Abstract
Voluntary surface electromyogram (EMG) signal is sometimes contaminated by spurious background spikes of both physiological and extrinsic or accidental origins. A novel method of muscle activity onset detection against such spurious spikes was proposed in this study based primarily on the sample entropy (SampEn) analysis of the surface EMG. The method takes advantage of the nonlinear properties of the SampEn analysis to distinguish voluntary surface EMG signals from spurious background spikes in the complexity domain. To facilitate muscle activity onset detection, the SampEn analysis of surface EMG was first performed to highlight voluntary EMG activity while suppressing spurious background spikes. Then, a SampEn threshold was optimized for muscle activity onset detection. The performance of the proposed method was examined using both semi-synthetic and experimental surface EMG signals. The SampEn based methods effectively reduced the detection error induced by spurious background spikes and achieved improved performance over the methods relying on conventional amplitude thresholding or its extended version in the Teager Kaiser Energy domain.
I. Introduction
Surface Electromyography (EMG) can be used to obtain physiological and clinical information about neuromuscular functions. In motor control, posture and movement studies, the onset of muscle activity is one of the most common parameters evaluated from surface EMG recordings [1], [2]. It is also of great importance to automatically and precisely detect the onset of a voluntary muscle contraction for realizing an active control, particularly in real-time myoelectic control systems [3]–[5]. Various computerized techniques for the determination of onset time of muscle activity have been proposed, in which the majority of utilized parameters were associated with the EMG signal amplitude, such as the envelope, the rectified value and root mean square of EMG time series [1], [3]–[5], [14]. However, such parameters associated with signal amplitude are very sensitive to various kinds of noises. The performance of these amplitude-based methods degrades as the signal to noise ratio (SNR) of the surface EMG signal is decreased [6].
Of different categories of noises that may contaminate voluntary surface EMG recordings, it is not uncommon to observe a series of spurious spikes, which dramatically affect the accuracy of muscle activity onset detection. The source of such spike-like noise varies. For example, the hyper-excitable motor unit discharges often seen in hemiparetic stroke or spinal cord injury subjects may lead to a series of involuntary tonic spikes, thus compromising the voluntary EMG recording. In studies of biomechanics and gait analysis with cyclic limb movements, the slight displacement of a skin-electrode interface might introduce motion artifacts in the form of spurious spikes. In addition, the ambient interference from radio transmission and electrical wire could also induce spurious spikes. For such applications, it is crucial to develop algorithms to overcome the influence of the spurious spikes for an accurate onset detection of muscle activity.
In this study, we propose a novel method that relies on nonlinear sample entropy (SampEn) analysis for robust muscle activity onset detection against the spurious spikes in surface EMG. The SampEn analysis can highlight bursts of surface EMG with low sensitivity to individual spikes and therefore facilitate the muscle activity onset detection when spurious background spikes are present in surface EMG recordings.
II. Methods
2.1. SampEn
Entropy is a measure of complexity and randomness of dynamic systems, describing the rate of information creation [7], [8]. So far, many methods have been proposed to estimate the entropy of a dynamic system represented by a time series. The approximate entropy (ApEn), introduced by Pincus [7], has proved to be an efficient measure of the regularity of a time series, especially in the field of physiology and medicine. Thereafter, the SampEn was originally proposed by Richman and Moorman [8] as a refinement of ApEn to reduce the bias induced by self-matching. Thus it is independent of the length of recording and displays relative consistency under various circumstances. Taking this advantage, SampEn has been applied in physiological time series analysis including diagnosis of cardiovascular diseases [9], [10] and detection and classification of motor behavior [11].
In order to compute the SampEn, the scalar time series {x1, x2, …, xi, …, xn} is first embedded in a delayed m-dimensional space, where vectors are constructed as:
| (1) |
The probability Bm(r) that two sequences match for m points is computed by counting the average number of vector pairs, for which the distance is lower than the tolerance r. Similarly, Am(r) is defined for an embedding dimension of m+1. The SampEn is then calculated as:
| (2) |
One issue of SampEn calculation is to determine the dimension m and tolerance r. The tolerance r can be chosen as (0.15~0.25) ×SD, the standard deviation of the original time series [7]–[11], while a larger tolerance is usually used for signals with short duration (e.g., 100 or less sampling points) [8]. Empirically, we set m = 2 and r = 0.25 × SD in this study.
