Abstract
BACKGROUND AND PURPOSE
Curcumin, the natural yellow pigment extracted from the rhizomes of the plant curcuma longa, has been demonstrated to exhibit a variety of potent beneficial effects, acting as an antioxidant, anti-inflammatory and anti-fibrotic. In this study we tested the hypothesis that curcumin attenuates maladaptive cardiac repair and improves cardiac function after ischaemia and reperfusion by reducing degradation of extracellular matrix (ECM) and inhibiting synthesis of collagens via TGFβ/Smad-mediated signalling pathway.
EXPERIMENTAL APPROACH
Sprague-Dawley rats were subjected to 45 min of ischaemia followed by 7, 21 and 42 days of reperfusion respectively. Curcumin was fed orally at a dose of 150 mg·kg−1·day−1 only during reperfusion.
KEY RESULTS
Curcumin reduced the level of malondialdehyde, inhibited activity of MMPs, preserved ECM from degradation and attenuated collagen deposition, as it reduced the extent of collagen-rich scar and increased mass of viable myocardium. In addition to reducing collagen synthesis and fibrosis in the ischaemic/reperfused myocardium, curcumin significantly down-regulated the expression of TGFβ1 and phospho-Smad2/3, and up-regulated Smad7 and also increased the population of α-smooth muscle actin expressing myofibroblasts within the infarcted myocardium relative to the control. Echocardiography showed it significantly improved left ventricular end-diastolic volume, stroke volume and ejection fraction. The wall thickness of the infarcted middle anterior septum in the curcumin group was also greater than that in the control group.
CONCLUSION AND IMPLICATIONS
Dietary curcumin is effective at inhibiting maladaptive cardiac repair and preserving cardiac function after ischaemia and reperfusion. Curcumin has potential as a treatment for patients who have had a heart attack.
Keywords: cardiac repair, curcumin, collagen, extracellular matrix, TGFβ/Smad pathway
Introduction
Death and disability after myocardial infarction (MI) continue to be a major public health problem (Ferdinandy et al., 2007; Liem et al., 2007). The current paradigm in the treatment of MI patients is to open the infarct-related artery through either percutaneous coronary intervention or coronary artery bypass graft surgery. However, the restoration of blood flow to an ischaemic myocardium may paradoxically exaggerate tissue injury and initiate a maladaptive tissue repair process (Vinten-Johansen et al., 2005; Prasad et al., 2009). During the early stages of MI, the degradation of extracellular matrix (ECM) by regional oxidative stress-activated MMPs results in progressive infarct expansion, ventricular wall thinning and chamber dilatation (Spinale et al., 2002; Tessone et al., 2005). This is followed by healing of the infarct area during which fibroblasts proliferate and deposit collagen to form a reparative fibrotic and non-contractile scar. These changes further result in the left ventricular global dilatation, cardiac dysfunction and heart failure (Brown et al., 2005).
It is generally agreed that, initially, the degraded pre-existing collagenous network leads to ventricular wall thinning and dilatation, while newly synthesized collagen results in scar tissue formation and left ventricle (LV) diastolic dysfunction (Brower et al., 2006). In this regard, activation of MMPs has been associated with degradation of the extracellular matrix (ECM), and TGFβ1/Smads signalling pathway has been suggested to play a major role in mediating collagen synthesis and scar tissue formation in the infarcted myocardium during cardiac repair (Spinale et al., 2002; Brower et al., 2006).
The increasing incidence of heart failure resulting from maladaptive cardiac repair after MI has stimulated investigative efforts to develop pharmacological strategies for protecting against tissue injury (Gallagher et al., 2007). However, few of the drugs that inhibit scar tissue formation, promote cardiac repair and thereby improve cardiac function after reperfusion have gained clinical acceptance for a routine treatment of MI patients (Downey and Cohen, 2009).
Curcumin (diferuloylmethane) is the natural yellow pigment extracted from the rhizomes of the plant curcuma longa (Ghosh et al., 2010). In several studies, curcumin has been demonstrated to exhibit a variety of potent beneficial effects such as antioxidant, anti-inflammatory and anti-fibrotic (Venkatesan, 1998; Ghosh et al., 2009; 2010; Smith et al., 2010). Although it has previously been shown that curcumin inhibits the development of myocardial cell hypertrophy and hypertension-induced heart failure by attenuating p300 histone acetyltransferase activity (Morimoto et al., 2008), it is not known whether curcumin, when it is fed on a daily basis after ischaemia followed by reperfusion, promotes cardiac repair and improves cardiac function by modulating ECM degradation and collagen synthesis.
Therefore, we tested the hypothesis that curcumin inhibits the fibrotic process and promotes cardiac function in a rat model of ischaemia-/reperfusion-induced heart failure. Specifically, the effects of curcumin on oxidative stress, MMP activity, ECM degradation and TGF-β1/Smad-mediated collagen synthesis were examined. Our results revealed that curcumin improves cardiac function and this is associated with a mechanism of action that balances ECM degradation and collagen synthesis in the infarcted myocardium.
Methods
Surgical preparation of animals
Male Sprague-Dawley rats weighing 400–450 g were anaesthetized with an initial i.p. injection of a mixture of ketamine (90 mg·kg−1) and zylaxine (10 mg·kg−1). If re-dosing was required during surgery, ketamine alone was given. The animals were intubated and mechanically ventilated with oxygen-enriched room air using a rodent respirator. Procedures used on the first surgical day were performed under sterile conditions. The chest was opened by a left thoracotomy through the fourth intercostal space. After pericardiotomy, a 6-0 proline ligature was placed under the left coronary artery (LCA) where it emerged from beneath the left atrium, and the ends of the tie were threaded through a small plastic tube to form a snare for reversible LCA occlusion. After ischaemia, this proline ligature was kept in the chest for re-ligation at the end of the study. The body temperature was maintained constant at 37°C by a heating pad.
All animals received humane care in compliance with ‘The Guide for the Care of Use of Laboratory Animals’ published by the US National Institute of Health (NIH Publication no. 85-23, revised 1996) and the study protocol was approved by the Institutional Animal Care and Use Committee of Mercer University. The results of all studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Experimental protocol
The rats were randomly assigned to one of four groups (Figure 1): (i) control (n= 8 for each time point): no intervention was given at reperfusion; (ii) curcumin treatment (n= 8 for each time point): curcumin (Sigma Chemical Co., St. Louis, MO, USA) was orally fed at a dose of 150 mg·kg−1·day−1 (in peanut paste) during reperfusion. The rats in the control and curcumin groups were subjected to 45 min of LCA occlusion followed by 7, 21 and 42 days of reperfusion, respectively; (iii) sham control (n= 6 for each time point): the ligature was placed under LCA without occlusion and the observation period lasted 42 days; (iv) sham + curcumin (n= 6 for each time point): no LCA occlusion was conducted and curcumin was fed at the same dose as that in the curcumin group during the experiment. At the end of the surgical operation, the incisions were closed in layers. The chest and endotracheal tubes were removed after the spontaneous breathing was recovered.
Figure 1.
