Abstract
The central histaminergic actions are mediated by H1, H2, H3 and H4 receptors. The histamine H3 receptor regulates the release of histamine and a number of other neurotransmitters and thereby plays a role in cognitive and homeostatic processes. Elevated histamine levels suppress seizure activities and appear to confer neuroprotection. The H3 receptors have a number of enigmatic features like constitutive activity, interspecies variation, distinct ligand binding affinities and differential distribution of prototypic splice variants in the CNS. Furthermore, this Gi/Go-protein-coupled receptor modulates several intracellular signalling pathways whose involvement in epilepsy and neurotoxicity are yet to be ascertained and hence represent an attractive target in the search for new anti-epileptogenic drugs. So far, H3 receptor antagonists/inverse agonists have garnered a great deal of interest in view of their promising therapeutic properties in various CNS disorders including epilepsy and related neurotoxicity. However, a number of experiments have yielded opposing effects. This article reviews recent works that have provided evidence for diverse mechanisms of antiepileptic and neuroprotective effects that were observed in various experimental models both in vitro and in vivo. The likely reasons for the apparent disparities arising from the literature are also discussed with the aim of establishing a more reliable basis for the future use of H3 receptor antagonists, thus improving their utility in epilepsy and associated neurotoxicity.
Keywords: histamine H3 receptor antagonists, epilepsy, neurodegeneration and neurotoxicity, H3 receptor signalling pathways
Histamine has been one of the most studied substances in medicine for a century, regulating a wide spectrum of activities, including its function in neurotransmission (Brown et al., 2001). The association between the histaminergic system with the pathogenesis of epilepsy, which is currently the subject of extensive evaluation, is still in its infancy, owing to the complex brain neurophysiology of histamine (Haas et al., 2008) and pleiotropic receptor ligand pharmacology (Leurs et al., 2005; Esbenshade et al., 2008; Tiligada et al., 2009). Our understanding of the pathophysiology of epilepsy is mostly confined to the conventional theory of deranged inhibitory GABAergic and protracted excitatory glutamatergic neurotransmission in excitotoxic neuronal death (reviewed by Naylor, 2010; Werner and Coveñas, 2011; Rowley et al., 2012). The imbalance could be modulated by various other neurotransmitter systems including the histaminergic system. The latter, through H3 heteroreceptors, modulates the release of a wide spectrum of vital neurotransmitters, for example, GABA, glutamate, dopamine, 5-HT, noradrenaline and acetylcholine, in a pathway-dependent manner (reviewed by Passani et al., 2007; Haas et al., 2008). Again, histamine release is not only regulated by its own H3 autoreceptor system but also by GABA via GABAA and GABAB receptors and by glutamate via NMDA receptors (Okakura et al., 1992; Okakura-Mochizuki et al., 1996; Sherin et al., 1998).
Excitotoxicity is considered to be the key mechanism underlying neurodegenerative processes including epileptic seizures (Choi, 1992). Neurodegeneration followed by neuronal cell death resulting from excessive activation of excitatory ionotropic glutamate receptors [NMDA, AMPA and kainic acid (KA)] or malfunctioning of the inhibitory mechanism due to altered functioning of GABAA receptor, usually underlies the dissemination of epileptic activity (reviewed by Acharya et al., 2008). The compensatory yet abnormal neuronal activities that follow, viz. mossy fibre sprouting, significantly modify the neuronal organization and alter the propagation of neurotransmission processes that is attributable to the epileptogenic mechanisms (Dichter, 2009). Epileptogenesis refers to the progressive transformation of normal brain to a hyperexcitable epileptic brain, after an initial precipitating injury (IPI), for example status epilepticus (SE), and continues even after the withdrawal of the inciting stimulus. This is a characteristic of temporal lobe epilepsy (TLE), the most intractable form of epilepsy (Acharya, 2002; Loscher and Brandt, 2010). The conventional and new antiepileptic drugs (AEDs) affect seizure expression, merely providing symptomatic treatment without having any influence on the course of the disease (epileptogenesis). The current consensus is to employ anti-epileptogenic strategies for treating TLE (Walker, 2007; Acharya et al., 2008; Pitkanen, 2010). Hence, there is a growing interest that the AEDs, besides controlling seizure activity, must also prevent concurrent injury responses that result in epileptogenesis in the hippocampal structures, a phenomenon linked to the transformation of a normal non-epileptic brain to a hyperexcitable epileptic brain in which recurrent, spontaneous seizures occur. In fact, epilepsy treatment has always been associated with the protection of neural tissue, since it intends to diminish the duration or totally suppress seizures. Even though the debate on the capacity of simple seizures to induce neuronal injury is still in progress, there is compelling evidence for the disastrous effects of prolonged episodes of status epilepticus (Willmore, 2005; Acharya et al., 2008). The role of the brain histaminergic system in neuroprotection remains a challenging area of research that is currently under consideration. Based on recent findings, which include changes in H1 and H3 receptor expression in a KA-induced epileptic model, it has been proposed that the brain histaminergic system is involved in experimental SE and subsequent neurodegeneration (Jin et al., 2005; Lintunen et al., 2005).
Histamine is considered to be an anticonvulsive neurotransmitter as its low levels are associated with convulsions and seizures (Kiviranta et al., 1995; Chen et al., 2003; Hirai et al., 2004). Until now, research on the anticonvulsive role of histamine has largely focused on electrically- or PTZ-induced seizures (Vohora et al., 2000; 2001; Yawata et al., 2004). However, an understanding of the possible role of histamine in temporal lobe epilepsy and the brain regions affected has begun to evolve (Jin et al., 2007; Kukko-Lukjanov et al., 2012). Prolonged release of histamine in response to brain ischaemic insults and alleviation of neuronal damage upon post-ischaemic histamine administration suggests it has a neuroprotective role (reviewed by Adachi, 2005). Histaminergic drugs, most importantly H3 receptor antagonists or inverse agonists, which are considered to be a class of perspective drugs for the treatment of diverse neurological and neuropsychiatric disorders (reviewed by Leurs et al., 2011; Passani and Blandina, 2011), in view of the findings from various basic experimental models of epilepsy in rodents, are envisaged to possess promising anticonvulsive properties (Vohora et al., 2000; 2001; 2010; Harada et al., 2004a,b).
In this review, an attempt has been made to scrutinize the recent experimental evidence that has evoked the possibility that the histaminergic system, via modulation of H3 receptor function, can be engaged to mediate a neuroprotective effect in epilepsy-related neurotoxicity and also to address the possible mechanisms involved, which would explain the above effects. Histamine and various agents, which act by modulating the histaminergic system, especially H3 receptor antagonists, and their impact on epileptogenesis are then examined with particular reference to the neuroprotective action of some of these molecules. The core of the review deals with the current status of the literature on the use of some of these inhibitors as antiepileptic and neuroprotective agents as well as on their possible role in intervening in disease modifications of the brain (epileptogenesis). Probable explanations for many of the conflicting results arising from the literature are discussed in view of establishing a more reliable platform for future use of histaminergic agents to treat epilepsy and associated neurotoxicity.
Histaminergic system in CNS
In the CNS, the synthesis of histamine [2-(4-imidazolyl)-ethylamine] from 1-histidine by the catalytic activity of the rate-limiting enzyme histidine decarboxylase (HDC, EC 4.1.1.22; Moya-Garcia et al., 2005) takes place in a restricted population of neurons located in the tuberomammillary nucleus (TMN) of the posterior hypothalamus (Panula et al., 1984; Watanabe et al., 1984; Wouterlood et al., 1986). They give rise to widespread and diffuse projections extending through the basal forebrain virtually to the entire brain including the cortex, striatum, thalamus, hippocampus, hypothalamus, locus coeruleus and spinal cord (Watanabe et al., 1984; Panula et al., 1990; Zimatkin et al., 2006). This morphology renders histamine to be able to act as a neurotransmitter and neuromodulator of a wide spectrum of physiological functions and behaviours of the CNS, such as the circadian rhythms, catalepsy, energy homeostasis, thermoregulation, neuroendocrine and cardiovascular control, drinking and feeding, learning and memory, locomotion, sexual behaviour, analgesia and emotion (reviewed by Brown et al., 2001; Passani et al., 2007; Yanai and Tashiro, 2007; Haas et al., 2008).
Histamine H3 receptor
The histamine neuroreceptor system is one of the major aminergic systems exerting key neurological functions via pharmacologically distinct subtypes of histamine receptors, which belong to a large superfamily of GPCRs that are characterized by the presence of seven putative transmembrane spanning domains (Leurs et al., 2005; Parsons and Ganellin, 2006). To date, histamine has been recognized to be an endogenous ligand for four subtypes of metabotropic histamine receptor (H1–4; Parsons and Ganellin, 2006; Nuutinen and Panula, 2010), of which H1–3 receptors are very widely expressed throughout the mammalian brain (Garbarg and Schwartz, 1985; Hill et al., 1997; Tashiro and Yanai, 2007). For the H3 receptor, so far, six isoforms have been reported with varying distribution (H3A-F; Lovenberg et al., 1999; Drutel et al., 2001; Bakker et al., 2006) and pharmacological properties (reviewed in Hancock et al., 2003). Both the H3A and H3D isoforms contain the full-length third intracellular loop but have different C-termini. All the other isoforms have deletions in that loop (H3B and H3E: 32 amino acids; H3C and H3F: 48 amino acids). Isoforms H3A-C have the same C-terminus, while isoforms H3E-F have an alternative C-terminus (reviewed in Leurs et al., 2005; Arrang et al., 2007).
Numerous studies have established that all subtypes (H1 and H2) are located postsynaptically and are found in the CNS and periphery (reviewed in Parsons and Ganellin, 2006), whereas the recently discovered H4 receptor is found predominantly in mast cells and leucocytes (reviewed by Oda and Matsumoto, 2001; Leurs et al., 2009), although their presence in the CNS has also been detected (Connelly et al., 2009); with the exception of the H3 receptor, which was originally characterized as a presynaptic autoreceptor that is located on histaminergic and other cell somata, dendrites and axonal varicosities (Arrang et al., 1983; Lovenberg et al., 1999). Although, later on, a detailed mapping of the H3 receptors in rat brain revealed their presence post-synaptically on the perikarya, dendrites and projections of many neuronal populations (Pillot et al., 2002), but their postsynaptic physiological role is yet to be elucidated (reviewed in Arrang et al., 2007). Both rat and human H3 receptors (hH3 receptors) again have the striking feature of being constitutively (or spontaneously) active, by virtue of their existence in their active conformation even in the absence of histamine (agonist), thereby negatively regulating the synthesis and release of histamine (Schwartz et al., 1990). Inverse agonists by attenuating this constitutive inhibitory effect enhance histamine release (Jansen et al., 1998; Morisset et al., 2000; Rouleau et al., 2002; Arrang et al., 2007).
The relative expression of histamine H3 receptors is very high in the CNS, where they participate in the modulation of arousal, learning and memory and food intake by means of their autoreceptor and heteroreceptor functions. So far, the H3 receptor has been postulated to be a good target for drug discovery for a variety of CNS conditions (Gemkow et al., 2009; Schwartz, 2011). The H3 receptor, being an auto- as well as hetero-receptor, was subsequently found to restrict the release of histamine and other neurotransmitters, including acetylcholine, dopamine, glutamate, noradrenaline and 5-HT (Schwartz et al., 1990; Schlicker et al., 1994). Consequently, specific ligands for the H3 receptor show potential therapeutic effects in models of obesity, depression, mood disorders, neuropathic pain, epilepsy, sleep–wake disorders including narcolepsy and cognitive and sensorimotor deficits that are considered to be the more common deleterious features of CNS disorders such as Parkinson's disease (PD), attention deficit hyperactivity disorder (ADHD), Alzheimer's disease (AD) and schizophrenia (Fox et al., 2005; Leurs et al., 2005; Nuutinen and Panula, 2010; Passani and Blandina, 2011; Schwartz, 2011).
