Abstract
There are exciting findings in the field of depression and coronary heart disease. Whether diagnosed or simply self-reported, depression continues to mark very high risk for a recurrent acute coronary syndrome or for death in patients with coronary heart disease. Many intriguing mechanisms have been posited to be implicated in the association between depression and heart disease, and randomized controlled trials of depression treatment are beginning to delineate the types of depression management strategies that may benefit the many coronary heart disease patients with depression.
1. Introduction
Depression is quickly becoming the leading cause of years of life lived with disability worldwide [1] and has a particularly large impact on compromised health when comorbid with a chronic medical disorder [2]. More than 17 million American adults survived after an acute coronary syndrome (ACS), with 1.2 million new surviving cases added per year [3]. More than 2 out of every 5 of these patients have significant patient-reported depressive symptoms [4], and these symptoms will remain long after discharge [5]. Thus, as many as 7 million of Americans living with coronary heart disease (CHD) are also suffering with clinically significant depression, and we add half of a million new cases to this public health burden annually. As the comorbidity between these two conditions is high, and both predict increased occurrence of the other, a whole field has emerged to study the complex inter-relations of depression and ACS. For example, presence of a depressive disorder and/or minimally elevated depressive symptoms appears to be a robust risk and prognostic marker of CHD recurrence and all-cause mortality [4, 6, 7]. In this paper, the definition, measurement, epidemiology, mechanisms, screening, and treatment recommendations for depression comorbid with coronary heart disease will be presented, as will the controversies and future research directions in this interesting intersection between mental and physical disease.
2. Defining Depression
Many researchers and much of the lay public assume that depression is a categorical disorder, and that those who suffer with symptoms typically associated with depression have a disease qualitatively different from everyday distress. Consequently, they consider that this cluster of symptoms should be viewed as one of a series of psychiatric illnesses, as described within the Diagnostic and Statistical Manual of Mental Disorders (DSM) [8]. However, there are others promoting continuous or dimensional views of these symptoms. Both conceptualizations are reviewed, starting with disease categories because some of the debates within psychiatric nosology may shed light on the depression—CHD association.
2.1. Depression as a Psychiatric Disorder
Depressive disorders are syndromal diagnoses based on subjective experience of psychological and somatic symptoms that are reported to a professional who evaluates these symptoms along continua of intensity, duration, and impact on daily functioning. Categories from the DSM-IV [8] include major depressive disorder, dysthymia, adjustment disorder with depressed mood, depression due to a general medical condition, and depression not otherwise specified (n.o.s.).
Differential depression disorder diagnosis is decided based on the duration of the illness, the number of symptoms present, and the presumed etiology of the illness—all of which are distinctions that should be reported when investigating the link between depression and CHD. Major Depressive Disorder (MDD) is the most commonly studied clinical diagnosis in relation to CHD. It is characterized by clinically significant impairment in at least five areas such as appetite or sleep that have persisted for at least two weeks, plus the presence of depressed mood or the loss of pleasure/interest for most of the day during those two weeks. In contrast, dysthymia is reserved for a cluster of symptoms that persisted over a longer period. The number of symptoms needed to diagnose dysthymia is less than in MDD, but persistent depressed mood has to be present throughout two years. Both MDD and dysthymia can be further specified according to the presence or absence of atypical symptoms such as reactive mood, hyperphagia (i.e., excessive eating), hypersomnia, leaden paralysis, or longstanding interpersonal rejection sensitivity.
The diagnosis of mood disorder due to a general medical condition describes a significant disturbance in mood that is directly related to the presence of a medical condition known to cause depression, as long as the mood symptoms do not meet criteria for other DSM-IV categories of depression. Thus, there must be evidence that the psychiatric symptoms are actually caused by the medical condition rather than being just a reaction to being ill. One of the most clearly documented examples of this type of depressive disorder is the depression that results from Parkinson's disease [9]. Although there is a high prevalence of elevated depressive symptoms after an acute coronary syndrome (ACS) event, no one has yet proposed that the ACS may cause these symptoms; however, depressive symptoms can result from cerebrovascular disease [10]. Thus, one intriguing possibility is that depression due to a medical condition (vascular depression in in this case) may be precipitated by the same CVD risk factors as those responsible for the ACS.
Next, adjustment disorder is characterized by the development of clinically significant emotional or behavioral symptoms in response to an identifiable psychological stressor. The symptoms must develop within three months after the onset of the stressor and must resolve within six months of the termination of the stressor [11]. Severe physical events, such as an ACS, can be a precipitant of an adjustment disorder with depressed mood, but extreme mood disturbance would still be classified as a major depressive disorder in DSM-IV, and symptoms lasting longer than six months would similarly be classified as meeting criteria for a mental disease. Might it be adjustment disorder that predicts ACS recurrence? Only well-trained diagnostic staff, alert to the sometimes subtle distinction between adjustment and other depressive disorders, could address this question, and no such study has classified sufficient numbers of ACS patients with adjustment disorder to test this intriguing hypothesis.
Depression n.o.s. allows the classification of less severe syndromes of depression (with fewer symptoms) of a shorter duration (less than 2 weeks) that do not meet criteria for major depressive disorder or dysthymia. It encompasses DSM-IV research categories such as minor depression and brief recurrent depressive disorder, which have also been labelled as “subthreshold” depression, a category that encompasses syndromes of depression that fail to meet criteria for major depressive disorder [12], but which show some significant impairment in social or occupational functioning [13]. “Subthreshold” depression has been proposed by investigators conducting longitudinal studies of mood disorders (e.g., [14, 15]) to reflect normal fluctuations in the longitudinal course of mood disorders, and a similar construct has been found to predict CHD recurrence [16, 17]. Thus, the longitudinal course of depressive symptoms, as well as subthreshold depression levels, must be carefully assessed to determine if one or both are critical to ACS prognosis.
More recent approaches to categorical classification have addressed the problem of comorbid anxiety and depressive illnesses. For example, a study by Kessler et al. [18] found that up to 58% of patients with a lifetime diagnosis of major depressive disorder may suffer from anxiety disorder(s), suggesting that the psychiatric diseases' underlying pathophysiologies may not respect DSM-IV boundaries. The broad implications of this highly prevalent comorbidity are that etiologies [19], neuroendocrine mechanisms [20], and sequelae may be common to both diseases, so ACS patients should be assessed for both anxiety and depressive disorders, as well as histories of both, to more fully understand the association of depression to ACS recurrence.
2.2. Assessment of Depression as a Psychiatric Disorder
Common assessment tools for reliable diagnosis of depressive disorders include the Structured Clinical Interview for DSM-IV (SCID; [21]) and the Diagnostic Interview Schedule (DIS; [22]). The SCID is a semistructured interview designed for administration by a clinician or trained mental health professional to provide a basis for accurate DSM-IV diagnoses. It exists in several versions (e.g., SCID-I for DSM Axis I diagnoses, SCID-II for DSM Axis II (personality disorders) diagnoses), and every diagnostic category (e.g., mood disorders) has its own module that can be used separately. A research version is available which can be modified, and research assistants without a clinical degree can reliably administer this version after extensive training. Test-retest reliability of the SCID ranges widely according to setting and study population (e.g., Williams et al. [23]). Its validity has not been extensively studied mostly because of the lack of a “gold standard” for psychiatric diagnoses. In contrast, the SCID itself is widely used as the gold standard against which the sensitivity and specificity of self-report measures is evaluated.
The DIS can also be administered by trained nonclinician interviewers. Its answers are completely precoded which enhances reliability; however, the DIS is rather inflexible and some have argued that it is insensitive to change [24]. The DIS served as one basis for the development of the Depression Interview and Structured Hamilton [24] (DISH: a semistructured interview developed for the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study).
The DISH was designed to assess the severity of depressive symptoms and enable trained interviewers to make a DSM-IV major depression, minor depression, and dysthymia diagnosis in medically ill patients. Furthermore, the lifetime and family history of these diagnoses is assessed. The DISH was validated against the SCID (88% diagnostic overlap) and in ENRICHD, clinicians agreed with 93% of diagnoses made by research nurses [24]. It is likely the best depression interview to be used with ACS patients, to accurately determine if depression exists, rather than somatic symptoms resulting from the cardiac disease.
2.3. Depression as Self-Reported Distress Symptoms
When professionally trained interviewers are not available and/or it is not feasible to conduct psychiatric diagnostic interviews, reliance on self-report measures of depression for diagnostic classification has been common in studies of CHD patients [25]. There are many such measures, each with its own merits, and some available in updated and/or shortened versions. Each of these self-report measures was designed to detect depressive disorders in various populations (such as primary care patients, or older adults, or population-based samples) and they detect depressive disorders and demonstrate item consistency, split-half reliability, and stability to varying, but generally satisfactory degrees [25, 26]. Two of the most frequently used, the Beck Depression Inventory (a trademarked inventory—both versions I and II (see http://www.pearsonassessments.com/ for ordering instructions)) [27, 28] and the Center for Epidemiological Studies—Depression subscale (CES-D [29]), are also frequently employed in studies of depressive symptoms and CHD, and both have shown good predictive validity to ACS events [30, 31]. However, of concern in choosing any of these measures for use in a depression—CHD study is their sensitivity and specificity for depression detection in older patients with comorbid medical illnesses, who have recently undergone a life-threatening medical crisis [32].
Unfortunately, discrepancies between what investigators mean by the term “depression,” and how they then assess it, may lead to erroneous conclusions, contradictory findings, and difficulty in summarizing the evidence-base of the inter-relationships between depression and CHD that has—or has not—accrued.
3. Epidemiology and Prognostic Significance of Depression Comorbid with Coronary Heart Disease
3.1. Depression in CHD Patients Is Prevalent
An Agency for Healthcare Research & Quality systematic review reported that up to 20% of CHD patients meet criteria for a major depressive disorder, and up to 47% have significant patient-reported depressive symptoms, and these diagnoses/symptoms remain long after discharge [5]. Thus, as noted in a comprehensive review by Carney, almost 2 out of every 5 ACS patients have clinically significant depression [4]. This contrasts starkly with the general population, where depression is seen in 4 to 7% persons [33]. When DSM criteria are used to establish a diagnosis, prevalence tends to be somewhat lower (i.e., 15 to 20%) in CHD patients, but even minor depression or elevated depressive symptoms are seen in 30 to 50% of these patients [34]. Importantly, both clinically diagnosed depression and elevated depressive symptoms predict increased cardiac risk, as described below.
