Abstract
mRNA chimeras from chromosomal translocations often play a role as transforming oncogenes. However, cancer transcriptomes also contain mRNA chimeras that may play a role in tumor development, which arise as transcriptional or post-transcriptional events. To identify such chimeras, we developed a deterministic screening strategy for long-range sequence analysis. High-throughput, long-read sequencing was then performed on cDNA libraries from major tumor histotypes and corresponding normal tissues. These analyses led to the identification of 378 chimeras, with an unexpectedly high frequency of expression (≈2 x 10-5 of all mRNA). Functional assays in breast and ovarian cancer cell lines showed that a large fraction of mRNA chimeras regulates cell replication. Strikingly, chimeras were shown to include both positive and negative regulators of cell growth, which functioned as such in a cell-type-specific manner. Replication-controlling chimeras were found to be expressed by most cancers from breast, ovary, colon, uterus, kidney, lung, and stomach, suggesting a widespread role in tumor development.
Introduction
Several chimeric transcripts have been discovered in human solid tumors, which derive from chromosomal translocations. These often encode structurally and functionally altered signaling molecules or transcription factors [1] or may also function as non-coding RNA [2]. More than half of prostate cancers harbor fusion sequences, mostly TMPRSS-ERG [3]. The SLC45A3-ELK4 (ETS family) fusion transcript can be generated both by chromosomal rearrangement and by trans-splicing, and it was found to be expressed in both normal prostate tissue and in prostate cancer. High levels of SLC45A3-ELK4 mRNA are restricted to a subset of prostate cancer samples [4]. A small inversion within chromosome 2p leads to the formation of a fusion gene comprising EML4 and ALK in non-small cell lung cancer [5]. The fusion of MAML2 with CRTC1 or CRTC3 has a role in the development of mucoepidermoid carcinomas [6]. Rearrangements of RAF pathway members occur in prostate and gastric cancers [7], and a para-centric inversion of chromosome 7q results in an in-frame fusion between exons 1 and 8 of the AKAP9 gene and between exons 9 and 18 of BRAF in radiation-induced papillary carcinomas [8]. Other thyroid carcinoma-specific events include fusion of the RET oncogene to various partners [9]. Further oncogenic fusions have been detected in other solid tumors [10,11].
Cancer transcriptomes also contain mRNA chimeras that arise as transcriptional (long intergenic transcription) or post-transcriptional (trans-splicing [12]) events that may play a role in tumor development. Previous findings showed that oncogenic transcripts can indeed be generated post-transcriptionally [13–15]. The fusion of CYCLIN D1 mRNA to TROP2 transcripts generates oncogenic CYCLIN D1-TROP2 chimeras, whose tumor-promoting function is induced with a dramatically increased mRNA stability [13]. The oncogenic JAZF1-JJAZ1 chimeric mRNA can be originated by trans-splicing as well as by a chromosomal translocation [14]. Similarly, the SLC45A3-ELK4 chimeric transcript can be generated in the absence of chromosomal rearrangements [4,16]. Intergenic splicing generates a ubiquitous chimeric mRNA between the P2Y11 and SSF1 transcripts [17]. The generation of these chimeras appears as a regulated event [13,14] and was shown to also occur in normal tissues [4,13,14,17–20]. Several of these chimeric transcripts have been used as diagnostic or prognostic [21] markers and as targets for anti-neoplastic therapy [10,13,22,23].
Screening strategies were previously developed for in silico identification of mRNA chimeras in cancer cells [24]. Next-generation sequencing (NGS) approaches now provide much larger sequence information for chimera discovery [7,19,20,25–27]. However, most second-generation NGS approaches generate highly multiplexed, short-tag sequence reads, which are then condensed in strings of base-call probabilities, through a probabilistic fitting of massively parallel data sets. This makes contig assemblies and target alignments correspondingly more difficult [19,25,27,28]. Alignments to complex genomes are even more hampered, because of higher sequence complexity [29] and homology within closely related gene families and pseudogenes.
These problems have led to significant efforts for achieving longer sequence reads and higher sequencing accuracy. In 2005, 454 launched the first NGS apparatus, which was able to generate 100-bp reads. Sequence reads extended to 200 bp in 2007 [30] and are close to 900 bp at present [31]. SOLID sequencing generated 35-bp reads in 2007 [30], and this extended to 75 bp in 2011 [32]. Illumina generated 36-bp sequence reads in 2006 to 2008 [30]. These extended to 100 bp in 2010 [31] and to 300 bp in 2012 (www.illumina.com). Ion Torrent introduced its first sequencer at the end of 2010, and this was capable of 100-bp-long reads. As of 2012, reads of 525-bp average length have been obtained (www.iontorrent.com/lib/images/PDFs/pe_appnote_v12b.pdf). Pacific Biosciences (www.pacificbiosciences.com) succeeded in obtaining even longer reads, which currently are up to 1500 bp.
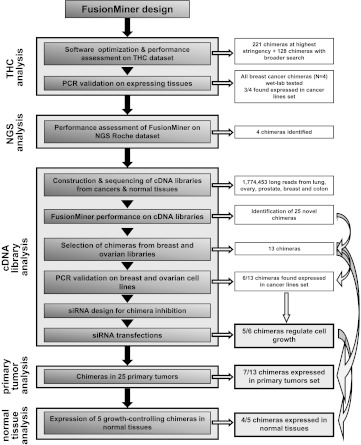
To take advantage of these technical advances, we have developed an analytical strategy for high-accuracy identification of mRNA chimeras in long-read DNA sequence data sets (Figure 1). This strategy was shown to work efficiently for chimera recognition (Tables S1–S7 and Figure S1). High-throughput, long-read sequencing was then performed on cDNA libraries from major tumor histotypes and corresponding normal tissues. This led to the identification of 378 chimeras, from both normal and transformed cells, indicating an unexpectedly high frequency of expression (≈2 x 10?5 of all mRNA). Functional assays in breast and ovarian cancer cell lines showed that a large fraction of mRNA chimeras regulate cell replication. Strikingly, chimeras were shown to include both positive and negative regulators of cell growth, which functioned as such in a cell-type-specific manner. Replication-controlling chimeras were found to be expressed by most cancers from breast, ovary, colon, uterus, kidney, lung, and stomach, suggesting selective pressure for a role in tumor development.
Figure 1.
Flow diagram of chimera identification and validation steps.
Materials and Methods
Cells
Human MCF-7, MCF-7/Almac, HBL-100, SK-BR-3, MDA-MB231, MDA-MB-361, MDA-MB-415, MDA-MB-453, MDA-MB468, HS578, and ZR751 breast cell lines and SKOV-3, IGROV-1, OVCAR-3, and OVCA-432 ovarian cancer cell lines were grown in RPMI 1640 medium supplemented with 10% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin (Euroclone, Milan, Italy). All cell lines were obtained from ATCC (LGC Standards, Teddington Middlesex, United Kingdom) where they were authenticated by standardized procedures (www.atcc.org).
Cell Growth Assays
MCF-7, HBL-100, SK-OV-3, IGROV-1, and OVCAR-3 cells were seeded at 1 x 103 to 10 x 103 cells/well in 96-well plates (five replicates per data point). Cell numbers were quantified by staining with crystal violet [33]. Standard growth curves for each cell line were generated by seeding two-fold serial dilutions of defined cell numbers. Crystal violet standard curves showed good linear responses (R2 > 0.998, in all cases) (Figure S2). To support the crystal violet readings, quantification was also performed by image analysis (ImageJ). Digital pictures were taken from 96-well plates after fixation. Picture noise was removed with GIMP software, after random sampling of cell-free pixels. ImageJ analysis was then performed by quantifying black areas in each culture well after image conversion to a gray scale (manuscript in preparation).
