Abstract
Lysosomal storage diseases (LSDs) are a family of disorders that result from inherited gene mutations that perturb lysosomal homeostasis. LSDs mainly stem from deficiencies in lysosomal enzymes, but also in some non-enzymatic lysosomal proteins, which lead to abnormal storage of macromolecular substrates. Valuable insights into lysosome functions have emerged from research into these diseases. In addition to primary lysosomal dysfunction, cellular pathways associated with other membrane-bound organelles are perturbed in these disorders. Through selective examples, we illustrate why the term “cellular storage disorders” may be a more appropriate description of these diseases and discuss therapies that can alleviate storage and restore normal cellular function.
Lysosomal storage disorders: A brief overview
Inborn errors of metabolism are a common cause of inherited disease (Burton, 1998), of which lysosomal storage diseases (LSDs) are a significant subgroup (Platt and Walkley, 2004; Fuller et al., 2006; Ballabio and Gieselmann, 2009). The combined incidence of LSDs is estimated to be approximately 1:5,000 live births (Fuller et al., 2006), but the true figure is likely greater when undiagnosed or misdiagnosed cases are accounted for. Common to all LSDs is the initial accumulation of specific macromolecules or monomeric compounds inside organelles of the endosomal–autophagic–lysosomal system. Initial biochemical characterization of stored macromolecules in these disorders led to the implication of defective lysosomal enzymes as a common cause of pathogenesis (Hers, 1963; Winchester, 2004). Although most LSDs result from acidic hydrolase deficiencies (Winchester, 2004), a considerable number of these conditions result from defects in lysosomal membrane proteins or non-enzymatic soluble lysosomal proteins (Saftig and Klumperman, 2009). Therefore, LSDs offer a window into the normal functions of both enzymatic and non-enzymatic lysosomal proteins.
Clinical phenotypes of LSDs
The age of clinical onset and spectrum of symptoms exhibited amongst different LSDs vary, depending on the degree of protein function affected by specific mutations, the biochemistry of the stored material, and the cell types where storage occurs. Apart from lysosomal diseases involving substrate storage in bone and cartilage (e.g., the mucopolysaccharidoses; Table 1) most babies born with these conditions appear normal at birth. The classical clinical presentation of an LSD is a neurodegenerative disease of infancy/childhood (Wraith, 2002), but adult-onset variants also occur (Spada et al., 2006; Nixon et al., 2008; Shapiro et al., 2008). A health surveillance program tasked with diagnosing all neurodegenerative disease cases in UK children has so far revealed that lysosomal disorders are amongst the most commonly confirmed diagnoses of neurodegeneration (45% of cases) and will provide a robust frequency of infantile/juvenile onset cases as the study gathers more data over the coming years (Verity et al., 2010). Key molecular and clinical features of the storage diseases mentioned in this review are summarized in Table 1. In addition, detailed medical descriptions on the various disorders are available on the Online Metabolic and Molecular Bases of Inherited Disease (OMMBID) website (Valle et al., 2012).
Table 1.
The causes of lysosomal storage diseases, the organelles affected, and major sites of pathology
| Mechanism of lysosomal storage | Disease examples | Lysosomal protein defect (gene symbol) | Substrate(s) stored | Major peripheral organ systems affected | CNS pathology |
| Lysosomal enzyme deficiencies | Aspartylglucosaminuria | Aspartylglucosaminidase (glycosylasparaginase, AGA) | aspartylglucosamine (N-acetylglucosaminyl-asparagine) | Skeleton, connective tissue | + |
| Fabry | α-Galactosidase (GLA) | (Lyso-)Globotriaosylceramide | Kidney, heart | − | |
| Gaucher types 1, 2, and 3 | β-Glucocerebrosidase (GBA) | Glucosylceramide, glucosylsphingosine | Spleen/liver, bone marrow | +a | |
| GM1-gangliosidosis | β-Galactosidase (GLB1) | GM1-ganglioside, oligosaccharides | Skeleton, heart | + | |
| Krabbe (globoid cell leukodystrophy) | Galactocerebrosidase (GALC) | Galactosylceramide | Heart | + | |
| Metachromatic leukodystrophy | Arylsulfatase A (ARSA) | Sulfogalactosylceramide | + | ||
| Mucopolysaccharidoses | Enzymes involve in mucopolysaccharide catabolism | Mucopolysaccharides | Cartilage, bone, heart, lungs | +b | |
| Multiple sulfatase deficiency | SUMF1 (Formylglycine-generating enzyme needed to activate sulfatases) | Multiple, including sulfated glycosaminoglycans | Spleen/liver, bone, skin | + | |
| Pompe | α-Glucosidase (GAA) | Glycogen | Skeletal muscle | − | |
| Sandhoff | β-hexosaminidase A and B (HEXB) | GM2-ganglioside | + | ||
| Trafficking defect of lysososomal enzymes | Mucolipidosis type II (I-cell disease) | N-acetyl glucosamine phosphoryl transferase α/β (GNPTAB) | Carbohydrates, lipids, proteins | Skeleton, heart | + |
| Mucolipidosis type IIIA (pseudo-Hurler polydystrophy) | N-acetyl glucosamine phosphoryl transferase α/β (GNPTAB) | Carbohydrates, lipids, proteins | Skeleton, heart | +/− | |
| Defects in soluble non-enzymatic lysosomal proteins | Niemann-Pick disease type C2 | NPC2 (soluble cholesterol binding protein) | Cholesterol and sphingolipids | Liver | + |
| Defects in lysosomal membrane proteins | Cystinosis | Cystinosin (cysteine transporter, CTNS) | Cystine | Kidney, eye | − |
| Danon disease | Lysosomal-associated membrane protein 2, splicing variant A (LAMP2) | Glycogen and other autophagic components | Cardiac and skeletal muscle | + | |
| Free sialic acid storage disorder | Sialin (sialic acid transporter, SLC17A5) | Free sialic acid | Liver/spleen, skeleton | + | |
| Mucolipidosis IV | Mucolipin-I (MCOLN1) | Mucopolysaccharides and lipids | Eye | + | |
| Niemann-Pick disease type C1 | NPC1 (membrane protein involved in lipid transport) | Cholesterol and sphingolipids | Liver | + | |
| Enigmatic lysosomal disorders | Neuronal ceroid lipofuscinoses (NCLs, including Batten disease) | Disparate group of diseases with genetic defects in apparently unrelated genes, not all of which are associated with the lysosomal system. Not known if these genes cooperate in common cellular pathways. | Autofluorescent lipofuscin is a common feature, with convergent clinical signs, e.g., visual system defects/blindness | + |
Listed are the diseases discussed in the main text. Mucopolysaccharidoses and neuronal ceroid lipofuscinoses refer to collections of related disorders.
Types 2 and 3.
Most mucopolysaccharidosis disorders.
Relatively few lysosomal diseases lack pathology in the central nervous system (CNS; Wraith, 2004). In the majority of LSDs, CNS involvement is common and neurodegeneration can occur in multiple brain regions (e.g., thalamus, cortex, hippocampus, and cerebellum). Neuropathology in LSDs involves unique temporal and spatial changes, which often entails early region-specific neurodegeneration and inflammation, before global brain regions are affected. The main reasons for this are threefold: (1) specific storage metabolites exert differential effects on neuronal subtypes, (2) varying proportions of macromolecules are synthesized in different neuronal populations, and (3) there is differential neuronal vulnerability to storage (e.g., Purkinje neurons degenerate in many of these diseases leading to cerebellar ataxia). Activation of the innate immune system is also prevalent in the brain of LSDs, which directly contributes to CNS pathology (Vitner et al., 2010). Astrogliosis (activation of astrocytes) is another common feature of LSDs, which damages neurons through an inflammatory process known as glial scarring (Jesionek-Kupnicka et al., 1997; Vitner et al., 2010). The additive detrimental effects that astrogliosis has on neuron function is recapitulated in animal models of lysosomal diseases (Farfel-Becker et al., 2011; Pressey et al., 2012).
A notable non-neuronopathic LSD is Type 1 Gaucher disease (β-glucocerebrosidase deficiency), which is a relatively common LSD, particularly within the Ashkenazi Jewish community. The major cell type affected by glucosylceramide storage in this disease is the macrophage (“Gaucher cells”), whose dysfunction affects the production and turnover of cells belonging to the hematopoietic system. Gaucher cells infiltrate into various organs and affect the immune system, bone strength, spleen, and liver function.
A key question currently challenging this field is how endosomal–lysosomal storage leads to pathogenesis and how expanding this knowledge will improve treatment for patients (Bellettato and Scarpa, 2010; Cox and Cachón-González, 2012). This review aims to delineate regulatory systems and organelles that become disrupted in these disorders, highlighting the complexity of cellular storage, its consequences on pathogenesis, and implications for therapy.
