Abstract
Motor unit number index (MUNIX) is a recently developed novel neurophysiological technique providing an index proportional to the number of motor units in a muscle. The MUNIX is derived from maximum M wave and voluntary surface electromyogram (EMG) recordings. The objective of this study was to address a practical question for computing MUNIX in the first dorsal interosseous (FDI), a multifunctional muscle that generates torque about the second metacarpophalangeal joint, i.e. how will different lines of muscle activation influence its MUNIX estimates? To address this question, the MUNIX technique was applied in the FDI muscle of 15 neurologically intact subjects, using surface EMG signals from index finger abduction and flexion, respectively, while the maximum M wave remained the same. Across all subjects, the average MUNIX value of the FDI muscle was 228 ± 45 for index finger abduction, slightly smaller than the MUNIX estimate of 251 ± 56 for index finger flexion. Different FDI muscle activation patterns resulted in an approximately 10% difference in MUNIX estimates. The findings from this study suggest that appropriate definition of voluntary activation of the FDI muscle should be kept to ensure consistency in measurements and avoid source of error. The current study is limited by only assessing neurologically intact muscles. It is important to perform a similar analysis for patients with amyotrophic lateral sclerosis (ALS), given that ALS is the primary intention of the MUNIX method as a potential follow-up measurement for motor unit loss.
I. INTRODUCTION
Since the introduction of the motor unit number estimation (MUNE) [10], the technique and its various forms of improvement have been used as an important tool in basic and clinical neurophysiology [2]. The traditional MUNE methods involve estimates of single motor unit action potential size using either incremental nerve stimulation or spike triggered averaging techniques, both potentially laborious and time-consuming. Thus, a motor unit number index (MUNIX) technique was developed [11]. The MUNIX measurement is based on surface electromyogram (EMG) and maximum M wave or compound muscle action potential (CMAP) recordings, which induces minimal discomfort and can be performed quickly. Because of these practical conveniences, the MUNIX measurement has attracted increasing attention. A number of its applications have been reported recently, focusing on detecting motoneuron loss and measuring disease progression in amyotrophic lateral sclerosis (ALS) and other neuromuscular disorders [1, 6, 7, 12–17].
The MUNIX technique requires recording of voluntary surface EMG signals for different muscle contraction levels. While “simple” muscles exert forces about a single degree-of-freedom joint, the body has many multifunctional muscles capable of producing joint torques in more than one direction. For example, biceps brachii, deltoid, and the interossei muscles can exert forces in multiple directions about their respective joints. Although the MUNIX measurement is convenient to implement and technically no major difficulties are anticipated, there is a practical question when computing MUNIX in multifunctional muscles, i.e., how will different lines of force generation influence the MUNIX estimates?
To answer this question, the objective of the current study was to assess directional dependence of MUNIX calculation in the first dorsal interosseous (FDI), a multifunctional muscle that generates torque about the 2nd metacarpophalangeal (MCP) joint. The plane of force production about the MCP joint consists of linear combinations of flexion, extension, abduction, and adduction of the index finger. The FDI muscle is a primary abductor and synergistic flexor about the MCP joint. These two lines of muscle action were used to determine whether there are systematic differences in MUNIX estimates of the FDI muscle according to the direction of force generation at the index finger.
II. METHODS
A. Subjects
Fifteen neurologically intact subjects (7 males, 8 females, 42.0 ± 13.5 years) participated in this study. The study was approved by the Institutional Review Board of Northwestern University (Chicago, IL, USA). All the subjects gave their written consent before the experiment.
B. Experimental Protocol
The experiments were performed in the FDI muscle of the dominant hand in all the subjects. Subjects were seated comfortably in a chair with the examined forearm placed in its natural, resting position on a height-adjustable table. They were instructed to relax, and the hand and forearm were held in a vertical half supinated position.