DNA Transfection
Cells were transfected with DNA in Lipofectamine 2000 (HBL-100, SKOV-3, IGROV-1, and OVCAR-3 cells) or LTX (Invitrogen, San Diego, CA), which was found to be optimal for MCF-7 cells (Figure 5C) [34], following the manufacturer's instructions. pEYFP transfection was used to quantify transfection efficiency [35] (EYFP expression, as measured by flow cytometry).
Figure 5.
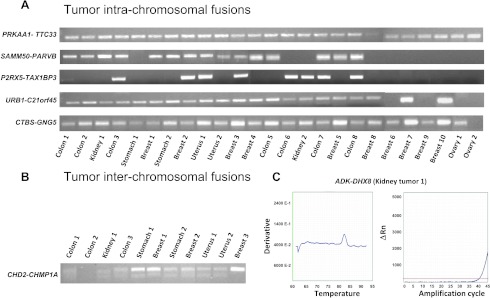
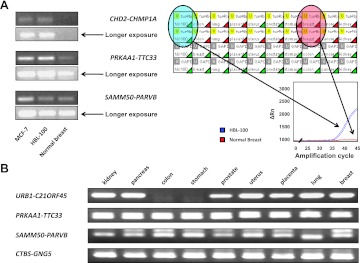
Expression of mRNA chimeras in primary tumors. (A, B) Agarose electrophoresis analysis of amplification products. Tumor origin and sample numbers are indicated. (A) Intrachromosomal chimeras as analyzed by real-time PCR. PRKAA1-TTC33 and CTBS-GNG5 were diagnosed in all 25 tumors; SAMM50-PARVB chimeric was found in 15 tumors, P2RX5-TAX1BP3 in 8 tumors, and URB1-C21orf45 in 21 tumors. (B) PCR amplicons of the interchromosomal CHD2-CHMP1A chimera. (C) Interchromosomal ADK-DHX8 chimeric. Melting temperature and real-time amplification curves.
Flow Cytometry Immunofluorescence
Flow cytometry analysis was performed as described previously [36,37], on fluorescence-activated cell sorters (FACSCalibur, Becton-Dickinson, Sunnyvale, CA). To improve the detection of EYFP transfectants, we performed subtraction of cell autofluorescence and displacement of true transfectants in the red channel as described [35,38].
Human Samples for Tumor Transcriptome Sequencing
Non-small cell lung cancer. Non-small cell lung cancer libraries were generated from a set of frozen tissue samples, comprising 65 tumor samples (30 adenocarcinomas, 20 squamous cell carcinomas, and 15 other morphologies) from the Roy Castle Lung Cancer Research Institute (University of Liverpool) and Queens University Belfast. To maximize chances of mRNA chimera discovery, we proceeded to generate libraries from both tumor and normal tissues. Normal lung RNA was obtained from multiple commercial suppliers (Clontech, Palo Alto, CA; Ambion, Austin, TX; BioCat, Heidelberg, Germany; Stratagene, La Jolla, CA; Cybridi, Rockville, MD; and OriGene, Rockville, MD), overall from 16 donors of different ethnicity.
Ovarian cancer. Ovarian library A comprised 64 ovarian tumors (31 serous, 14 endometroid, 6 mucinous, 5 clear cell, and 8 undifferentiated cancers; 52 were stage III/IV and 12 were stage I/II). For ovarian library B, RNA from normal ovarian tissue was obtained from commercial suppliers (Ambion and AMS Biotechnology [Bioggio, Switzerland]). The library was generated with equal quantities of RNA from different ethnicities (Asian, Caucasian, and African-American), with 23 donors overall. For ovarian library C, ovarian tumor total RNA was obtained from various commercial suppliers (Ambion, Clontech, Cytomyx [Lexington, MA], Biocat, and Asterand [Detroit, MI]). The library was composed of equal quantities of RNA of different ethnicity (Asian, African-American, and Caucasian), with 37 donors overall.
Prostate cancer. The prostate cancer library was constructed from 30 tumors (74% Caucasian and 26% African-American), 8 normal prostate RNA supplied by Clontech, AMS Biotechnology, and Cybridi, and 56 normal tissues adjacent to tumors obtained from St. Vincents Hospital (Dublin, Ireland).
Breast cancer. The breast cancer library was composed of 90 tumors and 18 normal samples [39–41].
Colorectal cancer. The colorectal library comprised 40 tumor samples and 40 normal tissues.
Tumor Validation Sample Set
cDNA was synthesized from 25 human primary tumors (10 breast, 6 colon, 3 stomach, 2 ovary, 2 kidney, and 2 uterus), which were independent from those used to construct the cDNA libraries. These 25 samples were used as a test set to validate chimera expression by both conventional polymerase chain reaction (PCR)/sequencing and real-time reverse transcription (RT)-PCR.
Normal Tissues
Normal breast, colon, uterus, prostate, placenta, lung, kidney, pancreas, and stomach RNA were obtained from Clontech.
cDNA Library Construction
All of the frozen tumor tissues were homogenized in RNA STAT-60 (Tel-Test, Friendswood, TX), and the RNA was extracted according to the manufacturer's instructions. Equal amounts of good quality total RNA were pooled, and the mRNA was isolated using µMACS mRNA isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany), as described by the manufacturer. Lung cDNA libraries were constructed from 3 µg of mRNA using the CloneMiner cDNA library construction kit (Invitrogen), according to the manufacturer's instructions. cDNA were inserted in the pDONR 222 vector from Invitrogen. Titer and average insert size in each cDNA library were determined according to the manufacturer's instructions. Plasmid preparations of individual clones were carried out using a modified Montáge alkaline lysis method (Millipore, Billerica, MA) that incorporates MultiScreen Plasmid 384 Miniprep clearing plates for centrifugal lysate clearing.
Sequencing of cDNA Libraries
Colony sequencing automation was implemented (QPix colony picker Biomek liquid handlers). Cycle sequencing reactions were performed in 10-µl volumes using a 1/16 dilution of Big Dye Terminator v3.1 ready reaction mix in Big Dye sequencing buffer (Applied Biosystems, Foster City, CA), 5 µM M13 primer, and 100 ng of template DNA. Cycle sequencing was performed for 40 cycles at 95°C for 10 seconds, 50°C for 5 seconds, and 60°C for 2.5 minutes. Excess dye terminators were removed using CleanSEQ (Agencourt Biosciences Corporation, Beverly, MA). Sequencing plates were analyzed on Applied Biosystems 3730/3730 x 1 DNA Analyzers using Applied Biosystems Sequence Analysis software. M13 forward primers were used for 5′ end sequencing of the colorectal and breast libraries; M13 reverse primers were used for 3′ end sequencing of the normal lung and prostate libraries; both M13 forward and reverse primers were used for 5′ and 3′ end sequencing of the lung tumor and ovarian cancer libraries.
Plasmids
The pEYFP expression vector (Clontech) was used to express YFP. The pSUPER vector [42] was used for RNA interference.
Small Inhibitory RNA (siRNA)
siRNA design followed four complementary strategies, i.e., Tuschl criteria (position in the mRNA, guanine-cytosine [GC] content, base composition, and flanking sequences) [43], Invitrogen algorithms (rnaidesigner.invitrogen.com/rnaiexpress/; sequence composition, nucleotide content, thermodynamic properties, and experimental validation), Whitehead Institute screening procedures (jura.wi.mit.edu/bioc/siRNAext/; Tuschl criteria, predictions of binding energies and BLAST filtering of cross-hybridizing sequences) [44], and Sonnhammer searches (www.sirnawizard.com/design_advanced.php; data mining on validated siRNA databanks, using motif rules and energy parameters) [45].
Annealed siRNA oligos were subcloned into the pSUPER vector. siRNA expression constructs were transiently transfected in MCF-7 and HBL-100 breast cancer cells and in SK-OV-3, IGROV-1, and OVCAR-3 ovarian cancer cells. siRNA-targeted transcript levels were quantified by real-time PCR. Negative-control siRNA directed toward irrelevant targets were used; these were chosen after extensive testing for lack of off-target influence on cell growth.