2.2. Muscle Activity Onset Detection using the SampEn Analysis
The neuromuscular system can be considered as a complex dynamic system, where the motor control information can be revealed by the EMG and force signals. When a burst of muscle activity occurs, it is usually accompanied by an instantaneous increase in the complexity of the system, whereas the system complexity may not change with spurious background spikes. Based on this fact, we explored the feasibility of using the SampEn analysis for muscle activity onset detection against those spurious background spikes by means of detecting the instantaneous complexity changes in the surface EMG signal. Three steps were involved in the analysis:
The original surface EMG time series was segmented into a series of overlapped analysis windows with a window length of 32 ms and a window increment of 4 ms.
The SampEn analysis was applied on the analysis windows to highlight the muscle activities in a manner that the SampEn may increase with burst of muscle activity while it may be insensitive to spurious background spikes.
A proper threshold Th in the SampEn domain was derived. The onset time of muscle activity was determined when SampEn of the signal exceeded the preset threshold.
For calculating the SampEn of a series of analysis windows, we also examined the performance of two schemes to choose the tolerance r. One scheme was to use the local tolerances that were set as 0.25 × SD of the time series in each of the analysis windows respectively. The other scheme was to employ a global tolerance that was set as 0.25 × SD of the original EMG signal for all analysis windows.
2.3. Performance Evaluation
A validation study was conducted to evaluate the performance of the proposed method using semi-synthetic surface EMG signals where the precise onset time was known a priori. All the semi-synthetic EMG signals were derived from the experimental surface EMG signals as part of investigating a novel myoelectric pattern recognition approach towards improved stroke rehabilitation. The study was approved by the Institutional Review Board of the Northwestern University. During the experiments, each stroke subject was asked to voluntarily perform (or intend to perform) a series of arm, hand, and finger/thumb movements with their affected arm, on which multi-channel surface EMG signals were recorded at a sampling rate of 2000 Hz. During our recording, we found that motor unit over-activity (stretch-insensitive or stretch-sensitive or both) were sometimes present as a background signal of the voluntary surface EMG of paretic muscles. Such involuntary background signal made it difficult to use the conventional amplitude-based methods for muscle activity onset detection.
To construct the semi-synthetic surface EMG signals, three types of experimental signals were first identified. Ten representative signal segments for each type were selected from the experimental dataset.
The first type represents clear EMG bursts recorded during subjects’ voluntary activation of the muscle. The duration of each segment was 1 s.
The second type represents quiescent baselines where no spurious spikes were present. The duration of each segment was 2 s.
The third type represents typical spurious background spikes, originated from muscle overactivity often triggered by external stimuli. The duration of each segment was 2 s.
The 10 segments of the EMG activity of the first type were added with each of the 10 segments of the second and third types (from 500 ms to 1.5 s) respectively, generating 100 segments of semi-synthetic surface EMG signals. Thus, two groups of semi-synthetic surface EMG signals were generated, with quiescent baseline, namely normal data group, or spurious background spikes, namely noisy data group. We also scaled the signal segments to derive semi-synthetic surface EMG signals with different SNRs of 2, 5, 8, 10, 12, 15, 18, 20 and 22dB, respectively. The precise onset time for all the semi-synthetic surface EMG signals was known as 500 ms over the entire 2 s recording period. Both groups of semi-synthetic surface EMG signals were used to examine the onset detection performance of the proposed method under different conditions.