Endosomal–autophagic–lysosomal function and dysfunction in storage diseases
Lysosomes play a central role in processing the clearance of cellular substrates from multiple routes within the endosomal–autophagic–lysosomal system (Fig. 1). Lysosomes are acidic organelles that contain enzymes required for the degradation of macromolecules, and efflux permeases that facilitate the inside-out translocation of small molecules generated through macromolecule catabolism. In comparison to endosomes and autophagosomes, lysosomes are smaller in size, are highly enriched in particular transmembrane proteins and hydrolytic enzymes (including proteases, glycosidases, nucleases, phosphatases, and lipases), have a higher buoyant density, an electron-dense appearance by transmission electron microscopy, and a high proton and Ca2+ content (Luzio et al., 2007; Saftig and Klumperman, 2009; Morgan et al., 2011). Lysosomes differ from endosomes in their degree of acidification and more abundant levels of lysosomal membrane proteins (LMPs) such as LAMP1 and LAMP2. Most nascent lysosomal enzymes bind to mannose-6-phosphate receptors (M6PRs) in the trans-Golgi network (TGN), which traffic the enzymes to early and late endosomes (Ghosh et al., 2003). Lysosomes in turn receive these enzymes when endosomal–lysosomal fusion occurs. Notably, dense lysosomes do not contain M6PRs. Acidotropic reagents such as Lysotracker are useful for labeling lysosomes; however, the mildly acidic interiors of late endosomes and autophagosomes also allows Lysotracker to label these organelles to varying degrees (Bampton et al., 2005).
Figure 1.
Lysosomes as catabolic centers of the cell. Lysosomes utilize four distinct pathways for the degradation of cellular material. (A) Macroautophagy begins with the formation of isolation membranes that sequester regions of the cytosol that include denatured proteins, lipids, carbohydrates, and old/damaged organelles into encapsulated vesicles known as autophagosomes. The dynamic kinetics of autophagosome production and clearance by lysosomes is known as autophagic flux. (B) Endosomal degradation by lysosomes predominantly targets late endosomes/multivesicular bodies. Fusion between late endosomes and lysosomes can occur by (i) full fusion/degradation or (ii) kiss-and-run content mixing, where transient endosomal docking occurs. (C) Microautophagy involves the pinocytosis of cytosolic regions surrounding lysosomes. (D) Chaperone-mediated autophagy (CMA) selectively targets proteins with a KFERQ motif for delivery to lysosomes using Hsc-70 as its chaperone and LAMP-2A as its receptor.
The biogenesis and functioning of endosomal and autophagosomal pathways is controlled by transcription factor EB (TFEB), which regulates the expression of 471 genes that constitute the CLEAR (coordinated lysosomal expression and regulation) gene network (Sardiello et al., 2009; Palmieri et al., 2011). Recent work indicates that non-active TFEB is highly phosphorylated and associates with late endosomes/lysosomes (Roczniak-Ferguson et al., 2011). Autophagy-inducing conditions (e.g., deprivation of glucose or amino acids) result in reduced and altered TFEB phosphorylation, leading to its translocation into the nucleus (Peña-Llopis et al., 2011) and transcriptional expression of CLEAR genes (Palmieri et al., 2011).
Degradation of endosomal and autophagosomal material takes place upon exchange of content (via transient “kiss-and-run” contacts) or fusion with lysosomes, forming endolysosomes (Tjelle et al., 1996; Bright et al., 1997, 2005; Mullock et al., 1998) and autolysosomes (Jahreiss et al., 2008; Fader and Colombo, 2009; Orsi et al., 2010), respectively (Fig. 1, A and B). Lysosomes can be regarded as storage compartments for acidic hydrolases that enter cycles of fusion and fission with late endosomes and autophagosomes, while the digestion of endocytosed and autophagic substrates takes place primarily in endolysosomes and autolysosomes (Tjelle et al., 1996; Luzio et al., 2007). Under physiological conditions, endolysosomes and autolysosomes are transient organelles.
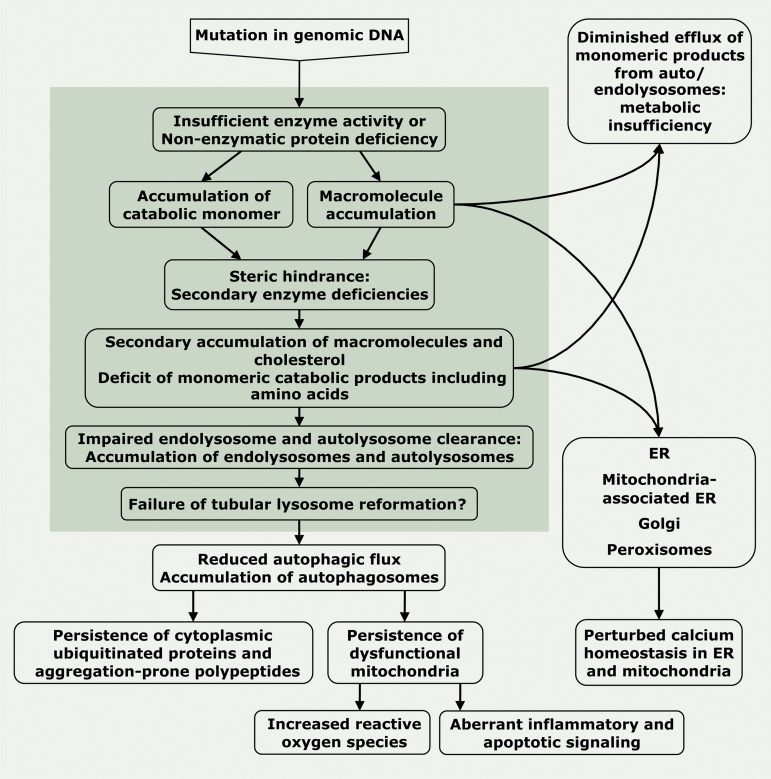
Cells deficient in lysosomal hydrolytic enzymes, lysosomal membrane proteins, or non-enzymatic soluble lysosomal proteins accumulate excessive levels of undegraded macromolecules (enzyme deficiency) or monomeric catabolic products (efflux permease deficiency) and contain numerous endo/autolysosomes (Fig. 2). When very high levels of macromolecules/monomers accumulate in endo/autolysosomes, they inhibit catabolic enzymes and permeases that are not genetically deficient, which results in secondary substrate accumulation (Walkley and Vanier, 2009; Lamanna et al., 2011; Prinetti et al., 2011). For example, lysosomal proteolytic capacity is reduced in fibroblasts from various LSDs, such as mucopolysaccharidoses I and VI, and GM1-gangliosidosis, which are themselves not caused by protease deficiency (Kopitz et al., 1993). The accumulation of primary and secondary substrates sets off a cascade of events that impacts not only the endosomal–autophagic–lysosomal system, but also other organelles, including mitochondria, the ER, Golgi, peroxisomes (Fig. 3), and overall cell function (Fig. 4).
Figure 2.

Subtypes of storage organelles accumulate in LSDs. In different LSDs, cells display a unique spectrum of dysfunctional organelles depending on the specific lysosomal enzyme or non-enzymatic protein affected. (A) In primary LSDs, deficiencies in degradative enzymes prevent the clearance of autophagic and endocytic substrates, resulting in the accumulation of (i) autolysosomes (LC3-II (+), LAMP-1 (+)), (ii) endolysosomes (CI-MPR (+), LAMP-1 (+)), and (iii), in the case of certain lipase deficiencies, lipid-rich multilamellar bodies (CI-MPR (+), LAMP-1 (+)). (B) In a secondary storage disease such as Niemann-Pick type C1, lysosomal enzyme function remains intact, but impaired heterotypic fusion of autophagic and endocytic organelles with lysosomes results in the accumulation of (iv) autophagosomes (LC3-II (+), LAMP-1 (−)), (v) late endosomes (CI-MPR (+), active cathepsin D (−)), and (vi) endosome-derived multilamellar bodies (lipid-rich, CI-MPR (+), active cathepsin D (−)). Note: many primary storage diseases also accumulate organelles seen in secondary storage diseases (see text).
Figure 3.

Summary of organelles affected in LSDs. Also shown are selective examples of LSDs. See Table 1 and main text for details.
Figure 4.
Hypothetical cascade of events in LSD pathology. How gene mutations in lysosomal enzymes and non-enzymatic lysosomal proteins could lead to LSDs. Endo/autolysosomal events are confined to the darker shaded background, whereas processes taking place in the cytoplasm that affect autophagosomes, the ER, Golgi, peroxisomes, and mitochondria are on the lighter background. Processes depicted have been observed in a number of LSDs but do not necessarily apply to all LSDs.
Autophagic pathways.
The autophagic (“self-eating”) pathway constitutively targets intracellular cytosolic components for lysosomal degradation, and is essential for maintaining cellular energy and metabolic homeostasis (Kuma and Mizushima, 2010; Singh and Cuervo, 2011). To date, three distinct forms of autophagy have been characterized: macroautophagy, microautophagy, and chaperone-mediated autophagy (Fig. 1, A, C, and D). All three autophagic processes culminate in lysosomal degradation; however, routes taken by substrates to the lysosome differ between each form. Macroautophagy involves the bulk sequestration of cytosolic regions into double- or multi-membrane bound autophagosomes, which are trafficked to lysosomes for content digestion (Fig. 1 A). A diverse range of cellular material is degraded via macroautophagy, including lipids, carbohydrates and polyubiquitinated proteins, RNA, mitochondria, and fragments of the ER (Eskelinen and Saftig, 2009). The most characterized protein associated with autophagosomes is the lipidated (phosphatidylethanolamine) form of microtubule-associated protein light chain 3 (MAP-LC3), known as LC3-II, which is generated early in the autophagic process but degraded in the final phase of autophagic digestion.
