Abstract
Background
Cancer screening programs must meet high standards for quality and effectiveness, because many healthy persons need to be screened to detect relatively few cases of cancer. In this study, we use the rate of interval cancers as an important surrogate indicator for evaluating the German mammography screening program (MSP).
Methods
All first-time participants in the MSP in the German federal state of North Rhine–Westphalia over the period 2005–2008 whose screening mammogram had been read as negative were followed over the next 24 months for the potential development of breast cancer (an “interval cancer” or IC). The screening data in the MSP database were compared in anonymized fashion with reports of cancer that were recorded at the Epidemiological Cancer Registry North Rhine–Westphalia.
Results
Among the 878 764 women with negative screening mammograms in the first screening round, 2036 (23.2 per 10 000) developed an IC in the ensuing 24 months. These ICs accounted for 40% of all T2–T4 breast cancers occurring in first-time participants in the screening program in the 2 years after screening. The relative rate of IC compared to the background incidence of breast cancer before introduction of the MSP was 27% in the first year and 58% in the second. Screening detected 78% of all breast cancers that occurred during a maximum of 2 years after screening.
Conclusion
The IC rates in the implementation phase of the MSP agree with those found in other, established European programs. The present study is the first one to assess this important surrogate parameter to characterize the effectiveness of the German MSP among women in North Rhine–Westphalia, Germany’s largest state by population.
Screening programs are a secondary cancer prevention measure. As a result of earlier diagnosis at a tumor stage with a more favorable prognosis, screening is intended to lead to less invasive treatment, longer lifespan, and, ideally, full recovery for those who test positive (1). Every population-wide cancer screening program involves testing a population of which the vast majority of individuals are healthy in order to reveal a very small percentage who have a disease, generally at a preclinical stage. The quality and benefit requirements for these programs must therefore be very high (2).
In Germany’s most populous federal region, North Rhine–Westphalia, the first screening units of the German mammography screening program (MSP) became operational as part of routine care on 24 October 2005. By 11 August 2008, all 23 screening units planned in North Rhine–Westphalia with at least one mammography unit were up and running. Comprehensive implementation of the screening program in North Rhine–Westphalia was completed on 23 December 2009.
Like similar programs in most European countries with population-wide mammography screening, the German screening program is geared towards the quality requirements established in the European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis (3). The EU guidelines distinguish between performance indicators and surrogate indicators. Since the German Mammography Cooperation Association (Kooperationsgemeinschaft Mammographie) put forward adapted performance and process parameters in its evaluation reports for the German MSP, the authors have analyzed the phase between screenings.
Performance indicators themselves give no direct information on the effect of screening on breast cancer mortality, but they do reflect the range and quality of individual steps within the screening process. In order to obtain initial evidence of the efficacy of the breast cancer screening program even before an expected long-term reduction in the breast cancer mortality rate occurs, the EU guidelines recommend that surrogate parameters be assessed and evaluated. The following parameters, among others, can be used for this purpose:
The breast cancer detection rate (the number of screening participants with breast cancer detected at screening as a percentage of all participants)
The stages of tumors detected at screening
The rate of interval cancers and their tumor stages.
Interval cancers are breast cancers detected independently of the cancer screening program in the interval before the next screening (24 months), in screening participants who had had negative screening mammography results. These surrogate parameters are a part of regular evaluation of the program, with the exception of the stage distribution of interval cancers. The implementation phase of the MSP in North Rhine–Westphalia has already been analyzed by our working group in terms of the above-mentioned parameters (4, 5) (Figure 1).
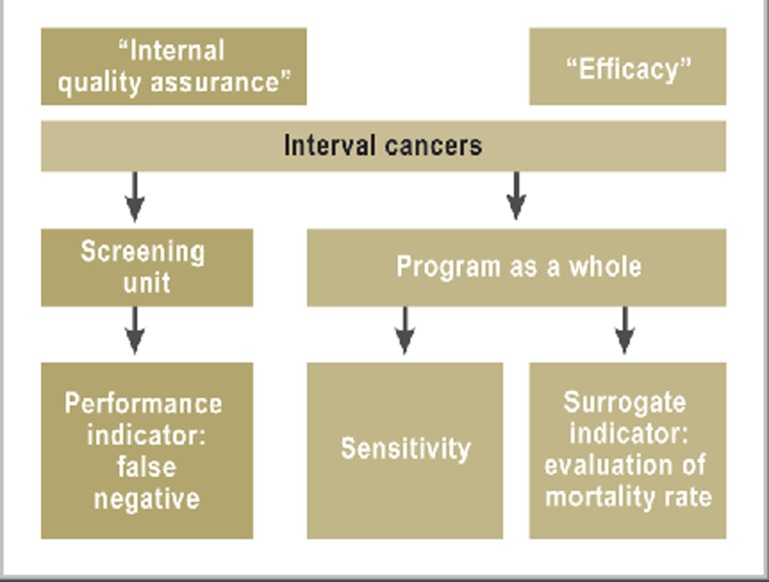
Figure 1.