Prior to the recording, each subject’s skin over the ulnar aspect of the wrist, the back of the hand, and the index finger were slightly abraded and cleaned with rubbing alcohol. To begin, the maximum M wave or CMAP was first recorded. The primary equipment used for this recording was a Sierra Wave EMG system (Cadwell Lab Inc, Kennewick, WA, USA). Electrode placement was similar to that for standard ulnar motor studies. The active surface electrode (10 mm silver/silver chloride disc) was positioned over the motor point of the FDI muscle with the reference surface electrode positioned over the second MCP joint. An adhesive ground electrode was placed on the back of the hand.
The ulnar nerve was stimulated about 2 cm proximal to the wrist crease with a remote handheld stimulator. The intensity of the stimulation pulse (200 μs in duration) started around 15–20 mA. The intensity was further increased in increments of approximately 20% above that until the stimulation intensity eliciting the maximal response was reached. Then, the stimulation intensity was increased to 120% of the final intensity to confirm no further enlargement in the peak-to-peak amplitude of the M wave. To ensure that the M wave amplitude is maximized and meanwhile the positive take-off (which is often created in FDI muscle M wave recording [26] and makes it difficult to define onset latency) is minimized, the electrode placement was optimized by testing several different locations. In addition, during the experiment re-cleaning of the skin and re-application of the electrode cream were performed as necessary to guarantee the best recording quality. The maximum M wave was recorded only once, and this maximum M wave was used for calculation of MUNIX with different voluntary muscle contraction tasks.
After the maximum M wave recording, with the EMG electrode maintained at the same position, the voluntary surface EMG signals were recorded from the FDI muscle while the subject generated an isometric contraction in two different directions (i.e., index finger abduction or flexion). For each direction, the different force levels were recorded using a single trial with graded contraction consisting of 5–10 interference EMG epochs representing minimal to maximal effort. A resistant force was provided to subjects by the examiner to ensure isometric voluntary contraction of the FDI muscle. For each direction this protocol was performed two times. Subjects were allowed substantial rest to avoid muscle fatigue during the recording. For all the subjects, the surface EMG was sampled at 2 kHz, with a band-pass filter setting at 10 Hz–500 Hz.
C. Data Analysis
The maximum M wave and different levels of surface interference pattern (SIP) EMG were used to compute the MUNIX for the FDI muscle. The MUNIX derivation was described in detail elsewhere [11, 12] and its procedures are outlined in brief here.
The area and power of the maximum M wave and different levels of SIP signals were first computed. The M wave onset and offset were defined from the baseline, and the tiny positive take-off part was not included for calculating the maximum M wave area and power. The area and power of the maximum M wave and each level of SIP EMG were then used to compute the “ideal case motor unit count (ICMUC)”, defined as the ratio of maximum M wave power to its area multiplied by the ratio of the SIP area to its power. Thus, each level of SIP gave two results: SIP area and ICMUC. Regression analysis was then used to define the relationship between the SIP area and the ICMUC by an exponential fitting: ICMUC = β(SIP Area)α The parameters β and α can be obtained from the regression using different levels of SIP. For each direction up to 20 different levels of SIPs (from combination of both trials) were used for this regression analysis. Finally, the SIP area of 20mVms was used to compute the MUNIX value from the established exponential fitting. The rationale for this selection was described by Nandedkar et al. [12]. To exclude the abnormally high MUNIX estimate induced by very low amplitude surface EMG signals (which may give very high ICMUC values), three criteria were imposed to accept an SIP epoch [11, 12]: (1) SIP area> 20mVms; (2) ICMUC<100; and (3) SIP area/CMAP area>1.
We measured the MUNIX values in the FDI muscle using voluntary surface EMG signals from index finger abduction and flexion, respectively, while the maximum M wave remained the same. We examined whether the MUNIX estimates were significantly different from varying directions of muscle activation. A dependent Student’s t test was used for statistical analysis. The significant level was defined as p<0.05.
III. RESULTS
Across all subjects, the maximum M wave amplitude from the FDI muscle was 14.1 ± 2.0 mV. Maximum M waves, in combination with voluntary surface EMG signals, were used to calculate the MUNIX measurement.
