Abstract
BACKGROUND AND PURPOSE
Current methods used to treat critical limb ischaemia (CLI) are hampered by a lack of effective strategies, therefore, therapeutic vasculogenesis may open up a new field for the treatment of CLI. In this study we investigated the ability of the DPP-4 inhibitor, sitagliptin, originally used as a hypoglycaemic agent, to induce vasculogenesis in vivo.
EXPERIMENTAL APPROACH
Sitagliptin were administered daily to C57CL/B6 mice and eGFP transgenic mouse bone marrow-transplanted ICR mice that had undergone hindlimb ischaemic surgery. Laser Doppler imaging and flow cytometry were used to evaluate the degree of neovasculogenesis and circulating levels of endothelial progenitor cells (EPCs) respectively. Cell surface markers of EPCs and endothelial NOS (eNOS) in vessels were studied.
KEY RESULTS
Sitagliptin elevated plasma glucagon-like peptide-1 (GLP-1) levels in mice subjected to ischaemia, decreased plasma dipeptidyl peptidase-4 (DPP-4) concentration, and augmented ischaemia-induced increases in stromal cell-derived factor-1 (SDF-1) in a dose-dependent manner. Blood flow in the ischaemic limb was significantly improved in mice treated with sitagliptin. Circulating levels of EPCs were also increased after sitagliptin treatment. Sitagliptin also enhanced the expression of CD 34 and eNOS in ischaemic muscle. In addition, sitagliptin promoted EPC mobilization and homing to ischaemic tissue in eGFP transgenic mouse bone marrow-transplanted ICR mice.
CONCLUSION AND IMPLICATIONS
Circulating EPC levels and neovasculogenesis were augmented by the DPP-4 inhibitor, sitagliptin and this effect was dependent on an eNOS-related pathway in a mouse model of hindlimb ischaemia. The results indicate that oral administration of sitagliptin has therapeutic potential as an inducer of vasculogenesis.
Keywords: dipeptidyl peptidase-IV, endothelial progenitor cells
Introduction
Critical limb ischaemia (CLI), due to occlusive atherosclerotic disease, is seen in end-stage peripheral artery disease and can severely compromise blood flow, resulting in chronic ischaemic rest pain, ulcers or tissue gangrene (Norgren et al., 2007). The incidence of CLI is estimated to increase at a rate of 500–1000 individuals per million per year (Verghese et al., 2007). Moreover, previous reports have shown that patients with CLI have a high 1-year amputation rate, up to 30–50%, and 1-year mortality rate, 19.1–25% (Bertele et al., 1999; Norgren et al., 2007); thus, the economic burden of CLI on the health care system is considerable (Varu et al., 2010). With the goal of opening occluded or nearly occluded vessels, current treatment modalities for CLI involve medical therapy with anti-platelet agents, statins (Schanzer et al., 2008), surgical bypass with or without thromboendarterectomy (Albers et al., 2006) or percutaneous angioplasty (Dorros et al., 2001; Faglia et al., 2005). However, all current treatment modalities have various limitations, to some degree, including a lack of effect or failure.
Therapeutic angiogenesis or vasculogenesis is a method used to create new blood flow to perfuse ischaemic areas and may open up a new field for the treatment of CLI. For example, stem and progenitor cells are capable of enhancing post-natal vasculogenesis (Tateishi-Yuyama et al., 2002), and endothelial progenitor cells (EPCs) (Asahara et al., 1997) have been demonstrated to be involved in post-natal vasculogenesis and re-endothelization. Indeed, clinical trials in which bone marrow mononuclear cells (Tateishi-Yuyama et al., 2002) or peripheral blood progenitor cells (Lenk et al., 2005) were injected into ischaemic limbs demonstrated various degrees of success as a treatment of CLI. In addition, intravascular or i.m. injection of growth factors or chemoattractive agents, that induce stem cell or progenitor cell mobilization, proliferation and homing, could potentially be used as an isolated or adjuvant treatment for therapeutic neovasculogenesis.
Dipeptidyl peptidase-4 (DPP-4), also known as lymphocyte cell surface marker CD26 or adenosine deaminase (ADA)-binding protein, is a 766-amino acid serine protease that preferentially cleaves peptide hormones, including endocrine peptides, neuropeptides and chemokines that contain an alanine or proline in position 2 (Drucker, 2007). In patients with type 2 diabetes or chronic hyperglycaemia circulating levels of active DPP-4 have been shown to be higher than those in healthy controls (Mannucci et al., 2005; Ryskjaer et al., 2006). Moreover, there is evidence that DPP-4 is capable of cleaving the incretin peptide glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), and inhibitors of DPP-4 prevent this degradation of GIP and GLP-1, which suggests that DPP-4 is a critical determinant of incretin inactivation (Mentlein et al., 1993). Thus, DPP-4 inhibitors could be used to maintain glucose homeostasis. DPP-4 inhibitors may not only enhance glucose homeostasis by increasing the serum levels of GIP and GLP-1 (Fadini et al., 2010; Oeseburg et al., 2010) but may also elevate serum concentrations of stromal cell-derived factor-1 (SDF-1) (Busso et al., 2005). Both GLP-1 and SDF-1 have been shown to increase the number of circulating EPCs and improve endothelial function (Fadini et al., 2010; Oeseburg et al., 2010), which suggests that DPP-4 could be a possible candidate for pharmacotherapy in therapeutic vasculogenesis. However, whether DPP-4 inhibitors regulate vasculogenesis by affecting EPC function still remains unclear. In this study, we demonstrated, for the first time, that inhibition of DPP-4 is able to enhance neovascularization in a mouse hindlimb ischaemia model, and we analysed the underlying mechanisms.
Methods
Reagents
Sitagliptin (Januvia®) was purchased from Merck, Sharp & Dohme (Italia) S.p.A. Co. (Pavia, Italy).
Animals and sitagliptin administration
All male C57CL/B6 and ICR mice were purchased from BioLASCO Taiwan Co., Ltd, Taipei, Taiwan; male B6.129P2-Nos3tm1Unc/J mice (an eNOS knock-out mouse; homozygous for the Nos3tm1Unc targeted mutation) were purchased from the Jackson Laboratory (JAX®, 002684, Bar Harbor, ME, USA); and eGFP transgenic mice were provided kindly by Dr Shinn-Chih Wu (Department of Animal Science and Technology, National Taiwan University). All animals were treated according to protocols approved by the Institutional Animal Care Committee of the Taipei Medical University (Taipei, Taiwan). The experimental procedures and animal care conformed to the ‘Guide for the Care and Use of Laboratory Animals’ published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All mice were kept in micro-isolator cages on a 12-h day/night cycle and fed a commercial mouse chow diet (Scientific Diet Services, Essex, UK) with water ad libitum. Forty C57CL/B6 mice and 24 B6.129P2-Nos3tm1Unc/J mice were used, and the animals were divided into several groups. C57CL/B6 mice were included in groups 1–5 and B6.129P2-Nos3tm1Unc/J mice were included in groups 6–8. Group 1 (C57CL/B6 mice; naïve control) was the naïve control group; group 2 (C57CL/B6 mice; IS) received a hindlimb ischaemia operation at the start of week 1 of the experiment; group 3 [C57CL/B6 mice; IS + SG 5 mg·kg−1 body weight (BW)] received a hindlimb ischaemia operation at the start of week 1 and oral gavage of 5 mg·kg−1 BW·day−1 of sitagliptin throughout the experiment (7 weeks); group 4 (C57CL/B6 mice; IS + SG 10 mg·kg−1 BW) received a hindlimb ischaemia operation and oral gavage of 10 mg·kg−1 BW·day−1 of sitagliptin throughout the experiment (7 weeks); group 5 (C57CL/B6 mice; IS + SG 20 mg·kg−1 BW) received a hindlimb ischaemia operation and oral gavage of 20 mg·kg−1 BW·day−1 of sitagliptin throughout the experiment (7 weeks); group 6 (B6.129P2-Nos3tm1Unc/J mice; eNOS knock-out control) was the eNOS knock-out control group; group 7 (B6.129P2-Nos3tm1Unc/J mice; IS) received a hindlimb ischaemia operation at the start of week 1 of the experiment; group 8 (B6.129P2-Nos3tm1Unc/J mice; IS + SG 20 mg·kg−1 BW) received a hindlimb ischaemia operation and oral gavage of 20 mg·kg−1 BW·day−1 of sitagliptin throughout the experiment (7 weeks).