The importance of interval cancers for the German mammography screening program Interval cancers are both an important performance indicator (“internal quality assurance”) and an important surrogate indicator for evaluation of mortality (“efficacy”).
This article analyses the incidence rates of interval cancers in women who had tested negative at initial screening between 2005 and 2008, on the basis of data from the Epidemiological Cancer Registry North Rhine–Westphalia (EKR NRW). The aim of this is to provide an early assessment of the efficacy of mammography screening.
Methods
Screening takes place in accredited mammography units. One or several mammography units, together with diagnosis units, form a screening unit.
North Rhine–Westphalia has 23 screening units: 13 in Westphalia–Lippe, and 10 in North Rhine. The number of women entitled to screening between the ages of 50 and 69 years was 2 251 008 (as of 31 December 2008).
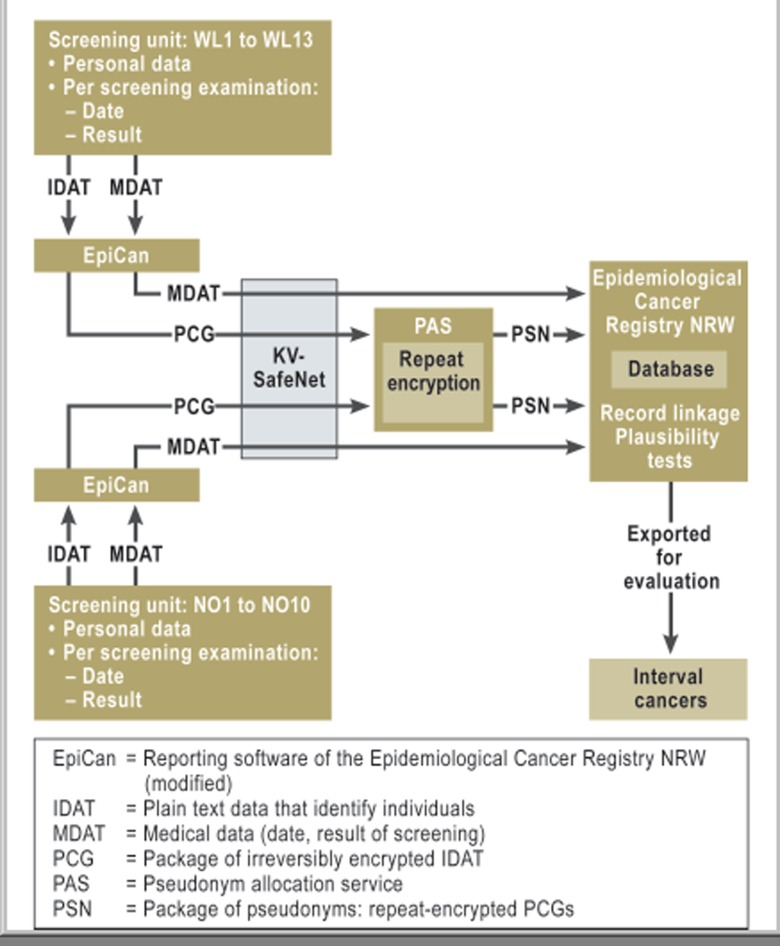
Data from North Rhine–Westphalia’s screening units were compared with those of the EKR NRW after being anonymized for the purpose. Full comparison was impossible for only one of the 23 units. This was because at that unit findings had initially been documented without using the MaSc software that was used throughout North Rhine–Westphalia. Data from the screening units were submitted to the EKR NRW in the same way as when reporting any incident cancer case, according to the current provisions of North Rhine–Westphalia’s cancer registry law (Figure 2).
Figure 2.