Quantitative RT-PCR
Hybrid sequences in cancer cell lines and tumor samples were amplified by quantitative RT-PCR. One microgram of total RNA was reverse transcribed with the M-MLV Reverse Transcriptase (Promega, Madison, WI) according to standard protocols. cDNA was quantified by ethidium bromide fluorescence in solution [46]. Quantitative RT-PCR was performed with an ABI-PRISM 7900HT Sequence Detection System (PE Applied Biosystems, Foster City, CA), using Sybr Green as the probe (Applied Biosystems). Samples were assayed as replicates (two or three independent samples), and the 1.83-ΔΔCT method was used to calculate the relative changes in gene expression [13]. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene was used as an internal control. For setup curves, ΔCT(CT, target gene - CT, GAPDH) was calculated for each cDNA dilution. The data were fit using least-squares linear regression analysis. As amplification efficiency was linear over the range of RNA amounts used, amplification curves were used to calculate crossover point values for siRNA-treated samples. To check for the correctness of amplified bands, amplification products were run on 3% agarose gels. Amplified products were purified and extensively sequenced (BMR Genomics, Padova, Italy). Quantitative RT-PCR was also performed with PrimeTime IDT (Integrated DNA Technologies, Bologna, Italy; www.idtdna.com) to reliably detect with higher sensitivity the interchromosomal CHD2-CHMP1A fusion in normal tissues.
Diagnostic PCR
Interchromosomal CHD2-CHMP1A and ADK-DHX8 and intrachromosomal PRKAA1-TTC33, SAMM50-PARVB and P2RX5-TAX1BP3, URB1-C21orf45, CTBS-GNG5, THC2538403 ZNF498-CUX1, THC2523555 C9orf47-S1PR3, and THC2668182 KLH22-SCARF were amplified in 10 breast and 4 ovarian cancer cell lines and in 25 tumor samples by nested PCR. Chimeric mRNA were amplified by 35 amplification cycles (30 seconds at 94°C for denaturation, 30 seconds at 60°C for annealing, and 30 seconds at 72°C for extension). Hot Master Taq-polymerase 0.7 units (Eppendorf) and 12.8 pmol of forward and reverse primers were used for the amplification reaction. All of the amplified products were purified and sequenced (BMR Genomics).
Statistical Analysis
Two-way analysis of variance and post-hoc Bonferroni t tests were used for growth curve comparisons. Data were analyzed using Sigma Stat (SPSS Science Software UK Ltd, Birmingham, United Kingdom) and GraphPad Prism (GraphPad Software Inc, La Jolla, CA).
Results
Chimeric mRNA Detection Procedure
A procedure (FusionMiner) was designed to process BLAST analyses of query sequences against genomic databanks, through sequential stages of analysis and exclusion and pass-or-fail tests, as described in the Supplemental Online Material (Figure 1 and Tables S1–S7). FusionMiner performance was assessed by screening the Dana Farber Cancer Institute Gene Index Project tentative human consensus (THC) collection (Figure S1) and long-sequence-read 454 Titanium data sets (Supplemental Online Material). Samples of the identified chimeras were then validated by diagnostic PCR and by real-time quantitative PCR analysis of cancer cell lines.
Transcriptome Sequencing for Growth Regulatory Chimera Discovery
To discover growth regulatory chimeras, we then performed a large-scale sequencing and analysis of tumor and normal tissue transcriptomes. To maximize chances of discovery of growth regulatory chimeras, both major tumor histotypes, i.e., non-small cell lung, breast, prostate, ovary, and colorectal cancers, and the corresponding normal tissues were analyzed. Long-sequence-read (900 bp on average) cDNA library data sets were obtained: 481,765 from ovary, 485,049 from prostate, 157,259 from breast, 46,445 from colon, and 603,935 from lung.
These sequences were run through FusionMiner. Twenty-five mRNA chimeras were identified (15 intrachromosomal and 10 interchromosomal; Table S8, Supplemental Sequence Data). All sequences were shown to possess the structural characteristics of bona fide chimeric mRNA [24] (Supplemental Sequence Data). Breast and ovarian chimeras were validated by RT-PCR and functional assays (see below).
These findings led to estimate absolute chimera frequencies as 1.4 x 10-5 of all mRNA. This was in remarkable agreement with NGS sequencing data (≈2 x 10-5) (Supplemental Online Material), indicating an unexpectedly high frequency of expression of chimeric mRNA.
Chimeric Transcript Expression in Cancer Cells
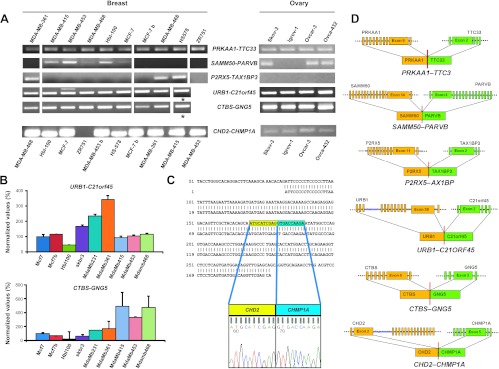
Expression of the nine chimeras from the breast library and of the four chimeras from the ovarian library was analyzed in breast (MCF-7, HBL-100, SK-BR-3, MDA-MB-231, MDA-MB-361, MDA-MB415, MDA-MB-453, MDA-MB-468, HS578, and ZR751) and ovarian (SKOV-3, IGROV-1, OVCAR-3, and OVCA-432) cancer cell lines (Figure 2 and Table S8). Six of the nine chimeras were successfully amplified by RT-PCR (Figure 2A and Table S9). Amplification from breast cancer cells was obtained for PRKAA-TTC33 (10/10 lines), SAMM50-PARVB (5/10 lines), P2RX5-TAX1BP3 (3/10 lines), and CHD2-CHMP1A (9/10 lines) (Figure 2A, left). All individual amplicons were sequence verified (Figure 2C). Three of these chimeras were also detected in ovarian cancer cells: PRKAA-TTC33 (4/4 lines); SAMM50-PARVB (3/4 lines), and CHD2-CHMP1A (4/4 lines) (Figure S3).
Figure 2.
Chimeric mRNA expression in cancer cell lines. (A) Expression of chimeras discovered from tumor and normal tissue library sequencing; agarose electrophoresis of nested or real-time PCR products. Breast and ovarian cancer cell lines are indicated; *SK-BR-3. (B) URB1-C21ORF45 (top) and CTBS-GNG5 (bottom) expression in breast cancer cell lines by quantitative RT-PCR; results are expressed as percent values (MCF-7 = 100); three replica samples were analyzed per data point. Bars, SD. (C) CHD2-CHMP1A. Sequence of the PCR amplicon versus that of the chimera isolated from the breast library. (D) Structure of validated chimeric mRNA; 5′ partners (orange) and 3′ partners (green) are shown; exon junctions are indicated.
The URB1-C21ORF45 and CTBS-GNG5 chimeras from the ovarian library were identified in all four ovarian cancer cell lines (Figure 2A, right). They were also detected in all breast cancer lines. Notably, different cancer cells expressed different steady-state levels of the chimeric mRNA, e.g., CTBS-GNGS was approximately 20 times less expressed in HBL-100 cells, as compared with MDA-MB-415 cells (Figure 2B).
Overall, 75% of the THC chimeras and 54% of the chimeras from breast and ovary libraries (Tumor Transcriptome Sequencing Project) were detected in breast and ovarian cancer cell lines/primary tumors.
Fusion Proteins Encoded by the Growth Regulatory Chimeras
CHD2-CHMP1A. CHD2 encodes the chromodomain helicase DNA-binding protein 2; CHMP1A encodes the chromatin-modifying protein 1A. Of interest, both of these chimera partners encode proteins with regulatory roles on chromatin/DNA structure. However, only the first 20 amino acids of helicase DNA-binding protein 2 are retained in the fusion-protein product (Table S11). This contains a casein kinase II phosphorylation site (prosite.expasy.org/). One out-of-frame C-terminal amino acid is provided by the chromatin-modifying protein 1A sequence (Table S11) and generates a hybrid N-glycosylation site, although it is not clear if this is processed in vivo.