Autophagic flux (the rate at which autophagic vacuoles are processed by lysosomes) is reduced in most LSDs (Ballabio, 2009; Ballabio and Gieselmann, 2009; Raben et al., 2009). This is evident from the combined elevation of autophagic substrates and autophagosome-associated LC3-II. LSD cells often display increased numbers of LC3(+) organelles, of which only a subgroup carry lysosomal markers, suggesting that both autophagosomes and autolysosomes persist in these conditions. For example, in mouse models of Batten disease (a neuronal ceroid lipofuscinosis [NCL] disorder; Table 1), most LC3-positive compartments are not positive for LAMP1 (Koike et al., 2005), and in multiple sulfatase deficiency and juvenile neuronal ceroid lipofuscinosis, LC3 and LAMP1 are predominantly localized in separate organelles, which is even more pronounced after starvation (Cao et al., 2006; Settembre et al., 2008). Endosome–lysosome and autophagosome–lysosome fusion is also impaired in mucolipidosis type IIIA and multiple sulfatase-deficient mouse embryonic fibroblasts (Fraldi et al., 2010).
Microautophagy does not involve de novo synthesis of nascent vacuoles, but rather occurs via the direct pinocytosis of cytosolic material by lysosomes (Fig. 1 C). The membrane dynamics regulating microautophagy are similar to those involved in the formation of intra-luminal vesicles (ILVs) found in multivesicular bodies/late endosomes (Sahu et al., 2011). Currently, little is known about the repercussions of lysosomal storage on microautophagy, but this process appears to be impaired in primary myoblasts from patients with the muscle-wasting condition Pompe disease (Takikita et al., 2009).
Chaperone-mediated autophagy (CMA) is a selective form of autophagic proteolysis that targets proteins containing a KFERQ motif for degradation (Dice et al., 1990; Cuervo and Dice, 2000). The eponymous chaperone that recognizes and binds to proteins destined for CMA is the heat shock cognate protein of 70 kD (Hsc70). Substrate-bound Hsc70 docks on lysosomes via contact with lysosomal-associated membrane protein 2A (LAMP-2A), allowing entry of proteins into lysosomes (Fig. 1 D). Mutations in LAMP-2A cause Danon disease, and specifically affect CMA (Eskelinen et al., 2003; Fidziańska et al., 2007). CMA is also known to be impaired in mucolipidosis IV, where mutations in transient receptor potential mucolipin-1 (MCOLN1) lead to reduced amounts of LAMP-2A and substrate uptake into lysosomes (Venugopal et al., 2009).
Lysosome reformation.
Both endolysosomes and autolysosomes extend tubular structures where lysosomal hydrolases and LMPs concentrate (Tjelle et al., 1996; Bright et al., 1997, 2005; Pryor et al., 2000; Yu et al., 2010). At the ends of these tubules, [LC3(−), LAMP1(+)] vesicles bud off and acidify, maturing into dense lysosomes, a fission process referred to as lysosome reformation. This event completes each cycle of endocytic and autophagic degradation, yielding dense lysosomes that are available to fuse with newly generated endosomes and autophagosomes.
Efficient processing of endo/autolysosomal substrates is essential for lysosome reformation. This is well illustrated in a study that monitored exogenous sucrose metabolism in rat kidney fibroblasts (Bright et al., 1997). Sucrose is a disaccharide composed of the monosaccharides glucose and fructose, and is itself indigestible by cells. In this study, sucrose-filled endosomes fused with lysosomes and formed large endolysosomes, which accumulated in the cytosol. A depletion of dense-core lysosomes was seen under these conditions; however, dissolution of the accumulated sucrose by uptake of exogenous invertase resulted in the reappearance of dense-core lysosomes. This study and another more recent one from Yu et al. (2010) indicate that lysosome biogenesis does not occur de novo, but is rather born out of a reformation/budding from endolysosomes. Lysosome reformation appears to be defective in sialic acid storage disease as skin fibroblasts from diseased individuals lack dense lysosomes, while lysosomal enzymes persist in intermediate or light organelles (Schmid et al., 1999).
Interestingly, impairment of lysosome reformation appears to be the primary cellular defect in Niemann-Pick type C2 (NPC2)-deficient cells, indicating that the NPC2 protein has a crucial role in this process (Goldman and Krise, 2010). Considering that NPC1 and NPC2 deficiencies have the same pathological consequences (Niemann-Pick type C disease; Table 1), this suggests that lysosome reformation is as essential as endosome/autophagosome–lysosome fusion, which is impaired in NPC1-deficient cells.
Recent reports have provided a mechanistic link between the failure of endo/autolysosomal clearance and the deficit of lysosome reformation. Central to this pathway is mTOR, a serine/threonine kinase that has an overarching role in coordinating cellular metabolism with nutritional status (Laplante and Sabatini, 2012). During the course of the autophagic process, mTOR goes through a cycle of phosphorylation-dependent inactivation and reactivation, with the latter being required for autophagic lysosome reformation (Yu et al., 2010). In turn, mTOR reactivation depends on the completion of autolysosomal substrate digestion, and sufficient levels of luminal amino acids (Zoncu et al., 2011). Limited information is currently available on the extent of lysosome reformation and mTOR reactivation in LSDs. However, inadequate autolysosomal degradation may preclude mTOR reactivation and, hence, also impede lysosome reformation, leaving affected cells deprived of dense lysosomes. Consequently, in addition to stalled autolysosomes, autophagosomes may persist due to a deficiency of dense lysosomes, explaining the low level of colocalization of autophagosomal and lysosomal markers. mTOR activity is reduced in the brain of a mouse model of juvenile neuronal ceroid lipofuscinosis (Cao et al., 2006), in fibroblasts from mucopolysaccharidosis type I S, Fabry disease and aspartylglucosaminuria subjected to starvation-induced autophagy (Yu et al., 2010), in NPC1- and NPC2-knockdown human umbilical vein endothelial cells (Xu et al., 2010), and in MCOLN1-deficient Drosophila pupae (Wong et al., 2012), but not in brain samples from Sandhoff, GM1-gangliosidosis, and NPC1 mice (Boland et al., 2010). Considering the myriad of cellular signaling pathways that mTOR is involved in (Laplante and Sabatini, 2012), it may be necessary to differentiate mTOR activity in affected cell populations of different brain regions. In addition, electron microscopy remains a powerful tool for the ultrastructural classification of autophagosomes and autolysosomes in LSD cells, and could also be used to monitor the extent of lysosome reformation.
Mitochondrial dysfunction and cytoplasmic protein aggregation.
In LSDs, a reduction of autophagic flux has a major impact on mitochondrial function and on cytoplasmic proteostasis. Constitutive macroautophagy maintains mitochondrial quality by selectively degrading dysfunctional mitochondria via a process known as mitophagy (Kim et al., 2007). Mitochondrial proteins are consistently found in the proteomes of highly purified autolysosomes, especially subunits of the mitochondrial ATPase (Schröder et al., 2010). Reduced autophagic flux in LSDs leads to the persistence of dysfunctional mitochondria, which is highly pronounced in Batten’s disease neurons (Ezaki et al., 1996). Several LSDs (mucolipidosis types IV, IIIA [pseudo-Hurler polydystrophy], and II [I-cell disease], late infantile neuronal ceroid lipifuscinosis [CLN2], mucopolysaccharidosis VI, and GM1 gangliosidosis) display mitochondrial abnormalities, including replacement of the extended filamentous mitochondrial network with high numbers of relatively short mitochondria, and loss of mitochondrial calcium-buffering capacity and membrane potential (Jennings et al., 2006; Settembre et al., 2008; Takamura et al., 2008; Tessitore et al., 2009). Studies into aging and autophagosome formation have shown that mitochondria are involved in signaling pathways regulating apoptosis and innate immunity, and that reduced autophagic flux and subsequent accumulation of dysfunctional, reactive oxygen species–generating mitochondria renders cells more sensitive to apoptotic and inflammatory stimuli (Terman et al., 2010; Green et al., 2011; Nakahira et al., 2011; Zhou et al., 2011). Therefore, the aberrant functioning of mitochondria may be responsible for apoptosis and inflammation in the CNS of multiple LSDs.
In addition, a lack of autophagy completion in LSDs leads to the persistence of ubiquitinated and aggregate-prone polypeptides in the cytoplasm, including p62/SQSTM1, α-synuclein, and Huntingtin protein (Ravikumar et al., 2002; Suzuki et al., 2007; Settembre et al., 2008; Tessitore et al., 2009). Alpha-synuclein itself contributes to neurodegeneration by reducing the efficiency of autophagosome formation (Winslow et al., 2010), and is also a main component of Lewy bodies that are notably elevated in Parkinson’s disease and other forms of dementia. Diminished quality control of cytosolic proteins may thus also contribute to LSD pathology.