Diagram showing the flow of data for anonymized comparison with data from the Epidemiological Cancer Registry NRW (North Rhine–Westphalia)
KV-SafeNet, a secure reporting intranet used by the German Associations of Statutory Health Insurance Physicians (KVen, Kassenärztliche Vereinigungen)
Double-encrypted personal data were used for data comparison in the EKR NRW, in the form of probabilistic record linkage. This whole procedure, including record linkage and its evaluation, has been described in detail elsewhere (6– 9). Probabilistic comparison of screening data with the reports in the EKR NRW was performed only for women in whom first-round mammography screening in 2005 to 2008 yielded normal results or subsequent diagnosis procedures did not confirm breast cancer (negative initial screening).
The regional background incidence rate was used as the reference value for evaluation of the effects of implementing the MSP. According to the EU guidelines background incidence is defined as the incidence rate of invasive breast cancers (ICD–10: C50) observed before implementation of screening in the target population of women aged between 50 and 69 years. Because the EKR NRW was not fully set up until 1 July 2005, data for 2000 to 2004 from the long-established cancer registry of the administrative area of Münster were used to calculate the regional background incidence rate for North Rhine–Westphalia (the completeness of breast cancer data was above 95% for the years 2000 to 2002, and above 90% for 2003 and 2004). The background incidence was 269.1 cases per 100 000 women eligible for screening (10).
The completeness of recorded cancer data is particularly important for the detection of interval cancers. Statewide registration of all new disease cases in North Rhine–Westphalia was achieved because all pathologists and breast centers, in particular, were connected electronically with the epidemiological cancer registry. As a result, a high level of breast cancer registration was attained from 2006 onwards in Westphalia–Lippe and from 2007 onwards in North Rhine, with completeness substantially above 90% (8).
The breast cancer detection rates for North Rhine–Westphalia have already been reported elsewhere (5). For comparison we therefore refer to the published statewide detection rate and T-category distribution in first-time screening participants for 2005 to 2008 (the number of breast cancer cases detected at screening as a percentage of the number of participants) based on MaSc documentation.
Interval cancers are all in situ cancers and invasive cancers (ICD–10: C50 plus D05) diagnosed after negative screening (including any subsequent diagnostic work-up) and before the next scheduled screening or less than two years after age-related exclusion from screening. This means that cancers diagnosed in an extended postscreening diagnosis process by short-term monitoring are not interval cancers. Instead, they are classified as cancers detected at screening with delayed diagnosis. The following types of interval cancers are distinguished:
True interval cancers with no visible correlate at screening and radiologically occult interval cancers that are also impossible to identify on a mammogram when diagnosed (i.e. true negatives)
Interval cancers with minimal mammographic evidence
Interval cancers with screening mammography classified as normal due to limited image quality or interpretation error (i.e. false negatives).
This type of interval cancer classification, which is particularly important as a further performance indicator for quality assurance, is currently impossible to carry out in North Rhine–Westphalia for reasons of data confidentiality. Current international publications (11) report that more than half of interval cancers should be classified as “true” in the above sense (12).
All participants identified in the MSP who had tested negative were compared with the data of the EKR NRW (as of 15 February 2012). For cases in which record linkage was reliable, the time interval (in days) was calculated on the basis of the date of negative screening and the date of diagnosis of incident breast cancer (interval cancer). Thanks to electronic reporting methods, in the EKR NRW more than 95% of all breast cancers are reported within 15 months of initial diagnosis (13). All interval cancers occurring up to 24 months after last screening in 2008 were therefore recorded.
No information regarding the numbers of women eligible for screening who moved to or from North Rhine–Westphalia is available to the EKR NRW. It was therefore not possible to use the exact length of time (in years) at risk (membership in the population covered by the EKR NRW or time to a new case of breast cancer during the interval) to calculate interval cancer rates (14). Because of this, they were calculated cumulatively, i.e. as percentages of the number of women who tested negative at screening, and divided into the intervals 1–12 months and 13–24 months after screening.