For performance comparison, two previously developed surface EMG onset detection methods were also implemented. One is based on amplitude threstholding of rectified surface EMG signals (denoted as AMP-based method). The other method applies Teager-Kaiser Energy (TKE) operation on raw EMG time series, defined as
| (3) |
where ψ is the discrete TKE operation in the time domain and x(i) represents the raw surface EMG time series. A threshold can be set in the TKE domain to determine the onset time of muscle activity when the signal’s TKE operation output exceeds the threshold. The details on the TKE method can be found in Li et al. [6]. The rationale for choosing AMP and TKE based methods for performance comparison is that both methods are commonly used for muscle activity onset detection due to their simple and quick implementation. The AMP and TKE based methods can be applied to individual raw EMG signal samples. In order to suppress the interference of spurious spikes, the moving average performed with a sliding window can also be applied in the AMP or TKE domains. Considering that the window length was set to 32ms for calculating the SampEn, we also applied a sliding window with the same length to perform the moving average in the AMP and TKE domains for improved onset detection. For statistical analysis, paired t-test was employed to compare the performance of different methods.
III. Results
3.1. SampEn Analysis of Semi-synthetic Surface EMG
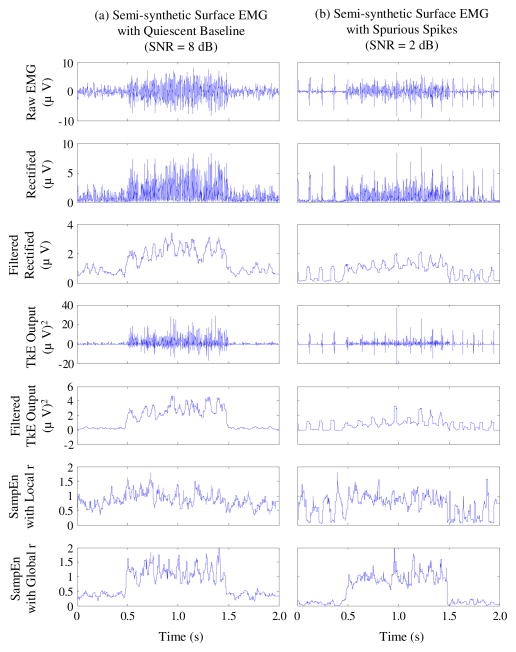
First, the SampEn analysis of semi-synthetic surface EMG signals from both normal and noisy data groups was performed with local and global tolerance schemes, respectively. Two representative semi-synthetic surface EMG signals from each group and their SampEn analysis are shown in Fig. 1, where their rectified values (amplitudes) and the TKE operation outputs (in the absence or presence of a 32-ms sliding window) are also shown for comparison. It was observed that with the global tolerance scheme, the SampEn shows a rapid increase at the onset time point (0.5 s), whereas there is no such a notable indication of onset time in SampEn results with the local tolerance scheme. Therefore, the global tolerance scheme was preferred in the following SampEn calculation for onset detection.
Fig. 1.
Semi-synthetic surface EMG signals and their corresponding rectified time series, TKE outputs (with absence or presence of a 32 ms sliding window, equivalent to a low-pass filter), and SampEn values (with local and global tolerance schemes), respectively. (a) represents the normal data group and (b) represents the noisy data group. The onset time of the muscle activity was set at 0.5 s for both groups.
For the semi-synthetic surface EMG signal from the normal group (Fig. 1a), all the methods including the rectified signal, the TKE output and the SampEn result (with global tolerance scheme) exhibit a distinct difference between the background noise and the muscle activity. However, when the signal is contaminated by a series of involuntary tonic spikes (Fig. 1b), it is difficult to detect the onset time by simply setting a threshold to distinguish muscle activity from spurious background spikes in rectified signal or in the TKE domain. This was also true when a 32ms sliding window was applied to AMP or TKE based methods. By contrast, the SampEn analysis of the EMG signal from the noisy group appears to differentiate the spurious background spikes from the voluntary muscle activity, thus facilitating the onset detection.
