Abstract
Background
Specialized community-based care (SCBC) refers to services that manage chronic illness through formalized links between primary and specialized care.
Objectives
The objectives of this evidence-based analysis (EBA) were as follows:
to summarize the literature on SCBC, also known as intermediate care
to synthesize the evidence from previous Medical Advisory Secretariat (now Health Quality Ontario) EBAs on SCBC for heart failure, diabetes, chronic obstructive pulmonary disease (COPD), and chronic wounds
to examine the role of SCBC in family practice
Results
Part 1: Systematic Review of Intermediate Care
Seven systematic reviews on intermediate care since 2008 were identified. The literature base is complex and difficult to define. There is evidence to suggest that intermediate care is effective in improving outcomes; however, the effective interventions are still uncertain.
Part 2: Synthesis of Evidence in Intermediate Care
Mortality
| • Heart failure | Significant reduction in patients receiving SCBC |
| • COPD | Nonsignificant reduction in patients receiving SCBC |
Hospitalization
| • Heart failure | Nonsignificant reduction in patients receiving SCBC |
| • COPD | Significant reduction in patients receiving SCBC |
Emergency Department Visits
| • Heart failure | Nonsignificant reduction in patients receiving SCBC |
| • COPD | Significant reduction in patients receiving SCBC |
Disease-Specific Patient Outcomes
| • COPD | Nonsignificant improvement in lung function in patients receiving SCBC |
| • Diabetes | Significant reduction in hemoglobin A1c (HbA1c) and systolic blood pressure in patients receiving SCBC |
| • Chronic wounds | Significant increase in the proportion of healed wounds in patients receiving SCBC |
Quality of Life
| • Heart failure | Trend toward improvement in patients receiving SCBC |
| • COPD | Significant improvement in patients receiving SCBC |
Part 3: Intermediate Care in Family Practice—Evidence-Based Analysis
Five randomized controlled trials were identified comparing SCBC to usual care in family practice. Inclusion criteria were 1) the presence of multiple chronic conditions, and 2) interventions that included 2 or more health care professions. The GRADE quality of the evidence was assessed as low for all outcomes due to the inconsistency and indirectness of the results.
Limitations
This review did not look at disease-specific studies on intermediate care in family practice.
Conclusions
Specialized community-based care effectively improves outcomes in patients with heart failure, COPD, and diabetes. The effectiveness of SCBC in family practice is unclear.
Background
Specialized community-based care refers to services that manage chronic illness through formalized links between primary and specialized care. Another term for specialized community-based care is intermediate care. Intermediate care and specialized community-based care are used interchangeably throughout this document.
Objective of Analysis
The objectives of this analysis were as follows:
to summarize the literature on specialized community-based care, also known as intermediate care
to synthesize the evidence from previous Medical Advisory Secretariat (now Health Quality Ontario) evidence-based analyses (EBAs) on intermediate care for heart failure, diabetes, chronic obstructive pulmonary disease (COPD), and chronic wounds
to examine the role of intermediate care in family practice
Clinical Need and Target Population
Chronic diseases have a large impact on the Ontario population. An estimated 1 in 3 Ontarians has a chronic disease, and among those over 65 years of age, 80% have at least 1 chronic disease and 70% have 2 or more chronic diseases. (1;2) Chronic diseases include heart failure, diabetes, cancer, COPD, and arthritis. In 2002, the World Health Organization estimated that medical treatment for chronic diseases and the resulting lost productivity would cost $80 million in Canada annually. (3)
Technology/Technique
For the purpose of this review, intermediate care refers to specialized community-based care (SCBC) that manages chronic illness through formalized links between primary and specialized care. Several other terms have been used to describe the concept of SCBC, including shared care, integrated care, chronic disease management, interdisciplinary primary care, care coordination, collaborative care, guided care, and care-and-case management, among others. Intermediate care aims to optimize health resource utilization by improving chronic disease management.
The intermediate care team can include primary care physicians, specialists, nurses, dietitians, pharmacists, social workers, caregivers, patients, and physiotherapists. Table 1 outlines some of the interventions used in intermediate care programs.