Results
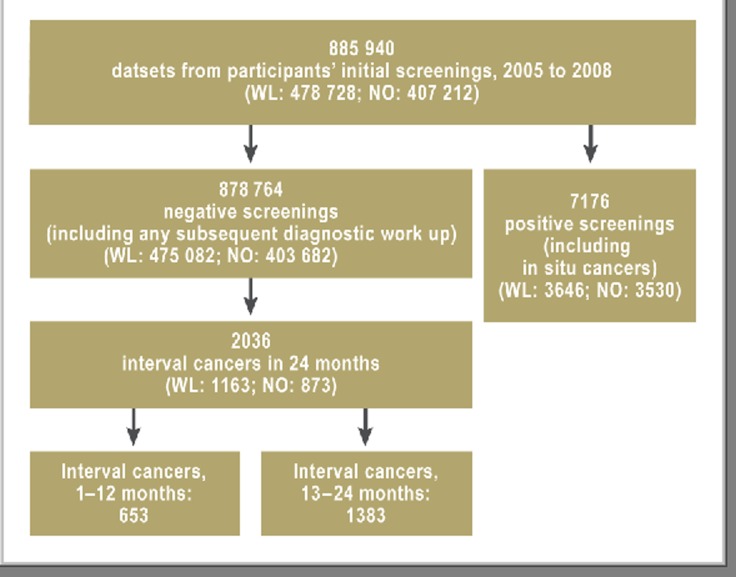
Analyses included 885 940 initial screenings in MSP participants. They had been carried out between October 2005 and December 2008 in North Rhine–Westphalia.
According to the results documented in the MaSc database, screening detected breast cancer (including ductal carcinoma in situ) in 7176 participants, and negative screening results were recorded for 878 764 women (Figure 3).
Figure 3.
Overall evaluation of first-time screening in the North Rhine–Westphalia mammography screening program, 2005 to 2008 (WL = Westphalia–Lippe; NO = North Rhine)
The breast cancers detected during the analysis period corresponded to an average detection rate of 81.0 per 10 000 screening participants. The detection rate was therefore 3.01 times higher than in the background incidence rate of 26.9 per 10 000 women (benchmark figure stated in the European Guidelines: ≥3).
Interval cancers
Within 24 months of initial screening, interval cancers occurred in 2036 women. Overall, this corresponded to an interval cancer rate (ICR) of 23.2 per 10 000 women who had tested negative at screening. The ICR for the first year was 7.4 per 10 000. For the second it was 15.7 per 10 000 (Table 1).
Table. Interval cancers (in situ plus invasive) at initial screening as part of the mammography screening program, 2005 to 2008.
| Region | Negative results at initial screening | Interval cancers, 1 to 12 months: n (per 10 000) | Interval cancers, 13 to 24 months: n (per 10 000) | Background incidence rate (invasive only) per 10 000 | Relative interval cancer rate* : 1st year/2nd year |
| WL | 475 082 | 371 (7.8) | 792 (16.7) | 26.9 | 0.29/0.62 |
| NO | 403 682 | 282 (7.0) | 591 (14.6) | 26.9 | 0.26/0.54 |
| NRW | 878 764 | 653 (7.4) | 1383 (15.7) | 26.9 | 0.27/0.58 |
WL = Westphalia–Lippe; NO = North Rhine; NRW = North Rhine–Westphalia; *[interval cancer/background incidence rate]
The statewide relative ICR (i.e. relative to the annual background incidence rate) was 0.27 for the first year and 0.58 for the second year. There were slight differences between the two regions of the state (Table 1).
The role of age in breast cancer incidence was demonstrated in the interval cancer rates of 21.0 per 10 000 in the age group 50 to 59 years and 25.8 per 10 000 in women aged between 60 and 69 years.
The number of cancers detected by the screening program as a percentage of all diagnosed breast cancers occurring in screening participants before their next screening or up to 24 months after screening was 78% (the program’s sensitivity for a two-year interval).