Biochemical measurements
Blood samples for biochemical measurements were collected from each animal before the start of the experiment and at the end of weeks 1, 3, 5 and 7. Samples collected from the mandibular artery into sodium citrate-containing tubes without sedation were used and separated by centrifugation. Serum blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using the SPOTCHEMTM automatic dry chemistry system (SP-4410; Arkray, Kyoto, Japan).
Mouse ischaemic hindlimb model
At the beginning of experimentation, unilateral hindlimb ischaemia of the 6-week-old mice was induced by ligation and excising the right femoral artery as previously described (Huang et al., 2010). Briefly, the animals were anaesthetized by i.p. injection of Xylocaine (2 mg·kg−1 of BW) plus Zoletil (containing a dissociative anaesthetic, Tiletamine/Zolazepam, at a ratio of 1:1; 5 mg·kg−1 of BW). Pain reflex and muscle tension were used to monitor the depth of anaesthesia of the mice during application of the ischaemic insult.
The proximal and distal portions of the femoral artery were ligated by silk thread, and cut the blood vessel approximately 0.2 cm between the two knots. Hindlimb blood perfusion was measured with a laser Doppler perfusion imager system (Moor Instruments Limited, Devon, UK) before and after the surgery and was then followed weekly. The animals were killed by cervical dislocation without sedation at the end of the seven experimental weeks. To avoid the influence of ambient light and temperature, the results are expressed as the ratio of perfusion in the right (ischaemic) versus left (non-ischaemic) limb.
Morphometry and immunohistochemistry
The liver, the kidney and the whole ischaemic limbs were harvested; the adhering tissues and femora were carefully removed and samples were immersion-fixed with 4% buffered paraformaldehyde, performed on serial 5-µm-thick paraffin-embedded sections. Haematoxylin/eosin staining was used for liver and kidney morphometry. Immunohistochemical staining was performed on mouse ischaemic thigh muscle (sartorius muscle, gracilis muscle, adductor muscle and semimembranosus muscle were included) using goat anti-Von Willebrand factor (vWF; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and rabbit anti-CD34 (Millipore, Billerica, MA, USA) antibodies, followed by counterstaining with Hoechst. The stained slides were observed using fluorescence microscopy. Three cross-sections were analysed for each animal and 10 different fields from each tissue preparation were randomly selected, and visible capillaries were counted. Capillary density was expressed as the capillary/myofibre ratio.
ELISA
Plasma was separated from fresh blood and stored at −80°C until use. ELISAs were performed to determine the plasma concentrations of SDF-1α (RayBiotech Inc., Norcross, GA, USA), GLP-1 (Epitope Diagnostics Inc., San Diego, CA, USA) and DPP-4 (USCN Life Science Inc., Los Angeles, CA, USA) according to the manufacturers' protocols. Absorbance was determined using a microplate reader.
Flow cytometry
To investigate the effects of sitagliptin on EPC mobilization in response to tissue ischaemia, a FACS caliber flow cytometer (Becton Dickinson, San Jose, CA, USA) was used to assess EPC mobilization. A volume of 300 µL of peripheral blood was incubated with FITC anti-mouse CD34 (eBioscience, San Diego, CA, USA) and phycoerythrin (PE) anti-mouse Flk-1 (VEGFR-2, eBioscience) antibodies. Isotype-identical antibodies served as controls (Becton Dickinson, Franklin Lakes, NJ, USA). After incubation for 30 min, cells were lysed (PharmLyse; BD Pharmingen), washed with PBS and fixed in 2% paraformaldehyde before analysis. Each analysis included 30 000 events. Circulating EPCs were considered to be from the mononuclear cell population and were gated with double positives for CD34 and Flk-1.
Western blotting analysis
Briefly, 0.5 g of skeletal muscle was ground and lysed in lysis buffer, and the protein lysates were subjected to SDS-PAGE, followed by transferring onto a PVDF membrane. Membranes were probed with monoclonal antibodies against endothelial NOS (eNOS) (Cell Signaling, San Diego, CA, USA) and β-actin (Chemicon, Temecula, CA, USA). Bands were visualized by chemiluminescence detection reagents.
Bone marrow transplantation model
Bone marrow transplantation was performed as previously described (Huang et al., 2010). Recipient wild-type ICR mice (Bltw:CD1 mouse) at 8 weeks of age were lethally irradiated with a total dose of 9.0 Gy. eGFP transgenic mice (ICR background) that ubiquitously expressed enhanced GFP were used as the donors. After being irradiated, a recipient mouse received unfractionated bone marrow cells (5 × 106) from an eGFP mouse by a tail vein injection. Six weeks after the bone marrow transplantation, the chimeric mice underwent unilateral hindlimb ischaemic surgery and were treated with sitagliptin 20 mg·kg−1 BW day−1 p.o. Repopulation by eGFP-positive bone marrow cells was 95%, as measured by flow cytometry. Five weeks after the induction of hindlimb ischaemia and sitagliptin treatment, ischaemic thigh muscles were harvested for histological analysis. Bone marrow-derived EPCs were stained with antibodies directed against eGFP (Chemicon) and CD34 (BD PharMingen). EPC density was observed by eGFP/CD34 double-positive cells (yellow colour) and evaluated by fluorescent microscopy at a magnification of 400×.
Statistical analysis
Values are expressed as means ± SEM. Statistical evaluation was performed using Student's t-test and one- or two-way anova, followed by Dunnett's test. A probability value of P < 0.05 was considered significant.
Results
Administration of sitagliptin did not affect the function and structural integrity of kidney and liver
To ensure that the dosage of sitagliptin used in the present animal study was harmless, biochemical measurements were taken, and morphometric analyses were performed. As shown in Table 1, blood glucose, serum BUN, creatinine, ALT and AST levels, and weight gain and final weight did not differ significantly among the experimental groups during the experimental period. The morphometric results indicated that liver samples from sitagliptin-treated mice did not exhibit feathery degeneration, micro- or macrocellular fatty changes, periportal fibrosis or vascular congestion (Figure S1A). Compared with the control group, 10 or 20 mg·kg−1 BWday−1 of sitagliptin (p.o.) for 7 weeks did not cause kidney injury; this included glomerulonephritis, compression of capillaries and narrowing of the Bowman space (Figure S1B). These results indicate that mice treated with 20 mg·kg−1 BW of sitagliptin had normal liver and kidney functions and histology.