3.2. Optimal Thresholding of the SampEn for Onset Detection
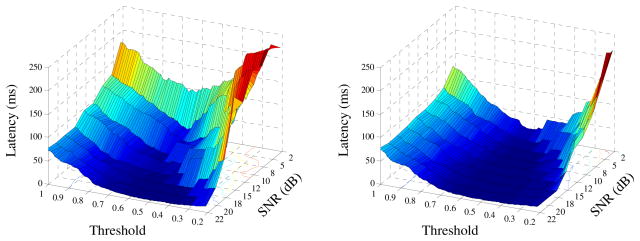
To optimize the threshold in the SampEn domain for muscle activity onset detection, the influence of different thresholds ranging from 0.2 to 1.0 on the detection performance was examined using the semi-synthetic surface EMG signals with different SNRs from both signal groups. The latency τ was defined as the absolute difference between the detected onset time td and true onset time t0 (i.e. 0.5s):
| (4) |
Fig. 2 shows the latency averaged across all the surface EMG signals from each of the normal and noisy data groups respectively, as the threshold increases from 0.2 to 1.0 and the SNR varies from 2 to 22 dB. Generally, from both the normal (Fig. 2a) and noisy (Fig. 2b) data groups, as the threshold increased, the latency tended to decrease to a minimum value and then steadily rose up, regardless of the SNR of the signals. Such a trend in latency variation with different thresholds resulted in a valley in each of the 3-dimensional shaded surface plots for both data groups as demonstrated in Fig. 2. For the signals with each SNR, the position of the valley indicated the optimal choice of the threshold with the smallest latency. By considering the optimal performance for the signals with different SNRs, it was observed that the threshold can be approximately chosen as 0.6–0.65 for the normal data group (Fig. 2a) and 0.50–0.65 for the noisy (Fig. 2b) data group, respectively.
Fig. 2.
The onset detection performance based on SampEn analysis using different thresholds and different SNRs of the semi-synthetic signals from the normal data group (left panel) and the noisy data group (right panel), respectively.
3.3. Performance Comparison using Different Methods
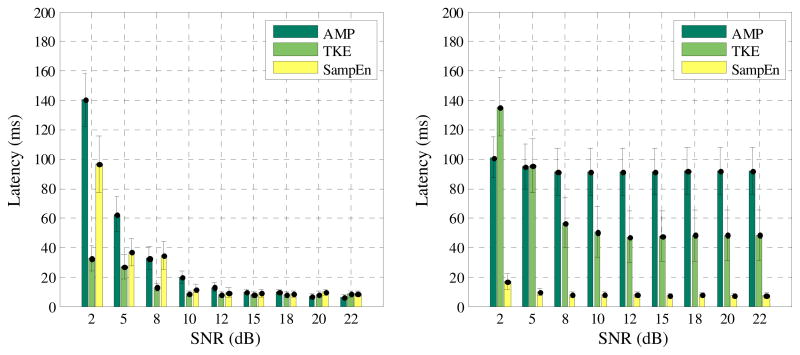
For both data groups, the onset detection results of the three different methods, as described by latencies, were summarized in Fig. 3. Determined by a pretest, the amplitude threshold was set to three times SD of the baseline activity for the AMP based methods, while the threshold was set to 8 times SD of the TKE domain baseline for the TKE based method. We acknowledge that for the spurious-noise case the latency as defined in (4) may not be the most appropriate measure of onset detection performance. For example, if there are several spurious spikes occurred before the muscle activity, onset detector may be triggered by the first spurious spike rather than the one closest to the muscle activity. To reduce such an effect, a searching range was limited within 250 ms before and after the real onset time (i.e. from 250 ms to 750 ms). In addition, it was found that for both AMP and TKE based methods, the application of moving average can improve the onset detection performance. For example, for the noisy data group at a SNR of 10 dB, the AMP and TKE based methods yielded the latencies of 199.5 ± 7.5 ms and 181.2 ± 8.0 ms, respectively, when the moving average was not applied. Such latencies were reduced to 91.3 ± 15.9 ms (p < 0.001) and 50.3 ± 17.0 (p < 0.001), respectively, with application of the moving average (32 ms sliding window). Thus, the latencies obtained from the moving average using the AMP or TKE methods were compared with the performance of the SampEn method with the same window size.
Fig. 3.