CTBS-GNG5. CTBS encodes chitobiase; GNG5 encodes the di-N- acetyl-binding and guanine-nucleotide-binding proteins. Chitobiase is a lysosomal glycosidase that is involved in degradation of asparagine-linked oligosaccharides on glycoproteins. It is also involved in the hydrolysis of N -acetyl-β-d-glucosamine. GNG5 encodes the γ chain of trimeric G proteins. A fusion mRNA between chitobiase and guanine-nucleotide- binding protein was also identified by Akiva et al. [47] and by Nacu et al. [26]. The CTBS-GNG5 is an “in-frame” fusion that preserves the first 319 amino acids from the N-terminal partner and the last 41 amino acids from the C-terminal partner (Table S11). CTBS provides an apparently functional chitinase catalytic domain, with a formal glycosylation site at S300. Most of Gγ5 is retained in the fusion (Supplemental Figure S4), which raises the possibility that the fusion protein can bind its Gβ partner, whether at the cell membrane or in the cytoplasm.
PRKAA1-TTC33. PRKAA1 encodes a 5′-AMP-activated protein kinase catalytic subunit α-1; TTC33 encodes tetratricopeptide repeat domain 33. PRKAA1 is a Ser/Thr protein kinase that protects cells from stress-dependent ATP depletion by switching off ATP-consuming biosynthetic pathways; PRKAA1 also regulates fatty acid and cholesterol synthesis. The N-terminal segment retained in the chimera contains most of the protein kinase A1 protein (478/559 amino acids; Table S11). This retains the full catalytic domain (46-279). However, it loses 12 phosphorylation sites (T488, T490, T522, S496, S502, S506, S508, S516, S520, S523, S524, and S527), which suggests the loss of at least some of its physiological regulation. The fusion protein contains 32 C-terminal amino acids from the 3′ partner mRNA (TTC33), which do not correspond to its canonical reading frame (out-of-frame fusion; Table S11).
SAMM50-PARVB. SAMM50 encodes the sorting and assembly machinery (SAM) component 50 homolog; PARVB encodes β-parvin. SAM-50 is part of the SAM complex, which has a role in integrating β-barrel proteins into the outer mitochondrial membrane. β-Parvin is an actin-binding protein that associates with focal contacts. Parvin is a key regulator of integrin-linked kinase (ILK) and of its downstream pathways. The encoded fusion protein contains an almost entire SAM-50, which only misses its last 15 C-terminal amino acids (Table S11). However, this may lead to disruption of the second major functionally relevant domain of SAM-50. Only two amino acids are contributed by the β-parvin mRNA, as an out-of-frame sequence (Table S11).
URB1-C21orf45. URB1 encodes the pre-ribosomal-associated protein 1 (Npa1p); C21orf45 encodes the kinetochore protein homolog A. Npa1p is a component of pre-60S ribosomal particles and associates with small nucleolar ribonucleoprotein particles (RNPs) that are required for peptidyl transferase center modification. The kinetochore protein homolog A is involved in mitosis and associates with chromatin. It also associates with centromeres in interphase cells, from late anaphase to G1. The fusion protein keeps essentially all the exons of the N-terminal partner (38/39), including its S1385 phosphorylation site (Table S11), suggesting a largely unaltered function. Albeit the C-terminal sequence is not in its native frame, it is unusually long (121 amino acids; Table S11) and may carry novel associated functions.
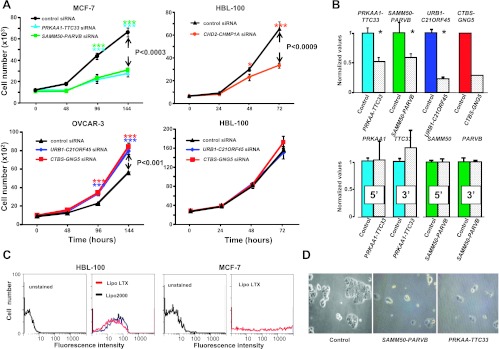
Chimeras Contain Positive and Negative Regulators of Cell Growth
The six chimeras that were found to be expressed by target cell lines in culture were assayed for a role in cell growth. siRNA targeting the chimeric joint (Table S11) were used to inhibit the expression of the corresponding chimeras in breast cancer cells (Figure 3). Transfection efficiency was optimized by using a co-transfected pEYFP reporter plasmid (Figure 3C, left); the vast majority of target cells appeared successfully transfected (Figure 3C). Transfected siRNA downregulated mRNA chimera levels by ≈75% (Figures 3B and S3). To ensure absence of off-target effects, due to artifactual reduction of chimera partner transcript levels, 5′ and 3′ chimeric partners were analyzed in parallel. Levels of partner transcripts of growth regulatory chimeras remained unaffected by siRNA targeting the chimera junction regions (Figure 3B).
Figure 3.
Cell growth modulation by chimeras. (A) Control (Table S10) and chimera-targeting siRNA are color coded. (Top, left) MCF-7; PRKAA-TTC33 (cyan), SAMM50-PARVB (green), control (black). (Top, right) HBL-100; CHD2-CHMP1A (orange), control (black). (Bottom, left) OVCAR-3; URB1-C21ORF45 (blue), CTBS-GNG5 (red). (Bottom, right) HBL-100 treated with siRNA for chimeras from ovarian libraries; URB1-C21ORF45 (blue), CTBS-GNG5 (red). Bars, SD. Brackets, P value of two-way analysis of variance; Bonferroni t test significance: *P ≤ .05; ***P ≤ .001. (B) Real-time PCR of siRNA-transfected cells. (Top) Chimeric RNA (left to right: PRKAA-TTC33 and SAMM50-PARVB in MCF-7; URB1-C21ORF45 and CTBS-GNG5 in OVCAR-3). (Bottom) Single-partner RNA expression after the indicated siRNA treatment (left to right: PRKAA-TTC33 and SAMM50-PARVB in MCF-7). (C) Flow cytometry analysis of transfected HBL-100 and MCF7 cells; YFP was used as a transfection efficiency benchmark. (Left) HBL-100; LTX (red) or Lipo-2000 (blue). (Right) MCF-7; LTX transfection. (D) MCF-7 cell growth blockade after PRKAA-TTC33-targeted or SAMM50-PARVB-targeted siRNA treatment (day 6 after transfection).
Remarkably, five of six tested chimeras appeared to regulate cell growth. The strongest growth inhibition in HBL-100 cancer cells was caused by down-regulation of CHD2-CHMP1A (Figure 3A). Parallel growth blockade in MCF-7 cells was observed on shutdown of PRKAA-TTC33 and SAMM50-PARVB (Figures 3A and S3B). Monitoring of cell growth inhibition by PRKAA-TTC33 and SAMM50-PARVB siRNA through optical microscopy (Figure 3D) and image analysis (Figure S3, B and C) confirmed a dramatic reduction of MCF-7 cell growth. Growth inhibition by PRKAA-TTC33 and SAMM50PARVB down-regulation was also demonstrated for HBL-100 cells.
We then went on to test URB1-C21ORF45-targeting siRNA in ovarian cell lines. Unexpectedly, an increase in cell growth was reproducibly observed in OVCAR-3 (Figure 3A) and IGROV-1 cells, which indicates a growth inhibitory role of the URB1-C21ORF45 chimera. Albeit URB1-C21ORF45 is expressed by SKOV-3 and HBL-100 cells, the corresponding siRNA had no effects on these cells, suggesting a cell-specific function of these growth inhibitory chimera (Figure 3A). These tests were repeated using CTBS-GNG5-targeted siRNA. These assays showed that the CTBS-GNG5 chimera also has a growth inhibitory function in OVCAR-3 and IGROV-1 cells (Figures 3 and S3B). Again, SKOV-3 and HBL-100 cancer cells were insensitive to the inhibitory function of CTBS-GNG5, consistent with a differential tuning of chimera-dependent growth-control circuitries in specific cell lines.