Table 1.
Plasma biochemical characteristics in experimental mice (n= 8)
| Kidney function | Liver function | ||||||
|---|---|---|---|---|---|---|---|
| Time point | Body weight (mg) | Glucose (mg 100 mL−1) | BUN (mg 100 mL−1) | Creatinine (mg 100 mL−1) | ALT (IU·L−1) | AST (IU·L−1) | |
| Control | Before experiment | 36.0 ± 2.8 | 120.5 ± 25.3 | 31.2 ± 5.4 | 0.54 ± 0.05 | 34.5 ± 8.4 | 25.3 ± 4.5 |
| End of 7 weeks | 40.3 ± 2.7 | 133.2 ± 32.5 | 28.6 ± 4.1 | 0.52 ± 0.08 | 37.5 ± 5.2 | 28.6 ± 5.2 | |
| Hindlimb ischaemia (IS) | Before ischaemia | 33.3 ± 3.1 | 115.3 ± 26.7 | 25.7 ± 2.1 | 0.65 ± 0.05 | 45.2 ± 5.8 | 26.58 ± 8.9 |
| Post-IS 7 weeks | 40.3 ± 2.0 | 128.5 ± 25.4 | 24.5 ± 3.7 | 0.84 ± 0.04 | 54.2 ± 8.2 | 35.6 ± 10.2 | |
| IS + SG 5 mg·kg−1 BW | Before ischaemia | 35.8 ± 2.1 | 123.3 ± 17.0 | 24.2 ± 4.8 | 0.72 ± 0.08 | 42.5 ± 5.7 | 29.5 ± 9.8 |
| Post-IS 7 weeks | 42.5 ± 3.2 | 143.4 ± 35.1 | 22.5 ± 5.6 | 0.91 ± 0.05 | 48.2 ± 5.6 | 32.8 ± 11.2 | |
| IS + SG 10 mg·kg−1 BW | Before ischaemia | 33.5 ± 2.5 | 135.5 ± 23.7 | 28.9 ± 4.8 | 0.72 ± 0.08 | 43.2 ± 6.4 | 35.6 ± 19.3 |
| Post-IS 7 weeks | 39.5 ± 1.5 | 125.2 ± 28.4 | 31.4 ± 3.6 | 0.53 ± 0.04 | 44.1 ± 7.1 | 33.4 ± 11.5 | |
| IS + SG 20 mg·kg−1 BW | Before ischaemia | 37.5 ± 2.2 | 149.5 ± 43.5 | 30.2 ± 4.4 | 0.84 ± 0.04 | 52.4 ± 5.8 | 36.5 ± 9.9 |
| Post-IS 7 weeks | 41.2 ± 3.2 | 126.2 ± 29.9 | 25.6 ± 4.5 | 0.71 ± 0.05 | 48.3 ± 6.1 | 34.9 ± 10.3 | |
Values are presented as mean ± SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; BW, body weight; IS, hindlimb ischaemia; SG, sitagliptin.
Sitagliptin enhances recovery of capillary density in C57CL/B6 mice
To evaluate the angiogenic effect of sitagliptin, we induced tissue ischaemia by performing unilateral hindlimb ischaemia surgery on wild-type male C57CL/B6 mice (n= 8 for each group). Ligation and excision of the femoral artery effectively blocked blood flow in C57CL/B6 mice (Figure 1A). The hindlimb ischaemic mice (IS) had delayed blood flow recovery after surgery compared with the sitagliptin-treated mice, as determined by laser Doppler imaging; daily administration of 10 and 20 mg·kg−1 BW of sitagliptin p.o. significantly improved blood flow in hindlimb ischaemia-treated mice 7 weeks post-ischaemic surgery (Figure 1B). Blood flow in ischaemic limbs was significantly recovered until the seventh week in the 10 mg·kg−1 BW sitagliptin-treated mice and until the fifth and seventh week in the 20 mg·kg−1 BW sitagliptin-treated mice when compared with the non-sitagliptin-treated group (Figure 1C). Seven weeks following hindlimb ischaemic surgery, the ischaemia/normal perfusion ratios were higher in the 10 and 20 mg·kg−1 BW sitagliptin-treated groups compared with the non-sitagliptin-treated group (Figure 1D). Figure 1E shows the results of immunohistochemistry before the operation (control) and 1 day after hindlimb ischaemia surgery (hindlimb ischaemia control). Consistent with the measurements obtained by laser Doppler imaging, anti-vwF immunostaining revealed that sitagliptin administration for 7 weeks at both 10 and 20 mg·kg−1 BW significantly increased the number of detectable capillaries in the ischaemic muscle of the treated mice (Figure 1F) compared with the untreated ischaemic mice. These results indicate that sitagliptin treatment enhances the recovery of capillary density after hindlimb ischaemia in C57CL/B6 mice.
Figure 1.
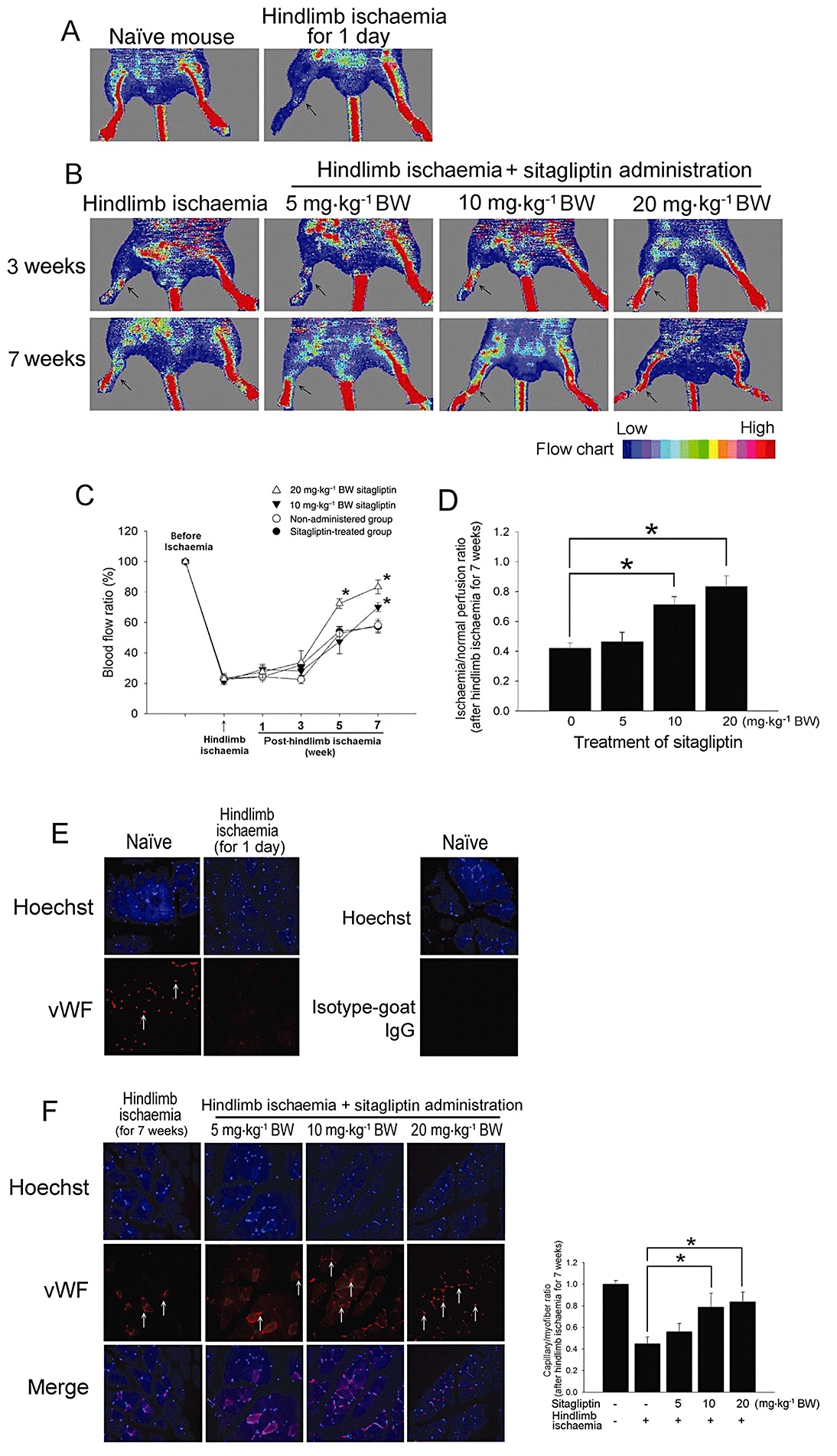
