Abstract
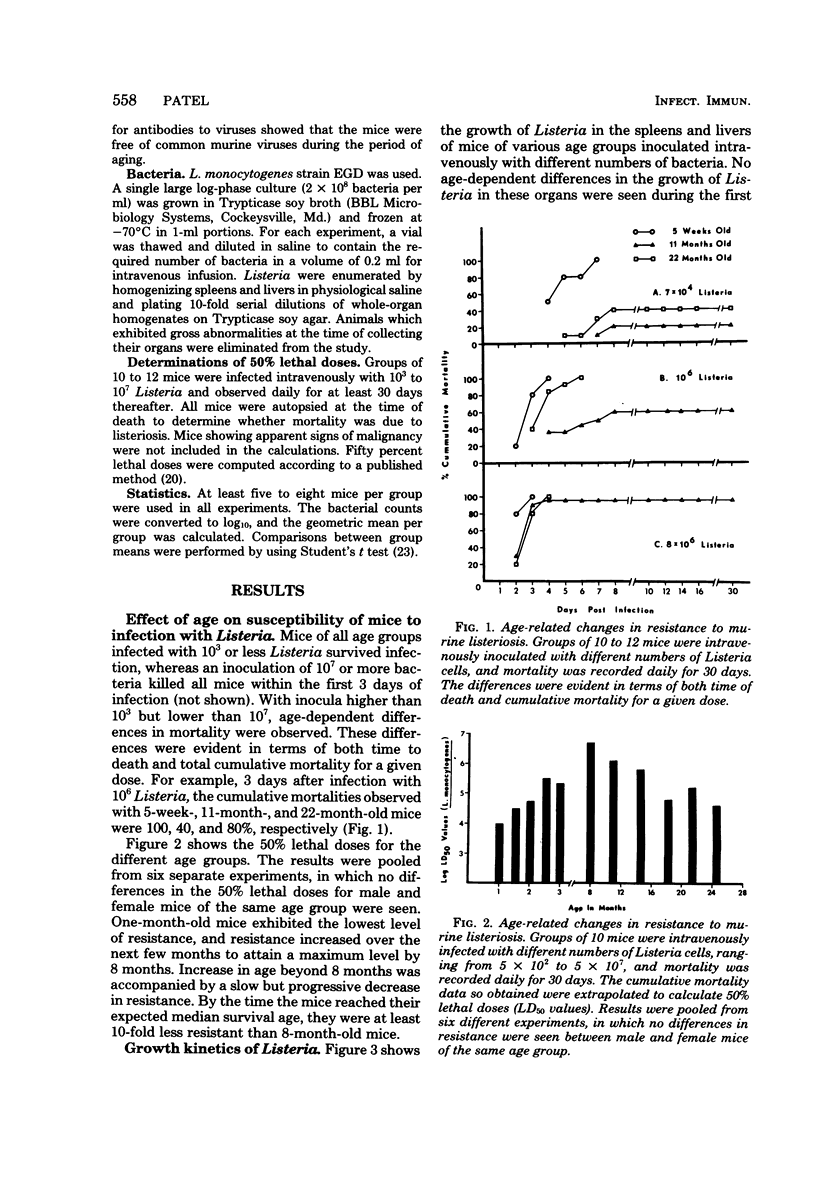
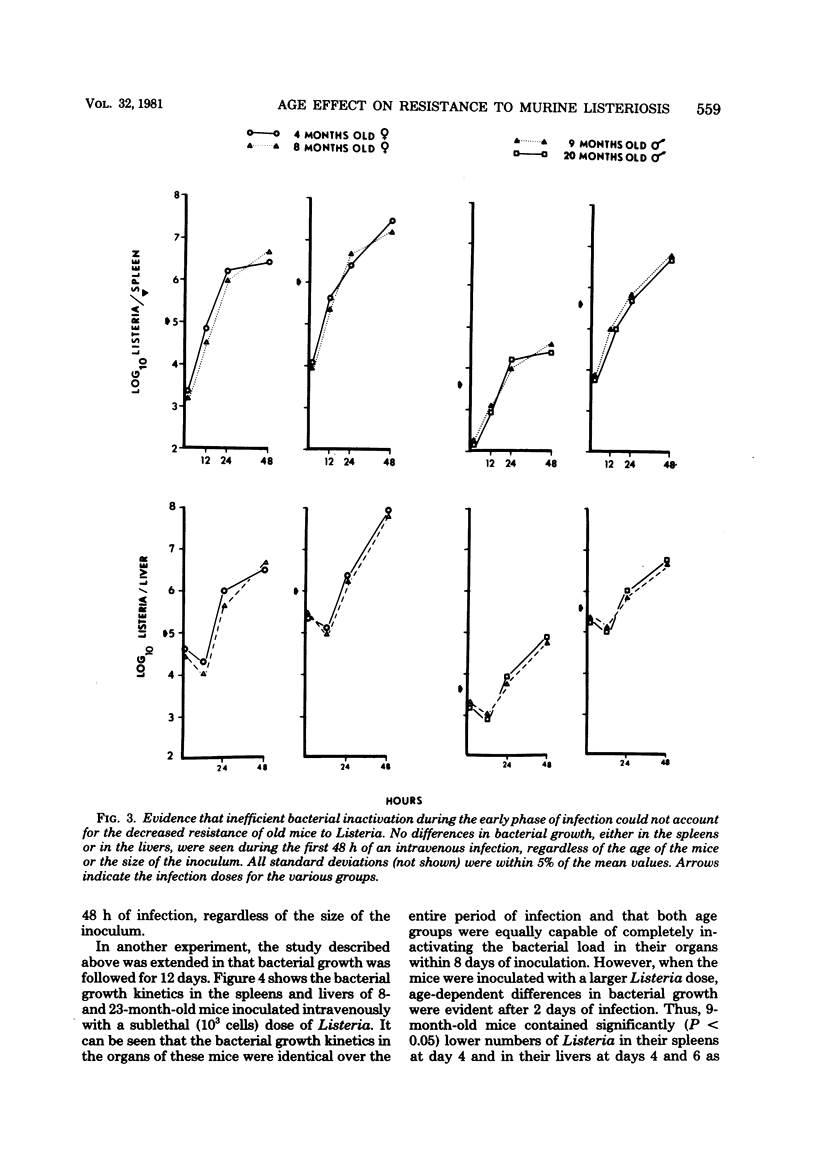
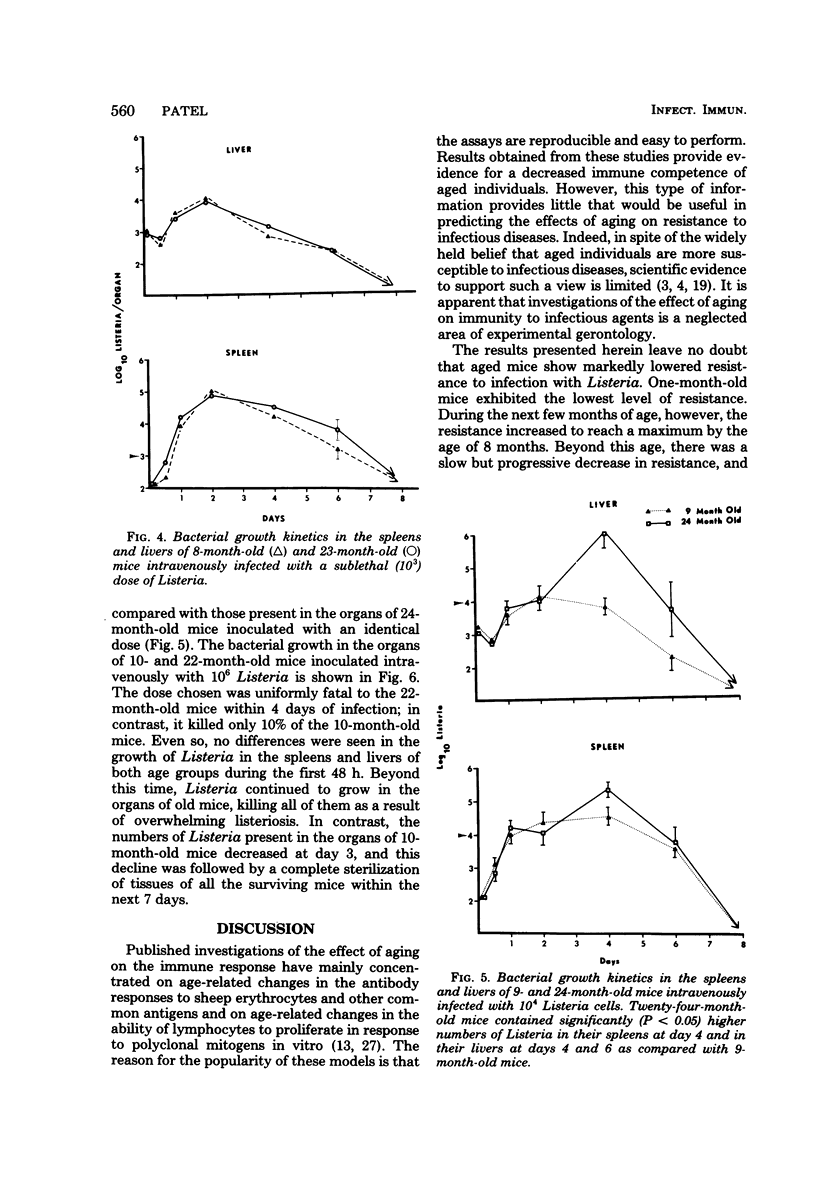
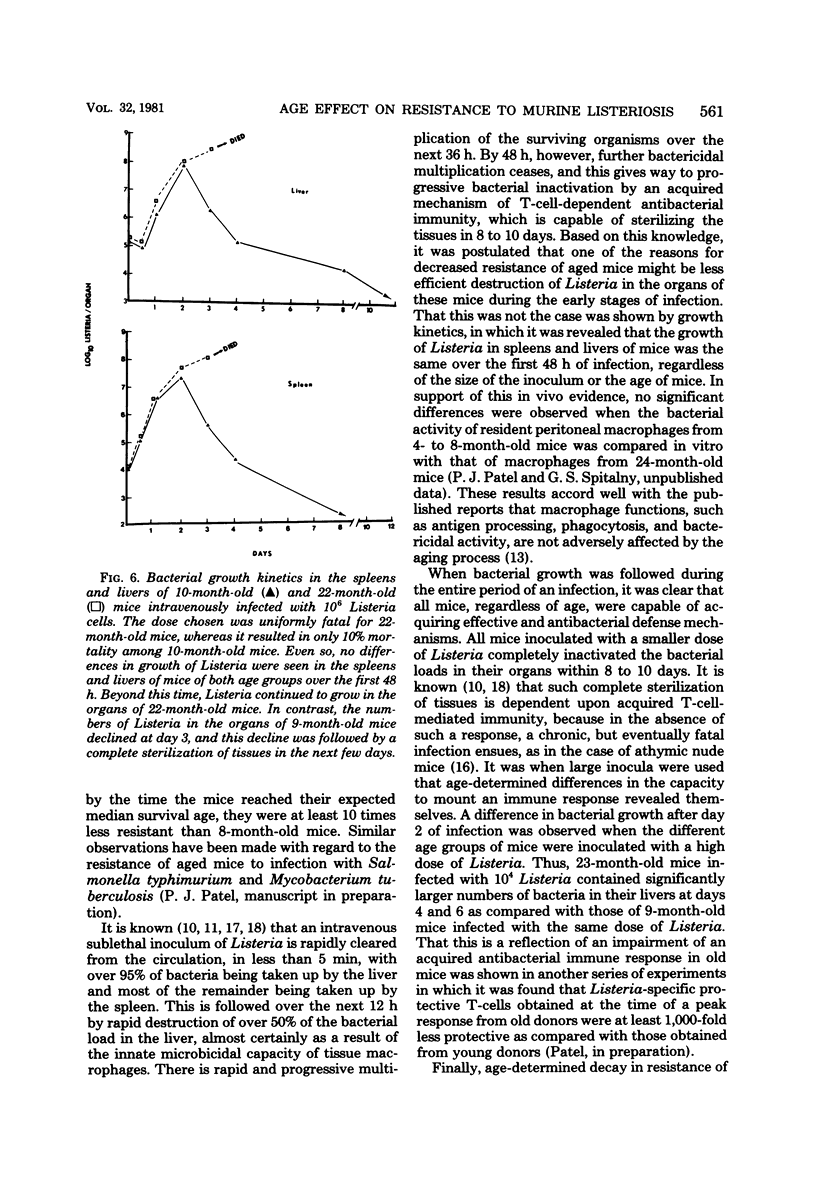
Age-related changes in resistance of mice to infection with Listeria monocytogenes were investigated. One-month-old mice exhibited the least resistance, and the resistance level increased over the first few months to reach a maximum by 8 months. Increase in age thereafter was accompanied by a slow but progressive decrease in resistance. Thus, 50% lethal doses for 1-, 8-, and 24-month-old mice were 10(4.2), 10(6.6), and 10(5.2), respectively. In spite of differences in resistance, the growth of Listeria in the organs of mice of different age groups was identical during the first 48 h of infection, regardless of the size of the inoculum. Moreover, both young (3- to 8-month-old) and old (22-month-old or older) mice inoculated with a small dose of Listeria were equally capable of inactivating the bacterial load in their spleens and livers within 8 to 10 days of infection. However, a difference in bacterial growth after day 2 of infection was observed when different age groups of mice were inoculated