Protein-encoding reading frames of the growth regulatory chimeras were analyzed (Table S11). In all cases but one, the downstream partners did not provide in-frame sequences, generating out-of-frame, mostly short chimeric tails. This suggested altered regulation and/or dominant-negative function of a truncated molecule as a mechanism of action of these chimeric products. However, the CTBS-GNG5 is an in-frame chimera that retains the first 319 amino acids from the N-terminal chitobiase and most of the C-terminal Gγ5 (41 amino acids), including its Gβ-binding interface (Figure S2). This suggested that the chimeric protein can bind its Gβ partner in trimeric G proteins (Supplemental Sequence Data).
Chimera Expression in Normal Tissues
We assessed the presence and expression levels of the five growth-controlling chimeras in mRNA from normal tissues (breast, lung, placenta, uterus, prostate, stomach, colon, pancreas, and kidney) by nested and real-time PCR. The four intrachromosomal chimeras (PRKAA-TTC33, SAMM50-PARVB, URB1-C21ORF45, and CTBSGNG5) were detected in all screened normal tissues (Figure 4). This was consistent with previous findings on the expression of oncogenic mRNA chimeras in normal tissues [4,13,14,17–20]. However, we found essentially no trace of the CHD2-CHMP1A interchromosomal chimera in normal tissues. CHD2-CHMP1A was expressed by almost all cancer cell lines (13/14), thus appearing as a cancer-related event.
Figure 4.
Expression of mRNA chimeras in normal tissues. (A) (Left) Agarose electrophoresis analysis of real-time PCR products (Sybr Green real-time PCR). (Right) Real-time PCR analysis of the CHD2-CHMP1A chimeric mRNA in normal tissues (PrimeTime real-time PCR). HBL-100 cell cDNA was used as a positive control. (Bottom) Amplification plots of CHD2-CHMP1A mRNA in the HBL-100 breast cancer cell line (blue) and normal breast tissue (red). CHD2-CHMP1A (top lanes) and GAPDH (bottom lanes) were amplified in duplicate. Green triangles, successful amplification; red triangles, no amplification. (B) Agarose electrophoresis of nested PCR products of PRKAA1-TTC33, SAMM50-PARVB, CTBS-GNG5, and URB1-C21orf45 in normal tissues.
Expression of Growth Regulatory Chimeras in Primary Tumors
In vitro cell growth regulatory chimeras are expressed by different cancer histotypes. Total RNA was extracted from breast, ovarian, gastric, colon, kidney, and uterine tumors [13,48], was reverse transcribed, and amplified. We took advantage of chimeric-band melting-temperature specificity peaks (Figure S3E) to select for bona fide amplification candidates. Amplified candidates were then systematically sequenced. PRKAA-TTC33 was detected in all 25 of these tumors, SAMM50PARVB in 15 tumors, P2RX5-TAX1BP3 in 8 tumors, and URB1-C21ORF45 in 21 tumors; CTBS-GNG5 was detected in almost all tumors (Figure 5A and Table 1); CHD2-CHMP1A was identified in 11 tumors (Figure 5B). ADK-DHX8 was diagnosed in two tumors (Figure 5C). Hence, growth regulatory chimeras are broadly expressed in human tumors but in heterogeneous manners. This suggests a positive selective pressure [49] for a fusion mRNA-based growth regulatory mechanism during tumor development, which appears to operate in a chimera and tumor-type-specific manner.
Table 1.
Expression of Chimeric mRNA by Tumor Type
| Chimera | Breast* [n/10 (%)] | Ovary [n/2 (%)] | Stomach [n/2 (%)] | Colon [n/7 (%)] | Kidney [n/2 (%)]> | Uterus [n/2 (%)] |
| PRKAA-TTC33 | 8 (80) | 2 (100) | 2 (100) | 7 (100) | 2 (100) | 2 (100) |
| SAMM50-PARVB | 5 (50) | - | 1 (50) | 5 (71) | 2 (100) | 1 (50) |
| P2RX5-TAX1BP3 | 2 (20) | - | - | 5 (71) | 1 (50) | 1 (50) |
| URB1-C21ORF45 | 8 (80) | - | 1 (100) | 7 (100) | 2 (100) | 2 (100) |
| CTBS-GNG5 | 8 (80) | 1 (50) | 2 (100) | 7 (100) | 2 (100) | 2 (100) |
| CHD2-CHMP1A | 3 (30) | - | 2 (100) | 2 (28) | 1 (50) | 2 (100) |
| ADK-DHX8 | - | - | - | 1 (14) | 1 (50) | - |
-, Not detected.
Tumors; total numbers are below each histotype.
Discussion
We have opened the field of the in silico identification of mRNA chimeras in cancer cells, through analysis of cDNA sequence databanks [24]. NGS approaches have enormously increased the amount of sequencing data of potential use for chimera discovery. However, short-read second-generation NGS analyses identify mRNA chimeras through a probabilistic fitting of highly multiplexed short-tag data sets [7,19,20,25–28,50–53], which severely affects both specificity and sensitivity of detection of mRNA chimeras. However, rapid progress is being made toward achieving longer sequence reads and higher sequencing accuracy, which allows to reduce sequence errors while improving contig assembly procedures. To permit high-throughput, high-specificity chimera discovery in long-read sequence data sets, we have developed the FusionMiner search strategy. This was shown to reach a 95.9% chimera identification specificity, with a low 4.1% false-negative classification rate. This search strategy was extensively validated by RT-PCR and cDNA sequencing (Table S1b).
Global chimera frequencies were computed for separate sequencing projects. Analysis of a human transcriptomic 454 data set of 19,527 contigs and 173,005 singletons led to the identification of four sequences as bona fide chimeras, for a chimera frequency of 4/192,532, i.e., 2 x 10-5 of all mRNA. High-throughput sequencing of cDNA libraries from tumors and corresponding normal tissues generated 1,774,453 long-read sequences. Twenty-five were identified by FusionMiner as bona fide chimeras, for a chimera frequency of 25/1,774,453, i.e., 1.4 x 10-5, in remarkable agreement with the NGS data. Taken together, these findings suggest a chimera frequency of ≈2 x 10-5 in cellular transcriptomes. Issues of data set size and of transcriptome tissue specificity suggest these to be minimal estimates. A proof of principle of this scenario was obtained, as one of the interchromosomal chimeras, which could not be detected in cell lines, and was identified in 2 of 10 primary breast cancers.
Most of the chimeras analyzed were shown to have a regulatory role in transformed cell growth [54,55]. Notably, tumor growth inhibitory mRNA chimeras, e.g., URB1-C21ORF45 and CTBS-GNG5, were also discovered. Of interest, these were shown to have inhibitory capacity on the growth of a subset of ovarian cancer cells, whereas other ovarian and breast cancer cells were not affected, suggesting different regulatory contexts for chimera-driven growth control in different cell lines. Most tumors were shown to express these growth regulatory chimeras, consistent with a positive selective pressure for exploiting this growth regulatory mechanism during tumor development.
Supplemental Material
Long-range transcriptome sequencing reveals cancer cell growth regulatory chimeric mRNAs
Roberto Plebani, Gavin R. Oliver, Marco Trerotola, Emanuela Guerra, Pamela Cantanelli, Luana Apicella, Andrew Emerson, Alessandro Albiero, Paul D. Harkin, Richard D. Kennedy and Saverio Alberti
Supplemental material includes:
Supplemental Material and Methods Supplemental Sequence Data
Supplemental References
Supplemental Material and Methods
Chimeric mRNA detection procedure. We designed the FusionMiner software workflow (Figure 1) to process BLAST analyses of query sequences against genomic databanks via sequential stages of analysis and exclusion, pass-or-fail tests. Candidate chimera were cross-validated versus experimentally verified sequences and by RT-PCR.