Effect of sitagliptin on blood flow recovery after hindlimb ischaemia in C57CL/B6 mice. (A) Representative results of laser Doppler measurements before operation (control) and 1 day after hindlimb ischaemia surgery. (B) Representative results of laser Doppler measurements 3 and 7 weeks after hindlimb ischaemia surgery in mice treated with 0, 5, 10 or 20 mg·kg−1 body weight (BW) of sitagliptin. Colour scale illustrates blood flow variations from minimal (dark blue) to maximal (red) values. Arrows indicate ischaemic (right) limb after hindlimb ischaemia surgery. (C) Doppler perfusion ratios (ischaemic/non-ischaemic hindlimb) over time in the different groups. Administration of 20 mg·kg−1 BW of sitagliptin and 10 mg·kg−1 BW of sitagliptin for 7 weeks enhanced beneficial blood flow recovery compared with the non-administered group 5 or 7 weeks after hindlimb ischaemia surgery. There was no significant difference in blood flow in the limb in the 5 mg·kg−1 BW sitagliptin-treated group compared with the non-administered group. (D) Seven weeks after ischaemic surgery, the ischaemia/normal perfusion ratio in the sitagliptin-treated group was higher than that in the non-sitagliptin-treated group. (E) Representative results of immunohistochemistry before operation (control) and 1 day after hindlimb ischaemia surgery. (F) Mice were killed 7 weeks after surgery, and capillaries in the ischaemic muscles were visualized by anti-vWF immunostaining (original magnification of 400×). The graph shows the quantification of capillary density in hindlimb ischaemia- and sitagliptin-treated mice. Results are expressed as mean ± SEM (*P < 0.05 was considered to be significant).
Sitagliptin increases circulation of EPC in blood and eNOS expression at sites of ischaemia
To investigate the effects of sitagliptin administration on EPC mobilization in response to tissue ischaemia, levels of CD34+/Flk-1+ cells in peripheral blood were determined using flow cytometry in C57CL/B6 mice. The basal number of EPCs did not differ significantly between sitagliptin-treated and untreated groups (data not showed). Three weeks after surgery, C57CL/B6 mice with hindlimb ischaemia had a higher circulating level of EPCs than the control (non-surgery) group. However, treatment with 20 mg·kg−1 BW of sitagliptin enhanced the mobilization of EPCs of C57CL/B6 mice with hindlimb ischaemia 3 weeks after the operation (Figure 2A). As seen in the diagram in Figure 2B, EPC mobilization was enhanced by tissue ischaemia in control mice (baseline vs. 1 week after surgery: 50.5 ± 25.3 cells vs. 135.7 ± 44.5 cells per 3 × 104 mononuclear cells). At the third and fifth week after hindlimb ischaemia surgery, C57CL/B6 mice from the 20 mg·kg−1 BW sitagliptin-treated group exhibited an increased capacity to mobilize EPCs into peripheral blood than the non-sitagliptin-treated group. As NO plays an important role in modulating EPC function, which is critical for blood vessel repair and angiogenesis (Yoder, 2005), we next examined phospho-eNOS, total-eNOS and CD31 expression with Western blotting in the ischaemic muscle. Total-eNOS and CD31 expression levels after hindlimb ischaemia for 5 weeks were significantly decreased compared with the control C57CL/B6 mice. Administration of sitagliptin increased the phospho-eNOS, total-eNOS and CD31 expression in C57CL/B6 mice with hindlimb ischaemia compared with non-sitagliptin-treated C57CL/B6 mice with hindlimb ischaemia (Figure 2C). After quantifying and normalizing the phospho-eNOS and total-eNOS expression using CD31 expression (phospho-eNOS/CD31 ratio and total-eNOS/CD31 ratio), the results show that sitagliptin induced the production of total eNOS and phosphorylation of eNOS in endothelial cells.
Figure 2.
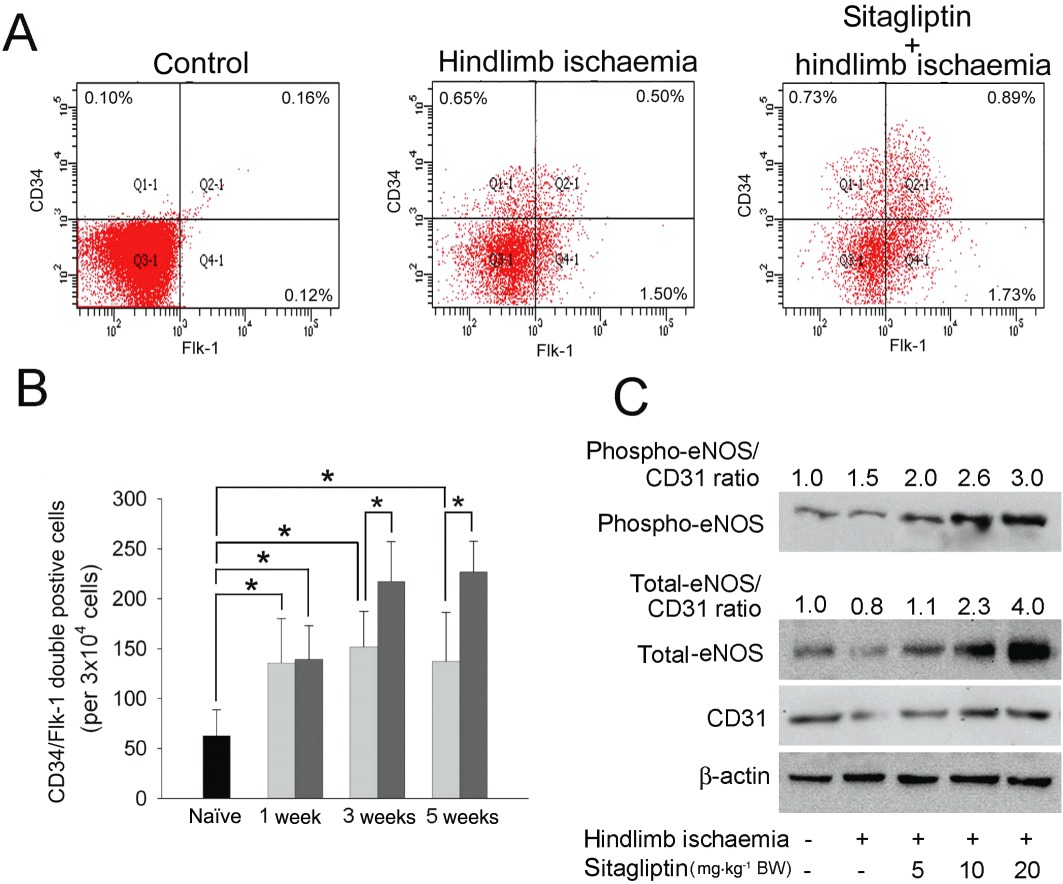
Effects of sitagliptin on EPC mobilization and eNOS expression at ischaemia site in hindlimb ischaemic mice. (A) EPC (defined as CD34+/Flk-1+ cells) mobilization 3 weeks after hindlimb ischaemia surgery was determined using flow cytometry. (B) The CD34+/Flk-1+ cells in control (black), non-sitagliptin (light gray) and 20 mg·kg−1 BW sitagliptin-treated (dark grey) mice 1 to 5 weeks after surgery. (C) The phospho-eNOS, total-eNOS and CD31 proteins levels in ischaemia muscle were analysed with Western blotting. β-Actin was used as a loading control. The phospho-eNOS and total-eNOS expression in endothelial cells were normalized using CD31 expression (phospho-eNOS/CD31 ratio and total-eNOS/CD31 ratio).