3.2. Depression in CHD Patients Is Observationally Associated with Diminished Health-Related Quality of Life
Depression in both stable CHD patients and those with a recent ACS clearly predicts impoverished health-related quality of life, independent of traditional predictors of quality of life [35]. For example, recent ACS patients with a history of depression have twice the rate of reported angina, triple the reported physical limitations, and are at almost triple the risk of diminished health-related quality of life [36]. Of multiple predictors of one-year quality of life in a study of myocardial infarction (MI) patients, including demographic and social variables, severity of disease, and other predictors, depression was the most important [37]. In another large study of CHD patients, depression was once again the most important correlate of diminished quality of life, while 2 traditional measures of cardiac function, ejection fraction and ischemia, were not [38]. In fact, a recent review demonstrated that depression was more highly associated with health-related quality of life & health status than angina, arthritis, asthma, or diabetes [39]. There have been calls to improve quality of life in post-ACS patients, rather than continue to focus on extending life of diminished quality [40]. Treating depression could answer this call.
3.3. Depression in CHD Patients Is Observationally Associated with High Costs
Depression has long been associated with high costs of medical utilization [42], many lost days of productivity, and reduced performance while at work [43]. Patients who have a chronic medical condition such as CHD, and comorbid depression, have significantly more ambulatory visits, emergency room visits, days in bed due to illness, and functional disability [44]. Five-year cardiovascular health costs were 15–53% higher in women with myocardial ischemia who were depressed compared to those who were not depressed [45]. In post-MI patients with elevated depressive symptoms, one year health care costs of out-patient, emergency room, and readmission visit costs (excluding mental health costs) were 41% higher than for post-MI patients with few depressive symptoms [46].
3.4. Depression in CHD Patients Is Observationally Associated with Poor Medical Prognosis
In addition to increased health care utilization, death and morbidity are societal costs associated with depression in post-ACS patients. Large epidemiological studies have demonstrated convincingly that depression is a predictor for occurrence and recurrence of CHD. Depressive symptoms alone also predict CHD risk, but stronger effect sizes have been observed for MDD compared to depressive mood, suggesting a dose-response relationship [31, 47, 48]. In addition to the enormous patient and caregiver burden, post-MI patients who are depressed have more medical comorbidities [49] and cardiac complications [50] and are at greater risk for mortality compared to nondepressed post-MI patients [16, 49–51]. Prospective observational studies show, among ACS patients, the hazard ratio associated with depressive symptoms is 1.80 (95% CI: 1.46–2.51) for all-cause mortality [52], and 1.95 (95% CI: 1.33–2.85) for MI recurrence [6]. A recent meta-analysis indicates that elevated depressive symptoms continue to strongly identify CHD patients at mortality risk (relative risk: 3.41 (95% CI: 2.19–5.31)) [52]. As can be seen in Figure 1, depressive symptoms in CHD patients is on par with more conventional CHD recurrence prognostic factors for predicting death and CHD recurrence.
Figure 1.
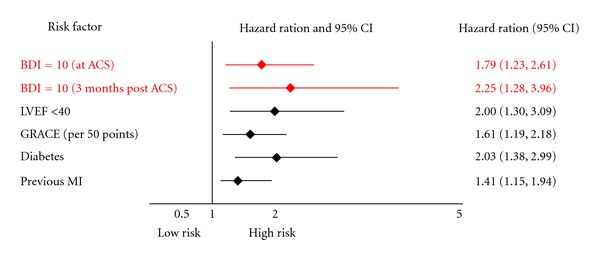
Hazard ratios of depressive symptoms and traditional cardiovascular risk factors.
The biology of depression suggests dysregulation of several physiological systems, some of which are implicated in the depression-CHD recurrence link [53]. However, the evidence remains equivocal regarding which biological dysregulations are responsible for the link between depression and CHD.
4. Mechanisms by Which Depression May Confer Coronary Heart Disease Recurrence and Death Risk
There are many behavioural and biological mechanisms that have been proposed to explain the association between depression and CHD [53, 54]. Biological mechanisms proposed to explain the depression-CHD prognosis association include platelet reactivity, inflammation, autonomic imbalance, sleep architecture disruption, circadian rhythm disruption anabolic/catabolic hormonal imbalance, as well as many others [55–58]. A selected review of the evidence available for many of the proposed mechanisms is provided below.
4.1. Evidence for the Link between Platelet Reactivity and Depression
Several case-control studies have demonstrated the existence of platelet hyperreactivity in CHD patients [59, 60]. In a study by Laghrissi-Thode et al. [60], higher levels of platelet factor 4 and β-thromboglobulin, markers of platelet aggregation, were described in MDD patients with CHD compared with nondepressed CHD patients. Serebruany et al. [61] pooled the data from 3 studies of post-ACS patients: ASSENT-2, PRONTO, and SADHART, the last of which specifically screened and enrolled MDD patients. Fifty nonsmoking, nondiabetic subjects without cardiovascular disease were also enrolled as controls. The study showed that patients with CHD and MDD exhibited the highest levels of platelet factor 4, β-thromboglobulin, and platelet/endothelial cell adhesion molecule-1 when compared with MI or unstable angina patients without MDD.
4.2. Evidence for the Link between Inflammation and Depression
Several cross-sectional studies have linked depression to chronic inflammation in otherwise healthy participants [62–64], as well as in post-ACS patients shortly after the index event [65, 66]. Elevated levels of inflammatory biomarkers including c-reactive protein (CRP), soluble intercellular adhesion molecule 1 (sICAM1), soluble vascular cell adhesion molecule-1, and tumor necrosis factor-α (TNF-α) are associated with an increased risk of cardiovascular events in patients with known CHD including survivors of a recent ACS [67–70].
CRP is significantly higher in depressed patients with no CHD compared with matched nondepressed controls. The cardiovascular health study enrolled patients aged 65+ years with no CHD history and found that participants with increased depressive symptoms had higher CRP levels, after adjusting for potential confounders [71]. Similarly, recent findings from the ATTICA study demonstrated that depressive symptoms were positively correlated with higher CRP in 853 participants [72]. A recent analysis of the National Health & Nutrition Examination (NHANES III) survey found that patients with a history of MDD had elevated CRP levels compared to patients with no history of depression (OR = 1.64), independent of other standard CHD risk factors [73]. Data also show that depressed patients have elevated peripheral white blood cell counts and increased B- and T-cell activity [74–77].
Similarly, sICAM1 has been cross-sectionally associated with depression [78]. A recent study showed that the expression of sICAM-1 on vascular endothelial cells in the brain was associated with depression development [79]. In addition, psychological stress (often associated with depressive symptoms) increases sICAM-1 expression in circulating granulocytes, lymphocytes, and monocytes [78].
Findings are similar for post-ACS patients. Four recent studies have examined the relation between depression and inflammatory biomarker levels after an ACS. Lespérance et al. [80] showed that levels of sICAM-1 was related to depression at a mean followup of 2 months after the ACS. Additional analyses revealed that a significant relationship was found between depression and elevated CRP levels only in patients not taking statins. Miller et al. [81] showed that depression was associated with increased CRP levels independent of several possible confounders including statin therapy in patients who had an acute coronary syndrome event or coronary revascularization ≥3 months prior to enrollment. There was no association between depression and levels of interleukin-6 (IL-6) or TNF-α. Similarly, we found that persistently elevated levels of depression 3 months following an acute coronary syndrome event were significantly associated with elevated CRP levels, independent of several possible confounders [82]. This was the case in both patients who were prescribed statins at any time during the three month period, and in those who were not. In contrast, only one study has shown no significant association between depression and elevated inflammatory biomarkers. Annique et al. [83] showed that there was no relation between several inflammatory markers such as CRP, TNF-α, IL-6, and neopterine in depressed versus nondepressed patients post-ACS at a mean followup of 6 months.
The immune system usually reacts to an inflammatory stimulus with an induction of a T helper 2/anti-inflammatory shift to protect the organism from excessive T helper 1/proinflammatory cytokine release. However, stress hormones such as cortisol are capable of facilitating inflammation through further inducing the release of proinflammatory cytokines without the adaptive simultaneous release of anti-inflammatory cytokines. In addition to an elevation in proinflammatory markers, a reduction in anti-inflammatory markers may also be associated with depression. Interleukin-10, an anti-inflammatory cytokine, was examined in chronic heart failure patients with and without depressive symptoms. Those with depressive symptoms exhibited significantly lower levels of interleukin-10 and higher levels of the proinflammatory cytokines TNF-α and IL-6 [84]. These findings suggest that depression is associated with a downregulation of anti-inflammatory cytokines with a simultaneous upregulation of proinflammatory cytokines.
In summary, several studies indicate that depression in CHD patients is associated with an increase in proinflammatory and a decrease in anti-inflammatory cytokines, which are both associated with an increased risk of recurrent cardiovascular events. Thus, a dysregulation in the inflammatory pathways may mediate the link between depression and recurrent CHD.
4.3. Evidence for the Link between Autonomic Dysregulation and Depression
Autonomic dysregulation is characterized by increased activation of the sympathetic nervous system (SNS), which usually acts in concert with a reduced activation of the parasympathetic nervous system (PNS). Both elevated SNS activity and reduced PNS activity have been implicated in depression and CHD recurrence.
Several studies have documented that depressed individuals compared to their nondepressed counterparts have lower heart rate variability (HRV), a well-established noninvasive index of cardiac autonomic regulation, although these differences may be seen in different frequency bands. Using data from the ENRICHD trial, Carney and colleagues demonstrated that very low frequency (VLF) HRV was lower in depressed post-MI patients compared to their nondepressed counterparts and partially mediated the relationship between depression and mortality after MI [85]. While the underlying physiology of VLF HRV is not well understood, the fact that it, along with low frequency (LF) and high frequency (HF) power, is virtually eliminated by the parasympathetic antagonist atropine suggests a substantial vagal contribution [86]. In another analysis from the ENRICHD dataset, HF, LF, VLF, and ultra-low frequency (ULF) HRV were all lower in depressed versus nondepressed post-MI patients [65]. After adjustment for covariates, these differences remained statistically significant for all but HF power. In healthy women from the Women's Health Initiative, several time domain indices of HRV were lower among depressed participants compared to those with no evidence of depression [87]. While there are exceptions [88, 89], these data suggest that depression is associated with reduced cardiac parasympathetic modulation.