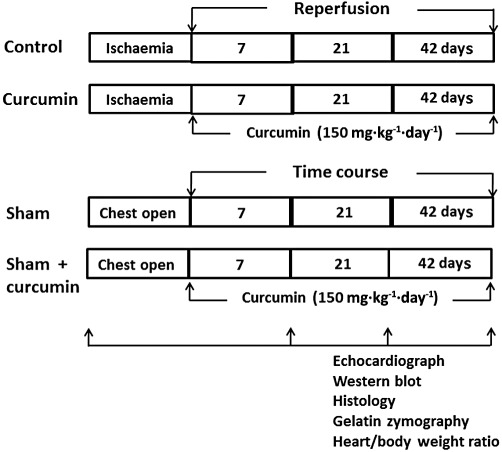
Experimental study group. In the control group (n= 8 for each time point), 45 min coronary occlusion was followed by 7, 21 and 42 days of reperfusion, respectively; in the curcumin group (n= 8 for each time point), curcumin was fed daily during the entire period of reperfusion; in the sham and sham + curcumin group (n= 6 for each time point, respectively), the chest was opened without coronary occlusion and curcumin was fed during the reperfusion. At each observational point, the heart was removed for histological analysis after cardiac function was measured.
Cardiac function and wall thickness by echocardiography
Two-dimensional (2D) guided M-mode ultrasound system (Acuson Sequoia, Siemens Medical Solutions USA, Inc., Mountain View, CA, USA) was used to assess LV systolic and diastolic function as we previously reported (Zhao et al., 2010). In brief, 2D images from a short-axis view of LV at the level of the papillary muscles were obtained using a 15 MHz linear transducer. LV % fraction shortening (FS) and ejection fraction (EF) were calculated. Pulsed-wave Doppler spectra of mitral inflow from the apical four-chamber view was used to assess LV diastolic flow characteristics by calculating a ratio of early diastolic filling (E-wave) velocity and atrial filling (A-wave) velocity. The 2D images, which were frozen at the end of diastole, were used to measure LV volume, interventricular septum and posterior wall thickness. The echocardiography was performed before opening the chest (baseline) and during the time course of the experiment in all groups. All measurements were averaged over three consecutive cardiac cycles.
Myocardial tissue preparation
At the end of each experiment, the LCA was re-ligated and 2 mL of 20% unisperse blue dye was injected into the carotid vein to outline the non-ischaemic and area at risk myocardium. The animal was killed and the heart was removed. After LV wet weight to body weight ratio was determined, infarct size was determined using triphenyl tetrazolium chloride staining as reported previously (Mykytenko et al., 2008) and calculated as a percentage of the area at risk myocardium. In other rats, a piece of transverse slice including the non-ischaemic and ischaemic zones was initially fixed in 10% phosphate-buffered formalin solution for 24 h, and then embedded in paraffin for further histological analysis. Cryosections (4 µm thick) of the tissue blocks for immunohistochemical staining were obtained using a Microtome cryostat (Leica RM2135, Meyer Instruments, Houston, TX, USA) as previously reported (Zhao et al., 2010). For Western blot analysis, the non-ischaemic and ischaemic zones were immediately frozen in liquid nitrogen and kept at −70°C until use.
Determination of lipid peroxidation
Malondialdehyde (MDA), a stable metabolite of the reactive oxygen species (ROS)-mediated lipid peroxidation cascade, was measured as previously reported (Sun et al., 2005). In brief, 100 mg of heart tissue from the non-ischaemic and ischaemic zones was minced in ice-cold Tris–HCl buffer and homogenized using a Teflon pestle. After centrifugation, the resulting supernatant fraction was used to determine the level of MDA using a lipid peroxidation assay kit (Calbiochem, San Diego, CA, USA). The absorbance of the supernatant was determined by a spectrophotometer (BioTek, Inc, Winooski, VT, USA) at 586 nm. The concentration of MDA was calculated using standard curves and is expressed as µM·g−1 protein.
Activity of MMP by gelatin zymography
Gelatin zymography was performed as described previously (Tessone et al., 2005). In brief, freshly frozen ischaemic tissue samples were homogenized in lysis buffer and gelatin (Sigma Chemical Co.) was used as a substrate. Samples (20 µg) and zymography standard (7 ng) of purified human MMP-9 and MMP-2 (Chemicon Inc., Temecula, CA, USA) were directly loaded onto standard polyacrylamide gels. After electrophoresis, the gels were soaked in Triton X-100, rinsed in water and incubated in zymogram development buffer (Bio-Rad). After incubation, the gels were stained in Coomassie blue R-250 and de-stained in methanol and acetic acid until clear bands were displayed. Molecular sizes of bands displaying enzymatic activity were characterized by comparison with purified standard MMP-9 or MMP-2. Zymographic activity was quantified using a digital image analyser (ImageJ, NIH).
Collagen deposition by Masson's trichrome staining
Masson's trichrome staining was used to evaluate collagen deposition within the scar muscle as previously reported (Zhao et al., 2010). In brief, the paraffin slides were deparaffinized, hydrated with distilled water and stained by Masson's trichrome method. The staining protocols produced collagen blue, nuclei black and viable muscle fibre red. Eight transmural sections in each heart including the infarct scar and the non-infarct myocardium were averaged to determine the collagen-rich area, calculated as a percentage of the area at risk myocardium (ImageJ, NIH).
Expression of TGFβ1, collagen and Smads by Western blotting
Western blotting was performed as described previously (Zhao et al., 2010). In brief, the freshly frozen non-ischaemic and ischaemic tissue samples were homogenized in lysis buffer and protein concentration was measured by the DC Protein Assay. The protein was then boiled and loaded onto gradient SDS-polyacrylamide gel using Mini Protean II Dual Stab Cell (Bio-Rad). Membranes were subsequently exposed to the following antibodies: a mouse monoclonal anti-TGFβ1 (Abcam, Inc., Cambridge, MA, USA), the goat polyclonal anti-collagen I, IV and a mouse monoclonal anti-collagen III (Sigma), the rabbit monoclonal phospho-Smad2 and Smad3 (Cell Signaling, Danvers, MA, USA), rabbit polyclonal anti-Smad4 and Smad7 (Santa Cruz Biotech, Santa Cruz, CA, USA) respectively. Bound antibody was detected by horseradish peroxidase conjugated anti-rabbit IgG. The membrane was incubated with chemiluminescence substrate and exposed to an X-ray film. The scanned images were imported into the ImageJ. Actin was used as a standard of protein-loading control for normalizing bands at different time points. The final results are calculated as the ratio of intensity of each band divided by actin intensity.
Detection of fibroblast differentiation with immunohistochemistry
Immunohistochemical staining of tissue sections was performed as described previously (Zhao et al., 2003). In brief, paraffin-embedded blocks were deparaffinized in xylene and dehydrated in graded ethanol. The transverse paraffin sections were stained using a monoclonal antibody against α-smooth muscle actin (α-SMA) (Sigma Chemical Co.). The slides were washed in PBS, incubated with an anti-mouse IgG and stained using the ABC-red and substrate kit (Vector Laboratories, Burlingame, CA, USA). The quality of the assay was controlled by either elimination of the primary antibody or incubation of the tissue with a non-immune IgG. Data were analysed using computed-assisted morphometry (ImageJ, NIH). Differentiation of fibroblasts is reported as mean number of α-SMA-expressing myofibroblasts from eight randomized high-powered fields (Zhao et al., 2010).