Sitagliptin also promotes new small vessel formation to ischaemic site
To extend the above observations, we next measured CD34 expression, which is considered a critical marker of endothelial cells in small blood vessels, on histological sections harvested from the ischaemic hindlimbs of C57CL/B6 mice and B6.129P2-Nos3tm1Unc/J mice. Consistent with our flow cytometry results, anti-CD34 immunostaining revealed that administration of 10 and 20 mg·kg−1 BW of sitagliptin for 7 weeks significantly increased the number of detectable capillaries in the ischaemic thigh muscle of treated compared with untreated ischaemic C57CL/B6 mice. In contrast, administration of 20 mg·kg−1 BW of sitagliptin did not increase the capillaries in the ischaemic hindlimb of B6.129P2-Nos3tm1Unc/J mice (Figure 3A). Further, daily administration of 20 mg·kg−1 BW of sitagliptin p.o. also did not improve blood flow in the ischaemic hindlimb of B6.129P2-Nos3tm1Unc/J mice 7 weeks post-ischaemic surgery, as determined by laser Doppler imaging (Figure 3B). These results indicate that eNOS expression is involved in the ability of sitagliptin to promote new vessel formation in the hindlimb of mice subjected to ischaemia.
Figure 3.
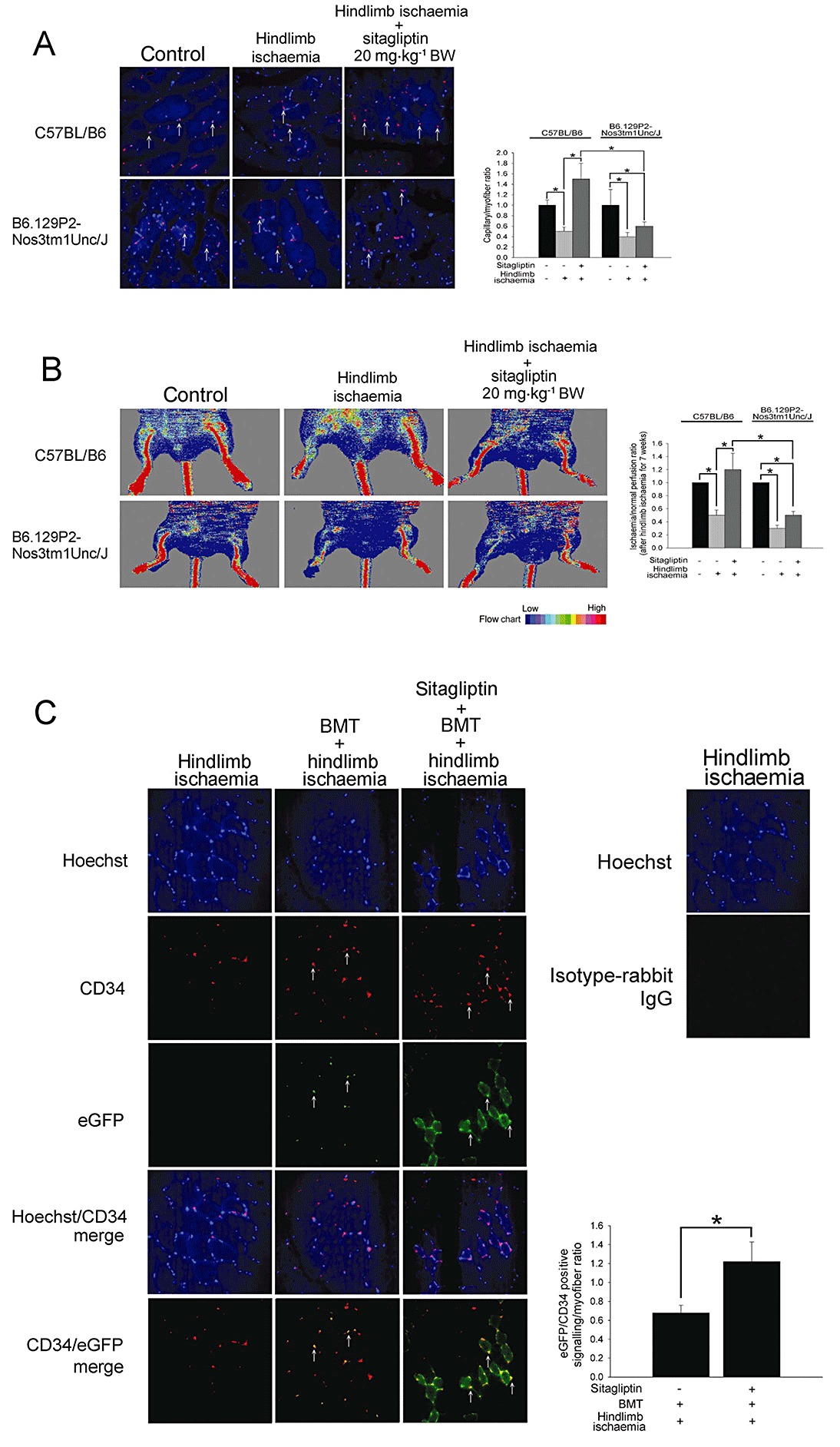
Effects of sitagliptin on EPC homing in hindlimb ischaemic mice. (A) Immunostaining of ischaemic hindlimb muscle with anti-CD34 conjugated Alex 633 (red) antibody in C57BL/B6 and B6.129P2-Nos3tm1Unc/J mice treated with 20 mg·kg−1 BW of sitagliptin. The CD34-postive homed hematopoietic stem precursor cells were indicated with arrows. The histogram shows the capillary/myofibre ratio. (B) Representative results of laser Doppler measurements 7 weeks after hindlimb ischaemia surgery in C57BL/B6 and B6.129P2-Nos3tm1Unc/J mice treated with 20 mg·kg−1 BW of sitagliptin. Colour scale illustrates blood flow variations from minimal (dark blue) to maximal (red) values. The histogram shows the ischaemia/normal perfusion ratio. (C) Hindlimb ischaemia was created in ICR mice that received eGFP mouse bone marrow cells. Immunofluorescence staining revealed, 20 mg·kg−1 BW sitagliptin-treated ICR mice in hindlimb ischaemia group had more GFP/CD34 double-positive cells (white arrow) in ischaemic muscle than those in the non-sitagliptin-treated group. The Hoechst was used to counterstain the nucleus. The ischaemic hindlimb tissue was evaluated by fluorescent microscopy at a magnification of 400×. The histogram shows the eGFP-CD34 double-positive signalling/myofibre ratio. Results are expressed as mean ± SEM (*P < 0.05 was considered to be significant).