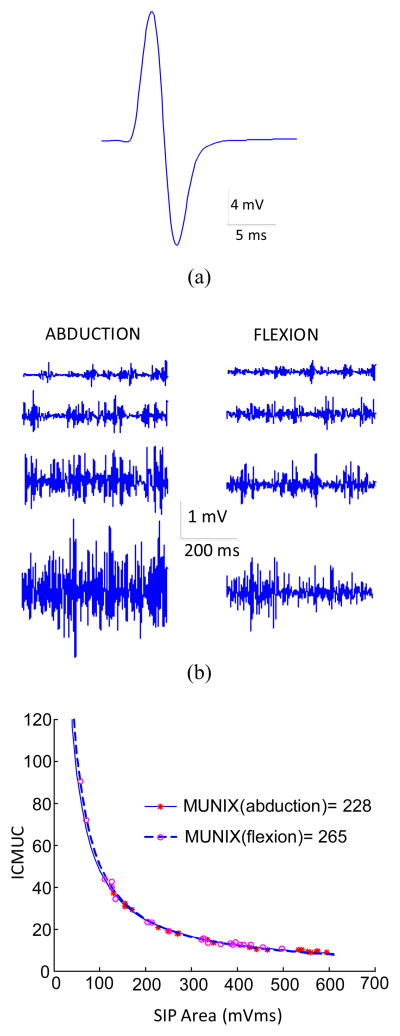
Figure 1 demonstrates an example of the MUNIX calculation from the FDI muscle using index finger abduction and flexion, respectively. Figure 1(a) shows the maximum M wave; figure 1b shows the surface EMG signals when muscle contraction force was increased from minimal to maximum during index finger abduction and flexion, respectively. The comparison of the MUNIX calculations from the two directions is presented in figure 1c. Analysis of SIP measurements (the individual data points in figure 1c) shows an excellent fit with the mathematical model used to calculate the MUNIX (the lines representing exponential fitting). It is noted that the voluntary surface EMG generated by the FDI muscle flexion was relatively small compared with that from the abduction, as indicated by the x-axis values of the individual data points used for the curve fitting. With the measured maximum M wave and different levels of SIP signals, this subject showed a MUNIX value of 228 for the FDI muscle abduction mode, and a MUNIX value of 265 for the flexion mode.
Figure 1.
The MUNIX calculation of the FDI muscle: (a) The maximum M wave. (b) The different levels of voluntary surface EMG from index finger abduction and flexion, respectively. (c) Comparison of the calculation of MUNIX using abduction and flexion surface EMG.
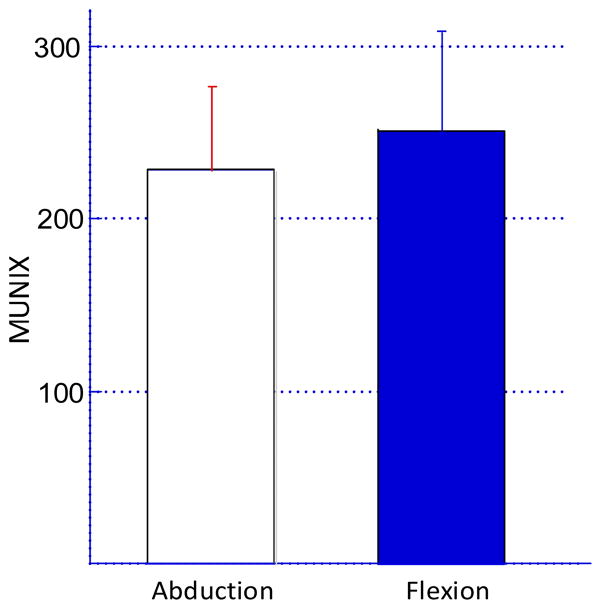
For all the subjects, exponential regression analysis showed a good fitting for the relationship between SIP area and ICMUC. As indicated in figure 2, across all subjects we observed an approximately 10% difference in MUNIX estimates using FDI muscle abduction or flexion mode. Fifteen subjects showed a MUNIX value of 228 ± 45 for the FDI abduction mode, which was slightly lower than the MUNIX value of 251 ± 56 for the FDI flexion mode (p<0.05).
Figure 2.
Comparison of the FDI MUNIX estimates from 15 neurologically intact subjects using index finger abduction and flexion, respectively.