Table 1: Frequently Reported Components of Intermediate Care Interventions.
| Component | Description |
|---|---|
| Disease-specific education | Education about the signs and symptoms and etiology of chronic condition |
| Medication education/review | Education about the side effects of medication, the relationship of medication to chronic disease management, and the importance of medication adherence |
| Medication titration | Assistance with appropriate dosing of specific medications |
| Diet counselling | Counselling on disease-specific diets |
| Physical activity counselling | Counselling on physical activity |
| Lifestyle counselling | Counselling on lifestyle choices, such as smoking cessation and alcohol intake |
| Self-care support behaviours | Encouragement for the patient to monitor weight, symptoms, and medications |
| Self-care tools | Patient dairies for recording weight, diet, and/or symptoms |
| Evidence-based guidelines | Clinical practice guidelines based on evidence |
| Regular follow-up | Regular follow-up visits between the beginning and end of the treatment phase |
Ontario Context
In Ontario, intermediate care is not a standardized, centralized health service. The province has several intermediate care programs for a variety of chronic conditions. Programs can be provided by hospitals, community care access centres, community health centres, family health teams (FHTs), or other groups of health care providers.
Diabetes Education Programs
According to the Ministry of Health and Long-Term Care’s Health Care Options website, there are 222 diabetes education programs (DEPs) in the province. (4) The DEPs are designed to provide the tools and skills for people with diabetes and their families. The services provided, which include counseling (group and one-on-one), self-management learning sessions, and education to minimize symptoms and prevent or delay complications associated with diabetes, are not standardized across all DEPs. Health care professionals at DEPs include diabetes nurse educators and registered dietitians.
The Programs for Assessment of Technologies in Health (PATH) Research Institute is currently reviewing the DEPs in the province. PATH will examine the impact of the DEPs on patient self-management, clinical outcomes (HbA1c, blood pressure, cholesterol), health care resource utilization, and quality of life. The group has recruited 9 DEPs to take part in the evaluation, with at least 1 DEP from each of the following sites: hospital, community health centre, and FHT. The results of the evaluation will be available in 2013.
Heart Failure Clinics
The Toronto Health Economics and Technology Assessment (THETA) Collaborative has assessed heart failure clinics in Ontario. The group identified the number of clinics, patients served, services provided, and staffing. Twenty-eight heart failure clinics were identified, mostly in community-based hospitals. Similar to the DEPs, heart failure clinics differ in services provided. The clinics are staffed by various health care professionals, including cardiologists, general practitioners, nurses, registered dietitians, counsellors, and physiotherapists. Approximately 80% of the staff have specialty training in heart failure. The research objective of THETA is to define the program elements that are most effective and cost-effective.
Wound Care Clinics
Wound care clinics in Ontario have also been evaluated by THETA. The group identified 49 wound care teams in the province, most led by a physician or a nurse. About a quarter of the clinics include allied health professionals such as dietitians or social workers, and almost two-thirds are affiliated with and funded through hospitals. The majority of patients who visit the clinics have chronic wounds. Some of the wide variety of services provided at these clinics (such as compression stockings and off-loading for foot ulcers) require patients to pay out of pocket or through private insurance, while others are funded through the Ontario Disability Support Program or the Assistive Device Program within the Ministry of Health and Long-Term Care.
Chronic Obstructive Pulmonary Disease Clinics
There are several Ontario clinics providing education and support to patients with COPD and their families. Most are within community-based hospitals, although some are located in physician practices and other independent sites. As with the other chronic disease clinics, the COPD clinics offer varied services. Some clinics require a referral from a physician or nurse practitioner, while others offer self-referral programs. Services offered include one-on-one counselling, group counselling, education sessions, and smoking cessation consultations.
Family Health Teams
There are about 200 FHTs in Ontario. By definition, FHTs are multidisciplinary teams of care providers. Each FHT must have a minimum of 3 family physicians, and can also have nurses, nurse practitioners, dietitians, social workers, and other allied health professionals as part of the team. The number of allied health professionals depends on the number of patients enrolled within the FHT. Some FHTs also employ specialized physicians (specialties including internal medicine, psychiatry, pediatrics, and geriatrics).
Each FHT must implement electronic health record software for “meaningful use,” rather than for appointment bookings only. There are 11 licensed vendors of electronic health record software for FHTs in Ontario. Ideally, these software programs are compatible with one another to enable efficient data transfer and access. The Waterloo Wellington LHIN has regionalized its health care information technology software for a pilot project for diabetes called Health eConnections. The project enables hospitals, FHTs, DEPs, and specialists to access the same patient information through compatible information systems.
The intent of FHTs and other family practice models is to provide chronic disease management to slow disease progression and reduce unnecessary hospital admissions. Several FHTs have specialized chronic disease management programs for diabetes, cardiovascular disease, and respiratory disease.