Research on the relation between the activity of the SNS and depression has a long history. Several studies have shown increased catecholamine levels in depression, although there have been exceptions [90, 91]. Esler et al. [92] found increased norepinephrine (NE) spillover. The rate of norepinephrine released to plasma from sympathetic nerves in some but not all depressed patients. Wong and colleagues measured cerebrospinal fluid (CSF) NE and plasma cortisol each hour for 30 consecutive hours in controls and patients with melancholic depression (MDD with specific additional features including anhedonia or loss of interest and pleasure in things or people) and demonstrated higher CSF NE and plasma cortisol levels around the clock in the depressed patients [93]. Veith and colleagues showed that depressed patients had an increased rate of NE entry into the circulation [94]. Other studies also have shown elevated levels of plasma NE in depressed patients [95–97]. Recently, Gold et al. demonstrated that severely depressed patients had increased heart rate (HR), blood pressure (BP), CSF NE, and plasma NE compared to a control group [98]. In a recent study of 91 women, higher levels of depression were positively associated with 24-hour urinary NE excretion [99]. The same pattern of results emerged from the Heart and Soul Study [100]. Among 598 patients with coronary heart disease, those with depressive symptoms (n = 106) had greater mean 24-hour urinary norepinephrine excretion levels and were more likely to have norepinephrine excretion levels in the highest quartile and above the normal range than those without depressive symptoms. Thus, there is substantial evidence that both serum and urinary norepinephrine are typically elevated in depressed patients.
4.4. Evidence for the Link between Autonomic Dysregulation and CHD
There is abundant evidence that the autonomic nervous system plays a significant role in the pathophysiology of CHD recurrence. By enhancing myocardial electrical stability, the parasympathetic nervous system reduces the risk of sudden cardiac death. Animal experiments confirm the capacity of higher levels of cardiac vagal regulation to protect against sudden death after experimentally induced MI [101], and HRV is a powerful predictor of sudden death in heart failure patients [102] and in patients at elevated risk for sudden death [103]. This effect may account for the repeated finding that low levels of HRV confer elevated risk of adverse events following an MI [104–106]. In post-CABG patients randomized to the placebo condition in a trial of gemfibrozil, low levels of HRV predicted the progression of coronary atherosclerosis after controlling for conventional risk factors [107].
Excess activity of the sympathetic nervous system produces many effects that contribute to CHD recurrence in post-ACS patients. It raises blood pressure, increases myocardial oxygen demand, activates platelets, induces myocyte apoptosis, and is arrhythmogenic. Administration of β-blockers, which protect against future events in post-MI patients, is evidence of the role of SNS in adverse events. Correspondingly, research suggests that diurnal changes in indices of SNS activity, for example, catecholamines, are associated with the diurnal pattern of the onset of cardiovascular events, which more frequently happen in the morning hours [108–112], with a meta-analysis concluding that 1 in 11 myocardial infarctions are attributable to the morning excess incidence [113]. Systemic a2-receptor blockade by yohimbine reduced the sympathetically-mediated orthostatic increase in platelet aggregation significantly, further suggesting the central role of the SNS in the risk of thrombotic vascular events in atherosclerosis [114]. Patients with evidence of coronary endothelial dysfunction show increased vasoconstriction in response to catecholamines [115].
4.5. Evidence for the Link between Sleep Architecture Disruption and Depression
Depression and sleep architecture disruption are closely linked, although identification of the specific polysomnographic parameters that are uniquely dysregulated in depression remains controversial [116].
Total sleep time is decreased in depressed patients and those prone to depressive episodes [117], and while prospective data are lacking, disturbances that shorten sleep duration (prolonged sleep latency, early morning awakening, sleep continuity disturbances, and insomnia) have all been suggested as possible contributors to depressed mood [118]. Cross-sectional studies of depressed versus nondepressed patients regularly find that total sleep time is almost always reduced in the depressed patients [116, 117, 119].
Reduced REM latency, the time from sleep onset to the first occurrence of REM is the most frequently reported sleep dysregulation that distinguishes MDD patients from normals, other psychiatric patients, and those with nonendogenous depression Benca 1992 [120]. A meta-analysis drew the same conclusion [116].
Depressed patients tend to have decreased slow wave sleep as reviewed by a meta-analysis incorporating results from 177 studies with data from 7151 patients and controls [116, 119, 121]. More recently, Armitage et al. reported that compared to healthy subjects, slow wave sleep was decreased in depressed outpatients [122]. Furthermore, antidepressant use appears to reverse these sleep markers of depression, by increasing total slow wave sleep and the slow wave sleep in the first sleep cycle, but not in the second cycle [123].
4.6. Evidence for the Link between Sleep Architecture Disruption and CHD
While prospective epidemiological studies relating many of the dimensions of sleep architecture to CVD recurrence are lacking, there is epidemiological evidence that short sleep duration is predictive of ACS incidence [101, 124]. REM sleep is characterized by pronounced surges of sympathetic activity [125] which may be of sufficient magnitude (a) to stimulate thrombotic processes, (b) to increase hemodynamic stress on vessel walls conducive to plaque rupture, and (c) to alter cardiac electrophysiological properties [126]. These autonomic surges could be responsible for cardiac events witnessed during REM sleep in humans [127, 128]. Importantly, this REM-induced cardiac sympathetic dominance is even more enhanced in individuals with recent MI [129]. There is no evidence addressing the possible role of slow wave sleep in predicting either ACS occurrence or recurrence, but lack of restorative vagal regulation could underly the association with ACS recurrence. Thus, we only have indirect evidence that sleep architecture may be implicated in CHD RECURRENCE/DEATH, but we do have theoretical foundations for positing this association.
4.7. Evidence for the Link between Circadian Rhythm Disruption and Depression
Endogenous circadian rhythms regulate daily variations in most of the hormonal, physiological, and psychological variables implicated in depression as well as ACS [130]. The systems with the most prominent variations are thermoregulation or core temperature, and melatonin.
Core temperature, defined as the operating temperature of an organism, specifically in deep structures of the body, is seen as the gold standard for measurement of circadian rhythm. Elevated nocturnal body temperature is one of the more consistently observed circadian abnormalities in depression [131–136]. Several studies show that during the depressed phase, average temperature rises and temperature amplitude (maximum temperature minus minimum temperature) decreases. Effects of antidepressants on the core body temperature of depressed patients include (a) lowering of the minimum temperature during the night, and (b) increasing temperature amplitude [133]. Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects [136].
Melatonin is a pineal indoleamine derived from serotonin and is a reliable marker of circadian rhythmicity in humans. Melatonin concentrations are increased at night and are undetectable during the day. Depression-related disturbances in the melatonin circadian profile in the form of advanced phases and/or decrease in nocturnal amplitude were demonstrated as early as 1979 [137–139] and have been subsequently replicated [140–149].
4.8. Evidence for the Link between Circadian Rhythm Disruption and CHD
There is evidence that the majority of cardiovascular events (including myocardial infarctions) show a marked circadian rhythmicity with a peak incidence between 6 am and 2 pm [113, 150]. However, in depressed patients, for whom there are often circadian dysregulations, the most prevalent time for myocardial infarctions is between 10 pm and 6 am [151]. Thus, circadian rhythm disruption in depressed patients may help elucidate some of the pathways by which these patients are at increased risk for recurrent cardiovascular events.
4.9. Evidence for the Link between Anabolic/Catabolic Hormone Imbalance and Depression
An adequate balance between catabolic processes (energy mobilization), which are induced by stress hormones such as catecholamines and cortisol, and anabolic processes (repair, healing, growth), which are induced by sex steroids, among others, is essential for health and survival. Dehydroepiandrosterone (DHEA), an anabolic sex steroid precursor, a marker frequently used in combination with cortisol to capture anabolic/catabolic hormone imbalance, has been implicated in protection against coronary artery disease [152–154].
The hypothalamic-pituitary-adrenal (HPA) axis, the major stress axis through which cortisol is released by the adrenal gland when stimulated by adrenocorticotrophin (ACTH), has been studied extensively in depression. Depressed patients exhibit elevated circulating plasma levels of ACTH and cortisol, and elevated urinary cortisol concentration [155]. 24-hour sampling studies have found increased cortisol secretion in depressed persons [155–158], as well as alterations in the circadian rhythm of cortisol [155–158]. Most studies on morning salivary cortisol have demonstrated an increased awakening response in depressed versus nondepressed subjects [159–161] but not all have found this [162]. In 781 subjects from the CARDIA study of heart disease in young adults, depression was associated with flatter diurnal cortisol rhythm [163]. Pharmacological challenge studies indicate diminished negative feedback in the HPA axis in depressed persons [164].
There are several studies on the association of anabolic/catabolic hormone balance with depression. The cortisol/DHEA ratio was greater in elderly patients with MDD compared with age-matched healthy controls [165]. In adolescents, high morning cortisol/DHEA ratios predicted persistent MDD [73]. In a nonclinical sample of older men, a high morning cortisol/DHEA ratio was associated with high anxiety, negative mood, and deficits in various aspects of cognitive function [166]. Young and colleagues showed that a high cortisol/DHEA ratio in the evening was associated with depression in an adult population [167]. In another study, patients with major depressive disorder comorbid with borderline personality disorder displayed elevated cortisol/DHEA ratios relative to normal controls [168]. In sum, there is evidence that anabolic/catabolic hormone imbalance is observed in depression. Variation in study results is likely to be due to the heterogeneity of depression as a phenotype and the lack of standard measurement of this phenotype.
4.10. Evidence for the Link between Anabolic/Catabolic Hormone Imbalance and CHD
Bain and colleagues prospectively investigated serum cortisol during hospitalization for acute MI and found that very high levels of cortisol (>2000 nmol/L) predicted mortality [169], a finding that was replicated by Karga and colleagues [170]. A high cortisol/DHEA ratio is thought to create an imbalance between protein synthesis and degradation favoring catabolism over anabolism in patients with chronic heart failure [171]. We know of no studies demonstrating that an imbalance is associated with CHD recurrence/death outcomes in post-ACS patients. Cortisol and DHEA are both secreted in response to ACTH released from the pituitary gland, hence, the two steroids tend to correlate. In a study in intensive care unit patients, age-adjusted correlations between early morning DHEA and cortisol ranged between 0.47 (surgical intensive care unit patients), 0.62 (medical intensive care unit patients), and 0.69 (neurologic intensive care unit patients) [172]. In contrast to cortisol, DHEA has been associated with antidepressive and neuroprotective properties, and the cortisol/DHEA ratio has been discussed as a measure of the relative activity of the two steroids with two types of hormone profiles posing an “endocrine risk”: higher cortisol levels with normal DHEA, and normal cortisol levels with lower DHEA leading to the same functional outcome [173].