Comparison of the onset detection performance using different methods (mean ± SD) for the normal data group (left panel) and the noisy data group (right panel), respectively. The mean latency was averaged across the semi-synthetic surface EMG signals with each SNR from each group.
For the normal data group as demonstrated in Figure 3a, when the SNR of the signal was relatively high, the onset detection performance of all three tested methods was comparable (p > 0.001, for SNRs higher than 12 dB). In the cases of relatively low SNRs, the method based on TKE operation exhibited the best performance among the three methods (p < 0.001 for both TKE vs. AMP, and TKE vs. SampEn, for SNRs lower than 10 dB). Comparison of the SampEn and AMP based methods indicated that the former tended to achieve better performance when SNR was relatively low (p < 0.001, for SNRs of 2, 5 and 10 dB).
For the noisy data group (where the spurious spikes were present in the signal) as demonstrated in Figure 3b, the performance of the AMP or TKE based methods dramatically decreased. The performance of the TKE based method was most severely affected when the SNR of the signals was relatively low. With an averaged latency of no more than 20 ms, the SampEn based approach achieved the best performance among all the three methods (p < 0.001 for both AMP vs. SampEn and TKE vs. SampEn, for all the tested SNRs). The performance of the SampEn based method was quite stable for a wide range of SNRs.
3.4. Onset Detection on Experimental Surface EMG Signals
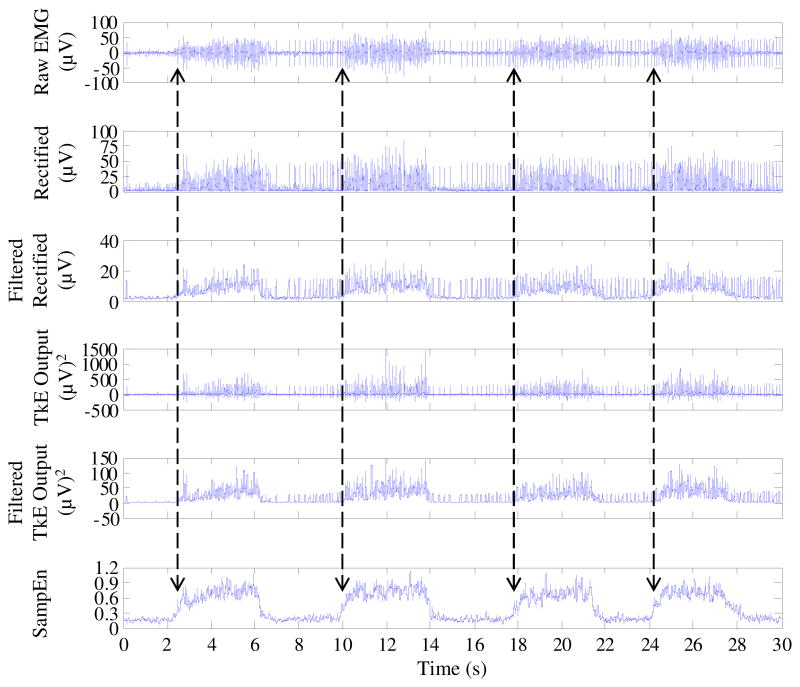
Finally, the performance of the proposed methods using an experimental surface EMG was demonstrated. The signal was recorded from the paretic forearm of a stroke subject in a 30 s time period during which the subject performed voluntary muscle contractions with four repetitions. The signal and comparison of the different methods were shown in Fig. 4. In this application, the SampEn threshold used for onset detection was set to 0.55. Compared to the raw surface EMG signal contaminated by involuntary tonic spikes, it is straightforward in the SampEn domain to determine each onset time (marked by vertical arrows in Fig. 4) of EMG burst associated with voluntary muscle contractions. By contrast, the outputs of the AMP or TKE based methods still maintain a series of spurious spikes in accordance with those on the raw signal, indicating that it is difficult to accurately detect the onset of voluntary muscle activity using the two methods.
Fig. 4.