Statistical analysis
All data are presented as the mean ± SEM. A one-way ANOVA followed by Student–Newman–Keul's post hoc test was used to analyse group differences in the intensity of TGFβ1, collagens, Smads and the percentage of collagen-rich area within the area at risk myocardium. Echocardiographic data were analysed by one-way repeated measures ANOVA followed by post hoc analysis with Student–Newman–Keul's test for multiple comparisons by SigmaStat (Systat Software Inc., Point Richmond, CA, USA). Statistical significance was set at a value of P < 0.05.
Results
A total of 93 rats were initially included in this study. Two rats in the control group and one rat in the curcumin group died immediately due to bleeding from an injured coronary artery. Two rats in the control group and two rats in the curcumin group died 1 day after the chest was closed due to a large area of infarction. One rat in the control group and one rat in the curcumin group were excluded because no evidence of ischaemic zone was found after coronary ligation. The final 84 rats were included for the histological examination and echocardiography. No animals died during the 42 days of observation. The curcumin dose selected was primarily based on a previous study (Ghosh et al., 2010). It has been reported that curcumin concentration after oral administration would reach a plateau in blood at 6 h, and remain at a significantly high level even at 24 h (Suresh and Srinivasan, 2010).
Curcumin treatment during reperfusion ameliorated oxidative stress
Detection of MDA has been used as an indicator of lipid peroxidation from damaged tissue stimulated by ROS. The profile of MDA levels measured from the lysed myocardium is presented in Figure 2A. MDA was detectable during the time course of the experiment in the sham and sham plus curcumin groups, but no significant difference among the groups was found, and, therefore, data were pooled. In addition, there was no significant difference in the MDA level in the non-ischaemic area among all the groups compared to the level seen in the sham group. Ischaemia/reperfusion resulted in a significant increase in MDA level during reperfusion relative to the sham control. Treatment with curcumin significantly decreased the levels of MDA during the course of reperfusion relative to the corresponding points in the control group.
Figure 2.
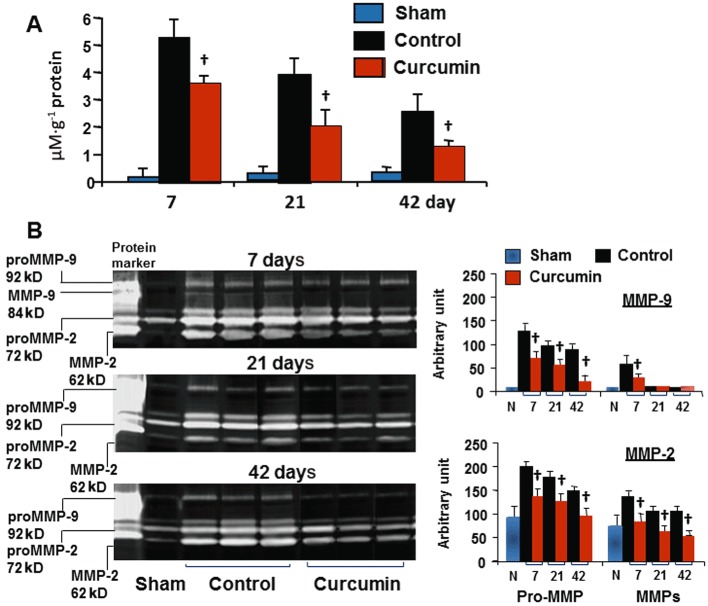
Tissue level of MDA (A) and activity of MMPs (B). MMP-9 was expressed at the 92-kD band (pro-form) and the 84-kD band (active form); MMP-2 was evident at the 72-kD (pro-form) and the 62-kD band (active form). Curcumin significantly reduced MDA levels during the course of the experiment, along with inhibition of both pro- and active forms of MMP-9 and MMP-2 as measured by an arbitrary unit. n= 8, values are mean ± SEM. †P < 0.05 versus control.
Curcumin treatment during reperfusion inhibited activity of MMPs
The activity of MMP-9 and MMP-2 on gelatin zymogram SDS-PAGE gel is illustrated in Figure 2B; proMMP-9 was not found in the sham and sham plus curcumin groups, but detected in the control and curcumin groups throughout the experiment. Consistent with a significant increase in expression of proMMP-9 at day 7, MMP-9 was significantly higher in the control group, but no MMP-9 was detected during 21 and 42 days. Curcumin treatment inhibited both proMMP-9 and MMP-9. ProMMP-2 was expressed in the sham and sham plus curcumin groups as well as in the non-ischaemic zones in the control and curcumin groups, but no group difference was detected. Consistent with enhanced proMMP-2, there was a significant increase in the expression of MMP-2 during the course of the experiment, suggesting that reperfusion triggers a constant activation of MMP2. However, both proMMP-2 and MMP-2 were significantly inhibited by daily administration of curcumin.
Curcumin treatment during reperfusion reduced infarct size and collagen deposition
No group difference in the area at risk myocardium was found, average 38 ± 6% in the control and 39 ± 4% in the curcumin group (P > 0.05) respectively. At 3 days after curcumin treatment, infarct size was significantly reduced relative to the control (45 ± 6% vs. 58 ± 8% of area at risk myocardium, P < 0.05, n= 6 for each group). The collagen-rich area was evaluated using Masson's trichrome staining. As shown in Figure 3, no newly synthesized collagen was detected in the non-ischaemic zones in the control and curcumin groups or in the sham and sham plus curcumin groups throughout the experiment. However, in the control animals, the collagen positive area was increased after 7 days of reperfusion, as determined by the size of collagen-rich area within the area at risk myocardium. The collagen-rich scar tissue was extended through the LV wall from endomyocardium to epimyocardium after 42 days of reperfusion. However, at this stage, the hearts treated with curcumin exhibited a significant reduction in the collagen-rich area within the area at risk myocardium, and more viable myocardium was detected, suggesting less degradation of pre-existing ECM. The ischaemic/reperfused myocardium appeared more organized and circumscribed, consistent with inhibition of MMPs by curcumin.
Figure 3.
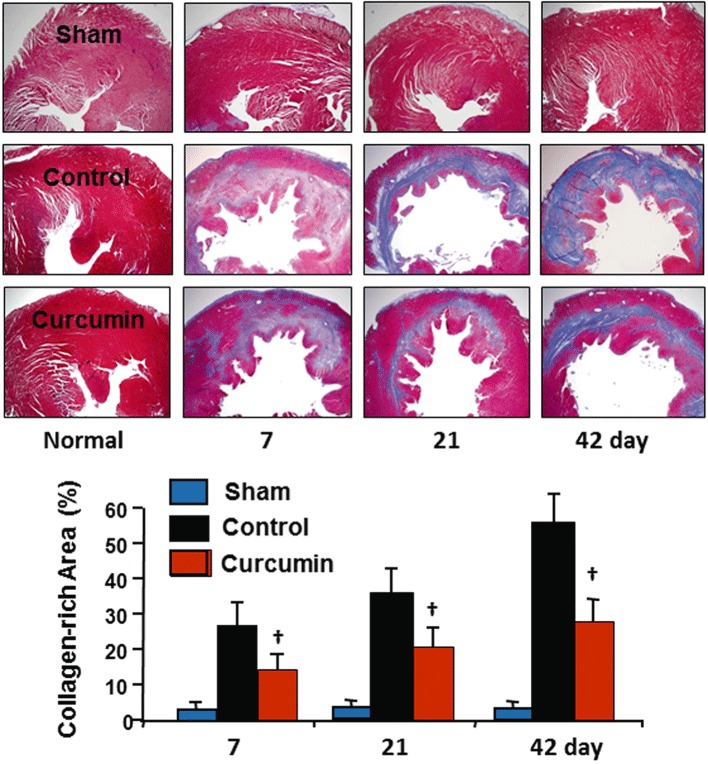
Collagen deposition in the myocardium. The scope of collagen deposition was measured by collagen-rich area calculated as the percentage of the area at risk myocardium. There was extensive loss of myocardial mass in the control group over time. Curcumin treatment significantly reduced collagen deposition with increased mass of viable myocardium within the LV anterior wall. No collagen deposition was detected in either of the sham groups. n= 8, values are mean ± SEM. †P < 0.05 versus control.