Tumor characteristics
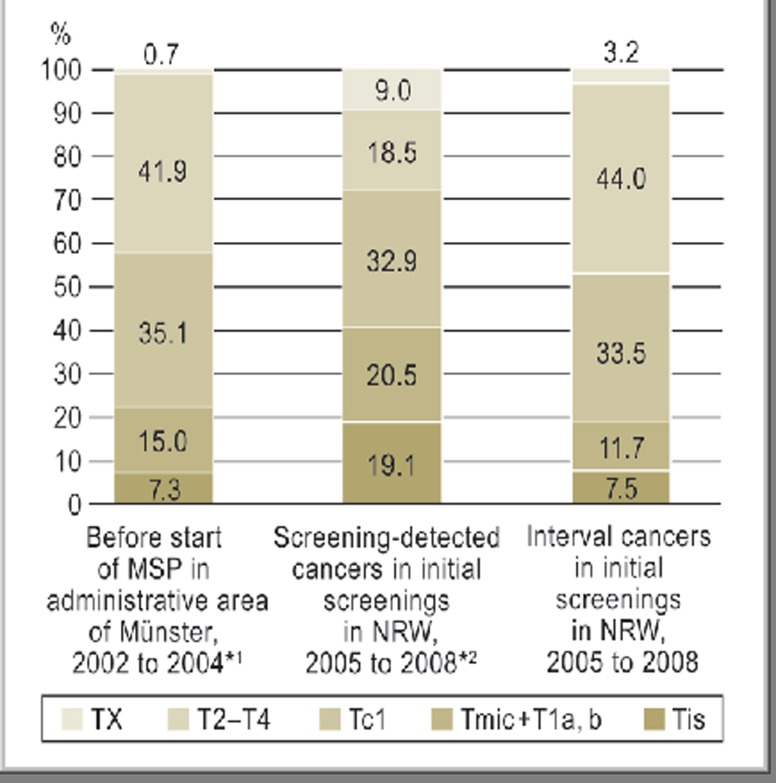
Figure 3 compares interval cancers’ T-category distribution to that of breast cancers diagnosed before the MSP was implemented (2002 to 2004) throughout the Münster district (according to [4]) and to that of breast cancers detected at screening among first-time screening participants in North Rhine–Westphalia from 2005 to 2008 (according to [5).
In situ cancers accounted for 7.5% of all interval cancers. The corresponding figure for cancers detected at screening was 19.1% (Figure 4).
Figure 4.
T-category distribution of breast cancers before implementation of the German mammography screening program (MSP) (2002 to 2004) in the administrative area of Münster, versus breast cancers in first-time screening participants in 2005 to 2008, divided into screening-detected cancers and interval cancers
In contrast, the percentage of malignant tumors of size T2 to T4 was significantly higher for interval cancers than for cancers detected at screening: 40% of all malignant tumors of size T2 to T4 diagnosed in first-time screening participants within a two-year observation period were interval cancers (Table 2).
Table 2. T-category distribution in first-time screening participants in North Rhine– Westphalia for 2005 to 2008, divided into screening-detected cancers and interval cancers (see eTable for data standardized for 10 000 participants).
| All T-categories (incl. Tx) | Tis/ T1 | T2 to T4 | |
| Total tumors (first-time participants, 2005 to 2008) | 9212 | 6276 | 2224 |
| Screening-detected cancers (%) | 7176 (78%) | 5203 (83%) | 1328 (60%) |
| Interval cancers (%) | 2036 (22%) | 1073 (17%) | 896 (40%) |
Lymph node-negative cancers accounted for 60% of invasive interval cancers (although there was no information on lymph node status in 18% of cases). They accounted for 75% of cancers detected at screening (5).
Differentiated analysis of M classification of interval cancers was not performed, as there was a high proportion of unknown M stages.
Discussion
The essential concept behind early breast cancer detection through mammography screening lies in the expectation that the number of breast cancers with fatal outcomes can be reduced (15).
In order to obtain initial evidence of the efficacy of the mammography screening program even before this expected long-term reduction in mortality occurs, surrogate endpoints are calculated to evaluate efficacy, using data from epidemiological cancer registries in accordance with the European guidelines (3). For the first time, this was done for a very large number of participants as part of the German mammography screening program for the largest German federal state, North Rhine–Westphalia, using the specific reporting structure of the EKR NRW.
Comparison with the detection rates of initial screening in other European countries (average: 60.4 per 10 000) (16) shows the detection rate of the mammography screening program implemented in North Rhine–Westphalia to be relatively high: 81.0 per 10 000 women. This corresponds to 3.01 times the background incidence rate, meeting the criteria stated in the European Guidelines (3). It should also be borne in mind that the German mammography screening program was launched later than those of other countries (October 2005), when the background breast cancer incidence rate in the target population was already high. Among other factors, this was ascribed to the widespread use of “grey screening” (intense early diagnostics outside an early detection programme). before the mammography screening program was implemented (17). Against this background it is understandable that although the detection rate at screening is high by international standards it only just meets the European guidelines’ requirement for its relationship to background incidence.