An example of the real surface EMG signal contaminated by a series of tonic spikes and its corresponding rectified time series, TKE outputs (with absence or presence of a 32 ms sliding window), and SampEn values (with global tolerance) used for onset detection. Each of the detected onset time from the SampEn analysis was marked by a vertical arrow.
IV. Discussion and Conclusion
The onset detection of muscle activity has been a topic for investigation for many years in surface EMG signal processing for various applications in motor control, posture and gait analysis, and myoelectric control. Both visual inspection of surface EMG and amplitude thresholding approach have been frequently used in such applications to determine the onset time of muscle activity. The visual inspection based onset detection requires expert interventions of the signal which is not suitable for computerized or automatic processing. On the other hand, the amplitude thresholding approach is routinely used for simple and fast implementation of the onset detection [1], [3], [14], but its performance may be compromised by low SNRs of the surface EMG considering that the signal amplitude is sensitive to all sorts of noises [6], [18]. In order to overcome this disadvantage, several methods have been developed. For example, wavelet template matching [17] was proposed for EMG onset detection by taking advantage of the morphological characteristics of the surface motor unit action potentials that can be represented by appropriate wavelet functions. The improved onset detection performance can be achieved in the condition that the shape of surface action potentials matches well with the selected wavelet template. However, for experimental EMG signals, such ideal condition cannot always be guaranteed. Another approach that applies some statistical criteria such as generalized likelihood ratio (GLR) and its modifications [15], [16], [19] was also developed to determine onset time of muscle activity. The performance of these statistical methods mainly depends on a priori knowledge of the signal. Thus, the correct estimation of a priori information of the surface EMG signal becomes a key factor for achieving optimal onset detection performance. Moreover, based on the fact that the firing of action potentials results in instantaneous increase of the signal amplitude as well as the frequency component, the TKE operation was recently proposed to highlight such an instantaneous increase, thus achieving improved performance for muscle activity onset detection especially when the SNR of the signal is relatively low [6], [12], [13]. However, the method based on TKE operation was mainly limited to the background noise with Gaussian distribution. A novel onset detection method was necessary to reduce the influence of spurious background spikes in different forms such as a series of involuntary tonic spikes, or recording artifacts induced from extrinsic interference. Such spurious spikes may compromise the difference between voluntary surface EMG and background noise in the time or energy domain, imposing a challenge for onset detection with previous methods.
In this study, we present a novel method for robust onset detection of muscle activity against spurious background spikes using the SampEn analysis of surface EMG. Entropy is a measure to quantify the complexity and randomness of a dynamic system represented by a time series. Since the introduction of SampEn [8], it has achieved a number of applications in analyzing and estimating the regularity of physiological time series for evaluation of the dynamic properties of biological systems [9], [10]. In the current study, surface EMG associated with voluntary muscle activation is considered as a specific dynamic process that is different from the spurious background spikes. It follows that voluntary EMG activity can be differentiated from such spurious spikes by measuring signal complexity. Mathematically the SampEn is computed by counting the number of “similar” vector pairs whose distances are just lower than a preset tolerance. As a result, the SampEn is not sensitive to instant large variations (e.g., spurious spikes) in the processed signal [7], [8]. Taking this advantage, the SampEn analysis can highlight bursts of surface EMG signals while suppressing the spurious background spikes, thus facilitating muscle activity onset detection. Indeed, it can be observed from Fig. 3 that at the similar SNR levels, the performance achieved by the SampEn analysis was even better for the noisy data group (surface EMG contaminated primarily by spurious spikes) than for the normal data group (surface EMG contaminated primarily by white noise).