Curcumin treatment during reperfusion reduced collagen synthesis
To further confirm the results demonstrated by Masson's trichrome staining, we measured the expression of collagen type I, III and IV in the infarcted tissue using Western blotting as shown in Figure 4. Collagen types I and III, but not collagen type IV, were expressed in the non-ischaemic zones in the control and curcumin as well as in both sham groups, but no significant difference among the groups was detected. However, the synthesis of these collagens was significantly increased at day 7, and continuously maintained at higher levels after 42 days of reperfusion relative to those in the sham group. The hearts treated with curcumin showed a significant reduction in synthesis of collagens relative to the control group at all time points measured. These data are consistent with the findings showing a reduction in fibrotic tissue in the infarcted myocardium identified by Masson's trichrome staining.
Figure 4.
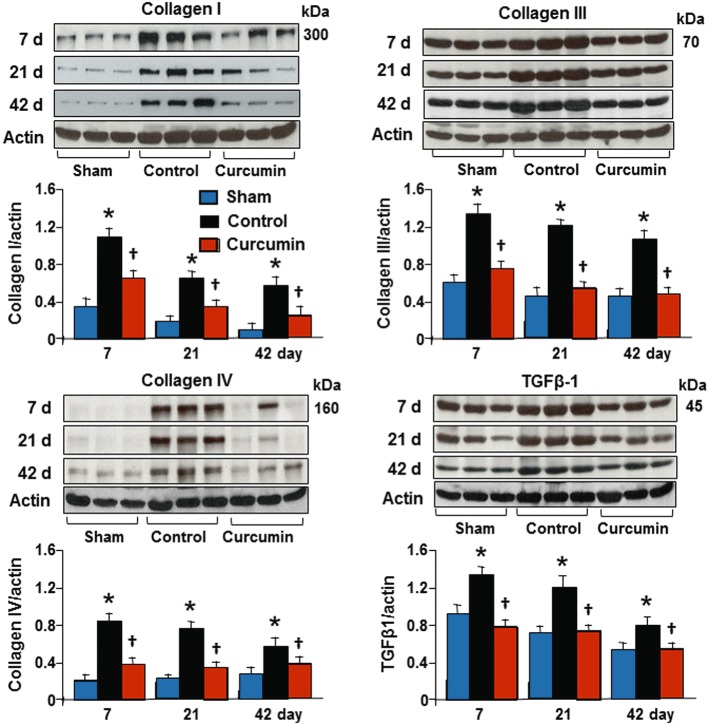
Levels of collagen I, III, IV and TGFβ1 protein. There was a significant increase in levels of collagen I, III IV and TGFβ1 protein relative to the sham and non-infarcted zone in the control group. Curcumin treatment significantly attenuated the changes in these proteins during the course of the experiment, as assessed by reduced intensity of bands shown at the bottom of each panel. All bands were normalized by actin as illustrated at 42 days. n= 8, values are mean ± SEM. *P < 0.05 versus sham; †P < 0.05 versus control.
Curcumin treatment during reperfusion inhibited the expression of TGFβ1 and phosphorylation of Smads
To demonstrate whether inhibition of collagen synthesis by curcumin is associated with TGFβ1-Smads signalling pathways, we measured TGFβ1 expression and Smads phosphorylation from the transmural tissue samples obtained from the infarcted myocardium using Western blotting assay. As shown in Figure 4, TGFβ1 was expressed in the sham and sham plus curcumin groups as well as in the non-ischaemic myocardium in the control and curcumin groups, but no significant difference among the groups was found. Ischaemia/reperfusion caused a constant increase in TGFβ1 expression during the course of the experiment, which was significantly attenuated by administration of curcumin. As shown in Figure 5, the phospho-Smad2 and Smad3 were expressed at a relatively lower level in the non-ischaemic zone and no significant difference was found between the sham, the sham plus curcumin, the control and the curcumin groups. Ischaemia/reperfusion caused a significant change in the phosphorylation of Smad2 and Smad3 at day 7, and they remained in an activated state until 42 days of reperfusion, consistent with an enhanced expression of Smad4. However, expression of Smad7 was down-regulated during the course of the experiment. Daily administration of curcumin enhanced the expression of Smad7 and attenuated the phosphorylation of Smad2 and Smad3 as well as the expression of Smad4, suggesting that Smad7 inhibits the phosphorylation of Smad2 and Smad3. These data are consistent with the significant reduction in collagen synthesis/deposition in the infarcted myocardium detected by Western blotting assay and Masson's trichrome staining.
Figure 5.
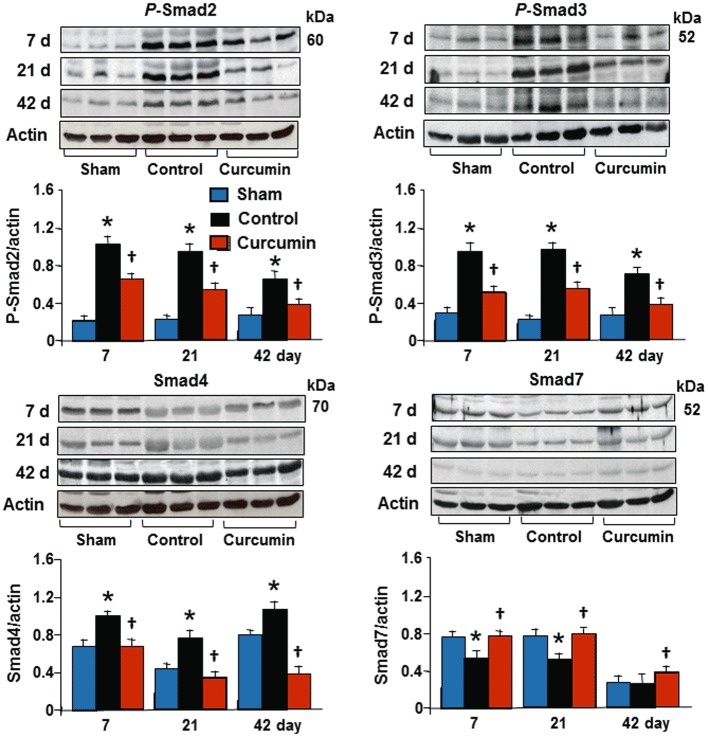
Expression of Smads protein. No significant changes in the total levels of Smad2 and Smad3 were detected (data not shown). However, relative to the sham and non-infarcted zone, ischaemia/reperfusion induced a significant increase in levels of phospho-Smad2, Smad3 and Smad4, and a significant reduction in Smad7 during the course of the experiment in the control group. Curcumin treatment significantly attenuated changes in these proteins as assessed by reduced intensity of bands; shown at the bottom of each panel. All bands were normalized to actin as illustrated at 42 days. n= 8, values are mean ± SEM. *P < 0.05 versus sham; †P < 0.05 versus control.