H3 receptor signalling pathways
The seminal work of Arrang et al. (1983) was the first to identify the third histamine receptor and this has opened a new chapter in the pharmacology of histamine. With the cloning of the hH3 receptor gene pioneered by Tim Lovenberg in 1999, H3 receptor research has received a tremendous boost and enormous progress has been made in the field ever since. At present, we are buoyed by the detailed knowledge of its modulation of several signalling pathways based on the findings in the last 10 years. The notion of G-protein (especially Gi/Go) involvement in the H3 receptor was confirmed by the heterologous expression of the H3 receptor (Lovenberg et al., 1999) and this was further substantiated by the reported inhibitory effects of G-protein toxins, such as cholera and Pertussis toxin, on H3 receptor function (Rouleau et al., 2002) and [35S]-GTPγS binding studies in rat brain (Clark and Hill, 1996).
Studies using recombinant cell systems have revealed that brain H3 receptors are coupled to Gi/o-proteins modulating protein kinases such as cAMP-dependent protein kinase (PKA), ERK2 (Giovannini et al., 2003) and GSK-3β (Bongers et al., 2007b). Thus, a number of signal transduction pathways have been identified as being modulated by H3 receptors (Figure 1), including inhibition of AC and activation of MAPK (reviewed by Leurs et al., 2005; Bongers et al., 2007a). H3 receptor activation of Gi/o-proteins might also lead to the activation of PLA2 to induce the release of arachidonic acid (AA; Rouleau et al., 2002), inhibition of the Na+/H+ exchanger (NHE; Silver et al., 2001) as well as modulation of intracellular Ca2+ ([Ca2+]i) levels (Silver et al., 2002). In view of the interest in the H3 receptor as a potential therapeutic target, each of these signalling pathways is discussed below and any evidence germane to epilepsy (if any) and neurodegeneration is cited.
Figure 1.
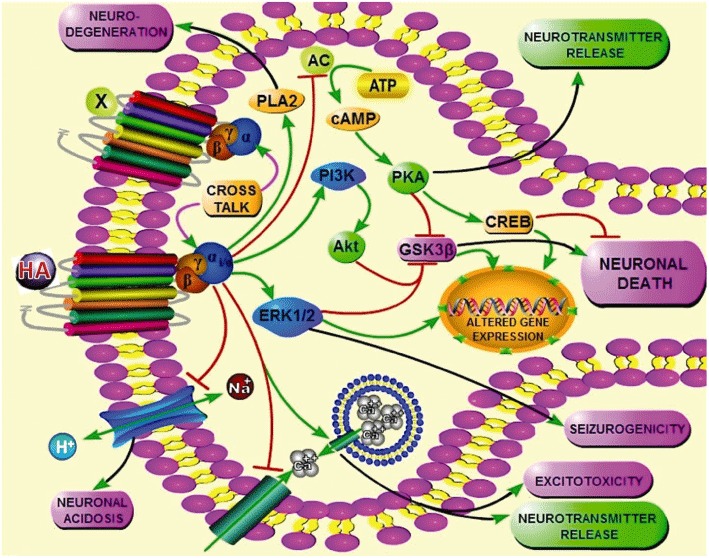
The schematic diagram is a hypothetical depiction of the H3 receptor-mediated signalling pathways in the CNS and their probable implications. Activation of H3 receptor, constitutively or in presence of an agonist, leads to the activation of many Gi/o-associated intracellular pathways. Active Gi/o-proteins negatively couple AC, thereby inhibiting the cAMP/PKA cascade and subsequent lowering of cAMP responsive element binding protein (CREB), a pro-survival transcription factor. Reduction in PKA activity may decrease release of neurotransmitters, for example histamine (HA), GABA, etc. Additionally, H3 receptor activation also activates PI3K and MAPK pathways. Activated PI3K then activates Akt, a serine threonine kinase, which phosphorylates and hence inactivates pro-apoptotic GSK-3β. In the MAPK pathway, especially p44 and p42 MAPK (ERK1/2) are coupled to H3 receptors; activated ERK has also been linked to epilepsy. The other three pathways known so far that are modulated upon H3 receptor activation include increased activity of the enzyme PLA2, whose inhibition confers neuroprotection in epileptic models; mobilization of intracellular Ca2+[Ca2+]i; and finally the inhibition of Na+/H+ exchanger (NHE), a protein that buffers intra-neuronal pH and it's defective functioning is also implicated in epilepsy. Also, H3 receptors possibly influence the function of other GPCRs and vice versa (e.g. dopamine D1 receptor) by virtue of cross talk in heteromer containing cells.
PKA/cAMP/CREB pathway
AC catalyses the formation of cAMP, which in turn, activates PKA and, subsequently, induces cAMP-responsive-element-binding protein (CREB) to modulate gene transcription. The H3 receptor negatively couples to AC; hence, H3 receptor activation lowers cAMP levels and reduces downstream events, such as CREB-dependent gene transcription (Lovenberg et al., 1999). The lowering of intracellular levels of cAMP through the activation of H3 autoreceptors (by an agonist or via a constitutive property) results in the modulation of histamine synthesis in histaminergic nerve terminals through the AC/PKA pathway (Gomez-Ramirez et al., 2002). Many classical H3 receptor antagonists, for example thioperamide, clobenpropit, ciproxifan, were found to reverse this process in various transfected cell lines (Morisset et al., 2000; Wieland et al., 2001). Similarly, ABT-239 (H3 receptor antagonist) restored cortical CREB level and increased its phosphorylation in mice (Bitner et al., 2011).
The transcriptional activation of CREB is induced by an array of kinases including PKA (Gonzalez and Montminy, 1989), Ca2+-activated calmodulin kinases (CaMKII) and MAPK-activated protein kinase 2 (MAPKAPK2; Davis et al., 2000) and was found to regulate many facets of neuronal functioning, including neuronal excitation, growth and survival, development and regeneration, circadian entrainment and long-term synaptic plasticity (reviewed by Lonze and Ginty, 2002; Borlikova and Endo, 2009). In rat cortical cells, an H3 receptor antagonist was found to alleviate NMDA-induced neurotoxicity by activating the cAMP/PKA pathway, which subsequently facilitated GABA release (Dai et al., 2007). It is well known that the signalling pathway of PKA/CREB has an anti-apoptotic effect (Lonze et al., 2002; Ao et al., 2006), and there is experimental evidence suggesting that CREB is actively involved in supporting neuroprotection (Lee et al., 2005, 2009; Kim et al., 2011; reviewed by Sakamoto et al., 2011) and that its disruption in the brain leads to neurodegeneration (Lee et al., 2002); thus, supporting a pivotal role for CREB in preventing neuronal death (reviewed by Ferrer, 2002). In transgenic mice, the gene profiling of degenerating hippocampal tissue with electron microscopy reveals that the sustained inhibition of CREB function is associated with neuron degeneration, whereas its strong chronic activation primarily causes excitotoxic hippocampal cell death and inflammation, indicating that a fine tuned control of CREB expression is critical for the viability of pyramidal neurons (Valor et al., 2010). Altered expression of CREB and ICER (inducible cAMP early repressor), an endogenous CREB antagonist, and their possible alteration of GABA receptor subunits in various epileptic models suggest a definitive role for the PKA/CREB pathway in epileptogenesis (reviewed by Borlikova and Endo, 2009; Brooks-Kayal et al., 2009). On the other hand, a number of experimental (Lee et al., 2007; Zhu et al., 2012) and clinical (Park et al., 2003; Rakhade et al., 2005) findings appear, in some respects, to contradict the established neuroprotective role of this pathway as they indicate that an increased expression of CREB is associated with seizure-induced pathological alterations in the CNS. Therefore, further studies with H3 receptor ligands are needed to elucidate the possible role of this particular pathway in epilepsy and the subsequent pathological remodelling of the hippocampus.
Activation of the MAPK pathway
G-protein-coupled activation of the MAPK pathway involves the Ras/Raf/MEK (MAP/ERK kinase) pathway that results in the phosphorylation of ERK1/2s. Activated ERKs phosphorylate several substrates, for example MAPK-activated protein kinases (MAPKAPKs; Wada and Penninger, 2004), of which MAPKAPK2 is associated with Akt phosphorylation (Rane et al., 2001). However, the occurrence of such cross-talk in the H3 receptor pathways remains to be further examined, as H3 receptor-mediated phosphorylation of Akt was observed to be independent of MAPK pathway in transfected SK-N-MC cells (Bongers et al., 2007b).
Notably, different components of the MAPK family are known to have opposing effects; the ERK/MAPK pathway is deemed to be pro-survival (Karmarkar et al., 2011), whereas the p38/MAPK and SAPK/JNK pathways are often implicated in cell death (de Lemos et al., 2010). More importantly, inhibition of ERK1/2 activation increases epileptiform activity and animal mortality in the pilocarpine model of status epilepticus (Berkeley et al., 2002) and ERK1/2 activation is thought to play a pivotal role in neuronal survival following hypoxic injury (Jin et al., 2002). Although the results of these studies indicated that ERK1/2 has a neuroprotective role, other studies have suggested ERK1/2 signalling has a detrimental role in neurotoxic responses (reviewed by Subramaniam and Unsicker, 2010). The role of ERK1/2 in status epilepticus is further complicated by evidence indicating that ERK1/2 activation is epileptogenic (Merlo et al., 2004) and triggers epilepsy in mice by augmenting NMDA receptor (GluN2B) function (Nateri et al., 2007).
Earlier, phosphorylation of the MAPK pathway induced by activation of rat H3 (H3A isoform) receptors in receptor transfected COS 7 cells (Drutel et al., 2001) led to the discovery that the MAPK pathway is linked to H3 receptors. However, in rat hippocampal CA3 pyramidal cells H3 receptor activation was also shown to be coupled indirectly to ERK2 phosphorylation, as thioperamide failed to alter phospho-ERK levels (Giovannini et al., 2003). The relevance of this H3 receptor signalling pathway in the aetiology of chronic epilepsies has yet to be ascertained.
Activation of the PI3K/Akt/GSK3β axis
H3 receptor activation in transfected cells leads to the activation of PI3K pathways and the subsequent phosphorylation–activation of another ubiquitous signalling molecule, Akt (PKB, serine/threonine kinase), which phosphorylates at serine residue and thereby inhibits the action of GSK-3β (pGSK-3βser9; Bongers et al., 2007b), a constitutively active, τ kinase. Advances in the understanding of signal transduction in the CNS regulated by Akt have paved the way to study the implications of the PI3K/Akt/GSK-3β axis (Brazil et al., 2004; Chuang, 2005). Akt has been shown to play an important role in preventing excitotoxic apoptosis by acting as a regulatory gate, preventing JNK activation (Kim et al., 2002).
In the CNS, the Akt/GSK-3β axis has emerged as a potential target for various neurological disorders. Activation of GSK-3β (pGSK-3βtyr216) promotes apoptosis in a wide variety of conditions, while its inhibition promotes cell survival (Rickle et al., 2004; Takashima, 2009). The role of H3 receptor-mediated activation of Akt/GSK-3β in the brain is still under investigation, but dysregulation of GSK-3β is linked to several neurodegenerative conditions including AD and other neurological disorders (Rickle et al., 2004; Takashima, 2009). The pro-apoptotic effect of activated GSK-3β suggests a potential role for its inhibitors in protection against neuronal cell death (Eldar-Finkelman, 2002; Takashima, 2009). Inhibition of GSK-3β was found to be critical in imparting neuroprotection in various models of neurodegeneration (Chin et al., 2005; Rosa et al., 2008), including KA-induced neurotoxicity (Goodenough et al., 2004). Selective GSK-3β inhibitors were found to reduce the development of complications in models of TLE, by limiting the extent of neuronal damage associated with TLE, without affecting the EEG parameters of KA-induced seizures (Busceti et al., 2007; Luna-Medina et al., 2007). Moreover, inhibition of GSK-3β has also been implicated in the mechanism of action of AEDs like valproic acid (Qing et al., 2008), suggesting a possible role for GSK-3β in the pathophysiology of epilepsy.