Impairment of autophagy and escalation of cytoplasmic protein aggregation are shared between neurodegenerative LSDs and more common neurodegenerative disorders, such as Alzheimer’s, Parkinson’s, Huntington’s disease, and amyotrophic lateral sclerosis (ALS; García-Arencibia et al., 2010; Wong and Cuervo, 2010). Mutations in presenilin-1, which cause a familial form of Alzheimer’s disease, is known to impair lysosomal clearance of autophagosomes (Esselens et al., 2004; Wilson et al., 2004; J.H. Lee et al., 2010). Different mechanisms have been proposed to explain how the partial loss of presenilin function impairs autophagic flux. Reports from J.H. Lee et al. (2010) indicate that presenilin 1 is need for the glycosylation and subsequent delivery of V0a1 protein to lysosomes, where it forms a subunit of lysosomal v-ATPase. This in turn is thought to impair lysosomal proteolysis by raising their pH above an optimal acidity of pH4–5. Alternatively, another recent report has indicated that mutations in presenilin 1 lead to a loss of lysosomal calcium regulation, which in turn affects fusion and clearance of autophagosomes (Coen et al., 2012). However, considering both groups confirmed that presenilin 1 mutations affect autophagic flux, Alzheimer’s disease is beginning to emerge as a neurodegenerative disorder that may share similarities in terms of underlying pathogenic mechanisms with lysosomal storage disorders.
Efflux of molecules from endo/autolysosomes.
Some storage molecules in LSDs (glycoconjugates, amino acids, or insoluble lipids) escape from cells and can be detected in blood and/or urine, which can be utilized for diagnostic purposes (Meikle et al., 2004). While glycoconjugates derived from storage cells in multiple tissues could escape as solutes in blood and urine, lipids extracted from urine are believed to be membrane associated and predominantly exosomal (Pisitkun et al., 2004).
At the cellular level, a big question that remains to be resolved concerns the way in which storage molecules escape the lysosomal system and affect the function of other organelles and cellular systems (Elleder, 2006). Theoretically, lipids can undergo redistribution within cells via membrane trafficking, fusion, or via altered trafficking pathways characteristic of these diseases (Chen et al., 1999). Endolysosomal macromolecules may also be disseminated via membrane contact sites between endolysosomes and the ER (Eden et al., 2010; Toulmay and Prinz, 2011), and by extracellular secretion of endolysosomal content, including exosome release. For example, primary kidney cells from arylsulfatase A–deficient mice secrete the accumulating lipid (sulfogalactosylceramide) into the culture medium (Klein et al., 2005), and NPC1-deficient cells release higher amounts of cholesterol-rich exosomes (Chen et al., 2010; Strauss et al., 2010). Accordingly, the possibility needs to be considered that exosomes containing storage molecules are taken up by recipient cells, and that these macromolecules and lipids affect recipient cell function by distributing to the plasma membrane and other organelles outside the endolysosomal system (Simons and Raposo, 2009).
Due to the extraordinarily high levels of lipids in the endo/autolysosomal system, even a minor redistribution to other cellular membranes could have functional implications. Over the past few years, multiple examples have emerged suggesting that this not only occurs but can actively contribute to the pathogenic cascade (Vitner et al., 2010). A key challenge is to demonstrate experimentally that particular storage macromolecules are indeed ectopically present in the membrane of other organelles. This is technically challenging due to the limitations of conventional cell fractionation techniques. Currently, the presence of storage components in non-lysosomal sites is either inferred indirectly or evidence has been provided by immunostaining methods. To date, the best examples come from studying the effects of lipid storage in the ER (Sano et al., 2009; Futerman, 2010).
Lysosomal calcium homeostasis.
Endosomes and lysosomes are regulated calcium stores (Morgan et al., 2011) that release calcium in response to the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP; Churchill et al., 2002). NPC1 disease is unusual in having a profound block in late endosome–lysosome fusion (Kaufmann et al., 2009; Goldman and Krise, 2010), a process known to be calcium dependent (Lloyd-Evans et al., 2008). In NPC1 patient cells and cultured cells deficient in NPC1 protein, calcium levels within acidic organelles are approximately 30% of wild-type cells (Lloyd-Evans et al., 2008; H. Lee et al., 2010). NPC1 cells do respond to NAADP, but, due to the reduced luminal calcium levels, release less calcium, thus leading to the fusion deficiency associated with this disorder (Lloyd-Evans et al., 2008). Therefore, NPC1 disease demonstrates that acidic calcium stores play a central role in the regulation of fusion and trafficking within the endocytic system itself (Morgan et al., 2011).
Endoplasmic reticulum defects.
In addition to the endoplasmic reticulum (ER) being the major site of the secretory pathway responsible for protein folding/quality control and N-glycosylation, it is also a regulated calcium store. The lipid and protein content of the ER is tightly regulated to maintain its essential quality-control functions. Surprisingly, very few examples of ER stress (e.g., unfolded protein response) have been reported among LSDs, with GM1 gangliosidosis being the only sphingolipid storage disorder in which this has been demonstrated to date (Tessitore et al., 2004; Sano et al., 2009; Vitner et al., 2010). Instead, the major impact in lipid storage disorders is on ER calcium regulation (Futerman and van Meer, 2004; Futerman, 2010). ER calcium homeostasis is perturbed in the sphingolipid storage disorders, Gaucher disease, GM1 and GM2 gangliosidoses, and Niemann-Pick type A (Ginzburg and Futerman, 2005), leading to elevated cytosolic calcium. In these diseases, the characteristic lipids being stored, glucosylceramide, GM1 and GM2 ganglioside, and sphingomyelin, respectively, may hypothetically escape from endolysosomes and affect ER calcium channel function. Interestingly, the mechanisms leading to defective ER calcium homeostasis are specific to each disorder and have recently been reviewed (Vitner et al., 2010). In turn, aberrant ER calcium regulation may impact mitochondria through ER–mitochondria contact sites, resulting in mitochondrial calcium excess and an induction of mitochondria-mediated apoptosis, as seen in GM1 gangliosidosis (Sano et al., 2009).
The Golgi.
Dysfunction of the Golgi is a common feature of many lipid storage disorders, and has traditionally been thought to arise from alterations in sphingolipid trafficking from the Golgi to the lysosome (Pagano et al., 2000). However, recently Golgi involvement has been demonstrated in mucopolysaccharidosis IIIB (Sanfillipo B syndrome; Vitry et al., 2010). Surprisingly, this study did not find any evidence that the endocytic and autophagic pathways were affected in Sanfillipo B syndrome; instead, they noticed that large storage bodies were enriched in the Golgi matrix protein, GM130, which is required for vesicle tethering in pre- and cis-Golgi compartments. Furthermore, the morphology of the Golgi apparatus was altered in cells with distended cisternae connected to LAMP1-postive storage bodies. This study therefore suggests that Golgi biogenesis may be affected in this disease and further studies will shed light on the molecular mechanisms that underpin Golgi involvement in this neurodegenerative disorder.
Peroxisomes.
There are reports of peroxisomal dysfunction occurring in some lipid lysosomal storage diseases, including Krabbe (globoid cell leukodystrophy; Haq et al., 2006) and NPC1 disease (Schedin et al., 1997). In Krabbe disease, the major storage lipid galactosylceramide is converted into its lysosomal metabolite, galactosylsphingosine, which down-regulates the peroxisome proliferator–activated receptor-α (PPAR-α). Loss of PPAR-α and subsequent cell death can be prevented using an inhibitor of secretory phospholipase A2, suggesting a novel therapeutic approach for Krabbe disease (Haq et al., 2006). In the NPC1 disease mouse model, peroxisomes appear normal at the ultrastructural level but have decreased peroxisomal β oxidation of fatty acids and catalase activity, which is an early event in disease pathogenesis (Schedin et al., 1997). In peroxisomal biogenesis disorders such as Zellweger syndrome and infantile Refsum disease, a-series gangliosides (e.g., GM1, GM2) and their precursor GM3 ganglioside are stored. As these gangliosides are common secondary storage metabolites in many LSDs, this raises the possibility that peroxisomal dysfunction underpins secondary ganglioside storage in LSDs and merits systematic study to test this hypothesis. How peroxisomal function affects ganglioside metabolism remains unknown but may be part of a broader lipid regulatory network in mammalian cells.
Cellular metabolic stress.
Considering that both endocytic and autophagic pathways are essential for maintaining cellular metabolic homeostasis, the diminished efflux of monomeric products from endo/autolysosomes is likely to induce a state of metabolic insufficiency, where key catabolic intermediates are unavailable to enter a variety of metabolic recycling pathways (Schwarzmann and Sandhoff, 1990; Walkley, 2007). For example, in some cell types, the majority of nascent glycosphingolipids are synthesized from endolysosome-derived sphingoid bases derived from ceramide catabolism (Tettamanti, 2004; Kitatani et al., 2008). Multiple endolysosomal exoglycosidases, including glucocerebrosidase, which is deficient in Gaucher disease, are involved in this process (Kitatani et al., 2009). The lack of reutilized sphingolipids/fatty acids that normally result from endolysosomal degradation would place such cells under significant metabolic stress. This may also apply to NPC disease, which is a particularly complex and enigmatic storage disease caused by mutations in either the NPC1 or NPC2 genes, with resulting storage of several lipids species including cholesterol and various sphingolipids (Lloyd-Evans and Platt, 2010). The NPC1 protein is an integral membrane protein of late endosomes that may function to efflux sphingosine (protonated at acidic pH) out of endolysosomes and into the sphingolipid salvage pathway or undergo phosphorylation to sphingosine-1-phosphate (S1P), raising the possibility that S1P deficiency contributes to NPC1 disease pathogenesis (Lloyd-Evans et al., 2008; Lloyd-Evans and Platt, 2010).
