543–546 10.1085/jgp.200709898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. 2012. Thinking in cycles: MWC is a good model for acetylcholine receptor-channels. J. Physiol. 590:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzomato V., Beato M., Groot-Kormelink P.J., Colquhoun D., Sivilotti L.G. 2004. Single-channel behavior of heteromeric alpha1beta glycine receptors: an attempt to detect a conformational change before the channel opens. J. Neurosci. 24:10924–10940 10.1523/JNEUROSCI.3424-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J.P. 1993. Allosteric proteins: from regulatory enzymes to receptors—personal recollections. Bioessays. 15:625–634 10.1002/bies.950150909 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. 1998. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125:923–947 10.1038/sj.bjp.0702164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. 2006. The quantitative analysis of drug-receptor interactions: a short history. Trends Pharmacol. Sci. 27:149–157 10.1016/j.tips.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A.G. 1977. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc. R. Soc. Lond. B Biol. Sci. 199:231–262 10.1098/rspb.1977.0137 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A.G. 1982. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 300:1–59 10.1098/rstb.1982.0156 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A.G. 1995a. A Q-matrix Cookbook. Single Channel Recording. Sakmann B., Neher E., Plenum Press, New York: 589–633 [Google Scholar]
- Colquhoun D., Hawkes A.G. 1995b. The principles of the stochastic interpretation of ion channel mechanisms. Single Channel Recording. Sakmann B., Neher E., Plenum Press, New York: 397–482 [Google Scholar]
- Colquhoun D., Sakmann B. 1981. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 294:464–466 10.1038/294464a0 [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. 1985. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J. Physiol. 369:501–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sigworth F.J. 1995. Fitting and statistical analysis of single-channel records. Single Channel Recording. Sakmann B., Neher E., Plenum Press, New York: 483–587 [Google Scholar]
- Colquhoun D., Gardner P., Ogden D.C. 1983. The conductance of ion channels opened by different cholinomimetic agonists in cultured rat muscle. J. Physiol. 345:139P [Google Scholar]
- Colquhoun D., Hawkes A.G., Merlushkin A., Edmonds B. 1997. Properties of single ion channel currents elicited by a pulse of agonist concentration or voltage. Philos. Trans. R. Soc. Lond. A. 355:1743–1786 10.1098/rsta.1997.0090 [DOI] [Google Scholar]
- Colquhoun D., Hatton C.J., Hawkes A.G. 2003. The quality of maximum likelihood estimates of ion channel rate constants. J. Physiol. 547:699–728 10.1113/jphysiol.2002.034165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dowsland K.A., Beato M., Plested A.J. 2004. How to impose microscopic reversibility in complex reaction mechanisms. Biophys. J. 86:3510–3518 10.1529/biophysj.103.038679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer P.J., Poitevin F., Prevost M.S., Sauguet L., Delarue M., Changeux J.-P. 2012. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure. 20:941–956 10.1016/j.str.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Del Castillo J., Katz B. 1957. Interaction at end-plate receptors between different choline derivatives. Proc. R. Soc. Lond. B Biol. Sci. 146:369–381 10.1098/rspb.1957.0018 [DOI] [PubMed] [Google Scholar]
- Edelstein S.J., Changeux J.P. 2010. Relationships between structural dynamics and functional kinetics in oligomeric membrane receptors. Biophys. J. 98:2045–2052 10.1016/j.bpj.2010.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Peskin C.S. 1984. Some unexpected consequences of a simple physical mechanism for voltage-dependent gating in biological membranes. Biophys. J. 46:549–558 10.1016/S0006-3495(84)84053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Parnas H., Dudel J. 1991. Kinetic constants of the acetylcholine (ACh) receptor reaction deduced from the rise in open probability after steps in ACh concentration. Biophys. J. 60:1008–1016 10.1016/S0006-3495(91)82138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E. 2004. Structure and function of AMPA receptors. J. Physiol. 554:249–253 10.1113/jphysiol.2003.054320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C. 2003. Free-energy landscapes of ion-channel gating are malleable: changes in the number of bound ligands are accompanied by changes in the location of the transition state in acetylcholine-receptor channels. Biochemistry. 42:14977–14987 10.1021/bi0354334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C., Auerbach A. 2000. Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: a single-channel study of second transmembrane segment 12′ mutants. J. Gen. Physiol. 115:621–635 10.1085/jgp.115.5.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C., Auerbach A. 2001. The dissociation of acetylcholine from open nicotinic receptor channels. Proc. Natl. Acad. Sci. USA. 98:14102–14107 10.1073/pnas.251402498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C., Zhou M., Auerbach A. 2000. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 403:773–776 10.1038/35001586 [DOI] [PubMed] [Google Scholar]