The novel finding that stimulation of H3 receptors triggers activation of the Akt/GSK-3β pathway and protects against NMDA-induced neurotoxicity, and that both are blocked by the H3-antagonist thioperamide (Bongers et al., 2007b; Mariottini et al., 2009), is contradicted by an in vivo study where inhibition of H3 receptors by the H3-antagonist ABT-239 conferred neuroprotection by elevating hippocampal GSK-3βser9 levels, which resulted in reduction in τ-phosphorylation in an Alzheimer model in mice (Bitner et al., 2011). These experimental anomalies require further elucidation to delineate this H3 receptor-linked pathway. Reducing neuronal death is an important therapeutic goal in the context of a number of neurodegenerative diseases including refractory epilepsies (Sloviter, 2011). Therefore, the emerging finding that H3 receptors are coupled to GSK-3β throws up an exciting avenue to explore a new therapeutic opportunity, if any, germane to TLE-induced neurodegeneration.
Activation of PLA2
H3 receptor-induced activation of Gi/o-proteins seems to stimulate PLA2 to induce the release of AA (Rouleau et al., 2002). Usually, activation of mammalian PLA2 cleaves membrane phospholipids liberating two major fatty acids of brain, AA and docosahexaenoic acid. Under pathological conditions, these metabolites act as precursors for various inflammatory compounds, for example PAF, prostaglandins, leukotrienes and lipid peroxides, which are crucial in mediating the oxidative and inflammatory responses in CNS pathologies such as stroke, AD, PD and multiple sclerosis (Sun et al., 2010). As PLA2 inhibitors confer neuroprotection in KA-induced neurodegeneration of hippocampal slices (Farooqui et al., 2004) and increased activity of this enzyme in the hippocampus is associated with human TLE (Gattaz et al., 2011), it would be interesting to investigate the significance of this pathway in epileptogenic mechanisms further by employing H3 receptor ligands.
Inhibition of the Na+/H+ exchanger
H3 receptor activation leads to the inhibition of the Na+/H+ exchanger (NHE; Silver et al., 2001) by an as yet unknown mechanism, although a direct interaction of Gαi/o-proteins has been proposed (Bongers et al., 2007a). Activation of the H3 receptor was shown to diminish neuronal NHE activity, and this pathway is proposed as the mechanism by which the H3 receptor inhibits the excessive release of noradrenaline during protracted myocardial ischaemia (Hashikawa-Hobara et al., 2012).
The NHE is of profound importance as it maintains intracellular physiological pH by the extrusion of one intracellular H+ in exchange for one extracellular Na+ across the plasma membrane and thereby prevents neural acidosis, as the loss of NHE function is hypothesised to lower seizure threshold (Cox et al., 1997). Earlier, the defective functioning or absence of the NHE1 gene was shown to contribute to the neuronal death in the brains of mutant mice with epilepsy (Cox et al., 1997; Zhao et al., 2005). In line with this, NHE is assumed to favourably regulate neuronal excitability and post-ictal recovery in different transgenic models (reviewed by Obara et al., 2008). However, in contrast, several studies have obtained experimental findings indicating that inhibitors targeting NHE are of significant therapeutic value in epilepsy (reviewed by Ali et al., 2008). In this context, the influence of H3 receptor ligands on NHE and subsequent implications in epilepsy need to be assessed.
Modulation of [Ca2+]i
Neuronal H3 receptor agonism decreases K+-induced extracellular calcium inflow, probably by impairing voltage operated ion channel (Silver et al., 2002; Seyedi et al., 2005), and inhibits histamine synthesis through the AC/PKA (Gomez-Ramirez et al., 2002) and CaMKII pathways (Torrent et al., 2005; Moreno-Delgado et al., 2009). Conversely, H3 receptor agonists, acting on recombinantly expressed H3 receptors, transiently mobilize calcium from intracellular depots (Bongers et al., 2006). To complicate this further, the H3 receptor antagonist clobenpropit was found to reverse NMDA-induced Ca2+ accumulation in rat cortical neurons (Dai et al., 2007). Also, it has been reported that both PKA and CaMKII tend to regulate phosphorylation and hence activation of the histamine synthesizing enzyme, HDC. Depolarization–stimulation of histamine synthesis is mediated by calcium entry and subsequent calmodulin and CaMKII activation, although it is unlikely that PKA-dependent stimulation of histamine synthesis is linked to depolarization (Gomez-Ramirez et al., 2002; Torrent et al., 2005). Previously, it was reported that, in rat brain cortical slices containing histaminergic nerve endings, the prototypical H3 receptor antagonist (now considered an inverse agonist) thioperamide stimulated histamine synthesis in a concentration-dependent manner (Gomez-Ramirez et al., 2002). These effects of thioperamide were mimicked by clobenpropit and A331440 (Moreno-Delgado et al., 2006).
Neuronal Ca2+ homeostasis and Ca2+ signalling via CaMKII regulate neuronal well-being. Ca2+ dyshomeostasis due to inhibition of CaMKII results in aberrant calcium and glutamate signalling, which can lead to neurodegenerative diseases including glutamate excitotoxicity and epilepsy (Ashpole et al., 2012). The implications of H3 receptor-mediated alteration in neuronal [Ca2+]i level pertaining to epileptogenesis still need to be elucidated.
Epilepsy and histamine H3 receptors
The possibility that the brain histaminergic system is involved in epilepsy is being driven by plethora of experimental and clinical findings suggesting that histamine acts as an antiepileptic neurotransmitter. Activation of the central histaminergic system increases seizure threshold and decreases seizure susceptibility in electrically and chemically induced seizures and kindling mediated by histamine H1 receptors (Tuomisto and Tacke, 1986; Tuomisto et al., 1987; Yokoyama et al., 1992; Iinuma et al., 1993; Kamei et al., 1998; Chen et al., 2003; Hirai et al., 2004; Ishikawa et al., 2007; Nishida et al., 2007; Zhu et al., 2007). In addition, high doses of several centrally acting H1 receptor antagonists when used as anti-allergic drugs occasionally induce convulsions in clinical settings (Yokoyama et al., 1993b; Jang et al., 2010; Takano et al., 2010; Miyata et al., 2011) and experimental models (Yokoyama et al., 1993a, 1996; Kamei et al., 2000; Singh and Goel, 2010), further supporting an association between the neuronal histaminergic system and epileptic seizure pathophysiology.
The involvement of histamine H3 receptors in epilepsy is currently under investigation. The pharmacological properties of H3 antagonists/inverse agonists with respect to their anticonvulsive potential has begun to receive increased attention, as mounting experimental evidence from both acute and chronic models of epilepsy indicate the effectiveness of H3 receptor antagonists. They were found to protect against experimental convulsions by increasing the release of histamine in the brain, which in turn interacts with H1 receptors (Yokoyama et al., 1993c; 1994; Kakinoki et al., 1998; Ishizawa et al., 2000; Vohora et al., 2000; 2001; Chen et al., 2002). In addition, they are assumed to mediate their anticonvulsive action by several other mechanisms (Table 1), such as facilitation of GABA release (Ishizawa et al., 2000; Vohora et al., 2001; Zhang et al., 2003a,b), increasing histidine decarboxylase (HDC) activity (Yokoyama et al., 1994; Hirai et al., 2004) and synergism with AEDs (Ishizawa et al., 2000; Vohora et al., 2001; Uma Devi et al., 2011). Buoyed by encouraging results in experimental epilepsy, many academic and industrial units have begun extensive research to increase the efficacy of possible H3 antagonists in clinical epilepsy. So far, the only success is in the form of pitolisant, which has progressed to phase II of a clinical trial for patients suffering from photosensitive epilepsy, a rare form of the disease where seizures are evoked by photic stimuli. The compound at a dose range of 20–60 mg, when administered alone as well as in combination with AEDs, dose-dependently improved the photoparoxysomal response. The onset of action was1–2 h post application and appreciably lasted from 8 h up to 36 h. Further progress in this direction has not emerged. (Tiligada et al., 2009; Kuhne et al., 2011).
Table 1.