In addition, the background incidence rate mentioned above, which is based only on data for Münster, cannot simply be extrapolated to all parts of North Rhine–Westphalia, a large federal state, as it stands. This may partly explain the differences between different parts of North Rhine–Westphalia. Finally, the incidence rate of breast cancer in the target group has been falling for several years independently of screening, which is in part thought to be due to the fact that fewer postmenopausal women are taking hormone replacement therapy (18, 19); this would imply that the background rate somewhat overestimates the current risk of disease.
The absolute interval cancer rate in North Rhine–Westphalia is higher than current findings from the pilot mammography screening program in Lower Saxony (20) and the mammography screening programs in the Netherlands, the UK, and Europe as a whole (pooled data of interval cancer rates in six European countries) (11, 16, 21). However, the relative interval cancer rate in relation to the annual background incidence rate was only 27% in the first year following a negative screening result, and 58% in the second year. Corresponding figures for comparison from established European screening programs were an average of 29% and 63% respectively (16), although the European guidelines stipulate values of <30% and <50% respectively (3). It should not be overlooked that the percentage of interval cancers that are in situ tumors, which as in other European countries is relatively high, may be largely caused by the regional popularity of spontaneous or “grey” screening (16). These tests, which are not part of the screening program and may also include radiation-free ultrasound examination of the breast, have a significant effect on the detection of asymptomatic cases that would otherwise not be diagnosed until the next scheduled screening.
A high percentage, 40%, of all tumors of size T2 to T4 detected in first-time screening participants from 2005 to 2008 were interval cancers. This confirmed findings from other mammography screening programs that more than half could be classified as “true” interval cancers, meaning that interval cancers included, in particular, aggressive and possibly fast-growing breast cancers. The high percentage of tumors of size T2 to T4 among interval cancers is also in line with observations from other screening programs (22, 23) and should be further examined in subsequent rounds of the mammography screening program.
The interval cancer rate is also used to calculate an indicator known as “program sensitivity” (3). In North Rhine–Westphalia, 22% of all breast cancers occurring in screening participants during the two-year screening interval were diagnosed as interval cancers; in other words, the sensitivity of the mammography screening program was 78%. This compares to an average sensitivity of 72% (range: 67% to 84%) in European screening programs (16).
Limitations
This population-based evaluation does have some limitations: Full, complete breast cancer data in the EKR NRW were obtained sooner in Westphalia–Lippe than in North Rhine. The results have therefore been presented separately for each of these areas. The somewhat lower interval cancers rates in North Rhine may therefore have been the result of slight initial underreporting.
If individual screening participants moved away from North Rhine–Westphalia during the two-year follow-up period, this might result in underestimation of interval cancer rates. However, no registration office information that could be related to specific cases is available to the EKR NRW. Nevertheless, the official annual rate of women aged between 50 and 69 years moving away from North Rhine–Westphalia between 2005 and 2010 was only 0.58% on average (source: North Rhine–Westphalia Regional Statistics Bureau for Information and Technology [Statistisches Landesamt IT.NRW]). Such a low rate should have only a minimal effect on calculated interval cancer rates.
The interval cancer rate is a surrogate indicator for the mammography screening program’s efficacy in achieving the expected reduction in breast cancer mortality. Other factors affecting the overall benefit of the program, such as the impact of additional exposure to radiation, the psychological burden caused by an additional test for clarification with normal results, or potential overdiagnosis (24), could not be examined in this study.
Conclusion
In North Rhine–Westphalia, population-based cancer registry data have allowed reliable interval cancer rates for the German mammography screening program to be determined for the first time. They have also made a further important surrogate parameter for evaluation of the program’s efficacy available.
Interval cancer rates have been calculated on the basis of a very high number of screening participants and are therefore very accurate. Detection rates at screening, interval cancer rates, and the resulting program sensitivity all compare favorably with findings from other European countries.
Key Messages.
This study was the first to determine the interval cancer rate in the largest German federal state, North Rhine–Westphalia, for the German mammography screening program (MSP) via comparison with population-based cancer registry data.
Screening participants with negative results at initial screening within the MSP in North Rhine–Westphalia in 2005 to 2008 had an absolute interval cancer rate of 23.2 per 10 000 over the next 24 months.
40% of all breast cancers of size T2 to T4 diagnosed in first-time screening participants during the two-year period examined were interval cancers.