To evaluate the effect of sitagliptin on bone marrow-derived EPC homing and differentiation to endothelial cells, ICR mice that had received eGFP transgenic mouse bone marrow cells (n= 6 in each group) were subjected to hindlimb ischaemia. Immunofluorescence staining revealed that 20 mg·kg−1 BW sitagliptin-treated mice had more GFP/CD34 double-positive cells in ischaemic muscle than the untreated group. This suggests that sitagliptin promotes EPC mobilization and homing to ischaemic tissue (Figure 3C).
Sitagliptin increases plasma GLP-1 and SDF-1α levels but decreases the DPP-4 level
Both GLP-1 and SDF-1α are the natural substrates for the DPP-4 peptide in vivo. Increasing plasma GLP-1 concentrations by inhibiting DPP-4 might have an effect on endothelial cells and EPCs through eNOS activation (Ban et al., 2008). Therefore, ELISA was performed to monitor the effect of sitagliptin on plasma GLP-1 and DPP-4 levels (Tables 2 and 3). In the non-sitagliptin(SG)-treated control and IS groups and in the IS + SG 5 mg·kg−1 BW group, GLP-1 and DPP-4 levels did not change significantly during the experiment. However, increasing GLP-1 levels were detected in the IS + SG 10 mg·kg−1 BW and IS + SG 20 mg·kg−1 BW groups at week 1, and these levels continued to increase significantly at 3 and 5 weeks. Additionally, administration of 10 or 20 mg·kg−1 BW of sitagliptin significantly increased GLP-1 levels compared with the untreated IS group at the same experimental time points after the first week. The plasma DPP-4 level were decreased significantly after 5 weeks of treatment in the IS + SG 5 mg·kg−1 BW group, after 3 weeks of treatment in the IS + SG 10 mg·kg−1 BW group, and after 1 week of treatment in IS + SG 20 mg·kg−1 BW group respectively. Previous reports have demonstrated that serum and tissue SDF-1α levels could be up-regulated and thus further stimulate the bone marrow to release EPCs in response to ischaemia. (Fadini et al., 2010) In this study, we performed ELISA to determine whether sitagliptin increases plasma SDF-1α levels in experimental animals. There was no significant difference in plasma SDF-1α levels at several time points in the control group. The amount of plasma SDF-1α was increased shortly after induction of ischaemia for 1 day (Table 4A). Administration of 20 mg·kg−1 BW of sitagliptin only did not affect the level of SDF-1α. Administration of 20 mg·kg−1 BW of sitagliptin induced significantly more plasma SDF-1α production 5 days after hindlimb ischaemia surgery. As shown in Table 4B, 1 week after hindlimb ischaemia surgery, the levels of plasma SDF-1α were increased and these increased levels persisted throughout the experimental period. However, administration of 10 and 20 mg·kg−1 BW of sitagliptin induced significantly more plasma SDF-1α production after hindlimb ischaemia surgery (Table 4B). These results suggest that sitagliptin enhances neovasculogenesis in our mouse hindlimb ischaemia model. We speculate that administration of sitagliptin induces eNOS expression and mobilization/homing of EPCs by increasing plasma levels of GLP-1 and SDF-1α; an effect which is independent of the blood glucose and plasma DPP-4 levels.
Table 2.
Plasma GLP-1 level (pmol·L−1) in experimental mice (n= 8)
| Before experiment | 1 week | 3 weeks | 5 weeks | |
|---|---|---|---|---|
| Control | 5.26 ± 0.52 | 4.25 ± 0.36 | 4.88 ± 0.21 | 4.10 ± 0.24 |
| Hindlimb ischaemia (IS) | 3.16 ± 0.50 | 5.16 ± 0.13 | 4.21 ± 2.03 | 4.09 ± 0.98 |
| IS + SG 5 mg·kg−1 BW | 6.04 ± 1.76 | 5.97 ± 0.57 | 8.85 ± 1.82 | 5.12 ± 2.52 |
| IS + SG 10 mg·kg−1 BW | 4.19 ± 1.58 | 7.06 ± 0.10† | 10.50 ± 0.10*,† | 15.76 ± 0.55*,† |
| IS + SG 20 mg·kg−1 BW | 5.49 ± 1.18 | 17.30 ± 1.46*,† | 11.96 ± 0.57*,† | 11.16 ± 1.25*,† |
Values are presented as mean ± SD.
P < 0.05 compared with the time point of before experiment at the same group.
P < 0.05 compared with the IS group at the same time point.
BW, body weight; GLP-1, glucagon-like peptide-1; IS, hindlimb ischaemia; SG, sitagliptin.
Table 3.
Plasma DPP-4 level (pg·mL−1) in experimental mice (n= 8)
| Before experiment | 1 week | 3 weeks | 5 weeks | |
|---|---|---|---|---|
| Control | 111.88 ± 1.14 | 109.66 ± 14.25 | 106.13 ± 8.34 | 110.29.01 ± 7.95 |
| Hindlimb ischaemia (IS) | 93.32 ± 0.20 | 88.763 ± 2.22 | 98.56 ± 4.38 | 94.81 ± 5.39 |
| IS + SG 5 mg·kg−1 BW | 102.75 ± 5.45 | 97.18 ± 3.23 | 73.05 ± 11.82 | 69.45 ± 5.89*,†,# |
| IS + SG 10 mg·kg−1 BW | 109.31 ± 3.43 | 109.46 ± 7.67 | 70.22 ± 6.92*,†,# | 65.09 ± 6.78*,†,# |
| IS + SG 20 mg·kg−1 BW | 97.61 ± 9.08 | 67.75 ± 7.27*,# | 64.77 ± 2.22*,†,# | 25.52 ± 12.80*,†,# |
Values are mean ± SD.
P < 0.05 compared with the time point of before experiment at the same group.
P < 0.05 compared with the IS group at the same time point.
P < 0.05 compared with the naïve group at the same time point.
BW, body weight; DPP-4, dipeptidyl peptidase-4; IS, hindlimb ischaemia; SG, sitagliptin.
Table 4.