Evidence-Based Analysis
This evidence-based analysis comprises 3 distinct parts:
a systematic review of intermediate care for chronic disease
a synthesis of evidence from MAS evidence-based analyses of SCBC
an examination of the role of SCBC in family practice
Part 1: Systematic Review of Intermediate Care
Research Questions
What is the current status of the literature base on intermediate care (also known as SCBC) for the management of multiple chronic conditions?
What intermediate care interventions are effective?
Research Methods
Literature Search
Search Strategy
A literature search was performed on May 11, 2011 (updated October 10, 2011) using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2008, to May 11, 2011 (updated October 11, 2011) on intermediate care. The search strategy is listed in Appendix 1.
Inclusion Criteria
Studies were limited to systematic reviews and meta-analyses focusing on intermediate care for the management of multiple chronic conditions.
Statistical Analysis
This is a narrative review of the systematic reviews identified; no statistical analyses were performed.
Results of Systematic Review
The search strategy identified over 2,500 citations. Seven systematic reviews and meta-analyses were included in this review. All reports were published between January 1, 2008, and May 11, 2011. Systematic reviews and meta-analyses that targeted specific chronic conditions beyond the scope of this review were not included.
A large volume of literature on the concept of intermediate care has been published. Intermediate care is a very broad term that encompasses all chronic conditions, different populations, and many types of interventions (as described in Table 1, above), and the terminology to define interventions is inconsistent. For instance, the terms “patient education” and “self-management” have been used to describe similar approaches to teaching patients and caregivers how to manage chronic conditions. (13) The Agency for Healthcare Research and Quality (AHRQ) review included 10 studies that used the term “disease management” to describe the intervention, each study with a different definition of the term. (14) Thus, effectively summarizing the literature on intermediate care presents a challenge.
Smith et al conducted a systematic review of shared care that was published in 2007 (12) and 2008 (11). They defined shared care as “joint participation of primary and specialty care providers in the planned delivery of care for patients with a chronic condition.” Shared care aims to establish a broader relationship between primary and specialty care providers than the routine discharge of patients and referral notices. The investigators identified 20 studies to include in their qualitative review. They concluded that, based on the limited quality of evidence, there was “insufficient evidence to support the introduction of shared care.” However, their overall negative conclusion was offset by some positive outcomes. For instance, patients receiving shared care had a statistically significant improvement in medication adherence compared with those receiving usual care. There was also a trend towards a reduction in hospitalizations in patients receiving shared care. Results for physical health outcomes, mental health outcomes, quality of life, and cost-effectiveness were inconclusive.
In 2009, Coleman et al (7) conducted a systematic review of intermediate care and attempted to stratify each of the studies they identified by the degree of health system redesign on a scale of 1 to 4. The investigators assigned a score of 1 to a study if there was no attempt at health system redesign and a score of 4 if there was a substantial attempt at health system redesign. They found much fewer studies attempting health system redesign than studies not attempting redesign. The latter often consisted of programs such as telephone interventions or education sessions offered by a third party. Of the 5 studies with no health system redesign, none reported an improvement in all outcomes (based on the outcomes reported in each of the studies). Of the 4 studies with limited redesign, 1 yielded an improvement in all outcomes. Eight of 17 studies with some health system redesign showed improvement in all outcomes, and 2 of the 3 studies with substantial redesign reported improvement in all outcomes. These results need to be interpreted with caution because the outcomes, study design, and quality varied across studies.
The review by Mitchell et al (10) from 2008 was derived from a larger review by the Australian Primary Health Care Research Institute from 2006 focused on multidisciplinary care planning. Of all the systematic reviews identified in the literature, the review by the Australian Primary Health Care Research Institute was the only one to clearly state that “devising and testing [intermediate care] strategies is a prerequisite for widespread, routine use of [intermediate care] in chronic disease management,” (10) even though several studies concluded that the components of an effective intermediate care program are unknown.
In 2008, the Health Services Assessment Collaboration (HSAC) in New Zealand published a systematic review of interventions to reduce ambulatory care–sensitive hospitalizations. (9) The review stratified interventions into 5 categories: disease management programs, education, telehealth, system level interventions, and specialist clinics (Table 2). Studies were grouped according to the following chronic disease categories: all conditions leading to ambulatory care–sensitive hospitalizations, asthma, heart failure, diabetes, and other. One hundred and forty-six studies met the inclusion criteria. The review’s greatest limitation was that hospitalization rates were not always the primary outcome, so the studies may not have been designed or powered to measure a change in hospitalizations.