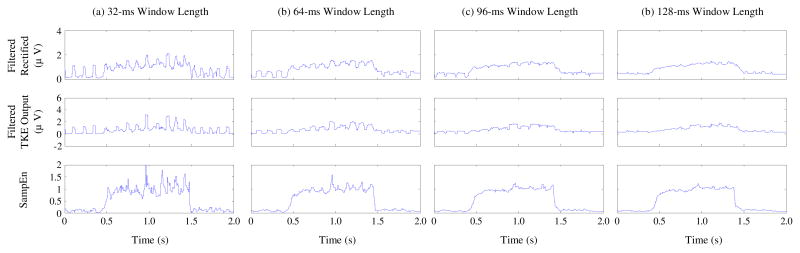
When using the SampEn or the moving average of AMP or TKE outputs for onset detection, a sliding window with certain length needs to be determined. While application of a sliding window in the AMP or TKE domain would reduce the effects of the spurious background spikes, it also induces a certain ambiguity for onset detection (i.e. the EMG information is from a specific window, not from an instantaneous timing). The same is also true for the SampEn based method. This effect is more evident with increasing of the window length, as demonstrated in Figure 5, where different domain (AMP, TKE, and SampEn) signals derived from varying window lengths were compared. For AMP and TKE based methods using a relatively small window length to perform moving average, sometimes it is difficult to reliably detect the onset because a small window may be not sufficient to suppress the spurious spikes. Depending on amplitude and duration of spurious spikes, it may be possible to suppress the spurious spikes with increasing of window length for AMP and TKE based methods. However, in such a case the time resolution for onset detection will be decreased (because of increased ambiguity). By contrast, as demonstrated in Figure 5, the muscle activity can be clearly differentiated from spurious background spikes with all the window lengths used for SampEn analysis. Although increasing the window length can smooth the SampEn curve, a 32-ms window was preferred in this study to reduce signal processing delay and maintain a relatively high time resolution for the onset detection. In addition to the window length, another parameter for SampEn analysis is the window increment/step, which determines the temporal resolution of the onset detection. Theoretically the 2000 Hz sampling rate used in this study induced the smallest window increment of 0.5 ms. Although the highest temporal resolution can be maintained with the smallest window increment, it also imposes a tremendous computational burden. The window increment of 4 ms used in this study can be viewed as a tradeoff between onset detection resolution and computational efficiency.
Fig. 5.
The effect of window length on the performance of onset detection with the three different methods examined in this study. The signal in Fig. 1b was processed with the window length set as (a) 32 ms, (b) 64 ms, (c) 96 ms and (d) 128 ms, respectively, for each method.
It is noteworthy that the tolerance r is a critical parameter in SampEn calculation. Both the local and global tolerance schemes were employed in pilot studies [9]–[11]. The local tolerance scheme was more suitable to the applications of quantifying the inherent signal structure of an analysis window in a way that the amplitude changes of the signal between different windows were not counted [11], whereas the global tolerance scheme considered the relative amplitude in measuring complexity by setting a uniform tolerance for the signal of all the analysis windows [10] [11]. It was found that the global tolerance scheme was suitable in the current study for onset detection. Indeed, the SampEn based onset detection with global tolerance achieved the best performance among all the three methods under the condition that the spurious spikes were present in the surface EMG signal.
Finally, it should be acknowledged that the choice of appropriate muscle activity onset detection method depends on the context of the application. For example, with sufficiently clean surface EMG signals in the absence of spurious background spikes, the traditional approach may achieve comparable performance to more complicated ones such as those based on TKE operation or SampEn analysis. The major motivation for us to develop a novel method for surface EMG onset detection against spurious background spikes was a requirement to determine the onset time of voluntary muscle activity performed by stroke patients. Between 40%–50% stroke survivors have spasm 12 months post stroke [20], which means that it is very likely the voluntary EMG signals are contaminated by involuntary background spikes, imposing detection errors with previous methods. The success of onset detection of voluntary muscle activity (which is indicative of the subjects’ intention of performing functional movements using their paretic limbs) is helpful to develop advanced active EMG-driven robot-aided therapeutic systems towards improved stroke rehabilitation. Moreover, the proposed onset detection method against spurious background spikes can also be used in many other applications (e.g. gait, posture, gesture and activity of daily living analysis) involving stroke survivors and patients with other neurological disorders (e.g., spinal cord injury) or motoneuron diseases (e.g., amyotrophic lateral sclerosis).