Alignments versus genomic assemblies were first parsed to remove spurious data on the basis of length and percent identity (=98%, over =95% of a candidate length). Individual filtered alignments were then clustered and concatenated across alignment breaks and intronic regions, based on a permissible gap criterion, to identify their genomic context. Sequences aligning to one or two chromosomes were segregated and processed separately, as intra-chromosomal (which most frequently derive from inter-genic transcription) and inter-chromosomal (which most frequently derive from chromosomal translocations and trans-splicing) chimeric candidates, respectively.
The fusion point (FP) for inter-chromosomal candidates was expected to correspond to the point in a clustered alignment where a sequence ceased to align with one chromosome and started to align with the second. Alignments on either side of a FP were assessed on the basis of length and percent identity (defaults =100 bp length, =98% identity), following clustering of the original alignments and weighted averaging of percent identities. This was to ensure that only high-quality alignment data were used in chimeras detection, while low quality or spurious alignments were dropped out. FPs were then examined and filtered, based on the degree of overlap at this position (default =10 bp). Allowing this degree of error ensured retention of true chimeras with small areas of fortuitous sequence homology across the other side of the FP. A parallel filtration procedure was applied for gaps at FP (default =10 bp), which allowed to compensate for possible sequencing errors at this position. Successful candidates were then checked for agreement of their FP with known exon boundaries, using genomic coordinate data from Ensembl, although non-canonical FP (i.e. recombinations within exons) were also identified and separately stored. Ensembl was chosen as genomic coordinate reference site as it contains confirmed gene predictions, which are integrated with external data sources, including the Sanger Institute HAVANA [1], RefSeq at NCBI [2], and the UCSC Genome Browser [3]. When candidate-chimera FPs were found to correspond to an exon-exon boundary, candidates were accepted. Intrachromosomal candidates were treated in a corresponding manner. A permissible error threshold (default 3 bp) was applied at this stage, to compensate for sequencing errors or alignment blurring due to small areas of local homology on either side of a boundary.
Both chimera partner mRNAs were selected for occurrence in the same reading orientation versus known mRNAs (i.e. plus orientation). Joining to a ‘minus’ strand is, indeed, most likely generated by cDNA recombination during library construction [4]. A special case is that of a gene that transcribes both the minus and the plus strands. Of note, both mRNA classes would be available for matching in transcript datasets. FusionMiner would then operationally qualify both mRNAs as ‘plus’.
Accepted candidates then entered a clustering step, whereby they were assessed and grouped if they shared the same FP. A disagreement of up to 10 bp was allowed during clustering, consistent with the errors permitted at the stage of FP definition. Chimeras were then sorted and presented in order of multiplicity of occurrence.
FusionMiner also allowed to identify and cluster candidates which were rejected during the exon-boundary checking stage. This was to permit the discovery of recurrent, non-canonical chimeras, which are expected to derive from DNA joining at recombination hot spots [5].
Prediction of reading-frame preservation at the FP was then performed.
FusionMiner search performance. The performance of the FusionMiner detection strategy (Figure S1) was assessed by screening the Dana Farber Cancer Institute (DFCI) Gene Index Project tentative human consensus (THC) collection (Figure S1). This led to the identification of 228 chimeras (105 inter-chromosomal and 123 intra-chromosomal), involving 414 genes (Tables S1–S3). Chimeras discovered by FusionMiner in the DFCI Gene Index Project encoded enzymes (16%), transcription factors/ chromatin modulators (11.5%), G proteins (5.8%), protein binding partners (5.8%), transporters (4.5%), cytoskeletal proteins (2.6%), receptors and proteases (1.9%). Curated sequence analysis [4] indicated that 221 of the 228 chimeric mRNAs candidates (96.9%) did fit all bona fide chimera criteria. Sixty-one of these chimeras were uniquely identified by FusionMiner (Table S3).
FusionMiner default settings were optimized to obtain maximum specificity in chimera detection. To provide differential estimates of performance (sensitivity versus specificity), FusionMiner analysis parameters were then systematically altered, and their impact on analysis outcomes was assessed. Splicing at exon/exon borders: exon-exon boundary settings were relaxed, by extending the tolerance up to 8 bp, i.e. exon boundaries were allowed to be identified within 8 bp of BLAST alignments borders. This can be useful for specific requirements, e.g. for short-sequence-length datasets, or for alignments to poor-quality genome sequence regions. Thirty-nine additional sequences were obtained as compared with the default 3 bp tolerance (Tables S1b, S4–S6). Thirty-seven of these appeared to be bona fide chimeras (96.6% specificity, versus 96.9% with optimal/ default parameters, -0.3% specificity; 228 + 39 = 267; +17.1% sensitivity). Bp gap: Extending the allowed gaps in FusionMiner to 30 bp, instead of the 10 bp default, resulted in identification of only 4 additional chimeras (Tables S1b, S4–S6). Three of these (75%) were bona fide fusion sequences.
Percent query identity (%ID): A low %ID might be due to gaps, bad sequencing, or real mismatch regions. The default requirement for 98% ID was thus relaxed to 94%, to allow detection of these problematic sequences. Thirty-four additional chimeras were detected versus default values (Tables S1b, S4–S6). Thirty two of these (91.4%) were confirmed to be true positive bona fide fusion sequences (-0.4% specificity; +14.9% sensitivity).
Query length: The FusionMiner strategy/ sequential validation/ parameter combination was optimized for the recognition of small, bona fide chimeric sequences from NGS analysis. We validated this by analyzing chimeras with 50-base matches around a fusion joint. By shortening the minimum allowed length for matches from 100 bp to 50 bp, we identified 59 additional THC sequences versus the default searches (Tables S1b, S4–S6). Strikingly, 56 out of these 59 (94.9%) were true positives (-0.4% specificity; +25.9% sensitivity). Remarkably, 349 of 364 (95.9%) chimeric mRNAs detected by FusionMiner from THC were shown to fit chimera identification criteria.
Validation of discovered THC chimeras. THC chimeras from breast cancer (4 sequences) were searched for in breast cancer cell lines (Figure S1). cDNAs were obtained from MCF-7, MCF-7/Almac, HBL-100, SK-BR-3, MDA-MB-231, MDA-MB-361, MDA-MB-415, MDA-MB-453, and MDA-MB468 cells, and each chimera was amplified by direct or nested PCR. All PCR amplified bands were verified by sequencing. Successful amplification was achieved for 3 out of 4 chimeras (75%), i.e. THC2538403 ZNF498-CUX1 (4/9 lines), THC2523555 (an additional long intergenic transcript was identified as ENST00000358157) C9orf47-S1PR3 (8/9 lines) and THC2668182 KLH22-SCARF2 (7/9 lines) (Figure S1). We then extended this analysis to SKOV-3, IGROV-1, OVCAR-3 and OVCA-432 ovarian cancer cells. Two of the chimeras from breast cancer, i.e. THC2523555 C9orf47-S1PR3 and THC2668182 KLH22-SCARF2, were identified in all four ovarian cancer cell lines (Figure S1), suggesting broad expression across different tumor histotypes. Chimeric sequence abundance was measured by real-time quantitative PCR (Figure S1B). Chimeras were detected at considerably different levels in the tested cell lines, consistent with a regulated expression [6–11].
FusionMiner performance on long-reads NGS datasets. FusionMiner was further validated on long-sequence read from 454-generated output files. A 454 Titanium dataset of 1,241,098 reads, 355.5 bp in average length (www.bmr-genomics.it/∼alex/ALBERTI/) was compiled by Newbler into 19,527 contigs and 173,005 singletons; 28,561 sequences were identified as outliers. FusionMiner identified one inter-chromosomal and three intra-chromosomal bona fide chimeras (Tables S1, S7, Supplemental Sequence Data). These findings reveal absolute chimeras frequencies of 2x10-5 (4 out of 192,532) in whole cell transcriptomes.submis
Supplemental Sequence Data
Sequences, BLAST alignments, and exons involved in fusions are indicated for the individual novel chimeras identified from specific sequencing datasets. The interaction networks of the proteins encoded by growth-regulatory chimera partners are shown.