Table 2: Summary of Existing Systematic Reviews and Meta-Analyses*.
| Study | Description |
|---|---|
| Foy et al, 2010 (5) |
This meta-analysis focused on the communication between primary care and specialists. The authors concluded that their results “suggest[ed] a potential role” for intermediate care. |
| Boult et al, 2009 (6) |
This systematic review specifically sought studies with “statistically significant positive outcomes.” One hundred and twenty-three studies met this criterion, but it is unclear how many were excluded due to negative outcomes. |
| Coleman et al, 2009 (7) |
This systematic review highlighted the many challenges and limitations of the intermediate care literature. Investigators opted for a qualitative review because “imprecise nomenclature and poor study design methodology limit[ed] quantitative analysis.” They found that interventions focusing on health system redesign had the highest impact on outcomes. |
| Dennis et al, 2008 (8) |
This qualitative synthesis of chronic disease management data reviewed over 150 studies and stratified results according to the Chronic Care Model. The review concluded that intermediate care was effective without providing much detail to support the conclusion. No outcomes on health services impact were reported. |
| HSAC, 2008 (9) |
This comprehensive narrative New Zealand review of ACSH identified 5 care patterns that reduced ACSH:
|
| Mitchell et al, 2008 (10) |
This review, derived from a larger review by the Australian Primary Health Care Research Institute from 2006, focused on multidisciplinary care planning. Investigators were cautious about recommending widespread adoption of intermediate care without devising and testing strategies first, stating that “the most effective participant numbers, discipline composition, source of initiation and timing of care planning teams is still to be determined.” |
| Smith et al, 2008 (11;12) |
This Cochrane review identified 20 studies for inclusion in their review of intermediate care. The review stratified the results by chronic disease condition and concluded that there was “insufficient evidence to demonstrate significant benefits from shared care apart from improved prescribing.” |
Abbreviations: ACSH, ambulatory care–sensitive hospitalization; HSAC, Health Services Assessment Corporation.
Table 3: Description of Studies in HSAC’s Systematic Review of Interventions to Reduce Ambulatory Care–Sensitive Hospitalizations*.
| Intervention | Definition | All ACSH | Heart Failure | Diabetes | |||
|---|---|---|---|---|---|---|---|
| Studies | Result | Studies | Result | Studies | Result | ||
| Disease management programs | Programs that included multidisciplinary teams offering a wide variety of chronic disease-related interventions, such as one-on-one treatments or group counselling. DMPs can be offered in a variety of settings: physician offices, hospitals, community centres, etc. | 2 SRs, 3 RCTs |
Effective† | 8 SRs, 20 RCTs |
Effective | 2 SRs, 1 RCT |
Unclear |
| Education | Education encompasses a wide array of educational opportunities, ranging from individual patient education in a physician’s office to group education in schools or other public settings. It also includes self-management education, which involves not only sharing information about the disease but also providing lessons and tools on management. | 2 SRs, 3 RCTs |
Unclear | 1 SR, 4 RCTs |
Effective | No studies | |
| Telehealth | Any interventions involving telephones, Internet interface, or any other mode of remote communication. | 2 SRs, 2 RCTs |
Unclear | 3 SRs, 8 RCTs |
Effective | 2 RCTs | Not effective |
| System level | Interventions aimed specifically at health delivery systems. Interventions that engage the medical practice, such as introduction of guidelines or clinical pathways, education targeting health care providers, or changes in how service is provided (advanced access, provided by nurse instead of physician, location of service, etc.). | 2 SRs, 1 RCT |
Unclear | 2 RCTs | Unclear | 1 SR | Unclear |
| Specialist clinics | Specialist clinics are defined as “provision of services by health care providers in an outpatient setting.” The term specialist is not limited to physician specialists. | No studies | 1 RCT | Unclear | 2 SRs, 1 RCT |
Unclear | |
Abbreviations: ACSH, ambulatory care–sensitive hospitalization; DMP, disease management program; RCT, randomized controlled trial; SR, systematic review.
In this case, “effective” means that disease management programs are effective at reducing hospitalizations in studies that investigated all ambulatory care–sensitive hospitalizations.
Source: HSAC, 2008 (9)
Part 2: Synthesis of Evidence on Intermediate Care
The purpose of this section was to synthesize the evidence from previous MAS (now Health Quality Ontario) EBAs on intermediate care (also known as SCBC) for heart failure, COPD, type 2 diabetes, and wound care.
