Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer and has a high rate of mortality. Emerging evidence indicates that hepatocyte growth factor receptor (or Met) pathway plays a pivotal role in HNSCC metastasis and resistance to chemotherapy. Met function is dependent on tyrosine phosphorylation that is under direct control by receptor-type protein tyrosine phosphatase β (RPTP-β). We report here that RPTP-β expression is significantly downregulated in HNSCC cells derived from metastatic tumors compared to subject-matched cells from primary tumors. Knockdown of endogenous RPTP-β in HNSCC cells from primary tumor potentiated Met tyrosine phosphorylation, downstream mitogen-activated protein (MAP) kinase pathway activation, cell migration, and invasion. Conversely, restoration of RPTP-β expression in cells from matched metastatic tumor decreased Met tyrosine phosphorylation and downstream functions. Furthermore, we observed that six of eight HNSCC tumors had reduced levels of RPTP-β protein in comparison with normal oral tissues. Collectively, the results demonstrate the importance of RPTP-β in tumor biology of HNSCC through direct dephosphorylation of Met and regulation of downstream signal transduction pathways. Reduced RPTP-β levels, with or without Met overexpression, could promote Met activation in HNSCC tumors.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, accounting for 6% of all cancers [1,2]. Despite advances in aggressive multidisciplinary treatments, the 5-year survival rate for HNSCC is lower than that for other cancers such as lymphoma, breast cancer, and malignant melanoma. The high mortality rate of HNSCC is due to frequent recurrence and early involvement of regional lymph nodes and subsequent metastasis [3,4]. HNSCC metastasis is a multistage process including cellular detachment, proteolytic degradation of the basement membrane, migration through extracellular matrix, and resistance to apoptosis in a new environment [5].
Met, also known as hepatocyte growth factor (HGF) receptor, is a member of the receptor protein tyrosine kinase (PTK) family. The Met signaling pathway has been shown to promote various stages of HNSCC metastasis [6–12]. Expression of Met and/or its ligand HGF increases during HNSCC progression [13–18]. Met expression is significantly increased in affected lymph nodes compared to corresponding primary tumors [15,18]. In addition, activating mutations of Met are specifically selected during HNSCC metastasis [19]. Increased expression of HGF has also been positively linked to lymph node metastasis of HNSCC [14,20–23]. Inappropriate completion of HGF/Met autocrine loop increases tumorigenesis and enhances metastatic activity [24]. Accordingly, Met expression often correlates with poor prognosis [25,26]. Consistent with the in vivo data, HGF stimulation of Met-expressing HNSCC cell lines promotes an invasive phenotype [7,27]. Collectively, compelling evidence exists that the HGF/Met axis is an important driving force in HNSCC metastasis.
Receptor-type protein tyrosine phosphatase β (RPTP-β) belongs to the family of RPTPs which have been implicated in cell-cell and cell-matrix interactions [28,29]. RPTP-β, encoded by the ptprb gene, is composed of an extracellular domain with cell adhesion molecule-like motif (multiple fibronectin type 3-like domains), a transmembrane domain, and a single intracellular protein tyrosine phosphatase domain [30,31]. RPTP-β is localized to chromosome 12 of human genome where abnormalities occur in some benign tumors whose cells have lost contact inhibition [32]. We have recently demonstrated that RPTP-β directly binds to and specifically dephosphorylates Met in vitro and in intact primary human keratinocytes [33].
We report here that RPTP-β is significantly reduced in cells derived from HNSCC metastatic tumors. Restoration of RPTP-β expression normalizes Met tyrosine phosphorylation, downstream signaling, proliferation, migration, and invasion. In addition, immunohistology reveals that RPTP-β is commonly reduced in HNSCC. These data indicate that down-regulation of RPTP-β may be a prominent mechanism of aberrant Met regulation in HNSCC.
Materials and Methods
Materials
Recombinant human HGF was purchased from R&D Systems (Minneapolis, MN). Met, extracellular-signal-regulated kinase (ERK), and RPTP-β antibodies and protein A/G agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Met (pY1349/1356) antibody was purchased from Rockland (Gilbertsville, PA). Phospho-Met (pY1356) antibody was purchased from Abgent (San Diego, CA). Mitogen extracellular kinase (MEK), phospho-ERK and phospho-MEK antibodies were purchased from Cell Signaling Technology (Beverly, MA). Grb2 antibody was purchased from BD Biosciences (San Jose, CA).
Cell Culture
HNSCC cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS under 5% CO2 at 37°C. The identities of all HNSCC cell lines used in the experiments have been confirmed by DNA genotyping using 10 short tandem repeats (STRs) in the Profiler Plus (Applied Biosystems, Foster City, CA) for human identification. UMSCC-17A and UMSCC-17B were established from a patient with squamous cell cancer of the larynx. UMSCC-17A was from the tumor within the larynx, and UMSCC-17B was from metastasis through the cartilage into the soft tissues of the neck [34]. UMSCC-22A was from a patient with squamous cell cancer of the hypopharynx, and UMSCC-22B was from a metastasis to a lymph node in the neck [35].
Preparation of Whole-Cell Lysates and Immunoprecipitation
After treatment, HNSCC cells were washed twice with ice-cold phosphate-buffered saline, scraped from the culture plates in ice-cold whole-cell extraction buffer [25 mM Hepes (pH 7.2), 75 mM NaCl, 2.5 mM MgCl2, 0.2 mM EDTA, 20 mM β-glycerophosphate, 0.5 mM DTT, and 0.1% Triton X-100], and supplemented with protease inhibitor cocktail (Cat. No. 11697498001; Roche Diagnostics, Indianapolis, IN) and 1 mM sodium orthovanadate (Sigma-Aldrich, St Louis, MO). Following 10 minutes of incubation at 4°C, whole-cell homogenate was centrifuged at 14,000g for 10 minutes. The resultant supernatant represented whole-cell lysate. For immunoprecipitation, whole-cell lysates were pre-cleared with protein A/G agarose and incubated with primary antibody overnight at 4°C, followed by incubation with protein A/G agarose for 2 hours at 4°C. After extensive washing with immunoprecipitation buffer [20 mM Hepes (pH 7.2), 100 mM NaCl, 10% glycerol, and 0.1% Triton X-100], agarose-bound proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blot.