H3 recepor ligands and the mechanisms entailed in their antiepileptic effect in diverse models of epilepsy
| H3 receptor ligands | Model used | Effect observed | Mechanism | Reference |
|---|---|---|---|---|
| Thioperamide (3.75, 7.5 and 15 mg·kg−1 i.p.) | MES seizure in mice | ↓ Duration of each phase of convulsion | H3 antagonism/inverse agonism: reversal of auto-inhibitory HA synthesis and release; H1-mediated seizure inhibition | Yokoyama et al., 1993c |
| Clobenpropit (0.1, 0.3, 1 and 3 mg·kg−1 i.p.) | MES seizure in mice | ↓ Duration of each phase of convulsion | As first mechanism; ↑ HDC activity | Yokoyama et al., 1994 |
| AQ-0145 | MES seizure in mice | ↓ Duration of each phase of convulsion | As first mechanism | Murakami et al., 1995 |
| Thioperamide (7.5 and 15 mg·kg−1 i.p.) | Proconvulsive effect of theophylline on MES seizure in mice | ↓ Mortality | As first mechanism | Yokoyama et al., 1997 |
| Clobenpropit (20–40 mg·kg−1 i.p.) | MES- and PTZ-induced seizure in mice | ↑ Threshold for tonic seizure in MES test ⊗ PTZ seizure threshold | As first mechanism; pharmacokinetic synergism with AEDs | Fischer and van der Goot, 1998 |
| Thioperamide (5–10 mg·kg−1 i.p.) and Clobenpropit (1, 5, 10 and 50 µg i.c.v.) | Amygdaloid kindled (electrically) rats | ↓ Seizure stage; ↓ AD duration | As first mechanism | Kakinoki et al., 1998 |
| Clobenpropit (0.5, 2, 5, and 10 mg·kg−1 i.p.) | Amygdaloid kindled (electrically) rats | ↓ Seizure stage; ↓ AD duration | As first mechanism; interaction with GABA actions | Ishizawa et al., 2000 |
| Thioperamide (3.75, 7.5 and 15 mg·kg−1 i.p.) R-α-methylhistamine (10 µg i.c.v.) | PTZ-induced seizure in mice | ↑ Latency of myoclonic jerks & clonic generalized seizures; ↓ % incidence; H3 agonism reversed the above | As first mechanism; | Vohora et al., 2000 |
| Thioperamide and AQ 0145 (each 5 and 10 mg·kg−1 i.p. and 10–50 µg i.c.v.) | Amygdaloid kindled (electrically) rats | ↓ Seizure stage; ↓ AD duration | As first mechanism | Kamei, 2001 |
| Thioperamide (3.75–7.5 mg·kg−1 i.p.); R-α-methylhistamine (10 µg i.c.v.) | MES- and PTZ-induced seizure in mice | ↑ Latency of myoclonic jerks and clonic generalized seizures; ↓ Duration of tonic and clonic phases; H3 agonism reversed the above | As first mechanism; potentiation of AEDs | Vohora et al., 2001 |
| Thioperamide (5, 10 and 20 µg; i.c.v.) | PTZ-induced kindling in rats | ↓ Duration of each phase of convulsion; ↑ seizure threshold | As first mechanism | Chen et al., 2002 |
| Clobenpropit (10 and 20 µg i.c.v.) Immepip (5 and 10 µg i.c.v.) | PTZ-induced kindling in rats | Delayed onset of kindling; ↑ latency of seizure stage; H3 agonism reversed the above | As first mechanism | Zhang et al., 2003a,b |
| Carcinine and Thioperamide (each 2–20 mg·kg−1 i.p.) | PTZ induced kindling in mice | ↑ Latency of seizures; ↓ Seizure stages | ↑ HDC activity; ↑ HA release | Chen et al., 2004 |
| Iodophenpropit, Clobenpropit, Thioperamide (each 10 mg·kg−1 i.p./i.c.v.), AQ0145 (20 mg·kg−1 i.p.); VUF5514, VUF5515 VUF4929 (i.c.v.) | Amygdaloid kindled (electrically) rats; MES seizure in rats | ↓ Seizure stages; ↓ AD duration; ↓ tonic seizure ⊗ Duration of clonic phase | As first mechanism | Harada et al., 2004a,b |
| Thioperamide (25 mg·kg−1 i.p.) | EL mice (model of human TLE) | ↑ Latency; ↓ % of seizure incidence | ↑ HDC activity; ↑ Brain HA release | Yawata et al., 2004 |
| Clobenpropit (i.c.v.) | PTZ-induced kindling in mice | ↑ Latency of myoclonic jerks and clonic generalized seizures; ↓ Seizure stages | As first mechanism | Zhang et al., 2004 |
| Thioperamide (10, 20 and 50 µg i.c.v.) | Imipramine-induced seizures in amygdaloid kindle (electrically) rats | ↓ Behavioural seizure; ↓ EEG seizure | As first mechanism | Ago et al., 2006 |
| Clobenpropit (0.75 and 1.5 mg·kg−1 i.p.) | MES seizure in mice | Inhibition of seizure | Pharmacodynamic synergism with pyridoxine | Uma Devi et al., 2011 |
| Thioperamide (2–10 mg·kg−1 i.p.) and R-α-methylhistamine (10–40 mg·kg−1 i.p.) | MES and PTZ induced seizure in mice | ⊗ Seizure threshold in both model | H3 antagonism and agonism | Scherkl et al., 1991 |
| Thioperamide and Burimamide | Picrotoxin induced seizure | ↑ Severity of clonic convulsions | H3 antagonism | Sturman et al., 1994 |
| Thiopermide (20 and 30 mg·kg−1 i.p.) Betahistine (200 and 400 mg·kg−1 i.p.) | Amygdaloid kindled rats | No anticonvulsive effect; mild effect only at very high dose | H3 antagonism | Yoshida et al., 2000 |
| Thioperamide (15 mg·kg−1 i.p.) | Methionine-sulfoximine induced convulsion in mice | ⊗ Seizure latency and mortality | H3 antagonism | Vohora et al., 2010 |
AD, after discharge; MES, Maximal electroshock; PTZ, pentylenetetrazole; ↑ increase; ↓ decrease; ⊗ no change.
By contrast, conflicting results have been published on the role of H3 receptors (either induce seizure or mainly have no effect) in various models of experimental convulsions (Scherkl et al., 1991; Sturman et al., 1994; Wada et al., 1996; Fischer and van der Goot, 1998). These paradoxes could be suggestive of the involvement of non-histaminergic mechanisms for the epileptic disposition in the model studied. All this notwithstanding, it is proposed that the intrinsic histaminergic system exerts a powerful inhibitory function during epileptic seizure episodes, evidently via an H1- and H3- dependent mechanism. In line with this, H3 receptor antagonists can be of therapeutic value in epilepsy, or at least a viable approach that can be combined with AEDs, especially in patients who are refractory or non-responsive to conventional therapies.
Histamine H3 receptor and neuroprotection
The status of the histaminergic system with regard to its neuroprotective role is still vague and inconclusive. The importance of histamine in neuroprotection is illustrated by the association of brain histamine levels with seizure threshold and duration (Yawata et al., 2004) and the ability of histaminergic neurons to protect the developing hippocampus from KA-induced neuronal damage, which is partly mediated by H1 and H3 receptors (Kukko-Lukjanov et al., 2006; 2012). Likewise, KA-induced neurotoxicity was aggravated in mice treated with an H1-receptor antagonist (Kukko-Lukjanov et al., 2010). At present, there is very little information regarding the effect of H3 antagonists on neuronal survival and function. A role for histamine H3 receptors in various neurodegenerative diseases, such as AD and PD, has been suggested (Alguacil and Pérez-García, 2003; Gemkow et al., 2009).
Numerous experimental findings have shown that histamine confers neuroprotection in degenerative changes due to ischaemia. For example, middle cerebral artery occlusion in the rat induces a long-lasting increase in neuronal histamine release in the striatum (Adachi et al., 1992). Moreover, histamine depletion with α-fluoromethylhistidine, an inhibitor of histidine decarboxylase (its synthesizing enzyme), significantly increases the number of necrotic pyramidal cells in the hippocampal CA1 region in rats subjected to cerebral ischaemia (Adachi et al., 1993). Post-infarction loading with histidine, a precursor of histamine, decreases the amount of neuronal damage in the rat brain (Adachi et al., 2005). In cultured cortical neurons, histamine protects against NMDA-induced necrosis via the histamine H2 receptor (Dai et al., 2006).
Unlike the anticonvulsive role attributed to H3 receptors in several studies, relatively little attention has been paid to their possible neuroprotective role especially in seizure-associated neurotoxicity. H3 receptor-dependent modulation of neuroprotective mechanisms has also been demonstrated in diverse studies. A robust up-regulation of H3 receptor mRNA was observed in certain brain areas that participate in epileptogenic processes after KA-induced seizures, indicative of a modulatory role of H3 receptors (Lintunen et al., 2005) as well as during experimental ischaemia (Lozada et al., 2005). Increased H3 receptor expression and subsequent constitutive activation of the downstream Akt/GSK-3β pathway could be the mechanism by which the H3 receptor exerts its endogenous neuroprotective role (Bongers et al., 2007b). On the flip side and in line with an antiepileptic mechanism, H3 receptor agonism aggravated delayed neuronal death in a rat model of cerebral ischaemia (Adachi et al., 1993). Moreover, H3 receptor antagonism conferred neuroprotection by elevating the hippocampal GSK-3βser9 level, which resulted in a reduction in τ phosphorylation in an Alzheimer model in mice (Bitner et al., 2011). Physiological chaperons like heat shock proteins, HSPs (HSP 27 and 70) are up-regulated in various neurodegenerative conditions including epilepsy, but their exact role is as yet uncertain as they may have a protective mechanism, but they have also been linked to deleterious effects (reviewed in Turturici et al., 2011). An H3 receptor antagonist was found to provide neuroprotection arising from their interaction with HSPs in a KA-induced neurotoxic model in teleost (Giusi et al., 2008).
The role of H3 receptor ligands in neuroprotection is further confounded by a number of discrepancies that have emerged (Table 2). H3 receptor antagonists have been found to be ineffective (Kukko-Lukjanov et al., 2006; Bongers et al., 2007b; Molina-Hernández and Velasco, 2008; Mariottini et al., 2009), and to reverse H3 receptor agonists-mediated neuroprotective parameters (Shen et al., 2007; Fu et al., 2008) in various models of neurodegeneration. Furthermore, a short lasting but very high up-regulation of the expression of mRNA for all of the H3 receptor isoforms (particularly H3A+D) was observed in the areas most vulnerable to the excitotoxic effect of KA (in CA1 and CA3), which correlated well with neuronal damage (Jin et al., 2005; Lintunen et al., 2005), suggestive of a dual role for this receptor. Neurodegeneration could occur by the activation of H3A receptor-mediated neuronal death through JNK pathways. However, this upregulation of H3A receptor could be a compensatory rise to carry out regenerative/cell survival process via H3A-coupled Ras-Raf-MEK pathways (Jin et al., 2005; Lintunen et al., 2005). Neurotoxic insult by 3-nitropropionic acid (3-NP) resulted in increased transcriptional activities of H3 receptors, which was comparable with deleterious neuronal effects in rat and hamster (Canonaco et al., 2005). In summary, H3 receptor antagonism cannot be deemed to be neuroprotective until the confounding factors, such as phenotype and isoforms specific variations among the different experimental paradigms used, are taken into consideration. Hence, it would be interesting to explore the modulatory effect, if any, of H3 receptor antagonists on the neurodegenerative processes associated with epilepsy.
Table 2.
H3 receptor ligands and the mechanisms entailed in neuroprotective effect in diverse models of neurotoxicity
| H3 receptor ligands | Model used | Effect observed | Mechanism | Reference |
|---|---|---|---|---|
| Thioperamide (40–400 mg·kg−1 i.p.) | Ibotenate-induced brain lesion in IL-9-treated mice | ↓ Brain lesion | H3 antagonism/inverse agonism | Patkai et al., 2001 |
| Thioperamide (10−8–10−5 M) | HA-induced neurotoxicity in cultured rat cerebellar granular neurons | ↓ % of cell death | As 1st mechanism | Gepdiremen et al., 2003 |
| Thioperamide (10 mg·kg−1 i.p.) | 3-NP-induced neurotoxicity in hamster | ↑ H1R mRNA, ↓ Motor behaviour | As 1st mechanism | Canonaco et al., 2005 |
| Thioperamide (10−7–10−5 M) | NMDA-induced neurotoxicity in cultured rat cortical neurons | ↓ NMDA-induced necrosis, ↑ Neuronal viability | As 1st mechanism, ↑ GABA release | Dai et al., 2006 |
| Thioperamide (1, 10, 102 & 103 nM) Clobenpropit (10, 102, 103 & 5000 nM) | KA-induced neurotoxicity in cultured rat hippocampal slices | ↓ Neuronal degeneration, Clobenpropit had no effect | As 1st mechanism | Kukko-Lukjanov et al., 2006 |
| Clobenpropit (10−11–10−7 M) | NMDA-induced neurotoxicity in cultured rat cortical neurons | ↓ NMDA-induced necrosis, ↑ Neuronal viability | As 1st mechanism; Activation of cAMP/PKA pathway; ↑ GABA release, ↓[Ca2+]i | Dai et al., 2007 |
| Thioperamide (10 mg·kg−1 i.p.) | Pb-induced neurotoxicity in teleost (T. pavo) | ↓ Behavioural hyperactivity, ↓ Neurodegeneration, ↓ mRNA expression of HSPs (70 and 90) | Cross-talk between H3R with HSPs | Giusi et al., 2008 |
| GSK 189254 (0.3 mg·kg−1 p.o.) | GSK 189254-treated rats | ↑ Survival of dentate gyrus neurons | ↑ NCAM-PSA expression | Foley et al., 2009 |
| ABT-239 (0.03, 0.1, 0.7, 1 mg·kg−1 i.p.) | Tg2576 and TAPP transgenic AD mice | ↑ p-CREB, ↑ p-GSK3βser9, ↓ Tau hyperphosphorylation | As 1st mechanism | Bitner et al., 2011 |
| Thioperamide (10−4& 10−4 M·L−1) | NMDA-induced neurotoxicity in rat PC12 cells | Reversal of carnosine induced neuroprotective effect and inhibition of glutamate release | As 1st mechanism | Shen et al., 2007 |
| Thioperamide (10 µM·L−1) Clobenpropit (100 µM·L−1) | SK-N-MC cells transfected with hH3R; Cultured rat cortical neurons and striatal slices | ↑ cAMP accumulation, ↓ pAktSer473, ↓ p-GSK3βSer9 | As 1st mechanism | Bongers et al., 2007b |
| Thioperamide (1 µM) | Cultured rat cortical stem cells | ⊗ Cell proliferation and differentiation | As 1st mechanism | Molina-Hernández and Velasco, 2008 |
| Thioperamide & Clobenpropit | A beta-42 induced neurotoxic rat PC12 cells | ↓ Cell viability; reversal of inhibition of glutamate release | As 1st mechanism | Fu et al., 2008 |
| Thioperamide (10 µM·L−1) Immepip (10 nM·L−1) | NMDA-induced neurotoxicity in cultured mouse cortical neurons | Blockade of H3 agonist induced- ↑ pAktSer473, ↑ Bcl-2 expression, ↓ LDH, GSK-3β and caspase 3 activity; ⊗per se on the above | As 1st mechanism | Mariottini et al., 2009 |
| – | KA in SD rats | Possible anti-seizure, neuroprotective role | Brief ↓ H3A+D mRNA (24 h); ↑↑ H3A+D mRNA (after 1 week) | Jin et al., 2005 |
| – | KA in SD rats | Variable (site & time dependent)- Both anti- and/or seizurogenicity; from neuronal death to survival/regeneration | Transient expression of- ↑↑ H3A+D mRNA followed by ↓ H3A+D mRNA | Lintunen et al., 2005 |
LDH, lactate dehydrogenase; pAktSer473, phosphorylation of Akt at serine 473 residue; p-GSK3βser9, phosphorylation of GSK-3β at serine 9 residue; NCAM-PSA, neural cell adhesion molecule-polysialylation; PC-12, pheochromocytoma cell line; SK-N-MC, a human neuroblastoma cell line that stably expresses the human H3R; SD, Sprague–Dawley; ↑ increase; ↑↑ very high increase; ↓ decrease; – no H3R ligand used; ⊗ no change.