The relative interval cancer rate as a percentage of the annual background incidence rate was 27% in the first year and 58% in the second.
22% of all breast cancers diagnosed among all MSP participants within two years of initial screening occurred in the interval after screening.
eTable. T-category distribution in first-time screening participants in North Rhine–Westphalia (NRW) for 2005 to 2008, divided into screening-detected cancers and interval cancers and standardized for 10 000 participants.
| Screening-detected cancers in NRW among first-time participants, 2005 to 2008 (per 10000 participants) | Interval cancers in NRW among first-time participants, 2005 to 2008 (per 10000 participants) | Incident breast cancers in the administrative area of Münster, 2002 to 2004 (per 10000 women aged 50 to 69 years) | |
| Tis | 15.5 | 1.7 | 2.0 |
| Stage T1 | 43.1 | 10.4 | 13.9 |
| Stage T2 to T4 | 15.0 | 10.1 | 11.3 |
| Total (including Tx) | 81.0 | 23.0 | 29.9 |
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
Dr. Heidinger is Manager of the Epidemiological Cancer Registry North Rhine–Westphalia (Epidemiologisches Krebsregister NRW GmbH). Dipl.-Soz. Batzler and Dr. Krieg are employees of the registry.
Dr. med. Weigel is a scientific employee of the Westphalia Wilhelm University of Münster, specializing in medicine. Her duties include scientific research and teaching, for example for the Mammography Reference Centre, a third party-funded project at Münster University Hospital.
Until his death, Dr. rer. nat. Biesheuvel worked as an epidemiologist for the Mammography Reference Centre, a third party-funded project at Münster University Hospital.
Prof. Dr. med. Heindel runs the Mammography Reference Centre, a third party-funded project at Münster University Hospital. He manages other third-party funding for research projects into breast cancer screening of the EU (High Resolution X–Ray Imaging for Improved Detection and Diagnosis of Breast Cancer [HighRex], EU project contract no. 037642) and the German Federal Office for Radiation Protection (BfS, Bundesamt für Strahlenschutz).
Prof. Dr. Hense manages third-party funding of the German Federal Office for Radiation Protection (BfS, Bundesamt für Strahlenschutz) for research projects into breast cancer screening.
All authors except Dr. Krieg were/are speakers for UKM Akademie GmbH at continuing professional development events on the qualification, ongoing training, and further professional development of mammography screening program doctors and radiologists and receive payment for their work.
References
- 1.Giersiepen K, Hense HW, Klug SJ, Antes G, Zeeb H. Planning, implementation and evaluation of cancer screening programs. Z Arztl Fortbild Qualitatssich. 2007;101:43–49. doi: 10.1016/j.zgesun.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Raffle AE, Gray JAM. Evidence and practice Oxford. Oxford University: Press; 2007. Screening. [Google Scholar]
- 3.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, van Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. European Communities. (Fourth Edition) 2006 doi: 10.1093/annonc/mdm481. [DOI] [PubMed] [Google Scholar]
- 4.Weigel S, Batzler WU, Decker T, Hense HW, Heindel W. First epidemiological analysis of breast cancer incidence and tumor characteristics after implementation of population-based digital mammography screening. RoFo. 2009;18:1144–1150. doi: 10.1055/s-0028-1109831. [DOI] [PubMed] [Google Scholar]
- 5.Biesheuvel C, Weigel S, Heindel W. Mammography Screening: Evidence, history and current practice in Germany and other European Countries. Breast Care. 2011:104–119. doi: 10.1159/000327493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieg V, Hense HW, Lehnert M, Mattauch V. Record Linkage mit kryptographierten Identitätsdaten in einem bevölkerungsbezogenen Krebsregister - Entwicklung, Umsetzung und Fehlerraten. Gesundheitswesen. 2001;63:376–382. doi: 10.1055/s-2001-15686. [DOI] [PubMed] [Google Scholar]
- 7.Meyer M. Kontrollnummern und Record Linkage. In: Hentschel S, Katalinic A, editors. Das Manual der epidemiologischen Krebsregistrierung. München, Wien, New York W: Zuckerschwerdt Verlag; 2008. pp. 57–68. [Google Scholar]
- 8.Epidemiologisches Krebsregister Nordrhein-Westfalen gGmbH (ed.) Report. www.krebsregister.nrw.de. 2011.