Western Blot Analysis
Equal amounts of protein (same concentration and volume) from whole-cell lysates were resolved by a 10% sodium dodecyl sulfate-polyacrylamide gel (Invitrogen, Carlsbad, CA) and transferred to Immobilon-P filter paper (Millipore, Bedford, MA). Immunoreactive proteins were visualized by enhanced chemifluorescence as per the manufacturers' instructions (GE Healthcare, Buckinghamshire, United Kingdom). Quantification of chemifluorescence was performed using a STORM PhosphorImager device (GE Healthcare) with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Sample loads, antibody concentration, and incubation times were adjusted to yield fluorescent signals within the linear range of detection.
Adeno-X Expression Vector Construction and Adenovirus Production
Wild-type human RPTP-β cDNA was cloned into pShuttle expression vectors, and RPTP-β expression cassette was subsequently ligated with linearized Adeno-X Viral DNA using the Adeno-X Expression System according to the manufacturer's protocol (Clontech Laboratories, Inc, Palo Alto, CA). Human embryonic kidney 293 (HEK293) cells were used for adenovirus production and purification.
Lentivirus-mediated shRNA Silencing of RPTP-β in HNSCC Cells
MISSION TurboGFP shRNA non-targeting (NT) control vector and shRNA constructs targeting RPTP-β (5′-CCGGCGGGTGTATCAGACTAATTATCTCGAGATAATTAGTCTGATACACCCGTTTTT-3′) were purchased from Sigma-Aldrich. Lentivirus particles were generated after transient transfection of RPTP-β shRNA construct DNA and helper plasmids into 293FT cells using the Superfection method according to the manufacturers' instructions (Qiagen, Hilden, Germany). Two days after transfection, cell culture medium was collected and used to infect human HNSCC cells to knockdown endogenous RPTP-β.
Quantitative Real-time Reverse Transcription-Polymerase Chain Reaction
Total cellular RNA was extracted from HNSCC cells using the Miniprep RNA isolation kit as per the manufacturers' instructions (Qiagen). Total cellular RNA (100 ng) was reverse transcribed with random primers using TaqMan Reverse Transcription Kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (PCR) was performed using a TaqMan GEx PCR Master Mix Kit (Applied Biosystems) and a 7300 Sequence Detector (Applied Biosystems). RPTP-β and housekeeping gene 36B4 mRNA levels were quantified and normalized to 36B4. Primers for real-time PCR were given as follows: RPTP-β sense primer, 5′-GGACCATCCGGGAGTTTAAGA-3′; RPTP-β antisense primer, 5′-AAGTGGCGGATGAGTCTGTGT-3′; 36B4 sense primer, 5′-ATGCAGCAGATCCGCATGT-3′; and 36B4 antisense primer, 5′-TTGCGCATCATGGTGTTCTT-3′.
Cell Migration Assay
Mitomycin (10 µg/ml)-treated confluent monolayers of HNSCC cells were scratched with a pipette tip to create a cell-free zone and incubated with or without 10 ng/ml HGF. Cells that migrated into the cell-free zones were photographed after staining with Cell Stain Solution (Cell Biolabs, San Diego, CA).
Matrigel Invasion Assay
Matrigel invasion assays were carried out according to the manufacturer's instructions (Cell Biolabs). Briefly, HNSCC cells were cultured in matrigel invasion assay wells with or without 10 ng/ml HGF. Cells that passed through the matrigel were stained with Cell Stain Solution (Cell Biolabs) and photographed.
Immunohistochemistry
HNSCC tumor tissue sections were purchased from US Biomax (Rockville, MD). After melting the wax at 65°C, tissue sections were deparaffinized in xylene three times, 3 minutes each. The sections were rehydrated through a series of methanol/water solutions three times, 3 minutes each. Antigen retrieval was performed by incubating the sections in antigen-retrieval buffer (6.5 mM sodium citrate, pH 6.0) at 60°C overnight. Endogenous peroxidase was quenched by immersing the sections in Peroxide Block (Biogenex, Fremont, CA) for 10 minutes, followed by incubation with Protein Block for 20 minutes to minimize nonspecific background staining. Sections were then incubated with 10 µg/ml Met C-12, RPTP-β H300 primary antibodies, or control IgG from Santa Cruz Biotechnology at room temperature for 1 hour. Sections were subsequently incubated with Multi-Link anti-(R+M) (Biogenex) for 10 minutes, followed by streptavidin HRP (Biogenex) for 10 minutes. Color was developed by incubating with AEC (Biogenex) for 3 minutes for detection. Sections were thoroughly washed, counterstained with hematoxylin, mounted, and photographed.
Results
RPTP-β Is Downregulated in Human Metastatic Tumor HNSCC Cells
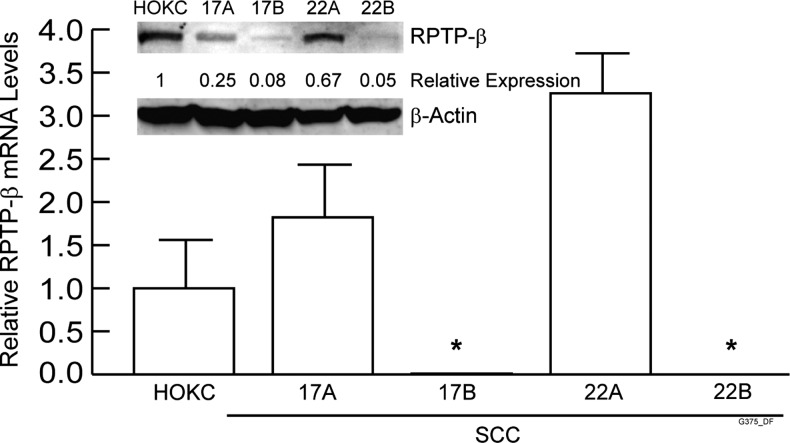
We initially quantified RPTP-β transcript levels in HNSCC cell lines established from eight individuals [34,36]. Cells derived from both primary and matched metastatic tumors were available from two individuals. RPTP-β mRNA levels in HNSCC cells derived from primary tumors were similar to normal human oral keratinocytes (HOKCs; data not shown). Importantly, RPTP-β mRNA and protein expression was nearly undetectable in both cell lines derived from metastatic tumors (UMSCC-17B and UMSCC-22B) (Figure 1).