IV. DISCUSSION
This study addresses a practical question for MUNIX calculation in the FDI, a multifunctional muscle that generates torque about the 2nd MCP joint. For a muscle with a single line of force generation, the muscle force production is governed largely by the biophysical properties of motoneurons. According to “size principle” [4], with increasing synaptic excitatory input to a given motoneuron pool, smaller motoneurons are known to be activated before large motoneurons. To calculate MUNIX, the voluntary surface EMG recording protocol for such muscles is straightforward since the force generation is in a single direction.
There are many muscles (e.g., the FDI muscle) that can produce forces in multiple directions about the respective joint. How such multifunctional muscles are controlled is still unclear. For these muscles, forces in different directions could be generated in different ways. For example, one strategy is to generate force by choosing groups of motor units that are tuned selectively to generate torque in a specified direction. This approach is mechanically simple but neurophysiologically complex, because the central nervous system has to pick out just those motor units that are suited to the task. Alternatively, the force generation of multifunctional muscles can be realized by pairing antagonist muscles or parts of the muscle against each other to generate a resultant force in the desired direction. This is a simple controller but energetically inefficient.
The neural control of multifunctional muscles has been a topic of investigation for at least 30 years [3, 5, 20–23]. How the multifunctional muscle is controlled is beyond the scope of this study. The focus of the study was to assess whether different directions of voluntary EMG generation may influence the MUNIX calculation. This is a practical question considering that the MUNIX calculation is sometimes performed with multifunctional muscles.
Experimental comparison of the MUNIX estimates with different directions of the FDI muscle activation was performed in this study. Considering that the primary line of the FDI muscle activation is in the index finger abduction–flexion plane, the choice of directions was focused on pure index finger abduction and flexion, respectively. Our findings indicate that on average the MUNIX values estimated from index finger flexion were slightly higher than estimates from index finger abduction. This may be primarily due to the relatively small surface EMG amplitude from the FDI muscle during index finger flexion compared with abduction [24, 25]. Such variation of surface EMG pattern with task direction potentially arises from different mechanisms. First, the abduction force produced at the MCP joint is generated largely by the FDI muscle, while the flexion force of the index finger is not generated by the FDI muscle alone. The flexor digitorum profundus and flexor digitorum superficialis may also be involved for index finger flexion [8]. Thus the FDI muscle was not the sole flexor about the MCP joint. Although biomechanical evaluation of the motor function of the index finger showed a similar strength in the direction of flexion and abduction [9], the surface EMG amplitude in the flexion mode was consistently lower than that in the abduction mode [24, 25]. On the other hand, results from a previous study characterizing motor unit recruitment patterns in the FDI muscle demonstrate that motor units can be recruited in flexible groupings that are dependent on direction motor tasks [18, 19]. The groups of motor units more suitable for abduction are located relatively superficial within the muscle compared with those more suitable for flexion. Thus the EMG amplitude in flexion mode was relatively low compared with that in the abduction mode. Moreover, the take-up area of the surface EMG is limited to approximately 2 cm [27]. This may further constrain surface EMG recording for the flexion mode.
The MUNIX calculation is based on exponential curve fitting derived from the relation between the M wave and different levels of voluntary surface EMG signals. In our MUNIX calculation using index finger abduction or flexion, the M wave was the same while the difference was solely induced by the voluntary surface EMG signals. It is noted that a bimodal distribution [12] of surface EMG may be more readily encountered with index finger flexion compared with index finger abduction. The small SIP at slight index finger flexion would generate modest ICMUC values, while at moderate and high flexion effort it may be possible to recruit more superficial motor units, generating very large SIPs. Because MUNIX is defined from a low SIP area, a bimodal distribution of SIPs may yield a higher than expected MUNIX value.
The MUNIX is an index of the number of motor units contained in a muscle, and not a direct estimation of the number, as provided by various MUNE methods. Therefore, when MUNIX methods are used, the objective of the study is always to compare the MUNIX changes in different situations. With the same definition for all parameters throughout the study, the absolute values of MUNIX are not important, in contrast to the changes seen from two different measurements (from the same muscle in a longitudinal study or different muscles in a cross-sectional study). According to this principle for MUNIX application, the direction of muscle contraction should keep consistent when comparing MUNIX in two multifunctional muscles. Therefore, the comparison will not be compromised by the difference induced from varying task directions.