- 9.Schmidtmann I, Hammer G, Sariyar M, Gerhold-Ay A. Abschlussbericht der Evaluation des Krebsregisters NRW Schwerpunkt Record Linkage. www.krebsregister.nrw.de/index.php?id=121 [Google Scholar]
- 10.Kooperationsgemeinschaft Mammographie (ed.) Ergebnisse des Mammographie-Screening-Programms in Deutschland. Berlin: 2012. Evaluationsbericht 2008-2009. [Google Scholar]
- 11.National Evaluation Team of Breast Cancer Screening (NETB) (ed.) Department of Public Health, Erasmus MC. 2009. National evaluation of breast cancer screenings in the Netherlands: 1990-2007 (XII); Twelfth evaluation report. Rotterdam. [Google Scholar]
- 12.Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krebserkrankungen (Krebsfrüherkennungs-Richtlinie/ KFE-RL) in der Fassung vom 16. Dezember 2010 (in Kraft getreten am 3. März 2011) www.g-ba.de. 2010.
- 13.Epidemiologisches Krebsregister Nordrhein-Westfalen gGmbH (ed.) Report. www.krebsregister.nrw.de.
- 14.Bulliard JL, Sasieni P, Klabunde C, De Landtsheer JP, Yankaskas BC, Fracheboud J. Methodological issues in international comparison of interval breast cancers. Int J Cancer. 2006;119:1158–1163. doi: 10.1002/ijc.21941. [DOI] [PubMed] [Google Scholar]
- 15.Verfahren zur Bewertung der Wirksamkeit des Deutschen Mammographie-Screening-Programms auf die Senkung der Sterblichkeit durch Brustkrebs. Stellungnahme des Wissenschaftlichen Gremiums des Beirats der Kooperationsgemeinschaft Mammographie vom. www.mammo-programm.de/fachinformationen/aktuelle-publikationen-details.php?id=15. 2011. Oct 17,
- 16.Törnberg S, Kemetli L, Ascunce N, Hofvind S, Anttila A, Seradour B, Paci E, Guldenfels C, Azavedo E, Frigerio A, Rodrigues V, Ponti A. A pooled analysis of interval cancer rates in six European countries. European Journal of Cancer Prevention. 2010;19:87–93. doi: 10.1097/CEJ.0b013e32833548ed. [DOI] [PubMed] [Google Scholar]
- 17.Giersiepen K, Haartje U, Hentschel S, Katalinic A, Kieschke J. Brustkrebsregistrierung in Deutschland: Tumorstadienverteilung in der Zielgruppe für das Mammographie-Screening. Dtsch Arztebl. 2004;101(30):2117–2121. [Google Scholar]
- 18.Katalinic A, Lemmer A, Zawinell A, Rawal R, Waldmann A. Trends in hormone therapy and breast cancer incidence - results from the German Network of Cancer Registries. Pathobiology. 2009;76:90–97. doi: 10.1159/000201677. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Decline in hormone replacement prescription and fall in breast cancer incidence - an epidemiologic discussion. Deutsches Ärzteblatt Int. 2008;105(16):303–309. doi: 10.3238/arztebl.2008.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbschat I, Kieschke J, Schlahnstedt-Jahn U, von Gehlen S, Thiel A, Jensch P. Beiträge bevölkerungsbezogener Krebsregister zur Evaluation des bundesweiten Mammographie-Screenings. Gesundheitswesen. 2005;67:448–454. doi: 10.1055/s-2005-858515. [DOI] [PubMed] [Google Scholar]
- 21.Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571–577. doi: 10.1038/bjc.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofvind S, Vacek PM, Skelly J, Weaver DL, Geller BM. Comparing screening mammography for early breast cancer detection in Vermont and Norway. J Natl Cancer Inst. 2008;100:1082–1091. doi: 10.1093/jnci/djn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofvind S, Skaane P. Stage distribution of breast cancer diagnosed before and after implementation of population-based mammographic screening. Fortschr Röntgenstr. 2012;184:437–442. doi: 10.1055/s-0031-1299352. [DOI] [PubMed] [Google Scholar]
- 24.Elmore JG, Fletcher SW. Overdiagnosis in breast cancer screening: time to tackle an underappreciated harm. Ann Intern Med. 2012;156:536–537. doi: 10.7326/0003-4819-156-7-201204030-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]