Figure 1.
RPTP-β is downregulated in metastatic HNSCC cell lines. Total RNA and whole-cell lysates were prepared from HOKCs, cells from primary HNSCC tumors (UMSCC-17A and UMSCC-22A), and cells from metastatic HNSCC tumors of the same patients (UMSCC-17B and UMSCC-22B). RPTP-β mRNA and internal control housekeeping gene 36B4 were quantified by real-time reverse transcription-PCR. RPTP-β mRNA was not detectable in cells from secondary metastatic tumors. Inset shows representative Western blot of RPTP-β protein, with β-actin used as loading control. N = 3.6, *P < .05 versus HOKCs.
RPTP-β Regulates Met Tyrosine Phosphorylation in HNSCC Cells
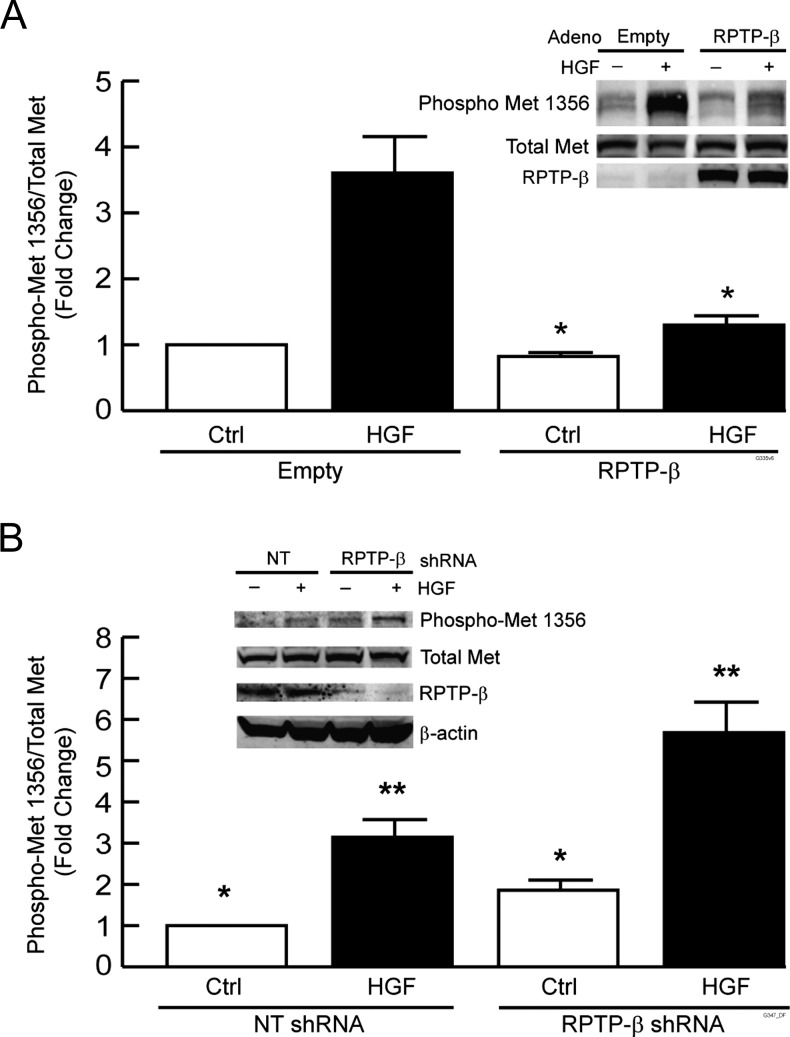
Loss of RPTP-β could drive Met activation. Therefore, we investigated the role of RPTP-β in regulating Met functions in HNSCC cell lines derived from both the primary and the metastatic tumors of the same individual (UMSCC-22A and UMSCC-22B, respectively). We either restored RPTP-β expression in metastatic tumor UMSCC-22B cells that lack endogenous RPTP-β protein or knocked down endogenous RPTP-β expression in UMSCC-22A primary tumor cells. We then determined the ability of RPTP-β to counteract both basal and HGF-induced Met tyrosine phosphorylation in these two HNSCC cell lines.
Restored expression of RPTP-β in metastatic tumor UMSCC-22B cells significantly reduced both basal and HGF-stimulated Met tyrosine 1356 phosphorylation. Basal tyrosine phosphorylation was reduced by 20% and HGF-stimulated phosphorylation was reduced by 70% (Figure 2A). In primary tumor UMSCC 22A cells, lentivirus shRNA-mediated knockdown reduced RPTP-β by 70%. This reduction increased both basal and HGF-stimulated Met tyrosine 1356 phosphorylation by two-fold (Figure 2B). Similar results were obtained with other cells, including primary normal human keratinocytes, UMSCC-9 and UMSCC-17B, and another shRNA construct targeting a different region of RPTP-β yielded similar results (data not shown). These data indicate that down-regulation of RPTP-β is functionally similar to activation of Met by HGF. Furthermore, it is notable that altered Met activity in response to reduced RPTP-β expression occurs without change in total Met expression. These data illustrate the importance of the Met/RPTP-β ratio in determining Met activation.
Figure 2.
RPTP-β regulates Met tyrosine 1356 phosphorylation in HNSCC cells. (A) UMSCC-22B metastatic HNSCC tumor cells were infected with empty adenovirus or RPTP-β-expressing adenovirus. (B) UMSCC-22A primary HNSCC tumor cells were infected with NT or RPTP-β-targeting shRNA lentivirus. Forty-eight hours post-infection, cells were treated with vehicle (-) or HGF (10 ng/ml, +) for 30 minutes. Equal amounts of whole-cell lysates were analyzed by Western blot probed with indicated antibodies. Immunoreactive bands were quantified by chemifluorescence using a STORM Image analyzer. Data are means ± SEM, N = 3; *P < .05. Inset shows representative Western blots.