Curcumin treatment during reperfusion inhibited the differentiation of fibroblasts in the myocardium
The differentiation of fibroblasts was identified as the accumulation of α-SMA-expressing myofibroblasts by immunohistochemical analysis. No α-SMA-expressing myofibroblasts were detected in either of the sham groups. A few α-SMA-expressing myofibroblasts were present only in vascular smooth muscle cells in the non-ischaemic myocardium in the control and curcumin groups. As shown in Figure 6, α-SMA-expressing myofibroblasts were significantly increased after 7 days of reperfusion in the control group. The majority of α-SMA-expressing myofibroblasts had aligned with the host myocardial fibres and along the ischaemic border and scar zones. After 42 days of reperfusion, the number of α-SMA-expressing myofibroblasts in these zones was still significantly higher in the control group relative to the non-ischaemic zone and sham control. However, the increase in the number of α-SMA-expressing myofibroblasts accumulated in the infarcted zone in the curcumin group was significantly reduced during the entire period of reperfusion, suggesting that treatment with curcumin inhibits differentiation of fibroblasts.
Figure 6.
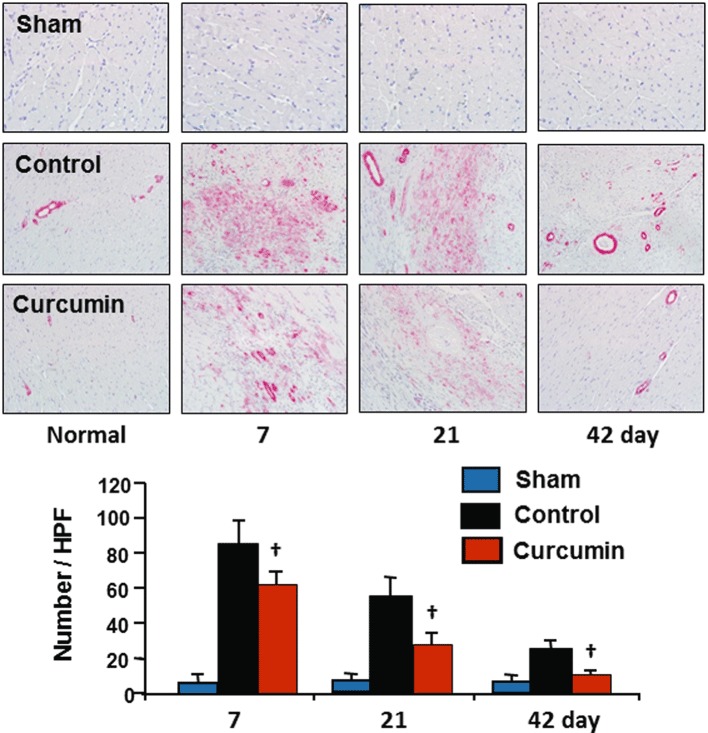
Differentiation of fibroblasts to myofibroblasts. No α-SMA expressing myofibroblasts were detected in the non-ischaemic zone in the control group or in either of the sham groups. Ischaemia/reperfusion caused a significant increase in the number of α-SMA expressing myofibroblasts accumulated during reperfusion, which subsequently declined to day 42 in the control group. However, this increase in accumulation of α-SMA expressing myofibroblasts was significantly reduced by curcumin relative to each time point during reperfusion in the control group. n= 8, values are mean ± SEM. *P < 0.05 versus sham; †P < 0.05 versus control.
Curcumin treatment during reperfusion enhanced cardiac repair
The LV wet weight to body weight ratio, LV end-diastolic volume and wall thickness were used to assess cardiac repair with curcumin treatment. As a sign of chronic heart failure, the heart : body weight ratio in the control group was significantly increased relative to the sham group (0.39 ± 0.01% vs. 0.24 ± 0.02 in Sham, P < 0.05) at day 42. Oral administration of curcumin for 42 days resulted in a significant reduction in this calculated ratio (0.26 ± 0.02%, P < 0.05 vs. control), suggesting a less marked hypertrophic response to ischaemia/reperfusion injury. As shown in Figure 7, the LV end-diastolic volume was not altered during experimental periods in either sham group (data were pooled). However, ischaemia/reperfusion caused a significant increase in the LV end-diastolic volume during the course of the experiment, when compared with baseline values in the sham and control groups. Furthermore, the wall thickness of the infarct middle anterior septum was significantly reduced relative to the posterior septum in the control group. Consistent with a reduction in the heart : body ratio in the curcumin group, the hearts treated with curcumin had smaller LV end-diastolic volume and greater wall thickness as measured from echocardiographic images.
Figure 7.
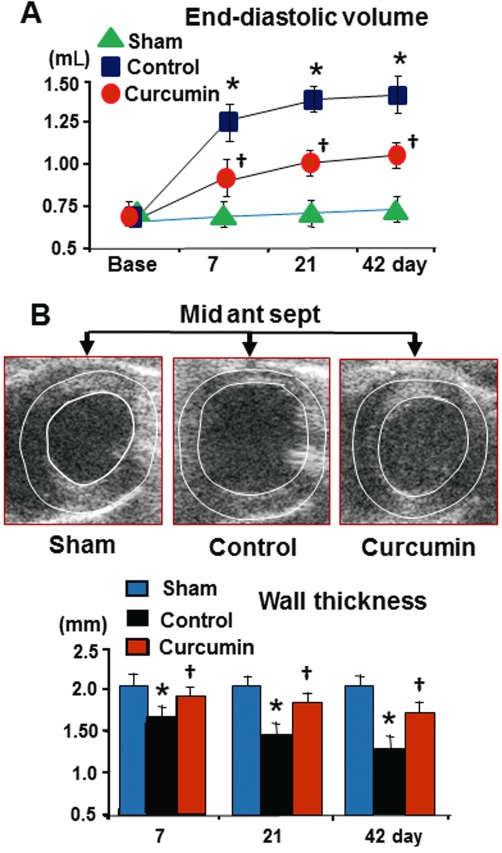
LV end-diastolic volume (LVEDV) and wall thickness. Relative to the sham control, ischaemia/reperfusion caused a significant increase in LVEDV (A) and reduced wall thickness (B) over time in the control group. Curcumin treatment significantly reduced the LVEDV and increased the wall thickness in the infarcted anterior and septum relative to the control. n= 8, values are mean ± SEM. *P < 0.05 versus normal; †P < 0.05 versus control.
Curcumin treatment during reperfusion improved cardiac systolic and diastolic function
Echocardiographic results of cardiac systolic and diastolic function among the groups are summarized in Table 1. No significant difference was found in echocardiographic baseline values in both the sham groups (data were pooled), control and curcumin groups. LVSd, LVSs, LVDd and LVDs were significantly higher during 21 and 42 days of reperfusion in the control group. In addition, the indices of systolic function, that is, the FS, EF and stroke volume, were significantly lower compared to the baseline value, suggesting cardiac contractile dysfunction (Figure 8A). Oral administration of curcumin over 42-day periods improved contractile function. EF values were increased by 25 ± 6% in the curcumin group compared to the control group at day 42.