Therapeutic implications
Over the past two decades there has been a remarkable expansion in the number of therapeutic strategies for LSDs that target different cellular organelles (Table 2). The first treatment that led to a licensed commercial product was enzyme replacement therapy (ERT) for type 1 Gaucher disease. The discoveries leading to that seminal therapeutic advance were recently reviewed by Roscoe Brady, who pioneered this approach (Brady, 2010). This therapy “replaces” the defective enzyme in the lysosome by delivering a fully functional wild-type enzyme that is endocytosed into macrophages via the macrophage mannose receptor. Wild-type glucocerebrosidase was initially purified from human placenta (now recombinant products are used) and typically given to patients every two weeks by intravenous infusion (Charrow, 2009). This strategy leads to a remarkable degree of therapeutic benefit and has transformed the lives of patients with this debilitating peripheral storage disease (Charrow, 2009). This success catalyzed the development of ERT for Fabry disease (Schiffmann and Brady, 2006; Angelini and Semplicini, 2012), Pompe disease (Angelini and Semplicini, 2012), and several of the mucopolysaccharide storage disorders (Kakkis, 2002). However, the clinical limitations of ERT are two-fold. First, product delivery is invasive and time-consuming to deliver, and second, lysosomal enzymes do not cross the blood–brain barrier to any significant extent, so cannot effectively treat CNS disease, which is characteristic of most LSDs. To circumvent this problem, bone marrow (BM) transplantation from healthy donors has been evaluated in some of these diseases. Microglia are of BM origin and over time a few donor-derived monocytes enter the CNS and serve as local sites of wild-type enzyme production, which can be taken up via secretion-recapture by neighboring host cells. On the whole, BM transplantation is only effective if it is performed in early infancy, does not show efficacy in all LSDs, and is not curative (Wraith, 2001). Further complications include the need for human leukocyte antigen (HLA) matched donors, the high rate of mortality associated with recipients, and the lack of standardization amongst different BMT regimens in different clinical centers.
Table 2.
Status of approved treatments and experimental therapies for LSDs with selected bibliography
| Therapy | Target organelle | In vitro POC | In vivo POC | Clinical trials | Regulatory approval | References |
| Enzyme replacement (ERT) | Lysosome | + | + | + | + | Brady, 2006b; Neufeld, 2011 |
| Bone marrow transplantation (BMT) | Lysosome | + | + | + | N/A | Krivit, 2002; Brady, 2006a |
| Substrate reduction therapy (SRT) | Golgi | + | + | + | + | Platt and Butters, 2004; Platt and Jeyakumar, 2008; Cox, 2010 |
| Enzyme enhancement therapy (EET) | ER/lysosome | + | − | In progress | − | Okumiya et al., 2007; Fan, 2008 |
| Gene therapy (GT) | Nucleus | + | + | In progress | − | Gritti, 2011; Tomanin et al., 2012 |
| Stop codon read-through | Nucleus | + | − | − | − | Brooks et al., 2006 |
| Calcium modulation therapy (CMT) | ER | + | + | − | − | Lloyd-Evans et al., 2008 |
| Enhanced exocytosis therapy (ExT) | Exosome | + | − | − | − | Strauss et al., 2010; Medina et al., 2011 |
| Chaperone therapy by HSp70 (CT) | Lysosome | + | − | − | − | Kirkegaard et al., 2010 |
| Proteostasis regulation therapy (PRT) | ER | + | − | − | − | Balch et al., 2008; Mu et al., 2008 |
| Cholesterol removal using cyclodextrin in NPC1 disease | Lysosome | + | + | − | − | Davidson et al., 2009; Ward et al., 2010; Aqul et al., 2011 |
POC, proof of concept.
Another therapy to be developed and subsequently approved for LSDs was substrate reduction therapy using the oral small molecule imino sugar drug, miglustat (Lachmann, 2006). This has been approved for type 1 Gaucher disease (worldwide) for over a decade, and in 2009 for treating neurological manifestations in Niemann-Pick type C disease (now approved in most countries/regions, except the USA; Patterson et al., 2007). Miglustat targets the Golgi enzyme, glucosylceramide synthase (Platt et al., 1994), and by partially inhibiting glycosphingolipid biosynthesis it reduces the catabolic burden of these molecules on lysosomes that cannot digest them. It has the potential to be used in diseases with glycosphingolipid storage, as miglustat inhibits the first committed step in the biosynthesis of this family of lipids. Also, miglustat crosses the blood–brain barrier, hence its disease-modifying benefit in Niemann-Pick type C disease (Patterson et al., 2007). Like all drugs, this compound has side effects, the primary one being inhibition of disaccharidases, which can lead to gastrointestinal symptoms, particularly in the first 1–2 months of therapy. More recently, eliglustat tartrate (Genz-112638) has entered clinical trials in type 1 Gaucher disease as an oral substrate reduction therapy. As this drug has a different chemistry to miglustat, it also has a different side-effect profile (Cox, 2010).
There are currently several alternative therapeutic strategies that have shown utility in tissue culture models and/or in animal models of these diseases and are summarized in Table 2. Many of these approaches target non-lysosomal organelles. No doubt as more is known about pathogenic cascades and their impact on cellular organelles, additional creative approaches to treatment will emerge and undergo pre-clinical testing. Due to the severity and complexity of these disorders it is likely that ultimately a combination therapy will be needed to target multiple steps/organelles in the pathogenic cascade.
Conclusion
In conclusion, we have provided some selective examples illustrating the complexity of how lysosomal dysfunction impinges upon multiple aspects of cell biology, often in unanticipated ways (summarized in Fig. 3). Many questions remain unanswered at the present time, and some of these are highlighted in Box 1. However, the study of these rare diseases (Table 1) fills two voids in our knowledge, namely providing fundamental insights into lysosomal biology and in leading to novel approaches to generate next-generation therapeutic interventions for treating these truly fascinating yet devastating disorders (Table 2). It is clear that although storage is primarily initiated in the late endosomal–autophagic–lysosomal system, it induces a pathogenic cascade that impacts on multiple cellular systems and organelles, suggesting that conceptually we should view these diseases as cellular storage disorders and use this broader knowledge for the design of therapeutic interventions.
Box 1. Open Questions
• How does storage affect other aspects of lysosomal function, independent of the primary storage metabolite?
• How does storage trigger innate immune activation?
• How does lysosomal storage affect cell signaling?
• How do storage lipids escape the lysosome and affect the function of other organelles?
• What is the hierarchy of the pathogenic cascade in these diseases, which steps should be targeted for optimal therapy?
• Do the genetic defects in the neuronal ceroid lipofuscinoses (NCL disorders) cause convergent symptoms by chance, or are the disparate genes functioning in common cell biological pathways?
Acknowledgments
Many thanks to Pak Phi Poon (Dalhousie University, Halifax, Nova Scotia, Canada) for stimulating discussions and expert editing.