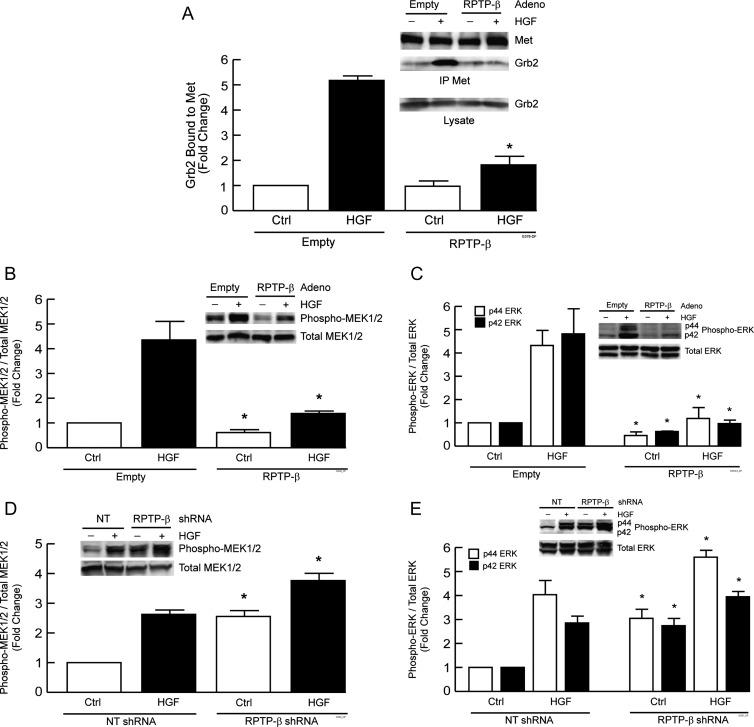
RPTP-β Regulates HGF-induced MEK and ERK Activation in HNSCC Cells
Met tyrosine 1356 phosphorylation serves as a docking site for Grb2 binding [37,38] that leads to activation of Ras/MAP kinase pathway. Because RPTP-β regulates tyrosine 1356 phosphorylation in UMSCC-22A and UMSCC-22B cells, we examined the effect of RPTP-β expression on basal and HGF-stimulated binding of Grb2 to Met. Expression of RPTP-β in metastatic tumor UMSCC-22B cells reduced HGF-stimulated Grb2 binding to Met by 70% (Figure 3A). Consistent with these data, expression of RPTP-β in metastatic tumor UMSCC-22B cells inhibited both basal and HGF-induced MEK1/2 activation by 40% and 70%, respectively (Figure 3B). Expression of RPTP-β inhibited basal p44 and p42 forms of ERK by 55% and 40%, respectively, and almost completely suppressed HGF-induced activation of both p44 and p42 ERK (Figure 3C). In primary tumor UMSCC-22A cells, knockdown of endogenous RPTP-β increased basal level of MEK1/2 to that of HGF stimulation and further potentiated HGF-induced MEK1/2 activation by 50% (Figure 3D). Knockdown of endogenous RPTP-β also increased basal p44 and p42 ERK to the level of HGF-stimulated activation and further potentiated HGF-induced p44 and p42 ERK activation by 30% and 40%, respectively (Figure 3E).
Figure 3.
RPTP-β regulates HGF-induced Grb2 binding to Met and downstream activation of MEK and ERK in HNSCC cells. (A) UMSCC-22B metastatic HNSCC tumor cells were infected with empty adenovirus or RPTP-β-expressing adenovirus. Forty-eight hours post-infection, cells were treated with vehicle (-, Ctrl) or HGF (10 ng/ml, +) for 15 minutes. Met was immunoprecipitated from whole-cell lysates, and immunoprecipitates were analyzed for Grb2 and Met by Western blot. (B and C) UMSCC-22B cells were treated as in A, except HGF treatment time was 30 minutes, and analyzed for MEK phosphorylation (B) and ERK phosphorylation (C). (D and E) UMSCC-22A primary HNSCC tumor cells were infected with NT or RPTP-β-targeting shRNA lentivirus. UMSCC-22A cells were treated as in A, except HGF treatment time was 30 minutes, and analyzed for MEK phosphorylation (D) and ERK phosphorylation (E). Immunoreactive bands were quantified by chemifluorescence using a STORM Image Analyzer. Data are means ± SEM, N = 3; *P < .05. Insets show representative Western blots.
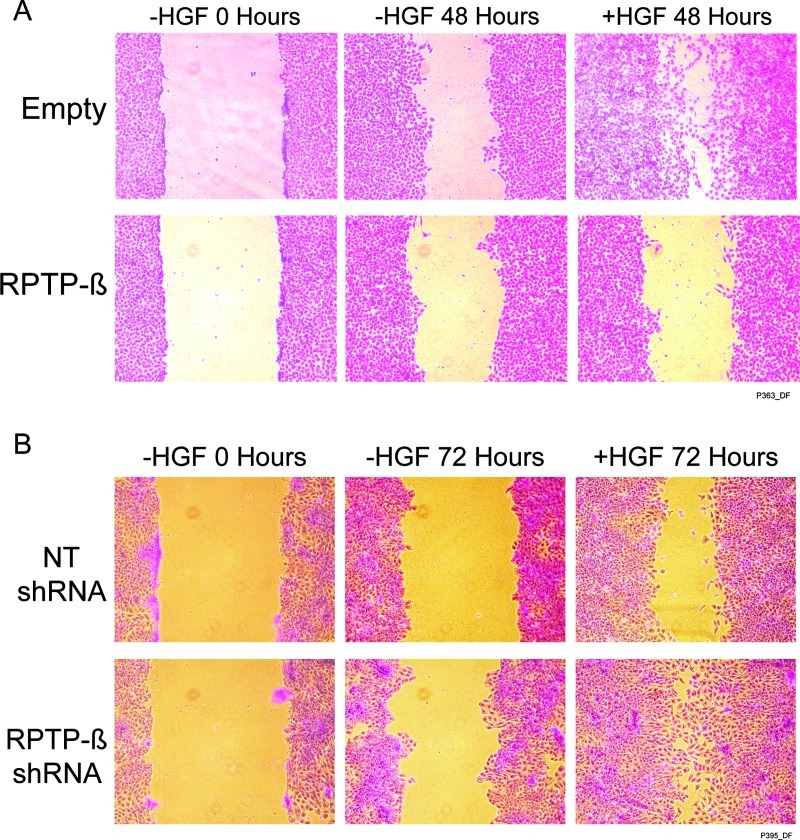
RPTP-β Regulates Met-mediated Migration, Proliferation, and Invasion of HNSCC Cells
MAP kinase activation by HGF has been shown to promote cell migration and proliferation in wound healing and tumor metastasis [39–41]. Therefore, we examined the impact of RPTP-β expression on migration and proliferation of primary tumor UMSCC-22A and metastatic tumor UMSCC-22B cells. Restored expression of RPTP-β, which inhibits HGF-induced MEK/ERK activation (Figure 3, A and B), abolished HGF-induced migration of UMSCC-22B, as measured by a scratch assay in the presence of proliferation inhibitor mitomycin (Figure 4A). Conversely, knockdown of endogenous RPTP-β in UMSCC-22A cells substantially potentiated both basal and HGF-stimulated UMSCC-22A cell migration (Figure 4B).