Acknowledgments
We thank M. Iacono for providing the Roche NGS data sets and C. Berrie for critical reading and editing of the manuscript.
Abbreviations
- FP
fusion point
- NGS
next-generation sequencing
- SD
standard deviation
Footnotes
This work was supported by Fondazione Cassa di Risparmio della Provincia di Chieti, Italian Ministry of Health (RicOncol grant RF-EMR-2006-361866), Fondazione Compagnia di San Paolo (grant 2489IT), Ministero dello Sviluppo-Made in Italy (contract No MI01_00424), and the Italian Foundation for Cancer Research (fellowship to M.T.).
This article refers to supplementary materials, which are designated by Tables S1 to S11 and Figures S1 to S4 and are available online at www.neoplasia.com. The Fusion-Miner software is freely available at FusionMiner.sourceforge.net.
References
- 1.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 2.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 3.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 4.Rickman DS, Pflueger D, Moss B, VanDoren VE, Chen CX, de la Taille A, Kuefer R, Tewari AK, Setlur SR, Demichelis F, et al. SLC45A3ELK4 is a novel and frequent erythroblast transformation-specific fusion transcript in prostate cancer. Cancer Res. 2009;69:2734–2738. doi: 10.1158/0008-5472.CAN-08-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Fehr A, Roser K, Heidorn K, Hallas C, Loning T, Bullerdiek J. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes Chromosomes Cancer. 2008;47:203–206. doi: 10.1002/gcc.20522. [DOI] [PubMed] [Google Scholar]
- 7.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, Han B, Cao Q, Cao X, Suleman K, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciampi R, Knauf JA, Kerler R, Gandhi M, Zhu Z, Nikiforova MN, Rabes HM, Fagin JA, Nikiforov YE. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture\ Eur J Endocrinol. 2006;155:645–653. doi: 10.1530/eje.1.02289. [DOI] [PubMed] [Google Scholar]
- 10.Edwards PA. Fusion genes and chromosome translocations in the common epithelial cancers. JPathol. 2010;220:244–254. doi: 10.1002/path.2632. [DOI] [PubMed] [Google Scholar]
- 11.Skotheim RI, Thomassen GO, Eken M, Lind GE, Micci F, Ribeiro FR, Cerveira N, Teixeira MR, Heim S, Rognes T, et al. A universal assay for detection of oncogenicfusion transcripts by oligo microarray analysis. Mol Cancer. 2009;8:5. doi: 10.1186/1476-4598-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruzik JP, Maniatis T. Spliced leader RNAs from lower eukaryotes are trans-spliced in mammalian cells. Nature. 1992;360:692–695. doi: 10.1038/360692a0. [DOI] [PubMed] [Google Scholar]
- 13.Guerra E, Trerotola M, Dell'Arciprete R, Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C, Lorenzini F, Rossi C, et al. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Wang J, Mor G, Sklar J. A neoplastic gene fusion mimics trans-splicing of RNAs in normal human cells. Science. 2008;321:1357–1361. doi: 10.1126/science.1156725. [DOI] [PubMed] [Google Scholar]
- 15.Terrinoni A, Dell'Arciprete R, Fornaro M, Stella M, Alberti S. Cyclin D1 gene contains a cryptic promoter that is functional in human cancer cells. Genes Chromosomes Cancer. 2001;31:209–220. doi: 10.1002/gcc.1137. [DOI] [PubMed] [Google Scholar]
- 16.Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, Han B, Jing X, Sam L, Barrette T, Palanisamy N, Chinnaiyan AM. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Communi D, Suarez-Huerta N, Dussossoy D, Savi P, Boeynaems J-M. Cotranscription and intergenic splicing of human P2Y11 and SSF1 genes. J Biol Chem. 2001;276:16561–16566. doi: 10.1074/jbc.M009609200. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Wang J, Ma X, Sklar J. Gene fusions and RNA trans-splicing in normal and neoplastic human cells. Cell Cycle. 2009;8:218–222. doi: 10.4161/cc.8.2.7358. [DOI] [PubMed] [Google Scholar]
- 19.Pflueger D, Terry S, Sboner A, Habegger L, Esgueva R, Lin PC, Svensson MA, Kitabayashi N, Moss BJ, Macdonald TY, et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2010;21:56–67. doi: 10.1101/gr.110684.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgren H, Murumagi A, Kangaspeska S, Nicorici D, Hongisto V, Kleivi K, Rye IH, Nyberg S, Wolf M, Borresen-Dale AL, et al. Identification of fusion genes in breast cancer by paired-end RNA-sequencing. Genome Biol. 2011;12:R6. doi: 10.1186/gb-2011-12-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrogi F, Biganzoli E, Querzoli P, Ferretti S, Boracchi P, Alberti S, Marubini E, Nenci I. Molecular subtyping of breast cancer from traditional tumor marker profiles using parallel clustering methods. Clin Cancer Res. 2006;12:781–790. doi: 10.1158/1078-0432.CCR-05-0763. [DOI] [PubMed] [Google Scholar]
- 22.Rabbitts TH, Stocks MR. Chromosomal translocation products engender new intracellular therapeutic technologies. Nat Med. 2003;9:383–386. doi: 10.1038/nm0403-383. [DOI] [PubMed] [Google Scholar]
- 23.Cimoli G, Malacarne D, Ponassi R, Valenti M, Alberti S, Parodi S. Meta-analysis of the role of p53 status in isogenic systems tested for sensitivity to cytotoxic antineoplastic drugs. Biochim Biophys Acta. 2004;1705:103–120. doi: 10.1016/j.bbcan.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Romani A, Guerra M, Trerotola M, Alberti S. Detection and analysis of spliced chimeric mRNAs in sequence databanks. Nucleic Acids Res. 2003;31:1–8. doi: 10.1093/nar/gng017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sboner A, Habegger L, Pflueger D, Terry S, Chen DZ, Rozowsky JS, Tewari AK, Kitabayashi N, Moss BJ, Chee MS, et al. FusionSeq: a modular framework for finding gene fusions by analyzing paired-end RNA-sequencing data. Genome Biol. 2010;11:R104–.. doi: 10.1186/gb-2010-11-10-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nacu S, Yuan W, Kan Z, Bhatt D, Rivers CS, Stinson J, Peters BA, Modrusan Z, Jung K, Seshagiri S, et al. Deep RNA sequencing analysis of read-through gene fusions in human prostate adenocarcinoma and reference samples. BMC Med Genomics. 2011;4:11. doi: 10.1186/1755-8794-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asmann YW, Hossain A, Necela BM, Middha S, Kalari KR, Sun Z, Chai HS, Williamson DW, Radisky D, Schroth GP, et al. A novel bioinformatics pipeline for identification and characterization of fusion transcripts in breast cancer and normal cell lines. Nucleic Acids Res. 2011;39:e100. doi: 10.1093/nar/gkr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyer MK, Chinnaiyan AM, Maher CA. ChimeraScan: a tool for identifying chimeric transcription in sequencing data. Bioinformatics. 2011;27:2903–2904. doi: 10.1093/bioinformatics/btr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carletti E, Guerra E, Alberti S. The forgotten variables of DNA array hybridization. Trends Biotechnol. 2006;24:443–448.. doi: 10.1016/j.tibtech.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 31.Loman NJ, Misra RV, Dallman TJ, Constantinidou C, Gharbia SE, Wain J, Pallen MJ. Performance comparison of benchtop high-throughput sequencing platforms. Nat Biotechnol. 2012;30:434–439. doi: 10.1038/nbt.2198. [DOI] [PubMed] [Google Scholar]
- 32.Mardis ER. A decade's perspective on DNA sequencing technology. Nature. 2011;470:198–203.. doi: 10.1038/nature09796. [DOI] [PubMed] [Google Scholar]