Way ahead
The rapid increase in our understanding of the range and mode of operation of different neurotransmitter systems and of synaptic neurophysiology is leading to landmark advancement in the treatment of epilepsy with the advent of new AEDs. However, improvement in terms of clinical outcome has fallen short of expectations, with up to one-third of patients continuing to experience seizures or unacceptable medication-related side effects in spite of efforts to identify optimal treatment regimes with one or more drugs. A plethora of experimental findings lend credence to the anticonvulsive and neuroprotective effects of H3 receptor ligands and they are currently in advanced stages of clinical development for a broad spectrum of central diseases (e.g. narcolepsy, AD, epilepsy and schizophrenia). As per the literature in hand, in spite of several experimental findings explicitly indicating an important role for central H3 receptors in the inhibition of seizures, the development of hH3 receptor ligands has been disappointingly slow, at least partly because of problems with selectivity. The only success met so far is in the form of pitolisant, which has reached phase II trial for photosensitive epilepsy. Nonetheless, the experimental findings accumulated in support of the involvement of H3 receptors in epilepsy and concomitant neurodegeneration should not be discounted.
On the basis of the present data, the following key points can be taken into consideration to absolve the discrepancies observed and hence to bridge the gap between experimental findings and success in clinical settings.
Averting the precipitation of status epilepticus is considered the most effective way to minimize neurodegeneration (Walker, 2007) and employment of immediate neuroprotective strategies could prevent epileptogenic changes (Acharya et al., 2008). Intriguingly, H3 receptor antagonists appear to possess both anticonvulsive and neuroprotective and/or disease modifying activity as suggested in numerous experimental findings. Strategically, a highly localized CNS expression of H3 receptors (Drutel et al., 2001) is likely to minimize peripheral side effects, making them a promising target for epilepsy and neurotoxicity.
The intrinsic activity of H3 receptor inverse agonists that reverse constitutive suppression of histamine and/or GABA release (Morisset et al., 2000; Dai et al., 2007) could contribute to but is unlikely to fully account for receptor-dependent seizure suppression and neuroprotection. The beneficial effects of some H3 antagonists observed could also be mediated through a non-histaminergic mechanism (Uma Devi et al., 2011). Hence, further investigations are warranted to reveal cross-talk with other cellular factors (Giusi et al., 2008; Moreno et al., 2011).
An explanation for H3 receptors being associated with the dual role of both cell death and survival could be the coupling of this receptor to MAPK pathways, different components of which can play an opposing role (Wada and Penninger, 2004). Moreover, ERK1/2 activation may initially defy oxidative damage, but prolonged oxidative stress leads to exhaustion of cellular defences and it serves as a signal to trigger cell death (Luo and DeFranco, 2006). Again, there are findings that indicate that H3 receptor agonism in vitro (Bongers et al., 2007b) leads to signalling changes in the Akt/GSK-3β pathway analogous to those observed with H3 receptor antagonism in vivo (Bitner et al., 2011). This paradox could be attributed to the possible phenotypic variation in the signalling pathways between the two experimental systems used. Hence, improved understanding of the signal transduction cascades that are activated by the H3 receptor is essential in order to elucidate the molecular mechanisms underlying the potential H3 receptor-mediated modulation of brain function. The neurotoxicity observed in the presence of high levels of histamine may be due to the interaction of the convulsant with other neurotransmitter receptors and/or other pathways (Díaz-Trelles et al., 1999).
Both H3 agonism and antagonism have been found to attenuate Ca2+ influx and [Ca2+]i levels. The possible explanation for these inconsistencies in H3 receptor-mediated Ca2+ signalling is speculated to be due to the presence of different ligand-bound active state conformations that couple differentially to the signalling system (Seyedi et al., 2005; Dai et al., 2007). A change in cell membrane composition, as in Apoe−/− mice, could also affect the structural conformation of H3 receptors and hence alter its ligand binding characteristics (van Meer et al., 2007).
Due to the advent of powerful tools like genetic engineering, the existence of more than 20 different species-distinct splice variants of H3 receptor have been identified, generating large molecular heterogeneity (reviewed in Hancock et al., 2003; Leurs et al., 2011). The resultant functional consequences with respect to their different CNS distribution patterns, biased ligand binding and their clinical relevance have yet to be determined. The dichotomous role of H3 receptors as demonstrated by the transient and variable expression of various H3 receptor isoforms in different brain regions that correlated with both neuronal survival and death in KA-treated rats (Jin et al., 2005; Lintunen et al., 2005) could be attributed to the existence of its pharmacological variants.
In addition, it is anticipated that the interaction of histamine receptors with the intracellular transduction pathways is evidently not straightforward. The complexity arisen from the emergence of various functional isoforms differentially coupled to various G-proteins, the possible intertwining of signalling pathways (Drutel et al., 2001) and interaction with other neurotransmitter receptors and/or pathways (Díaz-Trelles et al., 1999) must be taken into account by selective pharmacological targeting, possibly by devising isoform-specific ligands.
On the basis of the substantial experimental findings generated so far, H3 receptor antagonists can be envisaged as having a therapeutic effect on epileptic and associated neurodegenerative disorders. An enhanced understanding of the mechanisms of seizure control and neuroprotective effects mediated via H3 receptors in the light of the emerging signalling pathways coupled to it must be sought; as such mechanisms are likely to be the most valuable candidates for novel therapeutic interventions for improved seizure control. With that in mind, efforts should be taken to develop selective H3 receptor agonists, antagonists and inverse agonists, which could provide the lead for the potential exploitation of the histaminergic system in the treatment of epilepsy and epileptogenesis.
Acknowledgments
This work was supported by assistance from University Grants Commission Special Assistance Programme (UGC-SAP) to D V and UGC- RGNF to M B.
Glossary
- [35S]-GTPγS
[35S]-guanosine 5′-γ-thiotriphosphate
- [Ca2+]i
intracellular calcium
- 3-NP
3-nitropropionic acid
- AA
arachidonic acid
- AD
Alzheimer's disease
- ADHD
attention deficit hyperactivity disorder
- AEDs
antiepileptic drugs
- CaMKII
Ca2+-activated calmodulin kinases
- CREB
cAMP-responsive-element-binding protein
- HDC
histidine decarboxylase
- hH3
receptor, human H3 receptor
- HSPs
heat shock proteins
- ICER
inducible cAMP early repressor
- IPI
initial precipitating injury
- KA
kainic acid
- MAPKAPK2
MAPK-activated protein kinase 2
- MEK
MAP/ERK kinase
- NHE
Na+/H+ exchanger
- GluN2B
NMDA receptor subunit 2B
- PD
Parkinson's disease
- SE
status epilepticus
- TLE
temporal lobe epilepsy
- TMN
tuberomammillary nucleus
Statement of conflict of interest
The authors declare no conflict of interest.