Acknowledgments
This study was supported by the National Institute on Disability and Rehabilitation Research (NIDRR) of the U.S. Department of Education (H133G090093), the National Institutes of Health (Grants 1R21NS075463, 2R24HD050821), the Searle-Chicago Community Trust Foundation, and the Davee Research Foundation.
Biographies

Xu Zhang

Ping Zhou
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol. 1996 Dec;101(6):511–9. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 2.Vannozzi G, Conforto S, D’Alessio T. Automatic detection of surface EMG activation timing using a wavelet transform based method. J Electromyogr Kinesiol. 2010 Aug;20(4):767–72. doi: 10.1016/j.jelekin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Oskoei MA, Hu H. Myoelectric control systems—A survey. Biomedical Signal Processing and Control. 2007;2(4):275–294. [Google Scholar]
- 4.Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, Dewald JP, Kuiken TA. Decoding a new neural machine interface for control of artificial limbs. J Neurophysiol. 2007;98(5 ):2974–2982. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 5.Dalley S, Varol H, Goldfarb M. A Method for the Control of Multigrasp Myoelectric Prosthetic Hands. IEEE Trans Neural Syst Rehabil Eng. 2011 Dec 12; doi: 10.1109/TNSRE.2011.2175488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng. 2007 Sep;35(9):1532–8. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- 7.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci, USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–49. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 9.Costa M, Goldberger AL, Peng C-K. Multiscale entropy analysis of complex physiologic time series. Physical Review Letters. 2002;89(6):068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 10.Costa M, Healey JA. Multiscale entropy analysis of complex heart rate dynamics: discrimination of age and heart failure effects. Computers in Cardiology, 2003. 2003:705–708. [Google Scholar]
- 11.Zhou P, Barkhaus PE, Zhang X, Rymer WZ. Characterizing the complexity of spontaneous motor unit patterns of amyotrophic lateral sclerosis using approximate entropy. J Neural Eng. 2011;8(6) doi: 10.1088/1741–2560/8/6/066010. [DOI] [PubMed] [Google Scholar]
- 12.Solnik S, DeVita P, Rider P, Long B, Hortobágyi T. Teager-Kaiser Operator improves the accuracy of EMG onset detection independent of signal-to-noise ratio. Acta Bioeng Biomech. 2008;10(2):65–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer RT, Prosser LA. Use of the Teager-Kaiser Energy operator for muscle activity detection in children. Ann Biomed Eng. 2009 Aug;37(8):1584–93. doi: 10.1007/s10439-009-9727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang GC, Kang WJ, Luh JJ, Cheng CK, Lai JS, Chen JJ, Kuo TS. Real-time implementation of electromyogram pattern recognition as a control command of man-machine interface. Med Eng Phys. 1996;18(7):529–37. doi: 10.1016/1350-4533(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 15.Micera S, Sabatini AM, Dario P. An algorithm for detecting the onset of muscle contraction by EMG signal processing. Med Eng Phys. 1998;20(3):211–5. doi: 10.1016/s1350-4533(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 16.Staude G, Wolf W. Objective motor response onset detection in surface myoelectric signals. Med Eng Phys. 1999;21(6–7):449–67. doi: 10.1016/s1350-4533(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 17.Merlo A, Farina D, Merletti R. A fast and reliable technique for muscle activity detection from surface EMG signals. IEEE Trans Biomed Eng. 2003;50(3):316–23. doi: 10.1109/TBME.2003.808829. [DOI] [PubMed] [Google Scholar]
- 18.Allison GT. Trunk muscle onset detection technique for EMG signals with ECG artefact. J Electromyogr Kinesiol. 2003;13(3):209–16. doi: 10.1016/s1050-6411(03)00019-1. [DOI] [PubMed] [Google Scholar]
- 19.Micera S, Vannozzi G, Sabatini AM, Dario P. Improving detection of muscle activation intervals. IEEE Eng Med Biol Mag. 2001;20(6):38–46. doi: 10.1109/51.982274. [DOI] [PubMed] [Google Scholar]
- 20.Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16(5):515–22. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]