Footnotes
Abbreviations used in this paper:
- CNS
- central nervous system
- LSD
- lysosomal storage disease
- NPC
- Niemann-Pick type C
References
- Angelini C., Semplicini C. 2012. Enzyme replacement therapy for Pompe disease. Curr. Neurol. Neurosci. Rep. 12:70–75 10.1007/s11910-011-0236-5 [DOI] [PubMed] [Google Scholar]
- Aqul A., Liu B., Ramirez C.M., Pieper A.A., Estill S.J., Burns D.K., Liu B., Repa J.J., Turley S.D., Dietschy J.M. 2011. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 31:9404–9413 10.1523/JNEUROSCI.1317-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W.E., Morimoto R.I., Dillin A., Kelly J.W. 2008. Adapting proteostasis for disease intervention. Science. 319:916–919 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- Ballabio A. 2009. Disease pathogenesis explained by basic science: lysosomal storage diseases as autophagocytic disorders. Int. J. Clin. Pharmacol. Ther. 47(Suppl 1):S34–S38 [DOI] [PubMed] [Google Scholar]
- Ballabio A., Gieselmann V. 2009. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta. 1793:684–696 10.1016/j.bbamcr.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Bampton E.T., Goemans C.G., Niranjan D., Mizushima N., Tolkovsky A.M. 2005. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy. 1:23–36 10.4161/auto.1.1.1495 [DOI] [PubMed] [Google Scholar]
- Bellettato C.M., Scarpa M. 2010. Pathophysiology of neuropathic lysosomal storage disorders. J. Inherit. Metab. Dis. 33:347–362 10.1007/s10545-010-9075-9 [DOI] [PubMed] [Google Scholar]
- Boland B., Smith D.A., Mooney D., Jung S.S., Walsh D.M., Platt F.M. 2010. Macroautophagy is not directly involved in the metabolism of amyloid precursor protein. J. Biol. Chem. 285:37415–37426 10.1074/jbc.M110.186411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R.O. 2006a. Emerging strategies for the treatment of hereditary metabolic storage disorders. Rejuvenation Res. 9:237–244 10.1089/rej.2006.9.237 [DOI] [PubMed] [Google Scholar]
- Brady R.O. 2006b. Enzyme replacement for lysosomal diseases. Annu. Rev. Med. 57:283–296 10.1146/annurev.med.57.110104.115650 [DOI] [PubMed] [Google Scholar]
- Brady R.O. 2010. Benefits from unearthing “a biochemical Rosetta Stone”. J. Biol. Chem. 285:41216–41221 10.1074/jbc.X110.197954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright N.A., Reaves B.J., Mullock B.M., Luzio J.P. 1997. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 110:2027–2040 [DOI] [PubMed] [Google Scholar]
- Bright N.A., Gratian M.J., Luzio J.P. 2005. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr. Biol. 15:360–365 10.1016/j.cub.2005.01.049 [DOI] [PubMed] [Google Scholar]
- Brooks D.A., Muller V.J., Hopwood J.J. 2006. Stop-codon read-through for patients affected by a lysosomal storage disorder. Trends Mol. Med. 12:367–373 10.1016/j.molmed.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Burton B.K. 1998. Inborn errors of metabolism in infancy: a guide to diagnosis. Pediatrics. 102:E69 10.1542/peds.102.6.e69 [DOI] [PubMed] [Google Scholar]
- Cao Y., Espinola J.A., Fossale E., Massey A.C., Cuervo A.M., MacDonald M.E., Cotman S.L. 2006. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 281:20483–20493 10.1074/jbc.M602180200 [DOI] [PubMed] [Google Scholar]
- Charrow J. 2009. Enzyme replacement therapy for Gaucher disease. Expert Opin. Biol. Ther. 9:121–131 10.1517/14712590802573395 [DOI] [PubMed] [Google Scholar]
- Chen C.S., Patterson M.C., Wheatley C.L., O’Brien J.F., Pagano R.E. 1999. Broad screening test for sphingolipid-storage diseases. Lancet. 354:901–905 10.1016/S0140-6736(98)10034-X [DOI] [PubMed] [Google Scholar]
- Chen F.W., Li C., Ioannou Y.A. 2010. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS ONE. 5:e15054 10.1371/journal.pone.0015054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G.C., Okada Y., Thomas J.M., Genazzani A.A., Patel S., Galione A. 2002. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 111:703–708 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- Coen K., Flannagan R.S., Baron S., Carraro-Lacroix L.R., Wang D., Vermeire W., Michiels C., Munck S., Baert V., Sugita S., et al. 2012. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 198:23–35 10.1083/jcb.201201076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T.M. 2010. Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases. Curr. Opin. Investig. Drugs. 11:1169–1181 [PubMed] [Google Scholar]
- Cox T.M., Cachón-González M.B. 2012. The cellular pathology of lysosomal diseases. J. Pathol. 226:241–254 10.1002/path.3021 [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Dice J.F. 2000. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 1:570–583 10.1034/j.1600-0854.2000.010707.x [DOI] [PubMed] [Google Scholar]
- Davidson C.D., Ali N.F., Micsenyi M.C., Stephney G., Renault S., Dobrenis K., Ory D.S., Vanier M.T., Walkley S.U. 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 4:e6951 10.1371/journal.pone.0006951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J.F., Terlecky S.R., Chiang H.L., Olson T.S., Isenman L.D., Short-Russell S.R., Freundlieb S., Terlecky L.J. 1990. A selective pathway for degradation of cytosolic proteins by lysosomes. Semin. Cell Biol. 1:449–455 [PubMed] [Google Scholar]
- Eden E.R., White I.J., Tsapara A., Futter C.E. 2010. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat. Cell Biol. 12:267–272 [DOI] [PubMed] [Google Scholar]
- Elleder M. 2006. Glucosylceramide transfer from lysosomes—the missing link in molecular pathology of glucosylceramidase deficiency: a hypothesis based on existing data. J. Inherit. Metab. Dis. 29:707–715 10.1007/s10545-006-0411-z [DOI] [PubMed] [Google Scholar]
- Eskelinen E.L., Saftig P. 2009. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta. 1793:664–673 10.1016/j.bbamcr.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Eskelinen E.L., Tanaka Y., Saftig P. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13:137–145 10.1016/S0962-8924(03)00005-9 [DOI] [PubMed] [Google Scholar]
- Esselens C., Oorschot V., Baert V., Raemaekers T., Spittaels K., Serneels L., Zheng H., Saftig P., De Strooper B., Klumperman J., Annaert W. 2004. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J. Cell Biol. 166:1041–1054 10.1083/jcb.200406060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki J., Wolfe L.S., Kominami E. 1996. Specific delay in the degradation of mitochondrial ATP synthase subunit c in late infantile neuronal ceroid lipofuscinosis is derived from cellular proteolytic dysfunction rather than structural alteration of subunit c. J. Neurochem. 67:1677–1687 10.1046/j.1471-4159.1996.67041677.x [DOI] [PubMed] [Google Scholar]
- Fader C.M., Colombo M.I. 2009. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 16:70–78 10.1038/cdd.2008.168 [DOI] [PubMed] [Google Scholar]
- Fan J.Q. 2008. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol. Chem. 389:1–11 10.1515/BC.2008.009 [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T., Vitner E.B., Pressey S.N., Eilam R., Cooper J.D., Futerman A.H. 2011. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum. Mol. Genet. 20:1375–1386 10.1093/hmg/ddr019 [DOI] [PubMed] [Google Scholar]
- Fidziańska A., Walczak E., Walski M. 2007. Abnormal chaperone-mediated autophagy (CMA) in cardiomyocytes of a boy with Danon disease. Folia Neuropathol. 45:133–139 [PubMed] [Google Scholar]
- Fraldi A., Annunziata F., Lombardi A., Kaiser H.J., Medina D.L., Spampanato C., Fedele A.O., Polishchuk R., Sorrentino N.C., Simons K., Ballabio A. 2010. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 29:3607–3620 10.1038/emboj.2010.237 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fuller M., Meikle P.J., Hopwood J.J. 2006. Epidemiology of lysosomal storage diseases: an overview. Oxford: Oxford PharmaGenesis; 2006. Chapter 2 [PubMed] [Google Scholar]
- Futerman A.H. 2010. Calcium homeostasis in lysosomal storage diseases. Int. J. Clin. Pharmacol. Ther. 48:S6–S7 [Google Scholar]
- Futerman A.H., van Meer G. 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5:554–565 10.1038/nrm1423 [DOI] [PubMed] [Google Scholar]
- García-Arencibia M., Hochfeld W.E., Toh P.P., Rubinsztein D.C. 2010. Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. 21:691–698 10.1016/j.semcdb.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Dahms N.M., Kornfeld S. 2003. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 4:202–212 10.1038/nrm1050 [DOI] [PubMed] [Google Scholar]
- Ginzburg L., Futerman A.H. 2005. Defective calcium homeostasis in the cerebellum in a mouse model of Niemann-Pick A disease. J. Neurochem. 95:1619–1628 10.1111/j.1471-4159.2005.03534.x [DOI] [PubMed] [Google Scholar]
- Goldman S.D., Krise J.P. 2010. Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo. J. Biol. Chem. 285:4983–4994 10.1074/jbc.M109.037622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R., Galluzzi L., Kroemer G. 2011. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 333:1109–1112 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A. 2011. Gene therapy for lysosomal storage disorders. Expert Opin. Biol. Ther. 11:1153–1167 10.1517/14712598.2011.582036 [DOI] [PubMed] [Google Scholar]
- Haq E., Contreras M.A., Giri S., Singh I., Singh A.K. 2006. Dysfunction of peroxisomes in twitcher mice brain: a possible mechanism of psychosine-induced disease. Biochem. Biophys. Res. Commun. 343:229–238 10.1016/j.bbrc.2006.02.131 [DOI] [PubMed] [Google Scholar]