References
- Acharya JN. Recent advances in epileptogenesis. Curr Sci. 2002;82:679–688. [Google Scholar]
- Acharya MM, Hattiangady B, Shetty A. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N. Cerebral ischemia and brain histamine. Brain Res Rev. 2005;50:275–286. doi: 10.1016/j.brainresrev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Adachi N, Itoh Y, Oishi R, Saeki K. Direct evidence for increased continuous histamine release in the striatum of conscious freely moving rats produced by middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1992;12:477–483. doi: 10.1038/jcbfm.1992.65. [DOI] [PubMed] [Google Scholar]
- Adachi N, Oishi R, Itano Y, Yamada T, Hirakawa M, Saeki K. Aggravation of ischemic neuronal damage in the rat hippocampus by impairment of histaminergic neurotransmission. Brain Res. 1993;602:165–168. doi: 10.1016/0006-8993(93)90259-p. [DOI] [PubMed] [Google Scholar]
- Adachi N, Liu K, Arai T. Prevention of brain infarction by postischemic administration of histidine in rats. Brain Res. 2005;1039:220–223. doi: 10.1016/j.brainres.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Ago J, Ishikawa T, Matsumoto N, Ashequr Rahman M, Kamei C. Mechanism of imipramine-induced seizures in amygdala-kindled rats. Epilepsy Res. 2006;72:1–9. doi: 10.1016/j.eplepsyres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Alguacil LF, Pérez-García C. Histamine H3 receptor: a potential drug target for the treatment of central nervous system disorders. Curr Drug Targets CNS Neurol Disord. 2003;2:303–313. doi: 10.2174/1568007033482760. [DOI] [PubMed] [Google Scholar]
- Ali A, Ahmad FJ, Dua Y, Pillai KK, Vohora D. Seizures and sodium hydrogen exchangers: potential of sodium hydrogen exchanger inhibitors as novel anticonvulsants. CNS Neurol Disord Drug Targets. 2008;7:343–347. doi: 10.2174/187152708786441830. [DOI] [PubMed] [Google Scholar]
- Ao H, Ko SW, Zhuo M. CREB activity maintains the survival of cingulate cortical pyramidal neurons in the adult mouse brain. Mol Pain. 2006;2:15. doi: 10.1186/1744-8069-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci. 2007;28:350–357. doi: 10.1016/j.tips.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Song W, Brustovetsky T, Engleman EA, Brustovetsky N, Cummins TR, et al. Calcium/calmodulin-dependent protein kinase (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem. 2012;287:8495–8506. doi: 10.1074/jbc.M111.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RA, Lozada AF, van Marle A, Shenton FC, Drutel G, Karlstedt K, et al. Discovery of naturally occurring splice variants of the rat histamine H3 receptor that act as dominant- negative isoforms. Mol Pharmacol. 2006;69:1194–1206. doi: 10.1124/mol.105.019299. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Markosyan S, Nikkel AL, Brioni JD. In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer's disease. Neuropharmacology. 2011;60:460–466. doi: 10.1016/j.neuropharm.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Bongers G, Sallmen T, Passani M, Mariottini C, Wendelin D, Lozada A, et al. 2006. New signaling pathways for the histamine H3 receptor. In: Proceedings of the XXXVth Annual Meeting of European Histamine Research Societ. [DOI] [PubMed]
- Bongers G, Bakker RA, Leurs R. Molecular aspects of the histamine H3 receptor. Biochem Pharmacol. 2007a;73:1195–1204. doi: 10.1016/j.bcp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Bongers G, Sallmen T, Passani MB, Mariottini C, Wendelin D, Lozada A, et al. The Akt/GSK-3beta axis as a new signaling pathway of the histamine H (3) receptor. J Neurochem. 2007b;103:248–258. doi: 10.1111/j.1471-4159.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- Borlikova G, Endo S. Inducible cAMP early repressor (ICER) and brain functions. Mol Neurobiol. 2009;40:73–86. doi: 10.1007/s12035-009-8072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Raol YH, Russek SJ. Alteration of epileptogenesis genes. Neurother. 2009;6:312–318. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Busceti CL, Biagioni F, Aronica E, Riozzi B, Storto M, Battaglia G, et al. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. doi: 10.1111/j.1528-1167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Canonaco M, Madeo M, Alò R, Giusi G, Granata T, Carelli A, et al. The histaminergic signaling system exerts a neuroprotective role against neurodegenerative-induced processes in the hamster. J Pharmacol Exp Ther. 2005;315:188–195. doi: 10.1124/jpet.105.088153. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li WD, Zhu LJ, Shen YJ, Wei EQ. Effects of histidine, a precursor of histamine, on pentylenetetrazole-induced seizures in rats. Acta Pharmacol Sin. 2002;23:361–366. [PubMed] [Google Scholar]
- Chen Z, Li Z, Sakurai E, Izadi Mobarakeh J, Ohtsu H, Watanabe T, et al. Chemical kindling induced by pentylenetetrazol in histamine H(1) receptor gene knockout mice (H(1)KO), histidine decarboxylase-deficient mice (HDC(-/-)) and mast cell-deficient W/W(v) mice. Brain Res. 2003;968:162–166. doi: 10.1016/s0006-8993(03)02229-7. [DOI] [PubMed] [Google Scholar]
- Chen Z, Sakurai E, Hu W, Jin C, Kiso Y, Kato M, et al. Pharmacological effects of carcinine on histaminergic neurons in the brain. Br J Pharmacol. 2004;143:573–580. doi: 10.1038/sj.bjp.0705978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin PC, Majdzadeh N, D'Mello SR. Inhibition of GSK-3β is a common event in neuroprotection by different survival factors. Mol Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1267–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hill SJ. Sensitivity of histamine H3 receptor agonist-stimulated [35S]GTPγS binding to pertussis toxin. Eur J Pharmacol. 1996;296:223–225. doi: 10.1016/0014-2999(95)00800-4. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009;157:55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice Cell. Erratum in. Cell. 1997;91:861. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- Dai H, Zhang Z, Zhu Y, Shen Y, Hu W, Huang Y, et al. Histamine protects against NMDA-induced necrosis in cultured cortical neurons through H2 receptor/cAMP/protein kinase A and H3 receptor/GABA release pathways. J Neurochem. 2006;96:1390–1400. doi: 10.1111/j.1471-4159.2005.03633.x. [DOI] [PubMed] [Google Scholar]
- Dai H, Fu Q, Shen Y, Hu W, Zhang Z, Timmerman H, et al. The histamine H3 receptor antagonist clobenpropit enhances GABA release to protect against NMDA-induced excitotoxicity through the cAMP/protein kinase A pathway in cultured cortical neurons. Eur J Pharmacol. 2007;563:117–123. doi: 10.1016/j.ejphar.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Trelles R, Solana-López A, Fernández-González JR, Novelli A, Fernández-Sánchez MT. Terfenadine induces toxicity in cultured cerebellar neurons: a role for glutamate receptors. Amino Acids. 1999;16:59–70. doi: 10.1007/BF01318885. [DOI] [PubMed] [Google Scholar]
- Dichter MA. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol. 2009;66:443–447. doi: 10.1001/archneurol.2009.10. [DOI] [PubMed] [Google Scholar]
- Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, Timmerman H, et al. Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol Pharmacol. 2001;59:1–8. [PubMed] [Google Scholar]
- Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154:1166–1181. doi: 10.1038/bjp.2008.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Horrocks LA. Neuroprotection abilities of cytosolic phospholipase A2 inhibitors in kainic acid-induced neurodegeneration. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:85–96. doi: 10.2174/1568006043481239. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Cell signaling in the epileptic hippocampus. Rev Neurol. 2002;34:544–550. [PubMed] [Google Scholar]
- Fischer W, van der Goot H. Effect of clobenpropit, a centrally acting histamine H3-receptor antagonist on electroshock- and pentylenetetrazol-induced seizures in mice. J Neural Transm. 1998;105:587–599. doi: 10.1007/s007020050081. [DOI] [PubMed] [Google Scholar]
- Foley AG, Prendergast A, Barry C, Scully D, Upton N, Medhurst AD, et al. H3 receptor antagonism enhances NCAM PSA-mediated plasticity and improves memory consolidation in odor discrimination and delayed match-to-position paradigms. Neuropsychopharmacology. 2009;34:2585–2600. doi: 10.1038/npp.2009.89. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, et al. Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther. 2005;313:176–190. doi: 10.1124/jpet.104.078402. [DOI] [PubMed] [Google Scholar]
- Fu Q, Dai H, Hu W, Fan Y, Shen Y, Zhang W, et al. Carnosine protects against Abeta42-induced neurotoxicity in different rat PC12 cells. Cell Mol Neurobiol. 2008;28:307–316. doi: 10.1007/s10571-007-9235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarg M, Schwartz JC. Histamine receptors in the brain. N Engl Reg Allergy Proc. 1985;6:195–200. doi: 10.2500/108854185779045233. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Valente KD, Raposo NR, Vincentiis S, Talib LL. Increased PLA2 activity in the hippocampus of patients with temporal lobe epilepsy and psychosis. J Psychiatr Res. 2011;45:1617–1620. doi: 10.1016/j.jpsychires.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Gemkow MJ, Davenport AJ, Harich S, Ellenbroek BA, Cesura A, Hallett D. The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov Today. 2009;14:509–515. doi: 10.1016/j.drudis.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Gepdiremen A, Buyukokuroglu ME, Hacimuftuoglu A, Suleyman H. Contribution of the histaminergic receptor subtypes to histamine-induced cerebellar granular neurotoxicity. Pol J Pharmacol. 2003;55:383–388. [PubMed] [Google Scholar]
- Giovannini MG, Efoudebe M, Passani MB, Baldi E, Bucherelli C, Giachi F, et al. Improvement in fear memory by histamine-elicited ERK2 activation in hippocampal CA3 cells. J Neurosci. 2003;23:9016–9023. doi: 10.1523/JNEUROSCI.23-27-09016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusi G, Alò R, Crudo M, Facciolo RM, Canonaco M. Specific cerebral heat shock proteins and histamine receptor cross-talking mechanisms promote distinct lead-dependent neurotoxic responses in teleosts. Toxicol Appl Pharmacol. 2008;227:248–256. doi: 10.1016/j.taap.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramirez J, Ortiz J, Blanco I. Presynaptic H3 autoreceptors modulate histamine synthesis through cAMP pathway. Mol Pharmacol. 2002;6:239–245. doi: 10.1124/mol.61.1.239. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Conrad S, Skutella T, Behl C. Inactivation of glycogen synthase kinase-3beta protects against kainic acid-induced neurotoxicity in vivo. Brain Res. 2004;1026:116–125. doi: 10.1016/j.brainres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Esbenshade TA, Krueger KM, Yao BB. Genetic and pharmacological aspects of histamine H3 receptor heterogeneity. Life Sci. 2003;73:3043–3072. doi: 10.1016/j.lfs.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Harada C, Fujii Y, Hirai T, Shinomiya K, Kamei C. Inhibitory effect of iodophenpropit, a selective histamine H3 antagonist, on amygdaloid kindled seizures. Brain Res Bull. 2004a;63:143–146. doi: 10.1016/j.brainresbull.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Harada C, Hirai T, Fujii Y, Harusawa S, Kurihara T, Kamei C. Intracerebroventricular administration of histamine H3 receptor antagonists decreases seizures in rat models of epilepsia. Methods Find Exp Clin Pharmacol. 2004b;26:263–270. [PubMed] [Google Scholar]
- Hashikawa-Hobara N, Chan NY, Levi R. Histamine 3 receptor activation reduces the expression of neuronal angiotensin II type 1 receptorsin the heart. J Pharmacol Exp Ther. 2012;340:185–191. doi: 10.1124/jpet.111.187765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- Hirai T, Okuma C, Harada C, Mio M, Ohtsu H, Watanabe T, et al. Development of amygdaloid kindling in histidine decarboxylase deficient and histamine H1 receptor-deficient mice. Epilepsia. 2004;45:309–313. doi: 10.1111/j.0013-9580.2004.19303.x. [DOI] [PubMed] [Google Scholar]
- Iinuma K, Yokoyama H, Otsuki T, Yanai K, Watanabe T, Ido T, et al. Histamine H1 receptors in complex partial seizures. Lancet. 1993;341:238. doi: 10.1016/0140-6736(93)90099-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takechi K, Rahman A, Ago J, Matsumoto N, Murakami A, et al. Influences of histamine H1 receptor antagonists on maximal electroshock seizure in infant rats. Biol Pharm Bull. 2007;30:477–480. doi: 10.1248/bpb.30.477. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Chen Z, Okuma C, Sugimoto Y, Fujii Y, Kamei C. Participation of GABAergic and histaminergic systems in inhibiting amygdaloid kindled seizures. Jpn J Pharmacol. 2000;82:48–53. doi: 10.1254/jjp.82.48. [DOI] [PubMed] [Google Scholar]