Figure 4.
RPTP-β regulates HGF-induced HNSCC cell migration. (A) UMSCC-22B metastatic HNSCC tumor cells were infected with empty adenovirus or RPTP-β-expressing adenovirus. (B) UMSCC-22A primary HNSCC tumor cells were infected with NT or RPTP-β. targeting shRNA lentivirus. Forty-eight hours post-infection, confluent cells were treated with 10 µg/ml mitomycin for 30 minutes and scratched with a pipette tip to create a cell-free zone. Cells were then incubated with or without HGF (10 ng/ml) for 48 hours. A representative image of three independent experiments is shown.
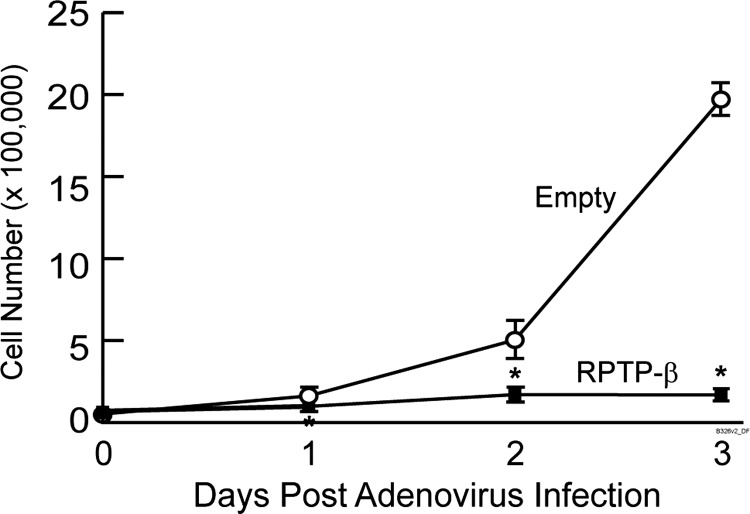
In addition to migration, we determined the effect of RPTP-β restoration on proliferation of metastatic tumor UMSCC-22B cells. Cells were plated at low density and infected with either empty or RPTP-β adenovirus. Cells infected with control adenovirus went through two population doublings between days 1 and 2 post-infection and more than two population doublings between days 2 and 3 post-infection. In stark contrast, cells infected with RPTP-β adenovirus failed to double after 3 days (Figure 5). This inhibition of proliferation by restoration of RPTP-β was similar to inhibition by a specific Met kinase inhibitor [42] (data not shown).
Figure 5.
RPTP-β inhibits proliferation of metastatic tumor-derived HNSCC cells. UMSCC-22B cells were infected with empty adenovirus or RPTP-β adenovirus. Cells were harvested at the indicated times post-infection and counted with a hemocytometer. Results are means ± SEM. N = 6; *P < .05.
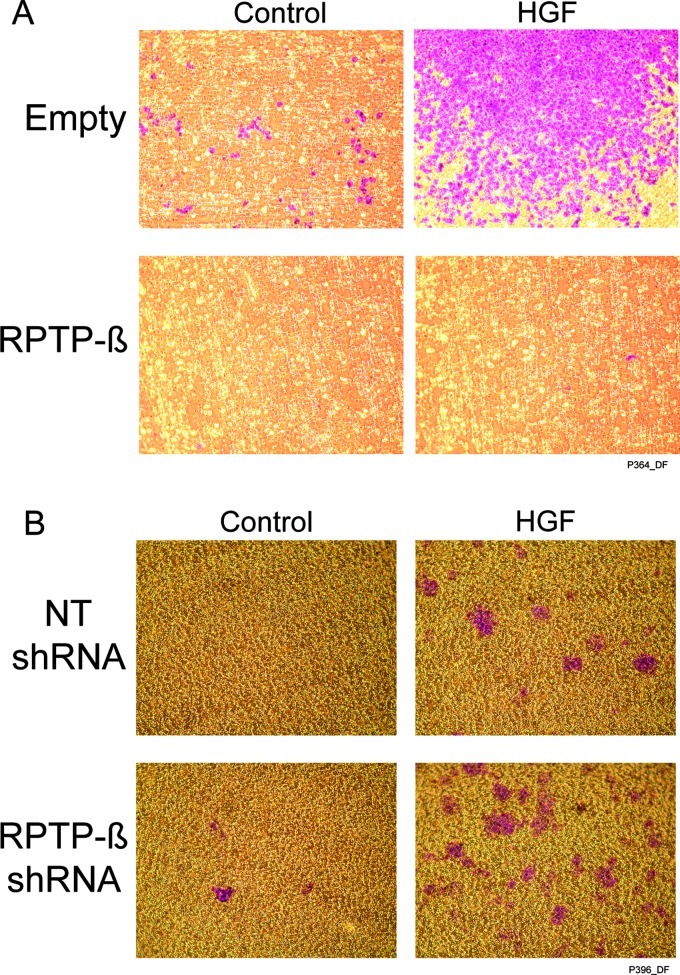
We next examined the role of RPTP-β in HNSCC cell invasion, using matrigel cell invasion assays. Metastatic tumor-derived UMSCC-17B and UMSCC-22B cells that do not express RPTP-β are highly invasive compared to primary tumor-derived UMSCC-17A and UMSCC-22A cells, which express RPTP-β (data not shown). Thus, loss of RPTP-β, which results in increased Met function, may be a mechanism for elevated metastatic potential in HNSCC. To test this possibility, we restored RPTP-β expression in metastatic tumor UMSCC-22B cells and reduced RPTP-β expression in primary tumor UMSCC-22A cells. As shown in Figure 6A, restoration of RPTP-β expression completely inhibited both basal and HGF-stimulated invasion of UMSCC-22B. Conversely, knockdown of endogenous RPTP-β in UMSCC-22A cells increased both basal and HGF-stimulated invasion (Figure 6B).