with a large dose of Listeria. These results suggest that the decreased capacity of aged mice to resist infection with Listeria is not due to deficiency in the innate mechanisms of antibacterial resistance, but instead is due to age-related decline in the capacity to acquire immunologically specific antibacterial immunity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL H. T., BERNS A. W. AUTOIMMUNITY AND AGING. Adv Gerontol Res. 1964;18:289–342. [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Age-related decline in the resistance of mice to infection with intracellular pathogens. Infect Immun. 1977 May;16(2):593–598. doi: 10.1128/iai.16.2.593-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Aging and the immune response. I. Antibody formation and chronic infection in Toxoplasma gondii-infected mice. J Immunol. 1978 Mar;120(3):939–943. [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Aging and the immune response. II. Lymphocyte responsiveness and macrophage activation in Toxoplasma gondii-infected mice. J Immunol. 1978 Mar;120(3):944–949. [PubMed] [Google Scholar]
- Goodman S. A., Makinodan T. Effect of age on cell-mediated immunity in long-lived mice. Clin Exp Immunol. 1975 Mar;19(3):533–542. [PMC free article] [PubMed] [Google Scholar]
- Ingram C. R., Phegan K. J., Blumenthal H. T. Significance of an aging-linked neuron binding gamma globulin fraction of human sera. J Gerontol. 1974 Jan;29(1):20–27. doi: 10.1093/geronj/29.1.20. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Effect of age on T cell differentiation. Fed Proc. 1978 Apr;37(5):1241–1244. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Adler W. H. Effects of aging on the differentiation and proliferation potentials of cells of the immune system. Fed Proc. 1975 Feb;34(2):153–158. [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Menon M., Jaroslow R. N., Koesterer R. The decline of cell-mediated immunity in aging mice. J Gerontol. 1974 Sep;29(5):499–505. doi: 10.1093/geronj/29.5.499. [DOI] [PubMed] [Google Scholar]
- Meredith P., Tittor W., DeLima M. G., Walford R. L. Age-related changes in the cellular immune response of lymph node and thymus cells in long-lived mice. Cell Immunol. 1975 Aug;18(2):324–330. doi: 10.1016/0008-8749(75)90060-x. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazmiño N. H., Yuhas J. M. Senescent loss of resistance to murine sarcoma virus (Moloney) in the mouse. Cancer Res. 1973 Nov;33(11):2668–2672. [PubMed] [Google Scholar]
- Shigemoto S., Kishimoto S., Yamamura Y. Change of cell-mediated cytotoxicity with aging. J Immunol. 1975 Jul;115(1):307–309. [PubMed] [Google Scholar]
- Smith G. S., Walford R. L., Mickey M. R. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 1973 May;50(5):1195–1213. doi: 10.1093/jnci/50.5.1195. [DOI] [PubMed] [Google Scholar]
- Stjernswärd J. Age-dependent tumor-host barrier and effect of carcinogen-induced immunodepression on rejection of isografted methylcholanthrene-induced sarcoma cells. J Natl Cancer Inst. 1966 Oct;37(4):505–512. [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B. Editorial: Aging and the regulation of immune reactivity. J Chronic Dis. 1975 Oct;28(9):437–440. doi: 10.1016/0021-9681(75)90054-5. [DOI] [PubMed] [Google Scholar]
- Tice R. R., Schneider E. L., Kram D., Thorne P. Cytokinetic analysis of the impaired proliferative response of peripheral lymphocytes from aged humans to phytohemagglutinin. J Exp Med. 1979 May 1;149(5):1029–1041. doi: 10.1084/jem.149.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]