- Hers H.G. 1963. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe’s disease). Biochem. J. 86:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahreiss L., Menzies F.M., Rubinsztein D.C. 2008. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 9:574–587 10.1111/j.1600-0854.2008.00701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.J., Jr, Zhu J.H., Rbaibi Y., Luo X., Chu C.T., Kiselyov K. 2006. Mitochondrial aberrations in mucolipidosis Type IV. J. Biol. Chem. 281:39041–39050 10.1074/jbc.M607982200 [DOI] [PubMed] [Google Scholar]
- Jesionek-Kupnicka D., Majchrowska A., Krawczyk J., Wendorff J., Barcikowska M., Lukaszek S., Liberski P.P. 1997. Krabbe disease: an ultrastructural study of globoid cells and reactive astrocytes at the brain and optic nerves. Folia Neuropathol. 35:155–162 [PubMed] [Google Scholar]
- Kakkis E.D. 2002. Enzyme replacement therapy for the mucopolysaccharide storage disorders. Expert Opin. Investig. Drugs. 11:675–685 10.1517/13543784.11.5.675 [DOI] [PubMed] [Google Scholar]
- Kaufmann A.M., Goldman S.D., Krise J.P. 2009. A fluorescence resonance energy transfer-based approach for investigating late endosome-lysosome retrograde fusion events. Anal. Biochem. 386:91–97 10.1016/j.ab.2008.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Rodriguez-Enriquez S., Lemasters J.J. 2007. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462:245–253 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard T., Roth A.G., Petersen N.H., Mahalka A.K., Olsen O.D., Moilanen I., Zylicz A., Knudsen J., Sandhoff K., Arenz C., et al. 2010. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature. 463:549–553 10.1038/nature08710 [DOI] [PubMed] [Google Scholar]
- Kitatani K., Idkowiak-Baldys J., Hannun Y.A. 2008. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20:1010–1018 10.1016/j.cellsig.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitatani K., Sheldon K., Rajagopalan V., Anelli V., Jenkins R.W., Sun Y., Grabowski G.A., Obeid L.M., Hannun Y.A. 2009. Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. J. Biol. Chem. 284:12972–12978 10.1074/jbc.M802790200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D., Büssow H., Fewou S.N., Gieselmann V. 2005. Exocytosis of storage material in a lysosomal disorder. Biochem. Biophys. Res. Commun. 327:663–667 10.1016/j.bbrc.2004.12.054 [DOI] [PubMed] [Google Scholar]
- Koike M., Shibata M., Waguri S., Yoshimura K., Tanida I., Kominami E., Gotow T., Peters C., von Figura K., Mizushima N., et al. 2005. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am. J. Pathol. 167:1713–1728 10.1016/S0002-9440(10)61253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz J., Arnold A., Meissner T., Cantz M. 1993. Protein catabolism in fibroblasts cultured from patients with mucolipidosis II and other lysosomal disorders. Biochem. J. 295:577–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivit W. 2002. Stem cell bone marrow transplantation in patients with metabolic storage diseases. Adv. Pediatr. 49:359–378 [PubMed] [Google Scholar]
- Kuma A., Mizushima N. 2010. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin. Cell Dev. Biol. 21:683–690 10.1016/j.semcdb.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Lachmann R.H. 2006. Miglustat: substrate reduction therapy for glycosphingolipid lysosomal storage disorders. Drugs Today (Barc). 42:29–38 10.1358/dot.2006.42.1.937457 [DOI] [PubMed] [Google Scholar]
- Lamanna W.C., Lawrence R., Sarrazin S., Esko J.D. 2011. Secondary storage of dermatan sulfate in Sanfilippo disease. J. Biol. Chem. 286:6955–6962 10.1074/jbc.M110.192062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. 2012. mTOR signaling in growth control and disease. Cell. 149:274–293 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Lee J.K., Min W.K., Bae J.H., He X., Schuchman E.H., Bae J.S., Jin H.K. 2010. Bone marrow-derived mesenchymal stem cells prevent the loss of Niemann-Pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing sphingosine-1-phosphate. Stem Cells. 28:821–831 10.1002/stem.401 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G., et al. 2010. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 141:1146–1158 10.1016/j.cell.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E., Platt F.M. 2010. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 11:419–428 10.1111/j.1600-0854.2010.01032.x [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Morgan A.J., He X., Smith D.A., Elliot-Smith E., Sillence D.J., Churchill G.C., Schuchman E.H., Galione A., Platt F.M. 2008. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14:1247–1255 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- Luzio J.P., Pryor P.R., Bright N.A. 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8:622–632 10.1038/nrm2217 [DOI] [PubMed] [Google Scholar]
- Medina D.L., Fraldi A., Bouche V., Annunziata F., Mansueto G., Spampanato C., Puri C., Pignata A., Martina J.A., Sardiello M., et al. 2011. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell. 21:421–430 10.1016/j.devcel.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle P.J., Fietz M.J., Hopwood J.J. 2004. Diagnosis of lysosomal storage disorders: current techniques and future directions. Expert Rev. Mol. Diagn. 4:677–691 10.1586/14737159.4.5.677 [DOI] [PubMed] [Google Scholar]
- Morgan A.J., Platt F.M., Lloyd-Evans E., Galione A. 2011. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 439:349–374 10.1042/BJ20110949 [DOI] [PubMed] [Google Scholar]
- Mu T.W., Fowler D.M., Kelly J.W. 2008. Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biol. 6:e26 10.1371/journal.pbio.0060026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B.M., Bright N.A., Fearon C.W., Gray S.R., Luzio J.P. 1998. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J. Cell Biol. 140:591–601 10.1083/jcb.140.3.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., et al. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12:222–230 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E.F. 2011. From serendipity to therapy. Annu. Rev. Biochem. 80:1–15 10.1146/annurev.biochem.031209.093756 [DOI] [PubMed] [Google Scholar]
- Nixon R.A., Yang D.S., Lee J.H. 2008. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 4:590–599 [DOI] [PubMed] [Google Scholar]
- Okumiya T., Kroos M.A., Vliet L.V., Takeuchi H., Van der Ploeg A.T., Reuser A.J. 2007. Chemical chaperones improve transport and enhance stability of mutant alpha-glucosidases in glycogen storage disease type II. Mol. Genet. Metab. 90:49–57 10.1016/j.ymgme.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Orsi A., Polson H.E., Tooze S.A. 2010. Membrane trafficking events that partake in autophagy. Curr. Opin. Cell Biol. 22:150–156 10.1016/j.ceb.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Pagano R.E., Puri V., Dominguez M., Marks D.L. 2000. Membrane traffic in sphingolipid storage diseases. Traffic. 1:807–815 10.1034/j.1600-0854.2000.011101.x [DOI] [PubMed] [Google Scholar]
- Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M., Ballabio A. 2011. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20:3852–3866 10.1093/hmg/ddr306 [DOI] [PubMed] [Google Scholar]
- Patterson M.C., Vecchio D., Prady H., Abel L., Wraith J.E. 2007. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 6:765–772 10.1016/S1474-4422(07)70194-1 [DOI] [PubMed] [Google Scholar]
- Peña-Llopis S., Vega-Rubin-de-Celis S., Schwartz J.C., Wolff N.C., Tran T.A., Zou L., Xie X.J., Corey D.R., Brugarolas J. 2011. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 30:3242–3258 10.1038/emboj.2011.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T., Shen R.F., Knepper M.A. 2004. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA. 101:13368–13373 10.1073/pnas.0403453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt F.M., Butters T.D. 2004. Inhibition of substrate synthesis: a pharmacological approach for glycosphingolipid storage disease therapy. Lysosomal Disorders of the Brain. Platt F.M., Walkley S.U., Oxford University Press, Oxford: 381–408 [Google Scholar]
- Platt F.M., Jeyakumar M. 2008. Substrate reduction therapy. Acta Paediatr. Suppl. 97:88–93 10.1111/j.1651-2227.2008.00656.x [DOI] [PubMed] [Google Scholar]
- Platt F.M., Walkley S.U. 2004. Lysosomal defects and storage. Lysosomal Disorders of the Brain. Platt F.M., Walkley S.U., Oxford University Press, Oxford: 32–49 [Google Scholar]
- Platt F.M., Neises G.R., Dwek R.A., Butters T.D. 1994. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 269:8362–8365 [PubMed] [Google Scholar]
- Pressey S.N., Smith D.A., Wong A.M., Platt F.M., Cooper J.D. 2012. Early glial activation, synaptic changes and axonal pathology in the thalamocortical system of Niemann-Pick type C1 mice. Neurobiol. Dis. 45:1086–1100 10.1016/j.nbd.2011.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinetti A., Prioni S., Chiricozzi E., Schuchman E.H., Chigorno V., Sonnino S. 2011. Secondary alterations of sphingolipid metabolism in lysosomal storage diseases. Neurochem. Res. 36:1654–1668 10.1007/s11064-010-0380-3 [DOI] [PubMed] [Google Scholar]
- Pryor P.R., Mullock B.M., Bright N.A., Gray S.R., Luzio J.P. 2000. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149:1053–1062 10.1083/jcb.149.5.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben N., Shea L., Hill V., Plotz P. 2009. Monitoring autophagy in lysosomal storage disorders. Methods Enzymol. 453:417–449 10.1016/S0076-6879(08)04021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B., Duden R., Rubinsztein D.C. 2002. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11:1107–1117 10.1093/hmg/11.9.1107 [DOI] [PubMed] [Google Scholar]