The current study is limited by only using neurologically intact muscles. It is acknowledged that ALS is the primary intention of the MUNIX method as a potential follow-up measurement for motor unit loss. The bimodal distribution of SIPs may be more likely in the FDI flexion mode of ALS patients considering that there might be erroneously small SIPs with low innervation and large SIPs with further innervation recruiting chronic neurogenic motor units. Further studies are required to investigate how the results of this study would change in muscles affected by ALS.
In conclusion, this study addresses a practical question for computing MUNIX in the FDI muscle using surface EMG signals from index finger abduction and flexion, respectively. Across all subjects, an approximately 10% difference in MUNIX estimates was observed with different FDI muscle activation patterns. The findings from this study suggest that appropriate definition of voluntary activation of the FDI muscle should be kept to ensure consistency in measurements and avoid source of error. The current study is limited by only assessing neurologically intact muscles. It is important to perform a similar analysis for ALS patients, given that ALS is the primary intention of the MUNIX method as a potential follow-up measurement for motor unit loss.
Acknowledgments
Funding: This work was supported in part by the National Institute on Disability and Rehabilitation Research of the U.S. Department of Education under Grant H133G090093 and Grant H133F110033, in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grant 1R21NS075463 and Grant 2R24HD050821, in part by the Brinson Stroke Foundation, the Searle-Chicago Community Trust Foundation, and the Davee Research Foundation.
Footnotes
Competing interests: None declared.
Ethical approval: The study was approved by the Institutional Review Board of Northwestern University, Chicago, IL, USA (Reference number: STU00017218).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn SW, Kim SH, Kim JE, Kim SM, Kim SH, Park KS, Sung JJ, Lee KW, Hong YH. Reproducibility of the motor unit number index (MUNIX) in normal controls and amyotrophic lateral sclerosis patients. Muscle Nerve. 2010;42:808–813. doi: 10.1002/mus.21765. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg MB. Updating motor unit number estimation (MUNE) Clin Neurophysiol. 2007;118:1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- 3.Desmedt JE, Godaux E. Spinal motoneuron recruitment in man: Rank deordering with direction but not with speed of voluntary movement. Science. 1981;214:933–935. doi: 10.1126/science.7302570. [DOI] [PubMed] [Google Scholar]
- 4.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motor neurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Herrman U, Flanders M. Directional tuning of single motor units. J Neurosci. 1998;18:8402–8416. doi: 10.1523/JNEUROSCI.18-20-08402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Rymer WZ, Li G, Zhou P. The effects of notch filtering on electrically evoked myoelectric signals and associated motor unit index estimates. J Neuroeng Rehabil. 2011;8(1):64. doi: 10.1186/1743-0003-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Wang YC, Suresh NL, Rymer WZ, Zhou P. Motor unit number reductions in paretic muscles of stroke survivors. IEEE Trans Inf Technol Biomed. 2011;15:505–512. doi: 10.1109/TITB.2011.2140379. [DOI] [PubMed] [Google Scholar]
- 8.Li ZM, Zatsiorsky VM, Latash ML. The effect of finger extensor mechanism on the flexor force during isometric tasks. J Biomech. 2001;34:1097–102. doi: 10.1016/s0021-9290(01)00061-6. [DOI] [PubMed] [Google Scholar]
- 9.Li ZM, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL. Multi-directional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003;18(10):908–15. doi: 10.1016/s0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 10.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–31. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nandedkar SD, Nandedkar DS, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX) IEEE Trans Biomed Eng. 2004;51:2209–11. doi: 10.1109/TBME.2004.834281. [DOI] [PubMed] [Google Scholar]