Table 1.
Echocardiographic data
| Sham | Control | Curcumin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index | Baseline | 7 | 21 | 42 day | Baseline | 7 | 21 | 42 day | Baseline | 7 | 21 | 42 day |
| LVSd (mm) | 2.0 ± 0.3 | 1.9 ± 0.4 | 1.9 ± 0.4 | 2.0 ± 0.2 | 2.0 ± 0.3 | 1.6 ± 0.4 | 1.4 ± 0.3 | 1.3 ± 0.3* | 1.9 ± 0.2 | 1.8 ± 0.4† | 1.7 ± 0.1† | 1.7 ± 0.2† |
| LVSs (mm) | 2.4 ± 0.2 | 2.4 ± 0.3 | 2.3 ± 0.6 | 2.3 ± 0.8 | 2.5 ± 0.5 | 1.5 ± 0.4 | 1.6 ± 0.2* | 1.4 ± 0.2* | 2.4 ± 0.2 | 2.2 ± 0.4† | 2.0 ± 0.2† | 2.1 ± 0.1† |
| LVDd (mm) | 7.2 ± 0.8 | 7.3 ± 0.7 | 7.5 ± 0.6 | 7.3 ± 0.7 | 6.8 ± 0.8 | 7.2 ± 0.7 | 9.0 ± 0.7* | 9.2 ± 0.5* | 7.4 ± 0.5 | 7.2 ± 0.7 | 7.3 ± 0.7 | 7.2 ± 0.7 |
| LVDs (mm) | 5.0 ± 0.9 | 5.2 ± 0.8 | 5.2 ± 0.4 | 5.1 ± 0.2 | 4.9 ± 0.9 | 5.6 ± 0.8 | 7.2 ± 0.5* | 7.5 ± 0.8* | 5.0 ± 0.8 | 4.9 ± 0.7 | 5.5 ± 0.7† | 5.5 ± 0.2† |
| LVPWd (mm) | 1.9 ± 0.1 | 2.0 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.3 | 2.0 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.5 | 2.1 ± 0.3 |
| LVPWs (mm) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.3 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2 |
| Ant-sep (mm) | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.3 | 2.1 ± 0.2 | 2.1 ± 0.2 | 1.6 ± 0.2* | 1.5 ± 0.5* | 1.4 ± 0.2* | 2.1 ± 0.1 | 1.8 ± 0.2† | 1.7 ± 0.5† | 1.6 ± 0.2† |
| Post-sep (mm) | 2.0 ± 0.1 | 1.9 ± 0.3 | 2.0 ± 0.1 | 1.9 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.5 | 2.0 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.2 |
Ant-sep, middle anterior septum; LVDd or LVDs, left ventricle volume in diastole or systole; LVPWd or LVPWs, left ventricular posterior wall in diastole or systole; LVSd or LVSs, interventicular septum thickness in diastole or systole; Post-sep, middle posterior septum.
Values are mean ± SEM.
P < 0.05 versus baseline values.
P < 0.05 versus control group.
Figure 8.
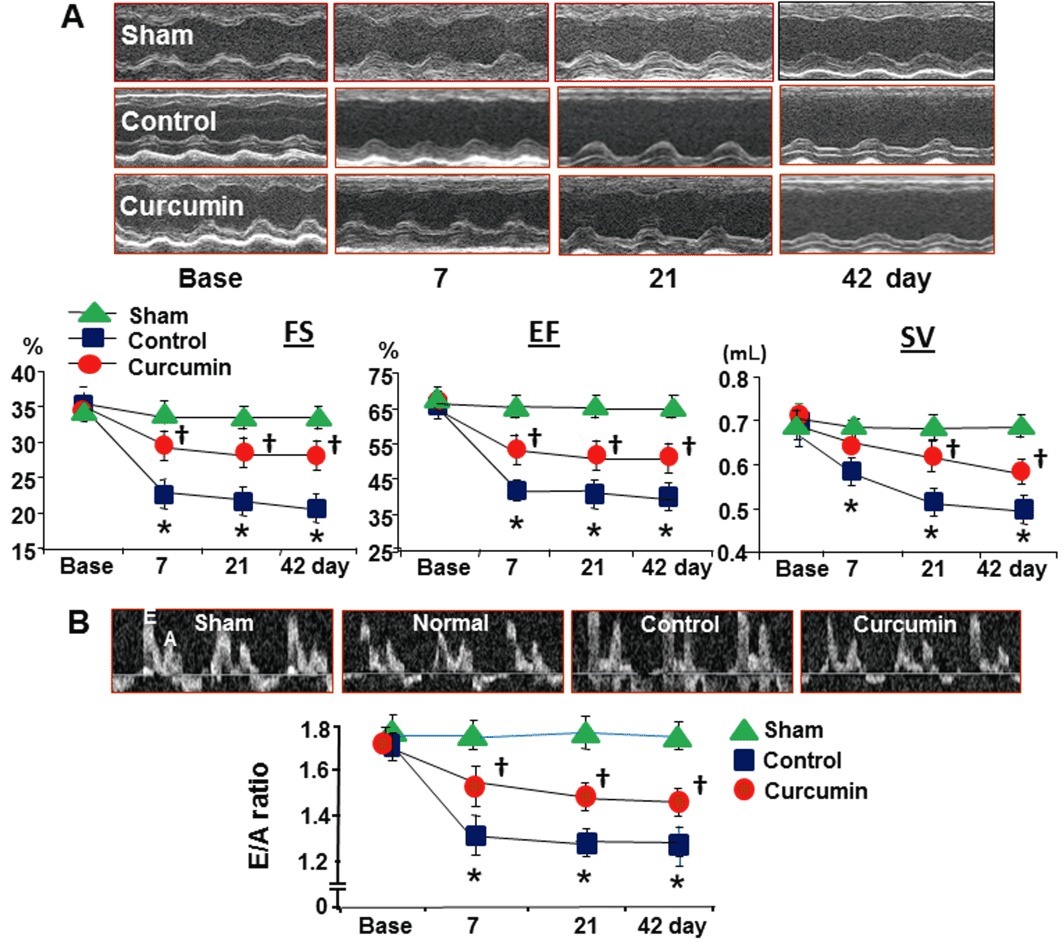
LV systolic and diastolic function. Ischaemia/reperfusion caused a significant reduction in cardiac systolic function measured by echocardiography (A) over the 42 days of reperfusion. Relative to these values in the control group, curcumin treatment significantly preserved cardiac systolic function; measured as FS, fraction shortening; SV, stroke volume; EF, ejection fraction. Cardiac diastolic function was measured by a pulsed-wave Doppler spectra of mitral inflow from the apical four-chamber view, that is, a ratio of early diastolic filling (E-wave) velocity and atrial filling (A-wave) velocity (panel B). Relative to the control, curcumin significantly preserved the E : A ratio over time. n= 8, values are mean ± SEM. *P < 0.05 versus baseline values in the sham and control group; †P < 0.05 versus control.