- Hatton C.J., Shelley C., Brydson M., Beeson D., Colquhoun D. 2003. Properties of the human muscle nicotinic receptor, and of the slow-channel myasthenic syndrome mutant epsilonL221F, inferred from maximum likelihood fits. J. Physiol. 547:729–760 10.1113/jphysiol.2002.034173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadey S., Auerbach A. 2012. An integrated catch-and-hold mechanism activates nicotinic acetylcholine receptors. J. Gen. Physiol. 140:17–28 10.1085/jgp.201210801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashia P., Lape R., Lodesani F., Colquhoun D., Sivilotti L.G. 2011. The long activations of α2 glycine channels can be described by a mechanism with reaction intermediates (“flip”). J. Gen. Physiol. 137:197–216 10.1085/jgp.201010521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R., Colquhoun D., Sivilotti L.G. 2008. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 454:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R., Krashia P., Colquhoun D., Sivilotti L.G. 2009. Agonist and blocking actions of choline and tetramethylammonium on human muscle acetylcholine receptors. J. Physiol. 587:5045–5072 10.1113/jphysiol.2009.176305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R., Plested A.J., Moroni M., Colquhoun D., Sivilotti L.G. 2012. The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors. J. Neurosci. 32:1336–1352 10.1523/JNEUROSCI.4346-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. 1985. Ionic channels with conformational substates. Biophys. J. 47:581–590 10.1016/S0006-3495(85)83954-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H.A., Dibas M.I., Dahan D.S., Leite J.F., Dougherty D.A. 2004. Cys-loop receptors: new twists and turns. Trends Neurosci. 27:329–336 10.1016/j.tins.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Milescu L.S., Akk G., Sachs F. 2005. Maximum likelihood estimation of ion channel kinetics from macroscopic currents. Biophys. J. 88:2494–2515 10.1529/biophysj.104.053256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Jacob F. 1961. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb. Symp. Quant. Biol. 26:389–401 10.1101/SQB.1961.026.01.048 [DOI] [PubMed] [Google Scholar]
- Monod J., Changeux J.P., Jacob F. 1963. Allosteric proteins and cellular control systems. J. Mol. Biol. 6:306–329 10.1016/S0022-2836(63)80091-1 [DOI] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.P. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12:88–118 10.1016/S0022-2836(65)80285-6 [DOI] [PubMed] [Google Scholar]
- Mukhtasimova N., Lee W.Y., Wang H.L., Sine S.M. 2009. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature. 459:451–454 10.1038/nature07923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P., Auerbach A. 2009. Unliganded gating of acetylcholine receptor channels. Proc. Natl. Acad. Sci. USA. 106:115–120 10.1038/pnas.0809272106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E.A., Miller C. 1990. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 247:1208–1210 10.1126/science.2156338 [DOI] [PubMed] [Google Scholar]
- Rothberg B.S., Magleby K.L. 2001. Testing for detailed balance (microscopic reversibility in ion channel gating. Biophys. J. 80:3025–3026 10.1016/S0006-3495(01)76268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone F.N., Zhou M., Auerbach A. 1999. A re-examination of adult mouse nicotinic acetylcholine receptor channel activation kinetics. J. Physiol. 516:315–330 10.1111/j.1469-7793.1999.0315v.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R., Ascher P. 1997. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 18:167–177 10.1016/S0896-6273(01)80055-6 [DOI] [PubMed] [Google Scholar]
- Wyllie D.J.A., Béhé P., Nassar M., Schoepfer R., Colquhoun D. 1996. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc. Biol. Sci. 263:1079–1086 10.1098/rspb.1996.0159 [DOI] [PubMed] [Google Scholar]
- Wyman J., Allen D.W. 1951. The problem of the heme Interactions in hemoglobin and the basis of the Bohr effect. J. Polym. Sci., Polym. Phys. Ed. VII:499–518 [Google Scholar]
- Wyman J., Gill S.J. 1990. Binding and Linkage Functional chemistry of biological macromolecules. University Science Books, Mill Valley, CA [Google Scholar]