- 12.Nandedkar SD, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX): principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle Nerve. 2010;42(5):798–807. doi: 10.1002/mus.21824. [DOI] [PubMed] [Google Scholar]
- 13.Nandedkar SD, Barkhaus PE, Stålberg EV. Reproducibility of MUNIX in patients with amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:919–922. doi: 10.1002/mus.22204. [DOI] [PubMed] [Google Scholar]
- 14.Neuwirth C, Nandedkar S, Stalberg E, Weber M. Motor unit number index (MUNIX): a novel neurophysiological technique to follow disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42:379–84. doi: 10.1002/mus.21707. [DOI] [PubMed] [Google Scholar]
- 15.Neuwirth C, Nandedkar S, Stålberg E, Barkhaus PE, Carvalho M, Furtula J, Dijk JP, Baldinger R, Castro J, Costa J, Otto M, Sandberg A, Weber M. Motor Unit Number Index (MUNIX): a novel neurophysiological marker for neuromuscular disorders; test-retest reliability in healthy volunteers. Clin Neurophysiol. 2011;122:1867–1872. doi: 10.1016/j.clinph.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Neuwirth C, Nandedkar S, Stålberg E, Barkhaus PE, Carvalho M, Furtula J, van Dijk JP, Baldinger R, Castro J, Costa J, Otto M, Sandberg A, Weber M. Motor Unit Number Index (MUNIX): reference values of five different muscles in healthy subjects from a multi-centre study. Clin Neurophysiol. 2011;122:1895–1898. doi: 10.1016/j.clinph.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Sandberg A, Nandedkar SD, Stålberg E. Macro electromyography and motor unit number index in the tibialis anterior muscle: differences and similarities in characterizing motor unit properties in prior polio. Muscle Nerve. 2011;43:335–341. doi: 10.1002/mus.21878. [DOI] [PubMed] [Google Scholar]
- 18.Suresh NL, Kuo AD, Heckman CJ, Rymer WZ. Differential recruitment of motor units in the FDI. Society for Neuroscience. 2001;27:168.11. [Google Scholar]
- 19.Suresh NL, Kuo AD, Heckman CJ, Ellis MD, Rymer WZ. Correlation of mechanical action with directional tuning in the first dorsal interosseous (FDI). Proceedings of the XIVth Congress of the International Society for Electrophysiology and Kinesiology; Vienna, Austria. June 22–25.2002. [Google Scholar]
- 20.Ter Haar Romeny BM, van der Gon JJ, Gielen CC. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol. 1982;78:360–368. doi: 10.1016/0014-4886(82)90054-1. [DOI] [PubMed] [Google Scholar]
- 21.Ter Haar Romeny BM, van der Gon JJ, Gielen CC. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol. 1984;85:631–650. doi: 10.1016/0014-4886(84)90036-0. [DOI] [PubMed] [Google Scholar]
- 22.Theeuwen M, Gielen CC, Miller LE, Doorenbosch C. The relation between the direction dependence of electromyographic amplitude and motor unit recruitment thresholds during isometric contractions. Exp Brain Res. 1994;98:488–500. doi: 10.1007/BF00233986. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CK, Ross BH, Stein RB. Motor unit recruitment in human first dorsal interosseous muscle for static contractions in three different directions. J Neurophysiol. 1986;55:1017–1029. doi: 10.1152/jn.1986.55.5.1017. [DOI] [PubMed] [Google Scholar]
- 24.Zijdewind I, Kernell D, Kukulka CG. Spatial differences in fatigue-associated electromyographic behaviour of the human first dorsal interosseus muscle. J Physiol. 1995;483(Pt 2):499–509. doi: 10.1113/jphysiol.1995.sp020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Suresh NL, Rymer WZ. Surface electromyogram analysis of the direction of isometric torque generation by the first dorsal interosseous muscle. J Neural Eng. 2011;8(3):036028. doi: 10.1088/1741-2560/8/3/036028. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Robinson LR. Can we accurately measure the onset latency to the first dorsal interosseous? Muscle Nerve. 2011;43(1):129–32. doi: 10.1002/mus.21864. [DOI] [PubMed] [Google Scholar]
- 27.Barkhaus PE, Nandedkar SD. Recording characteristics of the surface EMG electrodes. Muscle Nerve. 1994;17(11):1317–23. doi: 10.1002/mus.880171111. [DOI] [PubMed] [Google Scholar]