- Jang DH, Manini AF, Trueger NS, Duque D, Nestor NB, Nelson LS, et al. Status epilepticus and wide-complex tachycardia secondary to diphenhydramine overdose. Clin Toxicol (Phila) 2010;48:945–948. doi: 10.3109/15563650.2010.527850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen FP, Mochizuki T, Yamamoto Y, Timmerman H, Yamatodani A. In vivo modulation of rat hypothalamic histamine release by the histamine H3 receptor ligands, imepip and clobenpropit. Effects of intrahypothalamic and peripheral application. Eur J Pharmacol. 1998;362:149–155. doi: 10.1016/s0014-2999(98)00739-0. [DOI] [PubMed] [Google Scholar]
- Jin C, Lintunen M, Panula P. Histamine H(1) and H(3) receptors in the rat thalamus and their modulation after systemic kainic acid administration. Exp Neurol. 2005;194:43–56. doi: 10.1016/j.expneurol.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Jin CL, Zhuge ZB, Wu DC, Zhu YY, Wang S, Luo JH, et al. Lesion of the tuberomammillary nucleus E2-region attenuates postictal seizure protection in rats. Epilepsy Res. 2007;73:250–258. doi: 10.1016/j.eplepsyres.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Zhu Y, Greenberg DA. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J Neurochem. 2002;80:119–125. doi: 10.1046/j.0022-3042.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- Kakinoki H, Ishizawa K, Fukunaga M, Fujii Y, Kamei C. The effects of histamine H3-receptor antagonists on amygdaloid kindled seizures in rats. Brain Res Bull. 1998;46:461–465. doi: 10.1016/s0361-9230(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Kamei C. Involvement of central histamine in amygdaloid kindled seizures in rats. Behav Brain Res. 2001;124:243–250. doi: 10.1016/s0166-4328(01)00218-2. [DOI] [PubMed] [Google Scholar]
- Kamei C, Ishizawa K, Kakinoki H, Fukunaga M. Histaminergic mechanisms in amygdaloid-kindled seizures in rats. Epilepsy Res. 1998;30:187–194. doi: 10.1016/s0920-1211(98)00005-9. [DOI] [PubMed] [Google Scholar]
- Kamei C, Ohuchi M, Sugimoto Y, Okuma C. Mechanism responsible for epileptogenic activity by first generation H1 antagonists in rats. Brain Res. 2000;887:183–186. doi: 10.1016/s0006-8993(00)03041-9. [DOI] [PubMed] [Google Scholar]
- Karmarkar SW, Bottum KM, Krager SL, Tischkau SA. ERK/MAPK is essential for endogenous neuroprotection in SCN2.2 cells. Plos ONE. 2011;6:e23493. doi: 10.1371/journal.pone.0023493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, et al. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kim BS, Kim MY, Leem YH. Hippocampal neuronal death induced by kainic acid and restraint stress is suppressed by exercise. Neuroscience. 2011;194:291–301. doi: 10.1016/j.neuroscience.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Kiviranta T, Tuomisto L, Airaksinen EM. Histamine in cerebrospinal fluid of children with febrile convulsions. Epilepsia. 1995;36:276–280. doi: 10.1111/j.1528-1157.1995.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Kuhne S, Wijtmans M, Lim HD, Leurs R, de Esch IJ. Several down, a few to go: histamine H3 receptor ligands making the final push towards the market? Expert Opin Investig Drugs. 2011;20:1629–1648. doi: 10.1517/13543784.2011.625010. [DOI] [PubMed] [Google Scholar]
- Kukko-Lukjanov TK, Soin S, Taira T, Michelsen KA, Panula P, Holopainen IE. Histaminergic neurons protect the developing hippocampus from kainic acid induced neuronal damage in an organotypic coculture system. J Neurosci. 2006;26:1088–1097. doi: 10.1523/JNEUROSCI.1369-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukko-Lukjanov TK, Lintunen M, Jalava N, Lauren HB, Lopez-Picon FR, Michelsen KA, et al. Involvement of histamine 1 receptor in seizure susceptibility and neuroprotection in immature mice. Epilepsy Res. 2010;90:8–15. doi: 10.1016/j.eplepsyres.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kukko-Lukjanov TK, Grönman M, Lintunen M, Laurén HB, Michelsen KA, Panula P, et al. Histamine 1 receptor knockout mice show age-dependent susceptibility to status epilepticus and consequent neuronal damage. Epilepsy Res. 2012;100:80–92. doi: 10.1016/j.eplepsyres.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Dziema H, Lee KH, Choi YS, Obrientan K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol Dis. 2007;25:80–91. doi: 10.1016/j.nbd.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, et al. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–1265. doi: 10.1111/j.1471-4159.2008.05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Choi SS, Lee HK, Han KJ, Han EJ, Suh HW. Effects of MK-801 and CNQX on various neurotoxic responses induced by kainic acid in mice. Mol Cells. 2002;14:339–347. [PubMed] [Google Scholar]
- de Lemos L, Junyent F, Verdaguer E, Folch J, Romero R, Pallàs M, et al. Differences in activation of ERK1/2 and p38 kinase in Jnk3 null mice following KA treatment. J Neurochem. 2010;114:1315–1322. doi: 10.1111/j.1471-4159.2010.06853.x. [DOI] [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurs R, Vischer HF, Wijtmans M, de Esch IJ. En route to new blockbuster anti-histamines: surveying the offspring of the expanding histamine receptor family. Trends Pharmacol Sci. 2011;32:250–257. doi: 10.1016/j.tips.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Lintunen M, Sallmen T, Karlstedt K, Panula P. Transient changes in the limbic histaminergic system after systemic kainic acid-induced seizures. Neurobiol Dis. 2005;20:155–169. doi: 10.1016/j.nbd.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Loscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, et al. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- Lozada A, Munyao N, Sallmen T, Lintunen M, Leurs R, Lindsberg PJ, et al. Postischemic regulation of central histamine receptors. Neuroscience. 2005;136:371–379. doi: 10.1016/j.neuroscience.2005.06.079. [DOI] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, et al. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J Neurosci. 2007;27:5766–5776. doi: 10.1523/JNEUROSCI.1004-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281:16436–16442. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- Mariottini C, Scartabelli T, Bongers G, Arrigucci S, Nosi D, Leurs R, et al. Activation of the histaminergic H3 receptor induces phosphorylation of the Akt/GSK-3 beta pathway in cultured cortical neurons and protects against neurotoxic insults. J Neurochem. 2009;110:1469–1478. doi: 10.1111/j.1471-4159.2009.06249.x. [DOI] [PubMed] [Google Scholar]
- van Meer P, Pfankuch T, Raber J. Reduced histamine levels and H3 receptor antagonist-induced histamine release in the amygdala of Apoe-/- mice. J Neurochem. 2007;103:124–130. doi: 10.1111/j.1471-4159.2007.04705.x. [DOI] [PubMed] [Google Scholar]
- Merlo D, Cifelli P, Cicconi S, Tancredi V, Avoli M. 4-Aminopyridine-induced epileptogenesis depends on activation of mitogen-activated protein kinase ERK. J Neurochem. 2004;89:654–659. doi: 10.1111/j.1471-4159.2004.02382.x. [DOI] [PubMed] [Google Scholar]
- Miyata I, Saegusa H, Sakurai M. Seizure-modifying potential of histamine H1 antagonists: a clinical observation. Pediatr Int. 2011;53:706–708. doi: 10.1111/j.1442-200X.2011.03328.x. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández A, Velasco I. Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem. 2008;106:706–717. doi: 10.1111/j.1471-4159.2008.05424.x. [DOI] [PubMed] [Google Scholar]
- Moreno E, Hoffmann H, Gonzalez-Sepúlveda M, Navarro G, Casadó V, Cortés A, et al. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem. 2011;286:5846–5854. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Delgado D, Torrent A, GóMEZ-Ramírez J, de Esch I, Blanco I, Ortiz J. Constitutive activity of H3 autoreceptors modulates histamine synthesis in rat brain through the cAMP/PKA pathway. Neuropharmacology. 2006;51:517–523. doi: 10.1016/j.neuropharm.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Moreno-Delgado D, GóMEZ-Ramírez J, Torrent-Moreno A, González-Sepúlveda M, Blanco I, Ortiz J. Different role of cAMP dependent protein kinase and CaMKII in H3 receptor regulation of histamine synthesis and release. Neuroscience. 2009;164:1244–1251. doi: 10.1016/j.neuroscience.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneaux X, Gbahou F, Tardivel-Lacombe J, Stark H, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- Moya-Garcia AA, Medina MA, S'anchez-Jim'enez F. Mammalian histidine decarboxylase: from structure to function. Bioessays. 2005;27:57–63. doi: 10.1002/bies.20174. [DOI] [PubMed] [Google Scholar]
- Murakami K, Yokoyama H, Onodera K, Iinuma K, Watanabe T. AQ-0145, a newly developed histamine H3 antagonist, decreased seizure susceptibility of electrically induced convulsions in mice. Methods Find Exp Clin Pharmacol. 1995;17(Suppl. C):70–73. [PubMed] [Google Scholar]
- Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M, et al. ERK activation causes epilepsy by stimulating NMDA receptor activity. EMBO J. 2007;26:4891–4901. doi: 10.1038/sj.emboj.7601911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor DE. Glutamate and GABA in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia. 2010;51(Suppl. 3):106–109. doi: 10.1111/j.1528-1167.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- Nishida N, Huang ZL, Mikuni N, Miura Y, Urade Y, Hashimoto N. Deep brain stimulation of the posterior hypothalamus activates the histaminergic system to exert antiepileptic effect in rat Pentylenetetrazole model. Exp Neurol. 2007;205:132–144. doi: 10.1016/j.expneurol.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Panula P. Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Oda T, Matsumoto S. [Identification and characterization of histamine H4 receptor] Nihon Yakurigaku Zasshi. 2001;118:36–42. doi: 10.1254/fpj.118.36. [Article in Japanese] [DOI] [PubMed] [Google Scholar]
- Okakura K, Yamatodani A, Mochizuki T, Horii A, Wada H. Glutamatergic regulation of histamine release from rat hypothalamus. Eur J Pharmacol. 1992;213:189–192. doi: 10.1016/0014-2999(92)90680-3. [DOI] [PubMed] [Google Scholar]
- Okakura-Mochizuki K, Mochizuki T, Yamamoto Y, Horii A, Yamatodani A. Endogenous GABA modulates histamine release from the anterior hypothalamus. J Neurochem. 1996;67:171–176. doi: 10.1046/j.1471-4159.1996.67010171.x. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Airaksinen MS, Pirvola U, Kotilainen E. A histamine containing neuronal system in human brain. Neuroscience. 1990;34:127–132. doi: 10.1016/0306-4522(90)90307-p. [DOI] [PubMed] [Google Scholar]
- Park SA, Kim TS, Choi KS, Park HJ, Heo K, Lee BI. Chronic activation of CREB and p90RSK in human epileptic hippocampus. Exp Mol Med. 2003;35:365–370. doi: 10.1038/emm.2003.48. [DOI] [PubMed] [Google Scholar]