Figure 6.
RPTP-β regulates HGF-induced HNSCC cell invasion. (A) UMSCC-22B cells were infected with empty or RPTP-β-expressing adenovirus. (B) UMSCC-22A cells were infected with NT or RPTP-β-targeting shRNA lentivirus. Forty-eight hours post-infection, cells (1.5 x 105 cells/well) were harvested and seeded inmatrigel invasion assay wells in Dulbecco's modified Eagle's medium (with 2% FBS), with or without HGF (10 ng/ml) for 48 hours. Cells that passed through the matrigel were stained and photographed. Representative images from three independent experiments are shown. Cells stained purple. Matrigel stained yellow/orange.
RPTP-β Is Commonly Downregulated in HNSCC Tumors
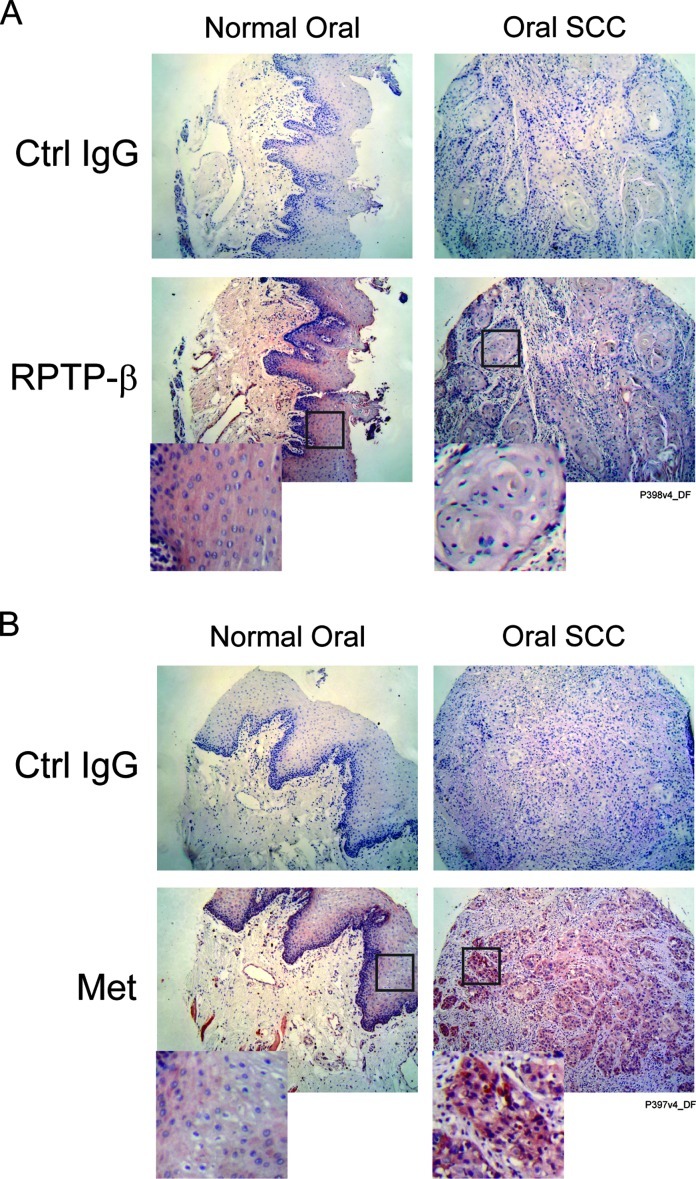
Collectively, the above data demonstrate the importance of RPTP-β in regulation of Met phosphorylation and key downstream cellular responses in HNSCC cells. As proof of concept, we assessed the relative protein levels of Met and RPTP-β in a small number of invasive HNSCC tumor biopsies from eight individuals (four T1N0M0 and four T3N0M0) and normal oral tissue samples from four individuals by immunohistochemistry. As shown in Table 1, six of eight HNSCC tumors showed deceased levels of RPTP-β, one had normal, and one had elevated expression compared to normal oral tissue. Among the six with reduced RPTP-β, four also had elevated Met levels, and the other two had normal Met levels (representative images are shown in Figure 7, A and B). These data demonstrate that imbalance of the Met/RPTP-β axis occurs in HNSCC tumors.
Table 1.
Staining Intensity of Met and RPTP-β Protein in HNSCC Tumor Samples.
| Core No. | Type | Stage | TNM | Met Staining | RPTP-β Staining |
| 1 | Malignant | 3 | T3N0M0 | +++ | - |
| 2 | Malignant | 3 | T3N0M0 | +++ | - |
| 3 | Malignant | 3 | T3N0M0 | ++ | - |
| 4 | Malignant | 3 | T3N0M0 | ++ | - |
| 5 | Malignant | 1 | T1N0M0 | + | - |
| 6 | Malignant | 1 | T1N0M0 | ++ | + |
| 7 | Malignant | 1 | T1N0M0 | + | - |
| 8 | Malignant | 1 | T1N0M0 | ++ | ++ |
| 9 | Normal | Normal tissue | Normal tissue | + | + |
| 10 | Normal | Normal tissue | Normal tissue | + | + |
| 11 | Normal | Normal tissue | Normal tissue | + | + |
| 12 | Normal | Normal tissue | Normal tissue | + | + |
+, Normal oral tissue level; ++ and +++, increased levels; ., reduced level.
Figure 7.
RPTP-β and Met protein expression levels are altered in HNSCC tumors. Formalin-fixed paraffin-embedded HNSCC tumor sections and normal oral tissue sections were stained with control IgG, (A) RPTP-β or (B) Met antibodies by immunohistochemistry. Representative images of normal tissue from four individuals and HNSCC tumors from eight individuals are shown.
Discussion
Our data indicate that down-regulation of RPTP-β results in overactivation of Met pathways and consequent HNSCC invasion/metastasis. This concept is supported by our finding that RPTP-β expression levels are substantially reduced in cell lines derived from metastasized secondary tumors compared to the primary tumors from the same patients. Consistent with this observation, the majority of invasive HNSCC tumor samples show either reduced levels of RPTP-β protein, increased levels of Met protein, or both.