Plasma SDF-1α level (pg·mL−1) in experimental mice (n= 8)
| A | ||||
|---|---|---|---|---|
| Before treatment | 1 day | 5 days | 10 days | |
| Control | 358.65 ± 29.64 | 368.21 ± 37.18 | 320.17 ± 40.87 | 287.41 ± 52.17 |
| Hindlimb ischaemia (IS) | 334.16 ± 25.32 | 687.52 ± 46.31* | 683.27 ± 49.55* | 701.42 ± 59.21* |
| SG 20 mg·kg−1 BW | 368.24 ± 39.99 | 421.31 ± 64.37 | 396.24 ± 58.37 | 346.12 ± 59.88 |
| IS + SG 5 mg·kg−1 BW | 399.66 ± 41.83 | 654.31 ± 60.58* | 612.45 ± 92.13* | 699.16 ± 78.54* |
| IS + SG 10 mg·kg−1 BW | 373.26 ± 41.54 | 570.32 ± 50.44* | 696.19 ± 60.21* | 970.19 ± 104.18*,† |
| IS + SG 20 mg·kg−1 BW | 331.52 ± 21.58 | 619.12 ± 81.47* | 899.88 ± 98.79*,† | 1146.54 ± 108.54*,† |
| B | ||||
|---|---|---|---|---|
| Before experiment | 1 week | 3 weeks | 5 weeks | |
| Control | 358.65 ± 29.64 | 301.44 ± 40.11 | 299.52 ± 28.97 | 294.01 ± 35.24 |
| Hindlimb ischaemia (IS) | 334.16 ± 25.32 | 651.23 ± 20.76*,‡ | 720.21 ± 75.54*,‡ | 648.53 ± 70.40*,‡ |
| IS + SG 5 mg·kg−1 BW | 399.66 ± 41.83 | 629.20 ± 52.74*,‡ | 869.57 ± 91.52*,‡ | 712.29 ± 52.41*,‡ |
| IS + SG 10 mg·kg−1 BW | 373.26 ± 41.54 | 1070.32 ± 50.44*,†,‡ | 622.25 ± 50.45*,‡ | 851.21 ± 58.57*,‡ |
| IS + SG 20 mg·kg−1 BW | 331.52 ± 21.58 | 1019.12 ± 81.47*,†,‡ | 1022.21 ± 70.44*,†,‡ | 1141.61 ± 86.52*,†,‡ |
Values are mean ± SD.
P < 0.05 compared with the time point of before experiment at the same group.
P < 0.05 compared with the IS group at the same time point.
P < 0.05 compared with the naïve group at the same time point.
BW, body weight; IS, hindlimb ischaemia; SDF-1α, stromal cell-derived factor-1α; SG, sitagliptin.
Discussion
The isolation of EPCs, which express the cell markers CD34+ and VEGFR-2+ on their cell membrane, by Asahara et al. (1997) suggests that vasculogenesis may also exist in well-developed mature individuals (Ribatti, 2007). Thus, therapeutic vasculogenesis, which can be induced by direct auto- or allograft transplantation or enhanced by gene therapy or pharmacotherapeutics, has become one of the most promising trends and future directions for treatment of patients with CLI. A delicate modulating system is required to handle the processes of EPC proliferation, mobilization from the bone marrow and homing to sites awaiting repair and differentiation (Solovey et al., 1997; Urbich et al., 2003; Ribatti, 2007). Knowledge of the mechanisms that enable the manipulation of EPC numbers and function could be applied to develop pharmacotherapies for therapeutic vasculogenesis. Unlike stem cells, EPCs are vulnerable to various kinds of stress (Jarajapu and Grant, 2010), including oxidative stress (Ingram et al., 2007; Thum et al., 2007), elevated blood sugar levels (Chen et al., 2007), reduced blood oxygenation (Balasubramaniam et al., 2007) or other inflammatory reactions (Gulati et al., 2003), which may reduce the number of circulating EPCs and impair EPC function. Moreover, the tissue environments in ill subjects may not be friendly for the engraftment or differentiation of progenitor cells. Hence, increasing EPC numbers, rejuvenating functionally impaired EPCs and re-vitalizing the tissue environment are keys to successful neovasculogenesis in patients at high risk of atherosclerosis (Jarajapu and Grant, 2010). Thus, gene therapy, stem or progenitor cell therapy, and pharmacotherapy could be applied individually or synergistically in protocols to optimize therapeutic vasculogenesis.
Christopherson et al. addressed the potential of DPP-4 in vasculogenesis (Christopherson et al., 2004). They demonstrated that endogenous DPP-4 expression in donor cells negatively regulates homing and engraftment. By inhibiting or deleting DPP-4, the transplantation and engraftment efficacy of haematopoietic stem cells was greatly enhanced; knock-down or knock-out of DPP-4 expression, thus increasing CXCL12 levels, may help to prime implanted progenitor cells. Additionally, GLP-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure (Menegazzi et al., 2004; Sokos et al., 2006). No hypoglycaemic episodes were observed in subjects with normal blood glucose levels. Our study demonstrated similar findings and showed that, when mice were treated with adequate doses of sitagliptin (10 and 20 mg·kg−1 BW), plasma GLP-1 levels were significantly elevated after 1or 3 weeks of treatment. This DPP-4 inhibitor does not affect BW or blood sugar levels in mice. Moreover, our biochemistry profiles and morphometric analysis of liver and renal function did not reveal any adverse effects of sitagliptin treatment. Thus, we believe that DPP-4 inhibitors could be applied to subjects with or without diabetes. As the GLP-1 receptor is expressed on endothelial cells (Nystrom et al., 2004), GLP-1 infusion may improve endothelial function in diabetes patients with stable coronary artery disease by increasing eNOS activation (Nystrom et al., 2004). In vitro studies using human umbilical vein endothelial cells showed that reactive oxygen species-induced senescence was attenuated by GLP-1 in a receptor-dependent manner involving downstream PKA signalling and induction of antioxidant genes (Oeseburg et al., 2010). Although we demonstrated that GLP-1-dependent eNOS activation was essential for sitagliptin-mediated EPC mobilization and differentiation, further studies are needed to clarify the possible roles of GLP-1 in EPC function.
In addition to GLP-dependent mechanisms, Fadini and Avogaro demonstrated other GLP-1-independent mechanisms that are involved in the cardiovascular effects of DPP-4 inhibitors (Fadini and Avogaro, 2011). SDF-1 is an 89-amino acid polypeptide that is expressed in cells from various tissues and CXCR4 is the only known receptor for SDF-1 (Bleul et al., 1996). The SDF-1/CXCR4 interaction has been reported to play an important physiological role in haematopoiesis (Nagasawa et al., 1996), vascular development, cardiogenesis (Tachibana et al., 1998) and cerebellar development (Zou et al., 1998) during embryo development. Several investigators have reported that CD34+ cells, classically considered hematopoietic stem cells, express CXCR4 (Christopherson et al., 2002), and that SDF-1 can induce CD34+ cell migration and trafficking in vitro (Mohle et al., 1998). Plasma SDF-1α levels serve as a predictor of EPC numbers, and a SDF-1 gene variant (rs2297630) may influence SDF-1α levels and circulating EPC numbers (Xiao et al., 2008). SDF-1 could act as a priming agent for ex vivo expansion of EPCs and further stimulate EPC proliferation and migration, inhibit EPC apoptosis, enhance matrix metalloproteinase (MMP)-2 and -9 expression, and induce eNOS activation and NO production (Hiasa et al., 2004; Shao et al., 2008). SDF-1 injection might also enhance EPC recruitment and vasculogenesis in hindlimb ischaemic mice (Yamaguchi et al., 2003). Fadini et al. demonstrated that administration of sitagliptin to patients with type 2 diabetes enhanced EPC mobilization through the SDF-1/CXCR-4 pathway (Fadini et al., 2010). In the present study, we demonstrated that oral administration of sitagliptin leads to the mobilization of EPCs and enhances neovasculogenesis.