- 33.Orsulic S, Li Y, Soslow RA, Vitale-Crosss LA, Gutkind JS, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberti S, Fornaro M. Higher transfection efficiency of genomic DNA purified with a guanidinium thiocyanate-based procedure. Nucleic Acids Res. 1990;18:351–353. doi: 10.1093/nar/18.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dell'Arciprete R, Stella M, Fornaro M, Ciccocioppo R, Capri MG, Naglieri AM, Alberti S. High-efficiency expression gene cloning by flow cytometry. J Histochem Cytochem. 1996;44:629–640. doi: 10.1177/44.6.8666748. [DOI] [PubMed] [Google Scholar]
- 36.Alberti S, Nutini M, Herzenberg LA. DNA methylation prevents the amplification of TROP1, a tumor-associated cell surface antigen gene. Proc Natl Acad Sci USA. 1994;91:5833–5837. doi: 10.1073/pnas.91.13.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti S, Herzenberg LA. DNA methylation prevents transfection of genes for specific surface antigens. Proc Natl Acad Sci USA. 1988;85:8391–8394. doi: 10.1073/pnas.85.22.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberti S, Parks DR, Herzenberg LA. A single laser method for subtraction of cell autofluorescence in flow cytometry. Cytometry. 1987;8:114–119. doi: 10.1002/cyto.990080203. [DOI] [PubMed] [Google Scholar]
- 39.Biganzoli E, Coradini D, Ambrogi F, Garibaldi JM, Lisboa P, Soria D, Green AR, Pedriali M, Piantelli M, Querzoli P, et al. p53 status identifies two subgroups of triple-negative breast cancers with distinct biological features. Jpn J Clin Oncol. 2011;41:172–179. doi: 10.1093/jjco/hyq227. [DOI] [PubMed] [Google Scholar]
- 40.Querzoli P, Coradini D, Pedriali M, Boracchi P, Ambrogi F, Raimondi E, La Sorda R, Lattanzio R, Rinaldi R, Lunardi M, et al. An immunohistochemically positive E-cadherin status is not always predictive for a good prognosis in human breast cancer. Br J Cancer. 2010;103:1835–1839. doi: 10.1038/sj.bjc.6605991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinari N, Lattanzio R, Natoli C, Cianchetti E, Angelucci D, Ricevuto E, Ficorella C, Marchetti P, Alberti S, Piantelli M, et al. Changes of topoisomerase IIα expression in breast tumors after neoadjuvant chemotherapy predicts relapse-free survival. Clin Cancer Res. 2006;12:1501–1506. doi: 10.1158/1078-0432.CCR-05-0978. [DOI] [PubMed] [Google Scholar]
- 42.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 43.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 44.Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert DN, Fesik SW. Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalk AM, Wahlestedt C, Sonnhammer EL. Improved and automated prediction of effective siRNA. Biochem Biophys Res Commun. 2004;319:264–274. doi: 10.1016/j.bbrc.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 46.Bonasera V, Alberti S, Sacchetti A. Protocol for high-sensitivity/long linear-range spectrofluorimetric DNA quantification using ethidium bromide. Biotechniques. 2007;43:173–176. doi: 10.2144/000112500. [DOI] [PubMed] [Google Scholar]
- 47.Akiva P, Toporik A, Edelheit S, Peretz Y, Diber A, Shemesh R, Novik A, Sorek R. Transcription-mediated gene fusion in the human genome. Genome Res. 2006;16:30–36.. doi: 10.1101/gr.4137606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Querzoli P, Pedriali M, Rinaldi R, Lombardi AR, Biganzoli E, Boracchi P, Ferretti S, Frasson C, Zanella C, Ghisellini S, et al. Axillary lymph node nanometastases are prognostic factors for disease-free survival and metastatic relapse in breast cancer patients. Clin Cancer Res. 2006;12:6696–6701. doi: 10.1158/1078-0432.CCR-06-0569. [DOI] [PubMed] [Google Scholar]
- 49.Alberti S. The origin of the genetic code and protein synthesis. J Mol Evol. 1997;45:352–358. doi: 10.1007/pl00006240. [DOI] [PubMed] [Google Scholar]
- 50.Ge H, Liu K, Juan T, Fang F, Newman M, Hoeck W. FusionMap: detecting fusion genes from next-generation sequencing data at base-pair resolution. Bioinformatics. 2011;27:1922–1928. doi: 10.1093/bioinformatics/btr310. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Wang K, He X, Chiang DY, Prins JF, Liu J. A probabilistic framework for aligning paired-end RNA-seq data. Bioinformatics. 2010;26:1950–1957. doi: 10.1093/bioinformatics/btq336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPherson A, Hormozdiari F, Zayed A, Giuliany R, Ha G, Sun MG, Griffith M, Heravi Moussavi A, Senz J, Melnyk N, et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-seq data. PLoS Comput Biol. 2011;7:e1001138. doi: 10.1371/journal.pcbi.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinsella M, Harismendy O, Nakano M, Frazer KA, Bafna V. Sensitive gene fusion detection using ambiguously mapping RNA-Seq read pairs. Bioinformatics. 2011;27:1068–1075. doi: 10.1093/bioinformatics/btr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, Lattanzio R, Piantelli M, Alberti S. The Trop-2 signalling network in cancer growth. Oncogene. 2012 doi: 10.1038/onc.2012.151. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 55.Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, Lattanzio R, de Lange R, Weidle UH, Piantelli M, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2012 doi: 10.1038/onc.2012.36. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
Supplemental References
- 1.Wilming LG, Gilbert JG, Howe K, Trevanion S, Hubbard T, Harrow JL. The vertebrate genome annotation (Vega) database. Nucleic Acids Res. 2008;36:D753–D760. doi: 10.1093/nar/gkm987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pruitt KD, Tatusova T, Klimke W, Maglott DR. NCBI Reference Sequences: current status, policy and new initiatives. Nucleic Acids Res. 2009;37:D32–D36. doi: 10.1093/nar/gkn721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karolchik D, Kuhn RM, Baertsch R, Barber GP, Clawson H, Diekhans M, Giardine B, Harte RA, Hinrichs AS, Hsu F, et al. The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 2008;36:D773–D779. doi: 10.1093/nar/gkm966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romani A, Guerra M, Trerotola M, Alberti S. Detection and analysis of spliced chimeric mRNAs in sequence databanks. Nucleic Acids Res. 2003;31:1–8. doi: 10.1093/nar/gng017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra E, Trerotola M, Dell' Arciprete R, Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C, Lorenzini F, Rossi C, et al. A bi-cistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Wang J, Mor G, Sklar J. A neoplastic gene fusion mimics trans-splicing of RNAs in normal human cells. Science. 2008;321:1357–61. doi: 10.1126/science.1156725. [DOI] [PubMed] [Google Scholar]
- 8.Terrinoni A, Dell'Arciprete R, Fornaro M, Stella M, Alberti S. The Cyclin D1 gene contains a cryptic promoter that is functional in human cancer cells. Genes Chromosomes Cancer. 2001;31:209–20. doi: 10.1002/gcc.1137. [DOI] [PubMed] [Google Scholar]
- 9.Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, Han B, Jing X, Sam L, Barrette T, Palanisamy N, Chinnaiyan AM. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101. doi: 10.1038/nature07638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickman DS, Pflueger D, Moss B, VanDoren VE, Chen CX, de la Taille A, Kuefer R, Tewari AK, Setlur SR, Demichelis F, et al. SLC45A3-ELK4 is a novel and frequent erythroblast transformation-specific fusion transcript in prostate cancer. Cancer Res. 2009;69:2734–8. doi: 10.1158/0008-5472.CAN-08-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Communi D, Suarez-Huerta N, Dussossoy D, Savi P, Boeynaems J-M. Cotranscription and Intergenic Splicing of Human P2Y11 and SSF1 Genes. J. Biol. Chem. 2001;276:16561–16566. doi: 10.1074/jbc.M009609200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.