Met is often constitutively activated in cancers, including HNSCC tumors [1,43]. This abnormal activation can be achieved by mutations of Met and/or overexpression of Met or HGF proteins. Our finding that RPTP-β negatively regulates Met provides a novel mechanism of Met activation, i.e., down-regulation of RPTP-β. In the basal state, tyrosine phosphorylation of Met by its intrinsic PTK activity is kept in check by RPTP-β protein tyrosine phosphatase activity. On HGF binding, transient activation of intrinsic tyrosine kinase results in increased Met tyrosine phosphorylation. In the absence of HGF, inhibition of RPTP-β activity similarly shifts the balance toward net accumulation of tyrosine phosphorylation of Met and consequent activation of downstream effectors [33].
Normal epithelial cells express Met but not HGF [44]. HGF is secreted by stromal cells to activate Met in a paracrine fashion. Completion of an autocrine loop by expressing HGF in epithelial cells or by overexpressing Met (i.e., overcoming the ability of RPTP-β to constrain Met activity) leads to transformation [24]. Our finding that RPTP-β is a negative regulator of Met identifies a novel mechanism for autocrine activation of Met, i.e., down-regulation of RPTP-β. A deficiency of RPTP-β relative to Met (i.e., insufficient RPTP-β to limit Met activity) would allow Met activation independent of stromal cell-produced HGF. Lower RPTP-β in tumor cells can result in higher Met activity and consequently higher metastatic potential. Identifying RPTP-β as a new component of the HGF/Met axis shifts the current paradigm of Met pathway regulation in cancer biology.
Given the importance of aberrant HGF/Met signaling, several different therapeutic strategies aimed at pathway inhibition have been developed and are currently being evaluated in clinical trials (phases 1 and 2). Ongoing clinical trials include HGF antagonists AM102 and c-Met inhibitors GSK1363089 (formerly XL880) and PF-02341066 (http://clinicaltrials.gov). The results from the present study could be used to inform investigators in clinical trials of these new drugs. For example, patients without apparent overexpression of either HGF or Met may still have tumors that are driven by the Met pathway because of down-regulation of RPTP-β. These patients may be good candidates for current and future therapeutic trials targeting the HGF/Met axis. Furthermore, on the basis of our data, RPTP-β expression may be a useful prognostic marker for HNSCC metastasis. RPTP-β expression may identify tumors that are best suited for organ sparing therapy versus surgical approaches. These decisions could affect patient quality of life and/or survival.
It is well recognized that PTK pathways are central to tumorigenesis and the progression of many types of epithelial cancer. RPTP-β has the potential to function as an important tumor suppressor through inhibition of Met in HNSCC metastasis. Understanding regulation of the Met pathway by RPTP-β will advance our mechanistic knowledge of carcinogenesis and progression and could enable identification of novel diagnostic and prognostic biomarkers.
Acknowledgments
We thank Diane Fiolek for graphic preparation and administrative support.
Footnotes
This research was partially funded by grants from the National Institutes of Health (RO1-ES-012920 to G.J.F. and Y.X.) and the University of Michigan Head and Neck Cancer Specialized Program of Research Excellence (1 P50 CA97248). The authors declare no conflict of interest.
References
- 1.De Herdt MJ, Baatenburg de Jong RJ. HGF and c-MET as potential orchestrators of invasive growth in head and neck squamous cell carcinoma. Front Biosci. 2008;13:2516–2526. doi: 10.2741/2863. [DOI] [PubMed] [Google Scholar]
- 2.Hardisson D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2003;260:502–508. doi: 10.1007/s00405-003-0581-3. [DOI] [PubMed] [Google Scholar]
- 3.Rogers SJ, Harrington KJ, Rhys-Evans P, O-Charoenrat P, Eccles SA. Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer Metastasis Rev. 2005;24:47–69. doi: 10.1007/s10555-005-5047-1. [DOI] [PubMed] [Google Scholar]
- 4.Werner JA, Rathcke IO, Mandic R. The role of matrix metalloproteinases in squamous cell carcinomas of the head and neck. Clin Exp Metastasis. 2002;19:275–282. doi: 10.1023/a:1015531319087. [DOI] [PubMed] [Google Scholar]
- 5.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 6.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanzawa M, Shindoh M, Higashino F, Yasuda M, Inoue N, Hida K, Ono M, Kohgo T, Nakamura M, Notani K, et al. Hepatocyte growth factor upregulates E1AF that induces oral squamous cell carcinoma cell invasion by activating matrix metalloproteinase genes. Carcinogenesis. 2000;21:1079–1085. [PubMed] [Google Scholar]
- 8.Kitajo H, Shibata T, Nagayasu H, Kawano T, Hamada J, Yamashita T, Arisue M. Rho regulates the hepatocyte growth factor/scatter factor-stimulated cell motility of human oral squamous cell carcinoma cells. Oncol Rep. 2003;10:1351–1356. [PubMed] [Google Scholar]
- 9.Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- 10.Seiwert TY, Jagadeeswaran R, Faoro L, Janamanchi V, Nallasura V, El Dinali M, Yala S, Kanteti R, Cohen EE, Lingen MW, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D, Wang CY. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFκB. J Biol Chem. 2002;277:25203–25208. doi: 10.1074/jbc.M201598200. [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Kim J, Kahng H, Choi EC. Change of E-cadherin by hepatocyte growth factor and effects on the prognosis of hypopharyngeal carcinoma. Ann Surg Oncol. 2007;14:1565–1574. doi: 10.1245/s10434-006-9320-5. [DOI] [PubMed] [Google Scholar]
- 13.Galeazzi E, Olivero M, Gervasio FC, De Stefani A, Valente G, Comoglio PM, Di Renzo MF, Cortesina G. Detection of MET oncogene/hepatocyte growth factor receptor in lymph node metastases from head and neck squamous cell carcinomas. Eur Arch Otorhinolaryngol. 1997;254(suppl 1):S138–S143. doi: 10.1007/BF02439745. [DOI] [PubMed] [Google Scholar]