In diabetic mice, SDF-1 expression and bone marrow eNOS phosphorylation are down-regulated, which reduces the number of circulating EPCs and this impaired EPCs mobilization and homing are reversed by SDF-1 treatment (Gallagher et al., 2007). Although hypoxia itself may induce up-regulation of SDF-1 in tissues (Ceradini et al., 2004), our results indicate that the augmentation of SDF-1 levels will be further increased in hindlimb ischaemic mice treated with adequate doses of oral sitagliptin. Although normal glycaemic mice were used in our experiments, the use of sitagliptin in diabetic subjects would probably not only improve blood sugar control but also reverse the decrease in SDF-1 level seen in diabetics, thus enhancing circulating EPC levels and neovasculogenesis. However, additional studies are needed to prove this hypothesis.
Our study demonstrates the therapeutic vasculogenesis potential of the oral administration of the DPP-4 inhibitor, sitagliptin, in a mouse model of hindlimb ischaemia. EPC mobilization, homing and differentiation may be enhanced by DPP-4 inhibition; an effect dependent on an elevation in plasma SDF-1 and GLP-1 but independent of blood glucose. Further clinical trials on CLI, especially in diabetics, could be initiated to prove the therapeutic vasculogenesis potential of sitagliptin in clinical practice.
Potential limitation of EPC characterization
There is an ongoing debate about the true nature of EPC. The situation in mice is even less clear, where several groups have used other criteria (e.g. Sca1+VEGF2R+; c-Kit+Tie-2+) to identify EPCs. However, according to many previous studies (Aicher et al., 2008; Feng et al., 2008; Schroder et al., 2009; Huang et al., 2010; O'Toole et al., 2010; Wheat et al., 2011), we originally used Sca-1, Flk-1, CD34 and c-kit to observe the circulating EPC in the mice blood.
Acknowledgments
We also thank Mr Tze-Liang Yang, Mr Chih-Hao Nien and Mrs Min-Yu Lo for excellent technical assistance. This work was supported by the Taipei Medical University (TMU 99-AE1-B23) and by the National Science Council (NSC 99-2314-B-038-007-MY3), Taiwan.
Glossary
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
serum blood urea nitrogen
- CLI
critical limb ischaemia
- DPP-4
dipeptidyl peptidase-4
- eNOS
endothelial NO synthase
- EPCs
endothelial progenitor cells
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- PE
phycoerythrin
- SDF-1
stromal cell-derived factor-1
- vWF
Von Willebrand factor
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Control mice treated with sitagliptin have normal liver and kidney histology. (B) Naïve mouse livershowing the central vein (indicated by black arrow) and hepatocytes arranged in the form of a cord. Livers of mice treated with sitagliptin or that have undergone hindlimb ischaemia also exhibit normal histology. (B) Naïve mouse kidney showing normal glomerulus (indicated by arrowheads). Following the naïve group, 10 or 20 mg·kg−1 BW of sitagliptin administration over 7 weeks did not harm the kidney. Tissue staining was performed on serial 5-µm-thick paraffin-embedded sections. Haematoxylin/eosin staining was used for morphometry (original magnification of 200×).
References
- Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, et al. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103:796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]
- Albers M, Romiti M, Brochado-Neto FC, De Luccia N, Pereira CA. Meta-analysis of popliteal-to-distal vein bypass grafts for critical ischemia. J Vasc Surg. 2006;43:498–503. doi: 10.1016/j.jvs.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Bertele V, Roncaglioni MC, Pangrazzi J, Terzian E, Tognoni EG. Clinical outcome and its predictors in 1560 patients with critical leg ischaemia. Chronic Critical Leg Ischaemia Group. Eur J Vasc Endovasc Surg. 1999;18:401–410. doi: 10.1053/ejvs.1999.0934. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Busso N, Wagtmann N, Herling C, Chobaz-Peclat V, Bischof-Delaloye A, So A, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166:433–442. doi: 10.1016/S0002-9440(10)62266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Dorros G, Jaff MR, Dorros AM, Mathiak LM, He T. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104:2057–2062. doi: 10.1161/hc4201.097943. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faglia E, Dalla Paola L, Clerici G, Clerissi J, Graziani L, Fusaro M, et al. Peripheral angioplasty as the first-choice revascularization procedure in diabetic patients with critical limb ischemia: prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003. Eur J Vasc Endovasc Surg. 2005;29:620–627. doi: 10.1016/j.ejvs.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Feng Y, Jacobs F, Van Craeyveld E, Brunaud C, Snoeys J, Tjwa M, et al. Human ApoA-I transfer attenuates transplant arteriosclerosis via enhanced incorporation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:278–283. doi: 10.1161/ATVBAHA.107.158741. [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- Huang PH, Tsai HY, Wang CH, Chen YH, Chen JS, Lin FY, et al. Moderate intake of red wine improves ischemia-induced neovascularization in diabetic mice – roles of endothelial progenitor cells and nitric oxide. Atherosclerosis. 2010;212:426–435. doi: 10.1016/j.atherosclerosis.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Krier TR, Mead LE, McGuire C, Prater DN, Bhavsar J, et al. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res. 2010;106:854–869. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk K, Adams V, Lurz P, Erbs S, Linke A, Gielen S, et al. Therapeutical potential of blood-derived progenitor cells in patients with peripheral arterial occlusive disease and critical limb ischaemia. Eur Heart J. 2005;26:1903–1909. doi: 10.1093/eurheartj/ehi285. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- Menegazzi JJ, Callaway CW, Sherman LD, Hostler DP, Wang HE, Fertig KC, et al. Ventricular fibrillation scaling exponent can guide timing of defibrillation and other therapies. Circulation. 2004;109:926–931. doi: 10.1161/01.CIR.0000112606.41127.D2. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl. 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- O'Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, et al. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–203. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- Ribatti D. The discovery of endothelial progenitor cells. An historical review. Leuk Res. 2007;31:439–444. doi: 10.1016/j.leukres.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Ryskjaer J, Deacon CF, Carr RD, Krarup T, Madsbad S, Holst J, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol. 2006;155:485–493. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47:774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- Schroder K, Kohnen A, Aicher A, Liehn EA, Buchse T, Stein S, et al. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, et al. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–1384. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Thum S, Schultheiss M, Daiber A, Wenzel P, et al. Differential effects of organic nitrates on endothelial progenitor cells are determined by oxidative stress. Arterioscler Thromb Vasc Biol. 2007;27:748–754. doi: 10.1161/01.ATV.0000258787.18982.73. [DOI] [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- Verghese M, Pathak S, Poston GJ. Increasing long-term survival in advanced colorectal cancer. Eur J Surg Oncol. 2007;33(Suppl. 2):S1–S4. doi: 10.1016/j.ejso.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, et al. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011;31:1598–1606. doi: 10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Ye S, Oberhollenzer F, Mayr A, Jahangiri M, Willeit J, et al. SDF1 gene variation is associated with circulating SDF1alpha level and endothelial progenitor cell number: the Bruneck Study. PLoS ONE. 2008;3:e4061. doi: 10.1371/journal.pone.0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yoder MC. NO role in EPC function. Blood. 2005;105:1846–1847. doi: 10.1182/blood-2004-12-4743. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