- Roczniak-Ferguson A., Petit C., Qian S., Ferguson S. 2011. The interaction of MiT/TFE transcription factors with lysosomes contributes to regulation of lysosomal homeostasis. In Annual Meeting, The American Scociety for Cell Biology, Denver, CO [Google Scholar]
- Saftig P., Klumperman J. 2009. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10:623–635 10.1038/nrm2745 [DOI] [PubMed] [Google Scholar]
- Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A.M., Santambrogio L. 2011. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. 20:131–139 10.1016/j.devcel.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., Forte M., d’Azzo A. 2009. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 36:500–511 10.1016/j.molcel.2009.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S., et al. 2009. A gene network regulating lysosomal biogenesis and function. Science. 325:473–477 [DOI] [PubMed] [Google Scholar]
- Schedin S., Sindelar P.J., Pentchev P., Brunk U., Dallner G. 1997. Peroxisomal impairment in Niemann-Pick type C disease. J. Biol. Chem. 272:6245–6251 10.1074/jbc.272.10.6245 [DOI] [PubMed] [Google Scholar]
- Schiffmann R., Brady R.O. 2006. Development of enzyme replacement therapy for Fabry disease. Fabry Disease: Perspectives from 5 Years of FOS. Mehta A., Beck M., Sunder-Plassmann G., , Oxford: [PubMed] [Google Scholar]
- Schmid J.A., Mach L., Paschke E., Glössl J. 1999. Accumulation of sialic acid in endocytic compartments interferes with the formation of mature lysosomes. Impaired proteolytic processing of cathepsin B in fibroblasts of patients with lysosomal sialic acid storage disease. J. Biol. Chem. 274:19063–19071 10.1074/jbc.274.27.19063 [DOI] [PubMed] [Google Scholar]
- Schröder B.A., Wrocklage C., Hasilik A., Saftig P. 2010. The proteome of lysosomes. Proteomics. 10:4053–4076 10.1002/pmic.201000196 [DOI] [PubMed] [Google Scholar]
- Schwarzmann G., Sandhoff K. 1990. Metabolism and intracellular transport of glycosphingolipids. Biochemistry. 29:10865–10871 10.1021/bi00501a001 [DOI] [PubMed] [Google Scholar]
- Settembre C., Fraldi A., Jahreiss L., Spampanato C., Venturi C., Medina D., de Pablo R., Tacchetti C., Rubinsztein D.C., Ballabio A. 2008. A block of autophagy in lysosomal storage disorders. Hum. Mol. Genet. 17:119–129 10.1093/hmg/ddm289 [DOI] [PubMed] [Google Scholar]
- Shapiro B.E., Logigian E.L., Kolodny E.H., Pastores G.M. 2008. Late-onset Tay-Sachs disease: the spectrum of peripheral neuropathy in 30 affected patients. Muscle Nerve. 38:1012–1015 10.1002/mus.21061 [DOI] [PubMed] [Google Scholar]
- Simons M., Raposo G. 2009. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21:575–581 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Singh R., Cuervo A.M. 2011. Autophagy in the cellular energetic balance. Cell Metab. 13:495–504 10.1016/j.cmet.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada M., Pagliardini S., Yasuda M., Tukel T., Thiagarajan G., Sakuraba H., Ponzone A., Desnick R.J. 2006. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 79:31–40 10.1086/504601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss K., Goebel C., Runz H., Möbius W., Weiss S., Feussner I., Simons M., Schneider A. 2010. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 285:26279–26288 10.1074/jbc.M110.134775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Iseki E., Togo T., Yamaguchi A., Katsuse O., Katsuyama K., Kanzaki S., Shiozaki K., Kawanishi C., Yamashita S., et al. 2007. Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses. Acta Neuropathol. 114:481–489 10.1007/s00401-007-0264-z [DOI] [PubMed] [Google Scholar]
- Takamura A., Higaki K., Kajimaki K., Otsuka S., Ninomiya H., Matsuda J., Ohno K., Suzuki Y., Nanba E. 2008. Enhanced autophagy and mitochondrial aberrations in murine G(M1)-gangliosidosis. Biochem. Biophys. Res. Commun. 367:616–622 10.1016/j.bbrc.2007.12.187 [DOI] [PubMed] [Google Scholar]
- Takikita S., Myerowitz R., Schreiner C., Baum R., Raben N., Plotz P.H. 2009. The values and limits of an in vitro model of Pompe disease: the best laid schemes o’ mice an’ men…. Autophagy. 5:729–731 10.4161/auto.5.5.8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A., Kurz T., Navratil M., Arriaga E.A., Brunk U.T. 2010. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 12:503–535 10.1089/ars.2009.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A., del P Martin M., Sano R., Ma Y., Mann L., Ingrassia A., Laywell E.D., Steindler D.A., Hendershot L.M., d’Azzo A. 2004. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol. Cell. 15:753–766 10.1016/j.molcel.2004.08.029 [DOI] [PubMed] [Google Scholar]
- Tessitore A., Pirozzi M., Auricchio A. 2009. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2:4 10.1186/1755-8417-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti G. 2004. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj. J. 20:301–317 10.1023/B:GLYC.0000033627.02765.cc [DOI] [PubMed] [Google Scholar]
- Tjelle T.E., Brech A., Juvet L.K., Griffiths G., Berg T. 1996. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J. Cell Sci. 109:2905–2914 [DOI] [PubMed] [Google Scholar]
- Tomanin R., Zanetti A., Zaccariotto E., D’Avanzo F., Bellettato C.M., Scarpa M. 2012. Gene therapy approaches for lysosomal storage disorders, a good model for the treatment of mendelian diseases. Acta Paediatr. 101:692–701 10.1111/j.1651-2227.2012.02674.x [DOI] [PubMed] [Google Scholar]
- Toulmay A., Prinz W.A. 2011. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr. Opin. Cell Biol. 23:458–463 10.1016/j.ceb.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D., Beaudet A.L., Vogelstein B., Kinzler K.W., Antonarakis S.E., Ballabio A.2012. The Online Metabolic and Molecular Bases of Inherited Diseases: http://www.ommbid.com/. Accessed Aug 7, 2012.
- Venugopal B., Mesires N.T., Kennedy J.C., Curcio-Morelli C., Laplante J.M., Dice J.F., Slaugenhaupt S.A. 2009. Chaperone-mediated autophagy is defective in mucolipidosis type IV. J. Cell. Physiol. 219:344–353 10.1002/jcp.21676 [DOI] [PubMed] [Google Scholar]
- Verity C., Winstone A.M., Stellitano L., Will R., Nicoll A. 2010. The epidemiology of progressive intellectual and neurological deterioration in childhood. Arch. Dis. Child. 95:361–364 10.1136/adc.2009.173419 [DOI] [PubMed] [Google Scholar]
- Vitner E.B., Platt F.M., Futerman A.H. 2010. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285:20423–20427 10.1074/jbc.R110.134452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitry S., Bruyère J., Hocquemiller M., Bigou S., Ausseil J., Colle M.A., Prévost M.C., Heard J.M. 2010. Storage vesicles in neurons are related to Golgi complex alterations in mucopolysaccharidosis IIIB. Am. J. Pathol. 177:2984–2999 10.2353/ajpath.2010.100447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley S.U. 2007. Pathogenic mechanisms in lysosomal disease: a reappraisal of the role of the lysosome. Acta Paediatr. Suppl. 96:26–32 10.1111/j.1651-2227.2007.00202.x [DOI] [PubMed] [Google Scholar]
- Walkley S.U., Vanier M.T. 2009. Secondary lipid accumulation in lysosomal disease. Biochim. Biophys. Acta. 1793:726–736 10.1016/j.bbamcr.2008.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., O’Donnell P., Fernandez S., Vite C.H. 2010. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 68:52–56 10.1203/PDR.0b013e3181df4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.A., Murphy D.D., Giasson B.I., Zhang B., Trojanowski J.Q., Lee V.M. 2004. Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons. J. Cell Biol. 165:335–346 10.1083/jcb.200403061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester B. 2004. Primary defects in lysosomal enzymes. Lysosomal Disorders of the Brain. Platt F.M., Walkley S.U., Oxford University Press, Oxford: 81–130 [Google Scholar]
- Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S., et al. 2010. α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 190:1023–1037 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.O., Li R., Montell C., Venkatachalam K. 2012. Drosophila TRPML Is Required for TORC1 Activation. Curr. Biol. 22:1616–1621 10.1016/j.cub.2012.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E., Cuervo A.M. 2010. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13:805–811 10.1038/nn.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith J.E. 2001. Advances in the treatment of lysosomal storage disease. Dev. Med. Child Neurol. 43:639–646 10.1017/S0012162201001165 [DOI] [PubMed] [Google Scholar]
- Wraith J.E. 2002. Lysosomal disorders. Semin. Neonatol. 7:75–83 10.1053/siny.2001.0088 [DOI] [PubMed] [Google Scholar]
- Wraith J.E. 2004. Clinical aspects and diagnosis. Lysosomal disorders of the brain. Platt F.M., Walkley S.U., Oxford University Press, Oxford: 50–77 [Google Scholar]
- Xu J., Dang Y., Ren Y.R., Liu J.O. 2010. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc. Natl. Acad. Sci. USA. 107:4764–4769 10.1073/pnas.0910872107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J., Mi N., Zhao Y., Liu Z., Wan F., et al. 2010. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 465:942–946 10.1038/nature09076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469:221–225 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D.M. 2011. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 334:678–683 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]