- 14.Kim CH, Moon SK, Bae JH, Lee JH, Han JH, Kim K, Choi EC. Expression of hepatocyte growth factor and c-Met in hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2006;126:88–94. doi: 10.1080/00016480510037014. [DOI] [PubMed] [Google Scholar]
- 15.Lo Muzio L, Leonardi R, Mignogna MD, Pannone G, Rubini C, Pieramici T, Trevisiol L, Ferrari F, Serpico R, Testa N, et al. Scatter factor receptor (c-Met) as possible prognostic factor in patients with oral squamous cell carcinoma. Anticancer Res. 2004;24:1063–1069. [PubMed] [Google Scholar]
- 16.Marshall DD, Kornberg LJ. Overexpression of scatter factor and its receptor (c-met) in oral squamous cell carcinoma. Laryngoscope. 1998;108:1413–1417. doi: 10.1097/00005537-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Morello S, Olivero M, Aimetti M, Bernardi M, Berrone S, Di Renzo MF, Giordano S. MET receptor is overexpressed but not mutated in oral squamous cell carcinomas. J Cell Physiol. 2001;189:285–290. doi: 10.1002/jcp.10010. [DOI] [PubMed] [Google Scholar]
- 18.Sawatsubashi M, Sasatomi E, Mizokami H, Tokunaga O, Shin T, Expression of c-Met in laryngeal carcinoma. Virchows Arch. 1998;432:331–335. doi: 10.1007/s004280050174. [DOI] [PubMed] [Google Scholar]
- 19.Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G, Comoglio PM. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 20.Chen YS, Wang JT, Chang YF, Liu BY, Wang YP, Sun A, Chiang CP. Expression of hepatocyte growth factor and c-met protein is significantly associated with the progression of oral squamous cell carcinoma in Taiwan. J Oral Pathol Med. 2004;33:209–217. doi: 10.1111/j.0904-2512.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 22.Uchida D, Kawamata H, Omotehara F, Nakashiro K, Kimura-Yanagawa T, Hino S, Begum NM, Hoque MO, Yoshida H, Sato M, et al. Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance. Int J Cancer. 2001;93:489–496. doi: 10.1002/ijc.1368. [DOI] [PubMed] [Google Scholar]
- 23.Kim CH, Lee JS, Kang SO, Bae JH, Hong SP, Kahng H. Serum hepatocyte growth factor as a marker of tumor activity in head and neck squamous cell carcinoma. Oral Oncol. 2007;43:1021–1025. doi: 10.1016/j.oraloncology.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka T, Takayama H, Sharp R, Celli G, LaRochelle WJ, Bottaro DP, Ellmore N, Vieira W, Owens JW, Anver M, et al. c-Met autocrine activation induces development of malignant melanoma and acquisition of the metastatic phenotype. Cancer Res. 1998;58:5157–5167. [PubMed] [Google Scholar]
- 25.Mazzone M, Comoglio PM. The Met pathway: master switch and drug target in cancer progression. FASEB J. 2006;20:1611–1621. doi: 10.1096/fj.06-5947rev. [DOI] [PubMed] [Google Scholar]
- 26.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–1285. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 27.Murai M, Shen X, Huang L, Carpenter WM, Lin CS, Silverman S, Regezi J, Kramer RH. Overexpression of c-met in oral SCC promotes hepatocyte growth factor-induced disruption of cadherin junctions and invasion. Int J Oncol. 2004;25:831–840. [PubMed] [Google Scholar]
- 28.Petrone A, Sap J. Emerging issues in receptor protein tyrosine phosphatase function: lifting fog or simply shifting? J Cell Sci. 2000;113(pt 13):2345–2354. doi: 10.1242/jcs.113.13.2345. [DOI] [PubMed] [Google Scholar]
- 29.Schaapveld R, Wieringa B, Hendriks W. Receptor-like protein tyrosine phosphatases: alike and yet so different. Mol Biol Rep. 1997;24:247–262. doi: 10.1023/a:1006870016238. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan R, Morse B, Huebner K, Croce C, Howk R, Ravera M, Ricca G, Jaye M, Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci USA. 1990;87:7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krueger NX, Streuli M, Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990;9:3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harder KW, Anderson LL, Duncan AM, Jirik FR. The gene for receptor-like protein tyrosine phosphatase (PTPRB) is assigned to chromosome 12q15→q21. Cytogenet Cell Genet. 1992;61:269–270. doi: 10.1159/000133419. [DOI] [PubMed] [Google Scholar]
- 33.Autero M, Saharinen J, Pessa-Morikawa T, Soula-Rothhut M, Oetken C, Gassmann M, Bergman M, Alitalo K, Burn P, Gahmberg CG, et al. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994;14:1308–1321. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carey TE, Van Dyke DL, Worsham MJ, Bradford CR, Babu VR, Schwartz DR, Hsu S, Baker SR. Characterization of human laryngeal primary and metastatic squamous cell carcinoma cell lines UM-SCC-17A and UM-SCC-17B. Cancer Res. 1989;49:6098–6107. [PubMed] [Google Scholar]
- 35.Balavenkatraman KK, Jandt E, Friedrich K, Kautenburger T, Pool-Zobel BL, Ostman A, Bohmer FD. DEP-1 protein tyrosine phosphatase inhibits proliferation and migration of colon carcinoma cells and is upregulated by protective nutrients. Oncogene. 2006;25:6319–6324. doi: 10.1038/sj.onc.1209647. [DOI] [PubMed] [Google Scholar]
- 36.Carey T. Head and neck tumor cell lines. In: Hay RJ, Park J-G, Gazdar A, editors. Atlas of Human Tumor Cell Lines. New York: Academic Press, Inc; 1994. pp. 79–120. [Google Scholar]
- 37.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Naujokas MA, Fixman ED, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]
- 39.Matsumoto K, Hashimoto K, Yoshikawa K, Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocyte growth factor. Exp Cell Res. 1991;196:114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- 40.Shimaoka S, Tsuboi R, Jindo T, Imai R, Takamori K, Rubin JS, Ogawa H. Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J Cell Physiol. 1995;165:333–338. doi: 10.1002/jcp.1041650214. [DOI] [PubMed] [Google Scholar]
- 41.Tajima H, Matsumoto K, Nakamura T. Regulation of cell growth and motility by hepatocyte growth factor and receptor expression in various cell species. Exp Cell Res. 1992;202:423–431. doi: 10.1016/0014-4827(92)90095-p. [DOI] [PubMed] [Google Scholar]
- 42.Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 43.Lesko E, Majka M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front Biosci. 2008;13:1271–1280. doi: 10.2741/2760. [DOI] [PubMed] [Google Scholar]
- 44.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]