The peak E-wave velocity/peak A-wave velocity ratio was selected to access diastolic function through the pulsed-wave Doppler recordings of mitral inflow. As shown in Figure 8B, ischaemia/reperfusion increased both early diastolic filling (E-wave) velocity and atrial filling (A-wave) velocity, and therefore resulted in a significant reduction in the E : A ratio relative to baseline values over 42-day periods in the control animals. However, this change in the E : A ratio was significantly attenuated by oral administration of curcumin. No change in the E : A ratio was found in either of the sham groups during the observation periods.
Discussion
The major new results of the present study demonstrated that oral administration of curcumin after ischaemia reduces tissue levels of MDA, protects the existing ECM from degradation by attenuating the activity of MMPs and attenuates collagen synthesis via the TGFβ1/Smads-mediated signalling pathways as well as inhibits accumulation of α-SMA-expressing myofibroblasts, indicative of a reduction in fibroblast differentiation. Consistent with these beneficial effects of curcumin on cardiac repair, cardiac function was improved, LV dilatation was reduced and wall thickness was increased. These results support the concept that curcumin could be an effective therapeutic candidate against ischaemia/reperfusion-induced heart failure (Srivastava and Mehta, 2009).
The preservation of original ECM after MI is one of the most important factors to retain tissue integrity and prevent maladaptive cardiac repair (Spinale et al., 2002). MMPs are members of a family of zinc endopeptidases and capable of cleaving all ECM components, resulting in degradation of collagens and formation of new fibrotic tissue in the infarcted myocardium. MMPs are synthesized as pro-enzymes and are usually activated by proteolytic cleavage of an inhibitory propeptide domain (Cheung et al., 2000). Although the cell types that synthesize and secrete MMPs are not yet clear, the stimulation of neutrophils, fibroblasts, endothelial cells and vascular smooth muscle cells by reactive oxygen species generated during reperfusion has been proposed to activate MMPs (Cheung et al., 2000; Falk et al., 2002). In the present study, proMMP-9 was detected during the entire period of the experiment, but its 84 kDa-activated form was only displayed at day 7, consistent with the highest tissue level of MDA at this time point. In contrast, both proMMP-2 along with a 62 kDa active form, MMP-2, were detected over the time of reperfusion. Daily curcumin treatment significantly reduced both the 84 kDa MMP-9 and 62 kDa MMP-2. This inhibition of MMPs with curcumin is potentially mediated through its potent antioxidant action, as demonstrated by a significant reduction in tissue MDA level.
Cardiac ECM turnover is primarily determined by the balance between collagen deposition and degradation. If collagen deposition predominates, the fibrosis can predispose the heart to undergo maladaptive tissue repair and failure. If collagen degradation predominates, the LV wall thinning can lead to aneurysm and rupture (Spinale et al., 2002; Tessone et al., 2005). Cardiac fibroblasts are known to synthesize and release collagen I, III, IV and many other ECM-related proteins (Brown et al., 2005). Differentiation of fibroblasts to myofibroblasts, characterized by the expression of α-SMA, is a key event in maladaptive cardiac repair. The simultaneous localization of myofibroblasts and of an excess of tissue collagens in the present study suggests myofibroblasts are a tissue source of extra collagen.
In the normal myocardium, types I and III collagen represent most of the newly synthesized collagen, and are the predominant fibrillar components of ECM with a small proportion of type IV collagen, a key component of the basement membrane (Cleutjens et al., 1995; Brown et al., 2005). We found that type I and type III collagens were expressed in the non-ischaemic myocardium by Western blotting, but their expression occurred predominantly in the infarcted zone at day 7 and remained elevated for 42 days, which is the time frame that the necrotic myocytes are entirely replaced by fibrous tissue (Sun, 2009). Type IV collagen was not detected in the non-infarcted zone but increased dramatically during the course of the experiment. Coincident with the reduction in collagen deposition identified by Masson's trichrome staining, the current study demonstrated that daily curcumin treatment significantly down-regulated the expression of types I, III and IV collagen. Although we cannot outline the exact time frame for collagen degradation and synthesis after ischaemia, previous studies have demonstrated that rapid collagen breakdown occurs during the early stages of reperfusion (Takahashi et al., 1990; 1999). We postulate that curcumin protects the ECM from degradation by attenuating MMPs during the early phases of reperfusion and reduces the fibrotic tissue by constantly inhibiting collagen synthesis over time.
The TGFβ1/Smad pathway stimulated by oxidative stress has been reported to be primarily involved in the stimulation of collagen synthesis in the heart (Sakata et al., 2008; Yuan and Jing, 2010; Euler-Taimor and Heger, 2006). We tested the hypothesis that inhibition of collagen by curcumin is associated with changes in the TGFβ1/Smad signalling pathway. Smads family can be categorized into three distinct proteins that function downstream from the TGFβ1 receptor: receptor-activated Smads (Smad1, Smad2, Smad3, Smad5 and Smad8), common mediator Smads (Smad4 and Smad10) and inhibitory Smads (Smad6 and Smad7). Upon activation of the cellular surface receptor by TGFβ1 in the heart, Smad2 forms a complex with Smad3 and Smad4. This complex then translocates from the cytoplasm into the nucleus, resulting in an alteration in the expression of TGFβ1-targeted collagen genes. The inhibitory Smad6 and Smad7 function as inhibitors of TGFβ1 signalling by preventing Smad2/3 phosphorylation and disrupting Smad complex formation (Yuan and Jing, 2010; Euler-Taimor and Heger, 2006; Bujak and Frangogiannis, 2007). The administration of exogenous Smad7 (adenoviral delivery) has previously been demonstrated to inhibit TGFβ1/Smad2/3-mediated collagen synthesis and fibrosis in in vivo and in vitro models (Wang et al., 2007). In the current study, we found that changes in the phosphorylation of Smad2/3 and down-regulation of Smad7 occur primarily during the first 21 days, suggesting they have a role in mediating collagen synthesis in the early stages of reperfusion. Given the fact that down-regulation of Smad7 is associated with enhanced expression of phosphorylated Smad2/3 (Wang et al., 2007), inhibition of phospho-Smad2/3 with curcumin was primarily associated with an up-regulation of Smad7. The data obtained in the present study are in agreement with the paradigm of suppression of TGFβ1/Smad pathway in mediating ECM production by curcumin in cultured fibroblasts, as reported recently (Gaedeke et al., 2004; Hsu et al., 2010; Hu et al., 2010). α-SMA-expressing myofibroblasts are thought to arise from the transdifferentiation of fibroblasts in response to TGFβ1 stimulation. At the site of damaged myocardium, myofibroblasts are responsible for the collagen secretion and contraction/realignment of the nascent collagen fibre (Dobazewski et al., 2010). In the present study, the accumulation of α-SMA expressing myofibroblasts was significantly attenuated by curcumin, in parallel with an inhibition in expression of TGFβ1, suggesting a role for TGFβ1 in fibroblast-to-myofibroblast transformation during periods of final fibrotic tissue formation.
Adequate replacement of necrotic tissue by granulation and scarless tissue is suggested to be beneficial because it can prevent the most common complications that occur during the healing of myocardial injury, such as wall thinning, chamber dilatation and cardiac dysfunction (Rohde et al., 1999; Payne et al., 2007). Current data confirm this hypothesis, because the faster healing and less scar formation induced by curcumin reduced wall thinning and inhibited ventricular lumen dilatation. Along with an increase in ventricular wall thickness and a reduction in LV end-diastolic volume at 42 days of reperfusion, curcumin significantly improved cardiac systolic and diastolic function compared with the control animals.