4.11. Summary of Biological Mechanisms
While many promising mechanisms have been reviewed, we have little direct human evidence that any of these are causally implicated in the pathogenesis associated with depression comorbid with CHD. A recent review of animal studies [174] suggests that most of the above mechanisms continue to be plausible, but until we move into human experiments or trials, there remains many future studies that are required to definitively answer which biological mechanisms are implicated in the depression—ACS recurrence association.
4.12. Evidence for the Link between Behavioral Mechanisms and Depression
Depression may also influence post-ACS outcomes through its effects on a patient's behaviors, such as adherence to prescribed medications [175, 176] and/or to secondary prevention recommendations after ACS [177]. In addition, there may be disparities in the way the healthcare system behaves towards depressed patients, and these differences, for example, the treatment they receive, may lead to worse outcomes [178]. Although it is now widely accepted that depression after ACS predicts poorer medical prognosis, there remains a gap in our knowledge about which of the proposed behavioral mechanisms might explain this association.
Prior research shows that overall, a diagnosis of depression is associated with poor adherence among patients with a number of chronic medical illnesses, including HIV [179], hypertension [180], diabetes [181], and ACS [182]. Our own research has shown that patients with persistent depressive symptoms after ACS are also less likely to adhere to secondary CHD prevention behaviors such as exercising regularly and quitting smoking [177]. Furthermore, we have data showing that as compared to nondepressed patients, patients with depression are at greatest risk for poor medication adherence [183].
In the case of ACS, not only secondary prevention behaviors, but behaviors pertaining to managing ACS symptoms have been shown to impact medical outcomes. For example, delay in the time from when patients develop symptoms of chest pain to the time that they present for medical care is clearly associated with worse post-ACS prognosis [184]. A recent publication indicates that depressed patients take on average 12 hours longer than nondepressed patients to present to ER [185]. While intermediary depression phenotypes were not differentiated in this sample, it may be the Melancholic depressed patients who largely explain this difference.
Finally, as a result of the cognitive, affective, and social characteristics of mental illnesses, patients with depression can be stigmatized by their illness [186]. This stigma may lead to lower rates of treatment for cardiac disease, or poorer communication about secondary prevention behaviors. Patients with depression tend to have flat affect and to be less engaging; they therefore may be most susceptible to such physician bias. Indeed, differences in the receipt of ACS treatment among patients with and without mental illness have been documented. Druss et al. showed that individuals with co-morbid mental disorders were less likely to undergo coronary revascularization procedures than those without mental disorders [178].
4.13. Evidence for the Link between Poor Adherence Behaviors and CHD
Poor adherence to behaviors recommended for managing medical illnesses is well established as an important factor in determining medical outcomes for a range of diseases. For example, in a meta-analysis comparing high adherers with low adherers across a broad group of illnesses, treatment adherence accounted for 26% of the difference in outcomes [187]. Nonadherence to cardiovascular medications after ACS is clearly linked with poor medical outcomes. For example, Biondi-Zoccai et al. published a meta-analysis of the hazards of not adhering to aspirin among 50,279 CAD patients and showed that aspirin nonadherence was associated with a 3-fold higher MACE risk [188]. Gehi et al. showed that self-reported nonadherence to medications independently predicted adverse cardiac events (hazard ratio 2.3, 95% CI 1.3–4.3) in a cohort of stable CAD patients followed for nearly four years [189]. Rasmussen et al. showed an advantage in long-term survival in response to improved cardiovascular drug adherence after MI in a population-based sample [176]. Discontinuation of aspirin, statins, and beta blockers was independently associated with higher mortality (HR 3.81; 95% CI 1.88–7.72) in a cohort of 1,521 post-MI patients when medication use was assessed by patient-report [175].
4.14. Evidence for the Link between Medication Adherence and Depression
Although there are a number of potential behavioral mechanisms linking depression and post-ACS outcomes, poor medication adherence represents the most promising and best-supported mechanism for explaining this association. For example, in a study of 940 outpatients with stable CHD, 14% of patients with major depression reported that they were not taking their medication as prescribed, compared to five percent of patients without depression [190]. In contrast, in this same population of patients with stable CHD, no association was found between inflammation markers, a potential biological mechanism linking depression with post-ACS outcomes, and the complex phenotype of depression [191]. While these studies strengthen the causal link between nonadherence to cardiovascular medications and outcomes in post-ACS patients, they assessed medication adherence by self-report or by administrative databases rather than by using electronic medication monitoring, which is the gold-standard for measuring adherence. Furthermore, these studies did not consider patients from across the full spectrum of ACS diagnoses.
Among post-ACS patients, we have reported that 42% of persistently depressed patients took their prescribed aspirin less than three-fourths of the time, while only 10.5% of nondepressed patients demonstrated this level of nonadherence (P < .001) [192]. In a separate analysis of this same group of patients using a cross-lagged path analytic model, we showed that improvements in depressive symptoms in the first month after the ACS were associated with improved aspirin adherence in the subsequent two months (standardized direct effect −0.32, P = .016) [183], further establishing the close relationship between depression and medication adherence.
4.15. Behavioral Mechanism Summary
Clearly, more detailed information on the mechanisms linking depression to CHD outcomes is needed to better tailor interventions. Confirming that electronically-monitored adherence is a mediator of this relationship in depressed patients will provide the evidence needed to develop tailored interventions for this subgroup of depressed post-ACS patients. Furthermore, assessing whether other patient and systems/physician behavioral factors additionally mediate poor outcomes can further inform the development of tailored interventions, and our understanding of how depression confers post-ACS prognostic risk.
5. Screening for Depression Comorbid with Coronary Heart Disease
5.1. Depression in CHD Patients Should Be Screened for and Treated, according to Numerous Guidelines
The strength of the depression to CHD outcome observational findings reviewed above has led many to advise routine depression screening for all CHD patients and referral for treatment when indicated, the approach incorporated into the recent advisory from the American Heart Association (AHA) [41] and endorsed by the American Psychiatric Association. Specifically, the advisory recommends administering a depression screening questionnaire to ACS patients and referring those who screen positive to a professional qualified to diagnose and manage depression (see Figure 2). We now have advisories/guidelines from AHA [41, 193], American Academy of Family Practitioners [194], European professional cardiology societies [195], and the British health care system [196] all endorsing depression screening in CHD patients, and then referral for treatment if depression is detected. However, there are no randomized controlled trials (RCTs) to inform this large, potentially expensive health care policy/guideline screening recommendation.
Figure 2.
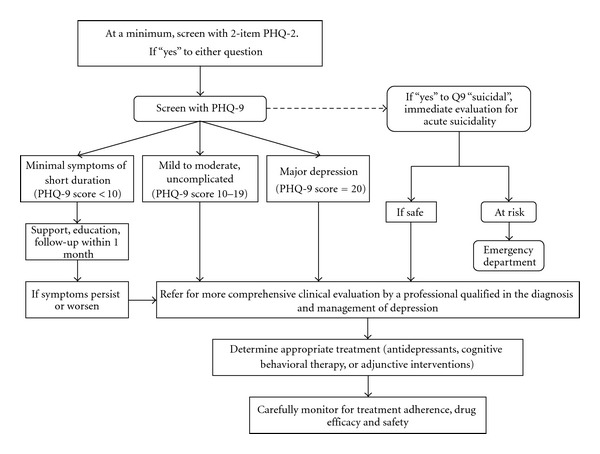
American Heart Association's Advisory for Depression Detection and Treatment (reprinted from Lichtman et al. [41]).
5.2. Perceived Barriers Interfere with Implementation of Depression Screening and Comprehensive Treatment in CHD Patients
In one recent examination of a quality initiative for depression screening, provider reported barriers to depression screening for CHD patients included: too little time, lack of professionals to refer to, and lack of education about logistics and usefulness [197]. Similarly, a 2012 education pretest offered by Medscape before a continuing medical education exercise on management of depression in patients with CHD revealed that the top three barriers reported by both cardiologists and primary care providers were: too little time, insufficient mental health network referrals, and lack of evidence to support this approach (January 2012; personal communication).
5.3. Health-Related Quality of Life Improvements and Cost Effectiveness of Providing CHD Patients with the AHA Depression Screen and Treat Algorithm Are Unknown
There is a paucity of information from either observational or randomized controlled trials to estimate the costs and benefits of screening and treating depression in post-ACS patients. Only one randomized sertraline placebo-controlled trial, SADHART [198] (n = 369) has been used to estimate costs, but this did not include cost-effectiveness and was not a depression screening RCT [199]. This analysis noted that many costs were not represented, as they were not collected.
A meta-analysis of RCTs testing depression screening with only notification of depression severity to primary care providers for primary care patients (NOT post-ACS patients) found that this strategy did not lead to increased detection or treatment of depression, and showed no impact on health-related quality of life, depressive symptoms, or other patient outcomes, including cost effectiveness [200]. An update of this Cochrane review found essentially the same disappointing results [201]. In a recent study examining the costs of conducting annual, lifetime, or every 5 year depression screening for primary care patients, the costs per QALY were unacceptable: the expected annual cost was $192,444 /QALY [202]. A nonstationary Markov model, using published literature, found that no depression screening was preferable over annual depression screening, and in the vast majority (99%) of scenarios, the cost per QALY was more than $50,000, with little expectation of patient benefit [202]. This heavy favoring of no screening makes sense in a primary care setting in which depression's association with clinical outcomes is less robust than in a post-ACS population. Thus, the evidence from RCTs in a primary care population have found that providing primary care patients with the depression screening and then treating have minimal quality of life improvements, and/or are cost ineffective.
5.4. The Importance of RCTs in Evidence-Based Clinical Guidelines
Evidence-based practice guidelines are often distinguished from consensus-based practice guidelines or advisories, as the former systematically review all available research on the specified topic, and then grade the level of evidence to make a clinical recommendation [203, 204]. However, not all research designs are given equal weight; standards of evidence for guidelines have evolved to place greater emphasis on RCTs [205]. The reason for this is that these designs are the most replicable, have the fewest sources of bias, and all else being equal, have the greatest power to detect evidence that a screening practice or treatment results in a net benefit or net harm [206].