- Parsons ME, Ganellin CR. Histamine and its receptors. Br J Pharmacol. 2006;147(Suppl. 1):S127–S135. doi: 10.1038/sj.bjp.0706440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passani MB, Blandina P. Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol Sci. 2011;32:242–249. doi: 10.1016/j.tips.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P. Histamine in the brain: beyond sleep and memory. Biochem Pharmacol. 2007;73:1113–1122. doi: 10.1016/j.bcp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Patkai J, Mesples B, Dommergues MA, Fromont G, Thornton EM, Renauld JC, et al. Deleterious effects of IL-9-activated mast cells and neuroprotection by antihistamine drugs in the developing mouse brain. Pediatr Res. 2001;50:222–230. doi: 10.1203/00006450-200108000-00010. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, et al. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Therapeutic approaches to epileptogenesis – hope on the horizon. Epilepsia. 2010;51(Suppl. 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models. J Exp Med. 2008;205:2781–2789. doi: 10.1084/jem.20081588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Yao B, Ahmed S, Asano E, Beaumont TL, Shah AK, et al. A common pattern of persistent gene activation in human neocortical epileptic foci. Ann Neurol. 2005;58:736–747. doi: 10.1002/ana.20633. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, et al. p38 kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer's disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- Rosa AO, Egea J, Martínez A, García AG, López MG. Neuroprotective effect of the new thiadiazolidinone NP00111 against oxygen-glucose deprivation in rat hippocampal slices: implication of ERK1/2 and PPARγ receptors. Exp Neurol. 2008;212:93–99. doi: 10.1016/j.expneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Rouleau A, Ligneaux X, Tardivel-Lacombe J, Morisset S, Gbahou F, Schwartz JC, et al. HistamineH3-receptor-mediated [35S]GTPγS binding: evidence for constitutive activity of the recombinant and native rat and human H3 receptors. Br J Pharmacol. 2002;135:383–392. doi: 10.1038/sj.bjp.0704490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley NM, Madsen KK, Schousboe A, Steve White H. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem Int. 2012 doi: 10.1016/j.neuint.2012.02.013. DOI: 10.1016/j.neuint.2012.02.013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherkl R, Hashem A, Frey HH. Histamine in brain – its role in regulation of seizure susceptibility. Epilepsy Res. 1991;10:111–118. doi: 10.1016/0920-1211(91)90003-x. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Malinowska B, Kathmann M, Göthert M. Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fundam Clin Pharmacol. 1994;8:128–137. doi: 10.1111/j.1472-8206.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JC. The histamine H3 receptor: from drug discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163:713–721. doi: 10.1111/j.1476-5381.2011.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Arrang JM, Garbarg M, Gulat-Marnay C, Pollard H. Modulation of histamine synthesis and release in brain via presynaptic auto and heteroreceptors. Ann N Y Acad Sci. 1990;604:40–54. doi: 10.1111/j.1749-6632.1990.tb31981.x. [DOI] [PubMed] [Google Scholar]
- Seyedi N, Mackins CJ, Machida T, Reid AC, Silver RB, Levi R. Histamine H3-receptor-induced attenuation of norepinephrine exocytosis: a decreased protein kinase A activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther. 2005;312:272–280. doi: 10.1124/jpet.104.072504. [DOI] [PubMed] [Google Scholar]
- Shen Y, Hu WW, Fan YY, Dai HB, Fu QL, Wei EQ, et al. Carnosine protects against NMDA-induced neurotoxicity in differentiated rat PC12 cells through carnosine-histidine-histamine pathway and H(1)/H(3) receptors. Biochem Pharmacol. 2007;73:709–717. doi: 10.1016/j.bcp.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RB, Mackins CJ, Smith NC, Koritchneva IL, Lefkowitz K, Lovenberg TW, et al. Coupling of histamine H3 receptors to neuronal Na+/H+ exchange: a novel protective mechanism in myocardial ischemia. Proc Natl Acad Sci U S A. 2001;98:2855–2859. doi: 10.1073/pnas.051599198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R. Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc Natl Acad Sci U S A. 2002;99:501–506. doi: 10.1073/pnas.012506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Goel RK. Proconvulsant potential of cyproheptadine in experimental animal models. Fundam Clin Pharmacol. 2010;24:451–455. doi: 10.1111/j.1472-8206.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Progress on the issue of excitotoxic injury modification vs. real neuroprotection; implications for post-traumatic epilepsy. Neuropharmacology. 2011;61:1048–1050. doi: 10.1016/j.neuropharm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Sturman G, Freeman P, Meade HM, Seeley NA. Modulation of the intracellular and H3-histamine receptors and chemically-induced seizures in mice. Agents Actions. 1994;41:C68–C69. doi: 10.1007/BF02007771. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010;12:133–148. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Sakaue Y, Sokoda T, Sawai C, Akabori S, Maruo Y, et al. Seizure susceptibility due to antihistamines in febrile seizures. Pediatr Neurol. 2010;42:277–279. doi: 10.1016/j.pediatrneurol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Takashima A. Drug development targeting the glycogen synthase kinase-3β (GSK-3β)- mediated signal transduction pathway: role of GSK-3β in adult brain. J Pharmacol Sci. 2009;109:174–178. doi: 10.1254/jphs.08r29fm. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Yanai K. [Molecular imaging of histamine receptors in the human brain] Brain Nerve. 2007;59:221–231. [Article in Japanese] [PubMed] [Google Scholar]
- Tiligada E, Zampeli E, Sander K, Stark H. Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs. 2009;18:1519–1531. doi: 10.1517/14728220903188438. [DOI] [PubMed] [Google Scholar]
- Torrent A, Moreno-Delgado D, GóMEZ-Ramírez J, Rodríguez-Agudo D, Rodríguez-Caso C, SáNCHEZ-Jiménez F, et al. H3 autoreceptors modulate histamine synthesis through calcium/calmodulin- and cAMP-dependent protein kinase pathways. Mol Pharmacol. 2005;67:195–203. doi: 10.1124/mol.104.005652. [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Tacke U. Is histamine an anticonvulsive inhibitory transmitter? Neuropharmacology. 1986;25:955–958. doi: 10.1016/0028-3908(86)90029-8. [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Tacke U, Willman A. Inhibition of sound-induced convulsions by metoprine in the audiogenic seizure susceptible rat. Agents Actions. 1987;20:252–254. doi: 10.1007/BF02074683. [DOI] [PubMed] [Google Scholar]
- Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011 doi: 10.1155/2011/618127. 2011: ID-618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uma Devi P, Manocha A, Khanam R, Vohora D. Beneficial interaction between clobenpropit and pyridoxine in prevention of electroshock-induced seizures in mice: lack of histaminergic mechanisms. Hum Exp Toxicol. 2011;30:84–88. doi: 10.1177/0960327110372398. [DOI] [PubMed] [Google Scholar]
- Valor LM, Jancic D, Lujan R, Barco A. Ultrastructural and transcriptional profiling of neuropathological misregulation of CREB function. Cell Death Differ. 2010;17:1636–1644. doi: 10.1038/cdd.2010.40. [DOI] [PubMed] [Google Scholar]
- Vohora D, Pal SN, Pillai KK. Thioperamide, a selective histamine H3 receptor antagonist, protects against PTZ-induced seizures in mice. Life Sci. 2000;66:PL297–PL301. doi: 10.1016/s0024-3205(00)00548-8. [DOI] [PubMed] [Google Scholar]
- Vohora D, Pal SN, Pillai KK. Histamine and selective H3 receptor ligands: a possible role in the mechanism and management of epilepsy. Pharmacol Biochem Behav. 2001;68:735–741. doi: 10.1016/s0091-3057(01)00474-9. [DOI] [PubMed] [Google Scholar]
- Vohora D, Khanam R, Pal SN, Pillai KK. Effect of combined treatment of thioperamide with some antiepileptic drugs on methionine-sulfoximine induced convulsions in mice. Indian J Exp Biol. 2010;48:858–860. [PubMed] [Google Scholar]
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- Wada Y, Shiraishi J, Nakamura M, Koshino Y. Biphasic action of the histamine precursor L-histidine in the rat kindling model of epilepsy. Neurosci Lett. 1996;204:205–208. doi: 10.1016/0304-3940(96)12358-2. [DOI] [PubMed] [Google Scholar]
- Walker M. Neuroprotection in epilepsy. Epilepsia. 2007;48(Suppl. 8):66–68. doi: 10.1111/j.1528-1167.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y, et al. Distribution of the histaminergic neuron system in the central nervous system of rats: a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- Werner FM, Coveñas R. Classical neurotransmitters and neuropeptides involved in generalized epilepsy: a focus on antiepileptic drugs. Curr Med Chem. 2011;18:4933–4948. doi: 10.2174/092986711797535191. [DOI] [PubMed] [Google Scholar]
- Wieland K, Bongers G, Yamamoto Y, Hashimoto T, Yamatodani A, Menge WM, et al. Constitutive activity of histamine H(3) receptors stably expressed in SK-N-MC cells: display of agonism and inverse agonism by H(3) antagonists. J Pharmacol Exp Ther. 2001;299:908–914. [PubMed] [Google Scholar]
- Willmore LJ. Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav. 2005;7:S25–S28. doi: 10.1016/j.yebeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Sauren YM, Steinbusch HW. Histaminergic neurons in the rat brain: correlative immunocytochemistry, Golgi impregnation, and electron microscopy. J Comp Neurol. 1986;252:227–244. doi: 10.1002/cne.902520207. [DOI] [PubMed] [Google Scholar]
- Yanai K, Tashiro M. The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies. Pharmacol Ther. 2007;113:1–15. doi: 10.1016/j.pharmthera.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Yawata I, Tanaka K, Nakagawa Y, Watanabe Y, Murashima YL, Nakano K. Role of histaminergic neurons in development of epileptic seizures in EL mice. Brain Res Mol Brain Res. 2004;132:13–17. doi: 10.1016/j.molbrainres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Onodera K, Maeyama K, Yanai K, Iinuma K, Tuomisto L, et al. Histamine levels and clonic convulsions of electrically-induced seizure in mice: the effects of a-fluoromethylhistidine and metoprine. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:40–45. doi: 10.1007/BF00167568. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Onodera K, Iinuma K, Watanabe T. Proconvulsive effects of histamine H1-antagonists on electrically-induced seizure in developing mice. Psychopharmacology (Berl) 1993a;112:199–203. doi: 10.1007/BF02244911. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Iinuma K, Yanai K, Watanabe T, Sakurai E, Onodera K. Proconvulsant effect of ketotifen, a histamine H1 antagonist, confirmed by the use of d-chlorpheniramine with monitoring electroencephalography. Methods Find Exp Clin Pharmacol. 1993b;15:183–188. [PubMed] [Google Scholar]
- Yokoyama H, Onodera K, Iinuma K, Watanabe T. Effect of thioperamide, a histamine H3 receptor antagonist, on electrically induced convulsions in mice. Eur J Pharmacol. 1993c;234:129–133. doi: 10.1016/0014-2999(93)90717-v. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Onodera K, Maeyama K, Sakurai E, Iinuma K, Leurs R, et al. Clobenpropit (VUF-9153), a new histamine H3 receptor antagonist, inhibits electrically induced convulsions in mice. Eur J Pharmacol. 1994;260:23–28. doi: 10.1016/0014-2999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Sato M, Iinuma K, Onodera K, Watanabe T. Centrally acting histamine H1 antagonists promote the development of amygdala kindling in rats. Neurosci Lett. 1996;217:194–196. [PubMed] [Google Scholar]
- Yokoyama H, Onodera K, Yagi T, Iinuma K. Therapeutic doses of theophylline exert proconvulsant effects in developing mice. Brain Dev. 1997;19:403–407. doi: 10.1016/s0387-7604(97)00051-x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Noguchi E, Tsuru N. Lack of substantial effect of the H(3)-antagonist thioperamide and of the non-selective mixed H(3)-antagonist/H(1)-agonist betahistine on amygdaloid kindled seizures. Epilepsy Res. 2000;40:141–145. doi: 10.1016/s0920-1211(00)00115-7. [DOI] [PubMed] [Google Scholar]
- Zhang LS, Chen Z, Huang YW, Hu WW, Wei EQ, Yanai K. Effects of endogenous histamine on seizure development of pentylenetetrazole-induced kindling in rats. Pharmacology. 2003a;69:27–32. doi: 10.1159/000071263. [DOI] [PubMed] [Google Scholar]
- Zhang LS, Chen Z, Ren K, Leurs R, Chen J, Zhang W, et al. Effects of clobenpropit on pentylenetetrazole-kindled seizures in rats. Eur J Pharmacol. 2003b;482:169–175. doi: 10.1016/j.ejphar.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Zhang LS, Shen HQ, Jin CL, Hu WW, Zhao MH, Chen Z. Mechanisms of the effect of brain histamine on chronic epilepsy induced by pentylenetetrazole [Article in Chinese] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004;33:201–204. doi: 10.3785/j.issn.1008-9292.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao P, Ma MC, Qian H, Xia Y. Down-regulation of delta-opioid receptors in Na+/H+ exchanger 1 null mutant mouse brain with epilepsy. Neurosci Res. 2005;53:442–446. doi: 10.1016/j.neures.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Zhu X, Han X, Blendy JA, Porter BE. Decreased CREB levels suppress epilepsy. Neurobiol Dis. 2012;45:253–263. doi: 10.1016/j.nbd.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YY, Zhu-Ge ZB, Wu DC, Wang S, Liu LY, Ohtsu H, et al. Carnosine inhibits pentylenetetrazol-induced seizures by histaminergic mechanisms in histidine decarboxylase knock-out mice. Neurosci Lett. 2007;416:211–216. doi: 10.1016/j.neulet.2007.01.075. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Kuznetsova VB, Strik ON. Spatial organization and morphometric characteristics of histaminergic neurons in the rat brain. Neurosci Behav Physiol. 2006;36:467–471. doi: 10.1007/s11055-006-0041-9. [DOI] [PubMed] [Google Scholar]