In summary, the current study demonstrates that daily treatment with curcumin during reperfusion protects against maladaptive tissue repair and improves cardiac function after ischaemia. Cardioprotection with curcumin was associated with attenuation of lipid peroxidation and active MMPs, inhibition of the TGFβ1-Smad signalling pathway and differentiation of fibroblasts and modulations in the balance between collagen degradation and synthesis. Through all these beneficial effects, curcumin could maintain the ECM integrity and stability responsible for cardiac recovery. Since curcumin has clinically been demonstrated as a safe food additive, it might be used as a potential add-on therapeutic drug to other conventional therapies, such as statins, β-blockers or ACE inhibitors for the treatment of patients after a heart attack.
Acknowledgments
This study was supported in part by a seed grant from the Mercer University School of Medicine and National Natural Science Foundation of China (81170145/H0203).
Glossary
- ECM
extracellular matrix
- EF
ejection fraction
- FS
fraction shortening
- LCA
left coronary artery
- LV
left ventricle
- MDA
malondialdehyde
- MI
myocardial infarction
- α-SMA
α-smooth muscle actin
Conflict of interest
No conflicts of interest are declared by the authors.
References
- Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, et al. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30:604–610. doi: 10.1016/j.ejcts.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Ann Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- Cleutjens JPM, Verluyten MJA, Smiths JFM, Daemen JAP. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- Dobazewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- Euler-Taimor G, Heger J. The complex pattern of Smad signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Falk V, Soccal PM, Grunenfelder J, Hoyt G, Walther T, Robbins RC. Regulation of matrix metalloproteinases and effect of MMP-inhibition in heart transplant related reperfusion injury. Eur J Cardiothorac Surg. 2002;22:53–58. doi: 10.1016/s1010-7940(02)00207-5. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int. 2004;66:112–120. doi: 10.1111/j.1523-1755.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Gallagher G, Menzie S, Huang Y, Jackson C, Hunyor SN. Regional cardiac dysfunction is associated with specific alterations in inflammatory cytokines and matrix metalloproteinases after acute myocardial infarction in sheep. Basic Res Cardiol. 2007;102:63–72. doi: 10.1007/s00395-006-0610-7. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Massey HD, Krieg R, Fazelbboy ZA, Ghosh DA, Fakhry SI, et al. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol. 2009;296:F1146–F1157. doi: 10.1152/ajprenal.90732.2008. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Salloum FN, Abbate A, Krieg R, Sica DA, Gehr TW, et al. Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol. 2010;299:H975–H984. doi: 10.1152/ajpheart.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC. Suppression of TGFβ1/Smad pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res. 2010;302:717–724. doi: 10.1007/s00403-010-1075-y. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liang H, Du Y, Zhu YL, Wang XD. Curcumin inhibits transforming growth factor-β activity via inhibition of Smad signaling in HK-2 cells. Am J Nephrol. 2010;31:332–341. doi: 10.1159/000287230. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem DA, Honda HM, Zhang J, Woo D, Ping P. Past and present course of cardioprotection against ischemia-reperfusion injury. J Appl Physiol. 2007;103:2129–2136. doi: 10.1152/japplphysiol.00383.2007. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Sunagawa YK, Awamura T, Takaya T, Wada H, Nagasawa A, et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytenko J, Reeves JG, Kin H, Zatta AJ, Jiang R, Guyton RA, et al. Persistent beneficial effect of postconditioning against infarct size: role of mitochondrial KATP channel activation during reperfusion. Basic Res Cardiol. 2008;103:472–484. doi: 10.1007/s00395-008-0731-2. [DOI] [PubMed] [Google Scholar]
- Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, et al. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- Prasad A, Stone GW, Holmes DR, Gersh B, Dphil C. Reperfusion injury, microvascular dysfunction, and cardioprotection: the ‘dark side’ of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- Rohde LE, Aikawa M, Cheng GC, Sukhova G, Solomon SD, Libby P, et al. Echocardiography-derived left ventricular end-systolic regional wall stress and matrix remodeling after experimental myocardial infarction. J Am Coll Cardiol. 1999;33:835–842. doi: 10.1016/s0735-1097(98)00602-0. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-β receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 2008;103:60–68. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, et al. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol (Lung Cell Mol Physiol) 2010;298:L616–L625. doi: 10.1152/ajplung.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinale FG, Gunasinghe H, Sprunger PD, Baskin JM, Bradham WC. Extracellular degradative pathways in myocardial remodeling and progression to heart failure. J Card Fail. 2002;8:S332–S338. doi: 10.1054/jcaf.2002.129259. [DOI] [PubMed] [Google Scholar]
- Srivastava G, Mehta JL. Currying the heart: curcumin and cardioprotection. J Cardiovasc Pharmacol Ther. 2009;14:22–27. doi: 10.1177/1074248408329608. [DOI] [PubMed] [Google Scholar]
- Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, et al. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–H1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- Sun Y. Myocardial repair/remodeling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh D, Srinivasan K. Tissue distribution and elimination of capsaicin, piperine and curcumin following oral intake in rats. Indian J Med. 2010;131:682–691. [PubMed] [Google Scholar]
- Takahashi A, Barry AC, Factor SM. Collagen degradation in ischemic rat hearts. Biochem J. 1990;265:233–241. doi: 10.1042/bj2650233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Greenen D, Nieves E, Iwazumi T. Collagenase degrades collagen in vivo in the ischemic heart. Biochim Biophys Acta. 1999;1428:251–259. doi: 10.1016/s0304-4165(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Tessone A, Feinberg MS, Barbash IM, Reich R, Holbova R, Richmann M, et al. Effect of matrix metalloproteinase inhibition by doxycycline on myocardial healing and remodeling after myocardial infarction. Cardiovasc Drugs Ther. 2005;19:383–390. doi: 10.1007/s10557-005-5201-6. [DOI] [PubMed] [Google Scholar]
- Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning – a new link in nature's armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100:295–310. doi: 10.1007/s00395-005-0523-x. [DOI] [PubMed] [Google Scholar]
- Wang B, Omer A, Angelovska T, Drobic V, Rattan SG, Jones SC, et al. Regulation of collagen synthesis by inhibitory Smad7 in cardiac myofibroblasts. Am J Physiol Heart Circ Physiol. 2007;293:H1282–H1290. doi: 10.1152/ajpheart.00910.2006. [DOI] [PubMed] [Google Scholar]
- Yuan SM, Jing H. Cardiac pathologies in relation to Smad-dependent pathways. Interactive cardiovascular and thoracic. Surgery. 2010;11:455–460. doi: 10.1510/icvts.2010.234773. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Puskas JD, Xu D, Wang NP, Guyton RA, Vinten-Johansen J, et al. Improvement in cardiac function with small intestine extracellular matrix is associated with recruitment of c-kit cells, myofibroblasts and macrophages after myocardial infarction. J Am Coll Cardiol. 2010;55:1250–1261. doi: 10.1016/j.jacc.2009.10.049. [DOI] [PubMed] [Google Scholar]