5.5. Screening Guidelines/Advisories in the Absence of RCT Evidence Have Recently Been Extensively Criticized (and Withdrawn)
As of the writing of this paper, a controversy is brewing over the reversal in one national screening guideline's recommendation [207–209]. Prostate cancer prevention screening had been considered equivocally beneficial by the U.S. Preventive Services Task Force (USPSTF) in 2002 [210] and 2008 [211], in large part because the observational data and common sense of this approach (the USPSTF found good evidence that PSA screening can detect early-stage prostate cancer). However, USPSTF on review of the most recent evidence, recently recommended against prostate-specific antigen-based screening for prostate cancer (grade D recommendation: there is moderate or high certainty that screening has no net benefit) for all men in the general US population, regardless of age [208]. They reversed their screening recommendation by reviewing the current body of evidence on the benefits and harms from recently conducted RCTs, data that were not available when screening was first thought to be useful. It is entirely possible that such a fate could await screening for depression in CHD patients. That is, current screening recommendations in clinical guidelines and AHA advisories are consensus-, rather than evidence-based, and the evidence, where it exists, does not favor depression screening [212].
6. Randomized Controlled Trials Treating Depression Comorbid with Coronary Heart Disease
The establishment of depression as a risk marker in patients with CHD prompted the National Heart, Lung, & Blood Institute to fund the Enhancing Recovery in CHD Patients (ENRICHD) trial, which randomized almost 2500 patients to determine whether treating depression and social isolation after acute MI improves event-free survival. There were no significant differences in all-cause mortality or nonfatal MI between the intervention and usual care arms of ENRICHD, nor in underpowered trials such as SADHART [198] and MIND-IT [213]. ENRICHD and these first generation phase II trials yielded only modest differences in depression between the treatment and control arms. Plausible reasons include: (1) the interventions were not efficacious, (2) the treatments were not well accepted, (3) the control conditions improved depression more than expected, and (4) the appropriate patient population was not studied. The ENRICHD investigators concluded that the next large, Phase III trial should be postponed until more efficacious depression treatments are available and the subtypes of depression that are most responsible for increased medical morbidity and mortality have been identified. Progress since then includes the STAR*D trial, [214] which demonstrated that aggressive, stepped-care delivery of existing therapies achieves better depression outcomes, several Phase II clinical trials that have demonstrated better depression outcomes in cardiac populations, and advances in our understanding of the characteristics of high-risk depression subtypes.
Freedland et al. [215] conducted an RCT in 123 patients with major or minor depression who had recently undergone CABG surgery. The primary purpose of the trial was to determine the efficacy of 2 behavioral treatments (cognitive behavioral therapy [CBT] or supportive stress management) compared with usual care. The depression effect size for CBT at treatment end of 3 months was yielded a BDI depression effect size of 0.85 (95% CI, 0.41–1.32). This effect was maintained 6 months after the end of the trial.
Huffman and others recently completed a randomized controlled trials of 175 hospitalized depressed cardiac patients [216]. They used a low-intensity depression collaborative care treatment, compared to usual care. The care was initiated in hospital, and then continued by telephone. Depression was assessed by the PHQ-9, using DSM-IV MDD criteria. All patients in the treatment arm received behavioral activation, and then by patient preference and/or prior treatment history received psychotherapy or pharmacotherapy. At 3 months (treatment end), treated patients had PHQ-9 score difference between group changes of −3.43 (95% CI: −5.41–−1.45), and this was not quite maintained at 6 months (when no treatment was offered for the prior 3 months): −1.77 (95% CI: −3.76–0.22).
The COPES II [217, 218] post-ACS trial tested the acceptability and efficacy of 6-month Stepped Depression Care, a patient preference-driven, stepped algorithm depression intervention, to Referred Depression Care, in which depression screening was followed by physician notification of depression and encouragement to initiate depression treatment. The protocol included 157 CHD patients with depressive symptoms, and was not limited to those who met DSM-IV criteria for major depressive disorder. This strategy targeted the ACS patients who are at greatest mortality risk based on recent studies of depression and cardiac outcomes, namely those with postdischarge depressive symptoms. COPES II employed an aggressive, stepped care, patient preference, symptom-driven approach that increased the acceptability and efficacy of the depression intervention. Depression treatment acceptability was three times higher in the stepped depression care group than in the referred care group. The differential change in depression between groups yielded an effect size of 0.59 (95% CI, 0.18–1.00).
Whether the COPES intervention can be delivered as intended in multiple clinical centers was then addressed by CODIACS I, a feasibility/vanguard study conducted at 5 U.S. sites [219]. In this vanguard, the COPES protocol has been streamlined, case-finding made more efficient, and treatment delivery centralized and conducted by a web-based interface and then by telephone. Leading experts have concluded that it is time to conduct the next Phase III depression trial in CHD patients [220–223], as to date, treating depression after ACS has not resulted in improved cardiovascular outcomes [213, 224], primarily because we do not have large RCTs that actually test this question.
7. Summary
CHD patients are quite likely to have subsyndromal or syndromal depression, which is associated with compromised quality of life, increased health care and societal costs, increased recurrence of CHD, and shortened life. We only have one early, adequately powered trial to test if depression reduction improved morbidity and mortality, and it did not [224]. We have few randomized controlled trials on which to base treatment decisions, but recent ones suggest that patient preference, collaborative care, and cognitive behavioral therapy all have promise for decreasing depression rates and improving quality of life. Clinicians should only screen for depression in their patients with CHD if they have the resources and system to conduct a thorough psychiatric diagnostic workup, refer or support patients found to be at imminent harm, and provide the depression treatment options needed to manage this relapsing, remitting disorder. For health care providers who do not have such a depression management system in place, referrals to appropriate sources of depression care is a better way to proceed. However, it behooves all of us to remain vigilant to the cardiovascular risk factor management issues that are faced by a depressed patient. Increasing structure, support systems, and other facilitators of adherence are important in these patients, as adherence is often a behavioral challenge they cannot manage. Finally, in the absence of more definitive tests of the biological and behavioral mechanisms by which depression may confer CHD risk in CHD patients, and in the absence of definitive trials testing if treating depression reduces this risk, we remain unsure if depression is a potent treatment target, or epiphenomena.
Conflict of Interests
The author has no conflict of interests to declare.
Acknowledgments
This work was supported by Grants HL-088117, HL-101663, and HL-84034 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics-2010 update: a report from the american heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Freedland KE. Depression in patients with coronary heart disease. American Journal of Medicine. 2008;121(11, supplement):S20–S27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial infarction depression. Evidence Report/Technology Assessment. 2005;(123):1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Van Melle JP, De Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosomatic Medicine. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic Medicine. 2005;67(supplement 1):S19–S25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC, USA: American Psychiatric Association; 1994. [Google Scholar]
- 9.McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biological Psychiatry. 2003;54(3):363–375. doi: 10.1016/s0006-3223(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulos GS. Vascular disease, depression, and dementia. Journal of the American Geriatrics Society. 2003;51(8):1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 11.Snyder S, Strain JJ, Wolf D. Differentiating major depression from adjustment disorder with depressed mood in the medical setting. General Hospital Psychiatry. 1990;12(3):159–165. doi: 10.1016/0163-8343(90)90074-m. [DOI] [PubMed] [Google Scholar]
- 12.Pincus HA, Davis WW, Mcqueen LE. ‘Subthreshold’ mental disorders: a review and synthesis of studies on minor depression and other ‘brand names’. British Journal of Psychiatry. 1999;174:288–296. doi: 10.1192/bjp.174.4.288. [DOI] [PubMed] [Google Scholar]
- 13.Broadhead WE, Blazer DG, George LK, Tse CK. Depression, disability days, and days lost from work in a prospective epidemiologic survey. Journal of the American Medical Association. 1990;264(19):2524–2528. [PubMed] [Google Scholar]
- 14.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders. 1998;50(2-3):97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 15.Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Archives of General Psychiatry. 1998;55(8):694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 16.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. American Journal of Cardiology. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 17.Penninx BW, Beekman ATF, Honig A, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Archives of General Psychiatry. 2001;58(3):221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. The British Journal of Psychiatry. Supplement. 1996;(30):17–30. [PubMed] [Google Scholar]
- 19.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological Psychiatry. 1999;46(11):1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000;886(1-2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC, USA: American Psychiatric Association; 1995. [Google Scholar]
- 22.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 23.Williams JBW, Gibbon M, First MB, et al. The structured clinical interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49(8):630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 24.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosomatic Medicine. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 25.Hickie IB, Andrews G, Davenport TA. Measuring outcomes in patients with depression or anxiety: an essential part of clinical practice. Medical Journal of Australia. 2002;177(4):205–207. doi: 10.5694/j.1326-5377.2002.tb04734.x. [DOI] [PubMed] [Google Scholar]
- 26.Maruish ME. Handbook of Psychological Assessment in Primary Care Settings. Mahwah, NJ, USA: Lawrence Erlbaum; 2000. Handbook of psychological assessment in primary care settings. [Google Scholar]
- 27.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, Tex, USA: Psychological Corporation; 1993. [Google Scholar]
- 28.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, Tex, USA: Psychological Corporation; 1996. [Google Scholar]
- 29.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 30.Lespérance F, Frasure-Smith N, Juneau M, Théroux P. Depression and 1-year prognosis in unstable angina. Archives of Internal Medicine. 2000;160(9):1354–1360. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 31.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. American Journal of Preventive Medicine. 2002;23(1):51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 32.Derogatis LR, Lynn LL., II . Handbook of Psychological Assessment in Primary Care Settings. Mahwah, NJ, USA: Lawrence Erlbaum; 2000. Screening and monitoring psychiatric disorder in primary care populations; pp. 115–152. [Google Scholar]
- 33.Eaton WW, Kalaydjian A, Scharfstein DO, Mezuk B, Ding Y. Prevalence and incidence of depressive disorder: the Baltimore ECA follow-up, 1981–2004. Acta Psychiatrica Scandinavica. 2007;116(3):182–188. doi: 10.1111/j.1600-0447.2007.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Canadian Journal of Psychiatry. 2006;51(12):730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 35.Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. Journal of Psychosomatic Research. 2007;62(4):401–410. doi: 10.1016/j.jpsychores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Rumsfeld JS, Magid DJ, Plomondon ME, et al. History of depression, angina, and quality of life after acute coronary syndromes. American Heart Journal. 2003;145(3):493–499. doi: 10.1067/mhj.2003.177. [DOI] [PubMed] [Google Scholar]
- 37.Lane D, Carroll D, Ring C, Beevers DG, Lip GYH. Mortality and quality of life 12 months after myocardial infarction: effects of depression and anxiety. Psychosomatic Medicine. 2001;63(2):221–230. doi: 10.1097/00006842-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the heart and soul study. Journal of the American Medical Association. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egede LE. Depression: greater effect on overall health than angina, arthritis, asthma or diabetes: commentary. Evidence-Based Mental Health. 2008;11(2, article 57) doi: 10.1136/ebmh.11.2.57. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RH, Montoye CK, Faul J, et al. Enhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use: the American College of Cardiology Acute Myocardial Infarction Guidelines Applied in Practice Project in Michigan: Flint and Saginaw Expansion. Journal of the American College of Cardiology. 2004;43(12):2166–2173. doi: 10.1016/j.jacc.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 41.Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment—a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 42.Von Korff M, Ormel J, Katon W, Lin EHB. Disability and depression among high utilizers of health care: a longitudinal analysis. Archives of General Psychiatry. 1992;49(2):91–100. doi: 10.1001/archpsyc.1992.01820020011002. [DOI] [PubMed] [Google Scholar]
- 43.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. Journal of the American Medical Association. 2003;289(23):3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 44.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. General Hospital Psychiatry. 2007;29(5):409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Rutledge T, Vaccarino V, Johnson BD, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia. Prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. Journal of the American College of Cardiology. 2009;53(2):176–183. doi: 10.1016/j.jacc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frasure-Smith N, Lespérance F, Gravel G, et al. Depression and health-care costs during the first year following myocardial infarction. Journal of Psychosomatic Research. 2000;48(4-5):471–478. doi: 10.1016/s0022-3999(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 47.Wulsin LR. Is depression a major risk factor for coronary disease? A systematic review of the epidemiologic evidence. Harvard Review of Psychiatry. 2004;12(2):79–93. doi: 10.1080/10673220490447191. [DOI] [PubMed] [Google Scholar]
- 48.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine. 2003;65(2):201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 49.Watkins LL, Schneiderman N, Blumenthal JA, et al. Cognitive and somatic symptoms of depression are associated with medical comorbidity in patients after acute myocardial infarction. American Heart Journal. 2003;146(1):48–54. doi: 10.1016/S0002-8703(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 50.Lauzon C, Beck CA, Huynh T, et al. Depression and prognosis following hospital admission because of acute myocardial infarction. CMAJ. 2003;168(5):547–552. [PMC free article] [PubMed] [Google Scholar]
- 51.Carney RM, Blumenthal JA, Catellier D, et al. Depression as a risk factor for mortality after acute myocardial infarction. American Journal of Cardiology. 2003;92(11):1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 53.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosomatic Medicine. 2004;66(3):305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 54.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. Journal of Psychosomatic Research. 2002;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 55.Shimbo D, Davidson KW, Haas DC, Fuster V, Badimon JJ. Negative impact of depression on outcomes in patients with coronary artery disease: mechanisms, treatment considerations, and future directions. Journal of Thrombosis and Haemostasis. 2005;3(5):897–908. doi: 10.1111/j.1538-7836.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- 56.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. Journal of the American College of Cardiology. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Goldston K, Baillie AJ. Depression and coronary heart disease: a review of the epidemiological evidence, explanatory mechanisms and management approaches. Clinical Psychology Review. 2008;28(2):288–306. doi: 10.1016/j.cpr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Parissis JT, Fountoulaki K, Filippatos G, Adamopoulos S, Paraskevaidis I, Kremastinos D. Depression in coronary artery disease: novel pathophysiologic mechanisms and therapeutic implications. International Journal of Cardiology. 2007;116(2):153–160. doi: 10.1016/j.ijcard.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 59.Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology. 2000;20(2):137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 60.Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and β-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biological Psychiatry. 1997;42(4):290–295. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 61.Serebruany VL, Glassman AH, Malinin AI, et al. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagulation and Fibrinolysis. 2003;14(6):563–567. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. American Journal of Cardiology. 2002;89(4):419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 63.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. European Heart Journal. 2004;25(6):492–499. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 64.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2004;164(9):1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 65.Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104(17):2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 66.Guinjoan SM, De Guevara MSL, Correa C, et al. Cardiac parasympathetic dysfunction related to depression in older adults with acute coronary syndromes. Journal of Psychosomatic Research. 2004;56(1):83–88. doi: 10.1016/S0022-3999(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 67.Biasucci LM. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110(25):e560–567. doi: 10.1161/01.CIR.0000148983.88334.80. [DOI] [PubMed] [Google Scholar]
- 68.Wallén NH, Held C, Rehnqvist N, Hjemdahl P. Elevated serum intercellular adhesion molecule-1 and vascular adhesion molecule-1 among patients with stable angina pectoris who suffer cardiovascular death or non-fatal myocardial infarction. European Heart Journal. 1999;20(14):1039–1043. doi: 10.1053/euhj.1999.1451. [DOI] [PubMed] [Google Scholar]
- 69.Mulvihill NT, Foley JB, Murphy RT, Curtin R, Crean PA, Walsh M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart. 2001;85(6):623–627. doi: 10.1136/heart.85.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 71.Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27(7):805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- 72.Goodyer IM, Herbert J, Altham PME, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine. 1996;26(2):245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- 73.Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychological Medicine. 2003;33(4):601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- 74.Maes M. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders. 1995;34(4):301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 75.Maes M. A review on the acute phase response in major depression. Reviews in the Neurosciences. 1993;4(4):407–416. doi: 10.1515/revneuro.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- 76.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 77.Kronfol Z, House JD. Lymphocyte mitogenesis, immunoglobulin and complement levels in depressed patients and normal controls. Acta Psychiatrica Scandinavica. 1989;80(2):142–147. doi: 10.1111/j.1600-0447.1989.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 78.Goebel MU, Mills PJ. Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and density. Psychosomatic Medicine. 2000;62(5):664–670. doi: 10.1097/00006842-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Schaefer M, Horn M, Schmidt F, et al. Correlation between sICAM-1 and depressive symptoms during adjuvant treatment of melanoma with interferon-α . Brain, Behavior, and Immunity. 2004;18(6):555–562. doi: 10.1016/j.bbi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Lespérance F, Frasure-Smith N, Théroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. American Journal of Psychiatry. 2004;161(2):271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 81.Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. American Journal of Cardiology. 2005;95(3):317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 82.Shimbo D, Rieckmann N, Paulino R, Davidson KW, Shimbo D. Relation between C reactive protein and depression remission status in patients presenting with acute coronary syndrome. Heart. 2006;92(9):1316–1318. doi: 10.1136/hrt.2005.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Annique S, Dorien T, Richel L, et al. Inflammatory markers in depressed post-myocardial infarction patients. Journal of Psychiatric Research. 2005;39(2):137–144. doi: 10.1016/j.jpsychires.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Parissis JT, Adamopoulos S, Rigas A, et al. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. American Journal of Cardiology. 2004;94(10):1326–1328. doi: 10.1016/j.amjcard.2004.07.127. [DOI] [PubMed] [Google Scholar]
- 85.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosomatic Medicine. 2005;67(supplement 1):S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 86.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98(6):547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 87.Kim CK, McGorray SP, Bartholomew BA, et al. Depressive symptoms and heart rate variability in postmenopausal women. Archives of Internal Medicine. 2005;165(11):1239–1244. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 88.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the heart and soul study. Archives of General Psychiatry. 2005;62(6):661–666. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rechlin T, Weis M, Claus D. Heart rate variability in depressed patients and differential effects of paroxetine and amitriptyline on cardiovascular autonomic functions. Pharmacopsychiatry. 1994;27(3):124–128. doi: 10.1055/s-2007-1014291. [DOI] [PubMed] [Google Scholar]
- 90.Carney RM, Freedland KE, Veith RC, et al. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biological Psychiatry. 1999;45(4):458–463. doi: 10.1016/s0006-3223(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 91.Koenigsberg HW, Teicher MH, Mitropoulou V, et al. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. Journal of Psychiatric Research. 2004;38(5):503–511. doi: 10.1016/j.jpsychires.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 92.Esler M, Turbott J, Schwarz R, et al. The peripheral kinetics of norepinephrine in depressive illness. Archives of General Psychiatry. 1982;39(3):295–300. doi: 10.1001/archpsyc.1982.04290030035006. [DOI] [PubMed] [Google Scholar]
- 93.Wong ML, Kling MA, Munson PJ, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(1):325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veith RC, Lewis N, Linares OA, et al. Sympathetic nervous system activity in major depression: basal and desipramine-induced alterations in plasma norepinephrine kinetics. Archives of General Psychiatry. 1994;51(5):411–422. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- 95.Louis WJ, Doyle AE, Anavekar SN. Plasma noradrenaline concentration and blood pressure in essential hypertension, phaeochromocytoma and depression. Clinical Science and Molecular Medicine. Supplement. 1975;2:239s–242s. doi: 10.1042/cs048239s. [DOI] [PubMed] [Google Scholar]
- 96.Roy A, Pickar D, De Jong J, Karoum F, Linnoila M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine. Relationship to hypothalamic-pituitary-adrenal axis function in depression. Archives of General Psychiatry. 1988;45(9):849–857. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- 97.Wyatt RJ, Portnoy B, Kupfer DJ, Snyder F, Engelman K. Resting plasma catecholamine concentrations in patients with depression and anxiety. Archives of General Psychiatry. 1971;24(1):65–70. doi: 10.1001/archpsyc.1971.01750070067009. [DOI] [PubMed] [Google Scholar]
- 98.Gold PW, Wong ML, Goldstein DS, et al. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. Journal of Psychosomatic Research. 2004;57(4):353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 100.Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the heart and soul study. American Journal of Psychiatry. 2005;162(11):2139–2145. doi: 10.1176/appi.ajp.162.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwartz PJ. The autonomic nervous system and sudden death. European Heart Journal. 1998;19:F72–F80. [PubMed] [Google Scholar]
- 102.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cadiac death in chronic heart failure patients. Circulation. 2003;107(4):565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 103.La Rovere MT, Pinna GD, Hohnloser SH, et al. Barorefiex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103(16):2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 104.Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 105.Camm AJ, Pratt CM, Schwartz PJ, et al. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109(8):990–996. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 106.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet. 1998;351(9101):478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 107.Huikuri HV, Jokinen V, Syvänne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(8):1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 108.Muller JE, Tofler GH. Triggering and hourly variation of onset of arterial thrombosis. Annals of Epidemiology. 1992;2(4):393–405. doi: 10.1016/1047-2797(92)90088-8. [DOI] [PubMed] [Google Scholar]
- 109.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 110.Smith M, Little WC. Potential precipitating factors of the onset of myocardial infarction. American Journal of the Medical Sciences. 1992;303(3):141–144. doi: 10.1097/00000441-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 111.Willich SN. Epidemiologic studies demonstrating increased morning incidence of sudden cardiac death. American Journal of Cardiology. 1990;66(16):15G–17G. doi: 10.1016/0002-9149(90)90387-g. [DOI] [PubMed] [Google Scholar]
- 112.Willich SN, Linderer T, Wegscheider K, Leizorovicz A, Alamercery I, Schroder R. Increased morning incidence of myocardial infarction in the ISAM study: absence with prior β-adrenergic blockade. Circulation. 1989;80(4):853–858. doi: 10.1161/01.cir.80.4.853. [DOI] [PubMed] [Google Scholar]
- 113.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. American Journal of Cardiology. 1997;79(11):1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 114.Andrews NP, Goldstein DS, Quyyumi AA. Effect of systemic alpha-2 adrenergic blockade on the morning increase in platelet aggregation in normal subjects. American Journal of Cardiology. 1999;84(3):316–320. doi: 10.1016/s0002-9149(99)00283-0. [DOI] [PubMed] [Google Scholar]
- 115.Vita JA, Treasure CB, Yeung AC, et al. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation. 1992;85(4):1390–1397. doi: 10.1161/01.cir.85.4.1390. [DOI] [PubMed] [Google Scholar]
- 116.Benca RM, Obermeyer WH, Thisted RA, Gillin JC, Kupfer DJ, Reynolds CF. Sleep and psychiatric disorders: a meta-analysis. Archives of General Psychiatry. 1992;49(8):651–670. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 117.John U, Meyer C, Rumpf HJ, Hapke U. Relationships of psychiatric disorders with sleep duration in an adult general population sample. Journal of Psychiatric Research. 2005;39(6):577–583. doi: 10.1016/j.jpsychires.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 118.Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth. 2005;32(3):187–193. doi: 10.1111/j.0730-7659.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 119.Reynolds CF, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10(3):199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 120.Benca RM, Obermeyer WH, Thisted RA, Gillin JC, Kupfer DJ, Reynolds III CF. Sleep and psychiatric disorders: a meta-analysis. Archives of General Psychiatry. 1992;49(8):651–670. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 121.Kupfer DJ, Thase ME. The use of the sleep laboratory in the diagnosis of affective disorders. Psychiatric Clinics of North America. 1983;6(1):3–25. [PubMed] [Google Scholar]
- 122.Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Research. 2000;95(3):201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 123.Shen J, Chung SA, Kayumov L, et al. Polysomnographic and symptomatological analyses of major depressive disorder patients treated with mirtazapine. Canadian Journal of Psychiatry. 2006;51(1):27–34. doi: 10.1177/070674370605100106. [DOI] [PubMed] [Google Scholar]
- 124.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Archives of Internal Medicine. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 125.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. New England Journal of Medicine. 1993;328(5):303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 126.Thompson C, Franey C, Arendt J, Checkley SA. A comparison of melatonin secretion in depressed patients and normal subjects. British Journal of Psychiatry. 1988;152:260–265. doi: 10.1192/bjp.152.2.260. [DOI] [PubMed] [Google Scholar]
- 127.Nowlin JB, Troyer WG, Collins WS, et al. The association of nocturnal angina pectoris with dreaming. Annals of Internal Medicine. 1965;63(6):1040–1046. doi: 10.7326/0003-4819-63-6-1040. [DOI] [PubMed] [Google Scholar]
- 128.Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovascular Research. 1996;31(2):181–211. [PubMed] [Google Scholar]
- 129.Vanoli E, Adamson PB, Ba L, Pinna GD, Lazzara R, Orr WC. Heart rate variability during specific sleep stages: a comparison of healthy subjects with patients after myocardial infarction. Circulation. 1995;91(7):1918–1922. doi: 10.1161/01.cir.91.7.1918. [DOI] [PubMed] [Google Scholar]
- 130.Kunz D, Herrmann WM. Sleep-wake cycle, sleep-related disturbances, and sleep disorders: a chronobiological approach. Comprehensive Psychiatry. 2000;41(2, supplement 1):104–115. doi: 10.1016/s0010-440x(00)80016-4. [DOI] [PubMed] [Google Scholar]
- 131.Avery DH, Shah SH, Eder DN, Wildschiødtz G. Nocturnal sweating and temperature in depression. Acta Psychiatrica Scandinavica. 1999;100(4):295–301. doi: 10.1111/j.1600-0447.1999.tb10864.x. [DOI] [PubMed] [Google Scholar]
- 132.Avery DH, Wildshiodtz G, Rafaelsen OJ. Nocturnal temperature in affective disorder. Journal of Affective Disorders. 1982;4(1):61–71. doi: 10.1016/0165-0327(82)90020-9. [DOI] [PubMed] [Google Scholar]
- 133.Duncan WC., Jr. Circadian rhythms and the pharmacology of affective illness. Pharmacology and Therapeutics. 1996;71(3):253–312. doi: 10.1016/s0163-7258(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 134.Souetre E, Salvati E, Belugou JL, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Research. 1989;28(3):263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 135.Souetre E, Salvati E, Wehr TA, Sack DA, Krebs B, Darcourt G. Twenty-four-hour profiles of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal control subjects. American Journal of Psychiatry. 1988;145(9):1133–1137. doi: 10.1176/ajp.145.9.1133. [DOI] [PubMed] [Google Scholar]
- 136.Szuba MP, Guze BH, Baxter LR. Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biological Psychiatry. 1997;42(12):1130–1137. doi: 10.1016/s0006-3223(97)00046-2. [DOI] [PubMed] [Google Scholar]
- 137.Wetterbuerg L, Beck-Friis J, Aperia B, Petterson U. Melatonin/cortisol ratio in depression. Lancet. 1979;2(8156-8157, article 1361) doi: 10.1016/s0140-6736(79)92837-x. [DOI] [PubMed] [Google Scholar]
- 138.Arendt J, Wirz-Justice A, Bradtke J, Kornemark M. Long-term studies on immunoreactive human melatonin. Annals of Clinical Biochemistry. 1979;16(6):307–312. doi: 10.1177/000456327901600182. [DOI] [PubMed] [Google Scholar]
- 139.Mendlewicz J, Linkowski P, Branchey L, Weinberg U, Weitzman ED, Branchey M. Abnormal 24 hour pattern of melatonin secretion in depression. Lancet. 1979;2(8156-8157, article 1362) doi: 10.1016/s0140-6736(79)92838-1. [DOI] [PubMed] [Google Scholar]
- 140.Nair NPV, Hariharasubramanian N, Pilapil C. Circadian rhythm of plasma melatonin in endogenous depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1984;8(4–6):715–718. doi: 10.1016/0278-5846(84)90044-7. [DOI] [PubMed] [Google Scholar]
- 141.Paparrigopoulos T. Melatonin response to atenolol administration in depression: indication of β-adrenoceptor dysfunction in a subtype of depression. Acta Psychiatrica Scandinavica. 2002;106(6):440–445. doi: 10.1034/j.1600-0447.2002.02342.x. [DOI] [PubMed] [Google Scholar]
- 142.Fountoulakis KN, Karamouzis M, Iacovides A, et al. Morning and evening plasma melatonin and dexamethasone suppression test in patients with nonseasonal major depressive disorder from northern Greece (latitude 40–41.5°) Neuropsychobiology. 2001;44(3):113–117. doi: 10.1159/000054928. [DOI] [PubMed] [Google Scholar]
- 143.Crasson M, Kjiri S, Colin A, et al. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology. 2004;29(1):1–12. doi: 10.1016/s0306-4530(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 144.Miles A, Philbrick DRS. Melatonin and psychiatry. Biological Psychiatry. 1988;23(4):405–425. doi: 10.1016/0006-3223(88)90291-0. [DOI] [PubMed] [Google Scholar]
- 145.Brown RP, Kocsis JH, Caroff S, et al. Depressed mood and reality disturbance correlate with decreased nocturnal melatonin in depressed patients. Acta Psychiatrica Scandinavica. 1987;76(3):272–275. doi: 10.1111/j.1600-0447.1987.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 146.Frazer A, Brown R, Kocsis J, et al. Patterns of melatonin rhythms in depression. Journal of Neural Transmission. Supplement. 1986;21:269–290. [PubMed] [Google Scholar]
- 147.Beck-Friis J, Von Rosen D, Kjellman BF. Melatonin in relation to body measures, sex, age, season and the use of drugs in patients with major affective disorders and healthy subjects. Psychoneuroendocrinology. 1984;9(3):261–277. doi: 10.1016/0306-4530(84)90005-2. [DOI] [PubMed] [Google Scholar]
- 148.Claustrat B, Chazot G, Brun J. A chronobiological study of melatonin and cortisol secretion in depressed subjects: plasma melatonin, a biochemical marker in major depression. Biological Psychiatry. 1984;19(8):1215–1228. [PubMed] [Google Scholar]
- 149.Branchey L, Weinberg U, Branchey M. Simultaneous study of 24-hour patterns of melatonin and cortisol secretion in depressed patients. Neuropsychobiology. 1982;8(5):225–232. doi: 10.1159/000117903. [DOI] [PubMed] [Google Scholar]
- 150.Muller JE, Stone PH, Turi ZG. Circadian variation in the frequency of onset of acute myocardial infarction. New England Journal of Medicine. 1985;313(21):1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 151.Carney RM, Freedland KE, Jaffe AS. Altered circadian pattern of acute myocardial infarction in patients with depression. Coronary Artery Disease. 1991;2(1):61–65. [Google Scholar]
- 152.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996;125(1):1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- 153.Beer NA, Jakubowicz DJ, Matt DW, Beer RM, Nestler JE. Dehydroepiandrosterone reduces plasma plasminogen activator inhibitor type 1 and tissue plasminogen activator antigen in men. American Journal of the Medical Sciences. 1996;311(5):205–210. doi: 10.1097/00000441-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 154.Williams MRI, Ling S, Dawood T, et al. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. Journal of Clinical Endocrinology and Metabolism. 2002;87(1):176–181. doi: 10.1210/jcem.87.1.8161. [DOI] [PubMed] [Google Scholar]
- 155.Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Archives of General Psychiatry. 1987;44(4):328–336. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- 156.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Archives of General Psychiatry. 1976;33(9):1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 157.Halbreich U, Asnis GM, Shindledecker R. Cortisol secretion in endogenous depression. I. Basal plasma levels. Archives of General Psychiatry. 1985;42(9):904–908. doi: 10.1001/archpsyc.1985.01790320076010. [DOI] [PubMed] [Google Scholar]
- 158.Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25(2):267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 159.Bhagwagar Z, Hafizi S, Cowen PJ. Increase in concentration of waking salivary cortisol in recovered patients with depression. American Journal of Psychiatry. 2003;160(10):1890–1891. doi: 10.1176/appi.ajp.160.10.1890. [DOI] [PubMed] [Google Scholar]
- 160.Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 161.Portella MJ, Harmer CJ, Flint J, Cowen P, Goodwin GM. Enhanced early morning salivary cortisol in neuroticism. American Journal of Psychiatry. 2005;162(4):807–809. doi: 10.1176/appi.ajp.162.4.807. [DOI] [PubMed] [Google Scholar]
- 162.Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community: interactions between social adversity, cortisol and serotonin neurotransmission. British Journal of Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- 163.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 164.Holsboer F, Gerken A, Stalla GK, Muller OA. Blunted aldosterone and ACTH release after human CRH administration in depressed patients. American Journal of Psychiatry. 1987;144(2):229–231. doi: 10.1176/ajp.144.2.229. [DOI] [PubMed] [Google Scholar]
- 165.Ferrari E, Mirani M, Barili L, et al. Cognitive and affective disorders in the elderly: a neuroendocrine study. Archives of Gerontology and Geriatrics. Supplement. 2004;(9):171–182. doi: 10.1016/j.archger.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 166.Van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26(6):591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 167.Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. American Journal of Psychiatry. 2002;159(7):1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- 168.Kahl KG, Bens S, Ziegler K, et al. Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biological Psychiatry. 2006;59(7):667–671. doi: 10.1016/j.biopsych.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 169.Bain RJI, Fox JP, Jagger J, Davies MK, Littler WA, Murray RG. Serum cortisol levels predict infarct size and patient mortality. International Journal of Cardiology. 1992;37(2):145–150. doi: 10.1016/0167-5273(92)90201-d. [DOI] [PubMed] [Google Scholar]
- 170.Karga H, Papaioannou P, Venetsanou K, et al. The role of cytokines and cortisol in the non-thyroidal illness syndrome following acute myocardial infarction. European Journal of Endocrinology. 2000;142(3):236–242. doi: 10.1530/eje.0.1420236. [DOI] [PubMed] [Google Scholar]
- 171.Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. Journal of the American College of Cardiology. 1997;30(4):997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 172.Folan MM, Stone RA, Pittenger AL, Stoffel JA, Hess MM, Kroboth PD. Dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and cortisol concentrations in intensive care unit patients. Critical Care Medicine. 2001;29(5):965–970. doi: 10.1097/00003246-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 173.Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. British Journal of Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- 174.Grippo AJ. Mechanisms underlying altered mood and cardiovascular dysfunction: the value of neurobiological and behavioral research with animal models. Neuroscience and Biobehavioral Reviews. 2009;33(2):171–180. doi: 10.1016/j.neubiorev.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Archives of Internal Medicine. 2006;166(17):1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 176.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Journal of the American Medical Association. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 177.Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. Journal of General Internal Medicine. 2006;21(11):1178–1183. doi: 10.1111/j.1525-1497.2006.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Druss BG, Bradford DW, Rosenheck RA, Radford MJ, Krumholz HM. Mental disorders and use of cardiovascular procedures after myocardial infarction. Journal of the American Medical Association. 2000;283(4):506–511. doi: 10.1001/jama.283.4.506. [DOI] [PubMed] [Google Scholar]
- 179.Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45(5):394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- 180.Bonnet F, Irving K, Terra JL, Nony P, Berthezène F, Moulin P. Depressive symptoms are associated with unhealthy lifestyles in hypertensive patients with the metabolic syndrome. Journal of Hypertension. 2005;23(3):611–617. doi: 10.1097/01.hjh.0000160219.71350.d2. [DOI] [PubMed] [Google Scholar]
- 181.Lin EHB, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 182.Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychology. 1995;14(1):88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- 183.Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes. An electronic medication monitoring study. Journal of the American College of Cardiology. 2006;48(11):2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 184.Nallamothu BK, Fox KAA, Kennelly BM, et al. Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events. Heart. 2007;93(12):1552–1555. doi: 10.1136/hrt.2006.112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bunde J, Martin R. Depression and prehospital delay in the context of myocardial infarction. Psychosomatic Medicine. 2006;68(1):51–57. doi: 10.1097/01.psy.0000195724.58085.f0. [DOI] [PubMed] [Google Scholar]
- 186.Penn DL, Martin J. The stigma of severe mental illness: some potential solutions for a recalcitrant problem. Psychiatric Quarterly. 1998;69(3):235–247. doi: 10.1023/a:1022153327316. [DOI] [PubMed] [Google Scholar]
- 187.Dimatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 188.Biondi-Zoccai GGL, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. European Heart Journal. 2006;27(22):2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 189.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Archives of Internal Medicine. 2007;167(16):1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Archives of Internal Medicine. 2005;165(21):2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the heart and soul study. Biological Psychiatry. 2007;62(4):314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rieckmann N, Kronish IM, Haas D, et al. Persistent depressive symptoms lower aspirin adherence after acute coronary syndromes. American Heart Journal. 2006;152(5):922–927. doi: 10.1016/j.ahj.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 193.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Journal of the American College of Cardiology. 2007;49(11):1230–1250. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 194.Green LA, Dickinson WP, Nease DE, et al. AAFP guideline for the detection and management of post-myocardial infarction depression. Annals of Family Medicine. 2009;7(1):71–79. doi: 10.1370/afm.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. European Heart Journal. 2007;28(19):2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 196.National Institute for Health and Clinical Excellence. Depression in Adults with Chronic Physical Health Problems. London, UK: National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 197.Smolderen KG, Buchanan DM, Amin AA, et al. Real-world lessons from the implementation of a depression screening protocol in acute myocardial infarction patients implications for the american heart association depression screening advisory. Circulation: Cardiovascular Quality and Outcomes. 2011;4(3):283–292. doi: 10.1161/CIRCOUTCOMES.110.960013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. Journal of the American Medical Association. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 199.Lattanzio F, Cherubini A, Furneri G, Di Bari M, Marchionni N. Sertraline treatment for depression associated with acute coronary syndromes: a cost analysis from the viewpoint of the Italian Healthcare System. Aging. 2008;20(1):76–80. doi: 10.1007/BF03324751. [DOI] [PubMed] [Google Scholar]
- 200.Gilbody S, House AO, Sheldon TA. Screening and case finding instruments for depression. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD002792.pub2.CD002792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178(8):997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The cost-utility of screening for depression in primary care. Annals of Internal Medicine. 2001;134(5):345–360. doi: 10.7326/0003-4819-134-5-200103060-00007. [DOI] [PubMed] [Google Scholar]
- 203.Guyatt GH, Oxman AD, Kunz R, et al. GRADE: going from evidence to recommendations. BMJ. 2008;336(7652):1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Grimshaw J, Eccles M, Russell I. Developing clinically valid practice guidelines. Journal of Evaluation in Clinical Practice. 1995;1(1):37–48. doi: 10.1111/j.1365-2753.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 205.Atkins D, Briss PA, Eccles M, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Services Research. 2005;5(article 25) doi: 10.1186/1472-6963-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users’ guides to patient care. Journal of the American Medical Association. 2000;284(10):1290–1296. doi: 10.1001/jama.284.10.1290. [DOI] [PubMed] [Google Scholar]
- 207.Payton S. Prostate cancer: new PSA screening guideline faces widespread opposition. Nature Reviews Urology. 2012;9(7, article 351) doi: 10.1038/nrurol.2012.124. [DOI] [PubMed] [Google Scholar]
- 208.Moyer VA. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Annals of Internal Medicine. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 209.Catalona WJ, D'Amico AV, Fitzgibbons WF, et al. What the U.S. preventive services task force missed in its prostate cancer screening recommendation. Annals of Internal Medicine. 2012;157(2):137–138. doi: 10.7326/0003-4819-157-2-201207170-00463. [DOI] [PubMed] [Google Scholar]
- 210.Allen K, Blascovich J, Mendes WB. Cardiovascular reactivity and the presence of pets, friends, and spouses: the truth about cats and dogs. Psychosomatic Medicine. 2002;64(5):727–739. doi: 10.1097/01.psy.0000024236.11538.41. [DOI] [PubMed] [Google Scholar]
- 211.Calonge N, Petitti DB, Dewitt TG, et al. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Annals of Internal Medicine. 2008;149(3):185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 212.Thombs BD, De Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. Journal of the American Medical Association. 2008;300(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 213.Van Melle JP, De Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. British Journal of Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 214.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. American Journal of Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 215.Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery a randomized controlled trial. Archives of General Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huffman JC, Mastromauro CA, Gillian S, Fricchione GL, Healy BC, Januzzi JL. Impact of a depression care management program for hospitalized cardiac patients. Circulation: Cardiovascular Quality and Outcomes. 2011;4(2):198–205. doi: 10.1161/CIRCOUTCOMES.110.959379. [DOI] [PubMed] [Google Scholar]
- 217.Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Archives of Internal Medicine. 2010;170(7):600–608. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemporary Clinical Trials. 2008;29(2):231–240. doi: 10.1016/j.cct.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Whang W, Burg MM, Carney RM, et al. Design and baseline data from the vanguard of the Comparison of Depression Interventions after Acute Coronary Syndrome (CODIACS) randomized controlled trial. Contemporary Clinical Trials. 2012;33(5):1003–1010. doi: 10.1016/j.cct.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Whang W, Davidson KW. Is it time to treat depression in patients with cardiovascular disease? Circulation. 2009;120(2):99–100. doi: 10.1161/CIRCULATIONAHA.109.881987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosomatic Medicine. 2006;68(5):645–650. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 222.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111(3):250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 223.Liu SS, Ziegelstein RC. Depression in patients with heart disease: the case for more trials. Future Cardiology. 2010;6(4):547–556. doi: 10.2217/fca.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. Journal of the American Medical Association. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]