Community-Based Care for the Specialized Management of Heart Failure: An Evidence-Based Analysis (15)
Community-Based Multidisciplinary Care for Patients With Stable Chronic Obstructive Pulmonary Disease (COPD): An Evidence-Based Analysis (16)
Community-Based Care for the Management of Type 2 Diabetes: An Evidence-Based Analysis (17)
Multidisciplinary Community-Based Care for Chronic Wound Management: An Evidence-Based Analysis (18)
These EBAs aimed to determine the effectiveness and cost-effectiveness of multidisciplinary team care for the management of the conditions they addressed. The full EBAs and associated economic analyses can be accessed from the Health Quality Ontario website at: www.hqontario.ca/en/mas/mas_ohtas_mn.html.
This report combines the results of the individual EBAs by outcome:
mortality (all-cause and disease-specific)
hospitalization (all-cause and disease-specific)
emergency department (ED) visits
length of stay
disease-specific patient outcomes
quality of life
Mortality
All-cause mortality data were reported in the EBAs on heart failure and COPD, and disease-specific mortality data were reported only in the heart failure EBA. Mortality was not a reported outcome in the EBAs of chronic wounds and diabetes.
All-Cause Mortality
Heart Failure
Eight studies investigating SCBC for heart failure reported all-cause mortality at 1-year follow-up (Figure 1). (19-26) When the results of all 8 studies were pooled, interventions involving SCBC yielded a statistically significant relative risk reduction (RRR) of 29% compared to those that did not involve SCBC, with moderate statistical heterogeneity between studies (I2 of 38%). The GRADE quality of evidence was assessed as moderate for this outcome.
Figure 1: Meta-Analysis of Outcomes for All-Cause Mortality for Heart Failure*.
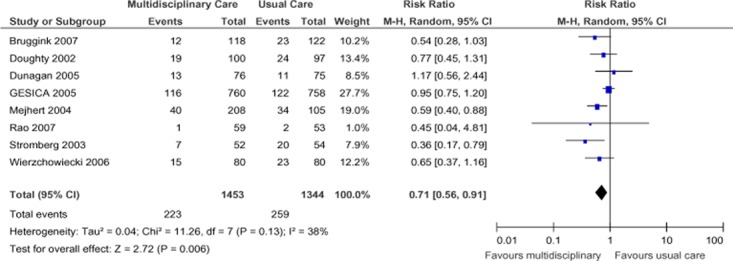
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Chronic Obstructive Pulmonary Disease
Three studies reported all-cause mortality for patients with COPD (Figure 2), (27-29) and estimates from these 3 studies were pooled to determine a summary estimate. There was a statistically nonsignificant reduction in mortality between treatment groups (relative risk [RR], 0.81; 95% confidence interval [CI], 0.52–1.27). The I2 value was 21%, indicating low statistical heterogeneity between studies. All studies had a 12-month follow-up period. The GRADE quality of evidence was assessed as very low for this outcome, indicating that the estimate of effect was uncertain.
Figure 2: Meta-Analysis of Outcomes for All-Cause Mortality for COPD*.
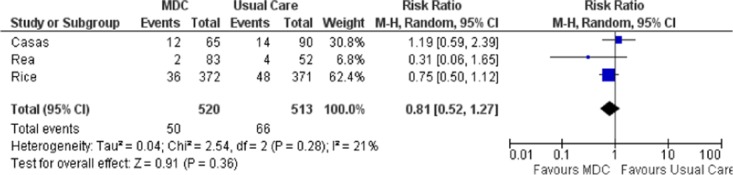
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; MDC, multidisciplinary care; M-H, Mantel Haenszel.
Disease-Specific Mortality
Heart Failure
Only the EBA on heart failure identified studies reporting disease-specific mortality. Three studies reported heart failure–specific mortality at 1-year follow-up (Figure 3). (21;24;25) When the results of these studies were pooled, SCBC yielded a nonsignificant RRR of 42% (compared to no SCBC), with high statistical heterogeneity between studies (I2 of 60%). The GRADE quality of evidence for the pooled analysis was assessed as moderate.
Figure 3: Meta-Analysis of Outcomes for Heart Failure–Specific Mortality*.

Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Hospitalization
Only the EBAs on heart failure and COPD identified studies reporting hospitalizations as an outcome.
All-Cause Hospitalization
Heart Failure
Seven studies investigating heart failure reported all-cause hospitalization at 1-year follow-up (Figure 4). (19;21-26) When pooled, their results showed a statistically nonsignificant 4% decrease in hospitalizations in the SCBC group compared with the non-SCBC group, with high statistical heterogeneity between studies (I2 of 45%). The GRADE quality of evidence for the pooled analysis was found to be low.
Figure 4: Meta-Analysis of Outcomes for All-Cause Hospitalization for Heart Failure*.
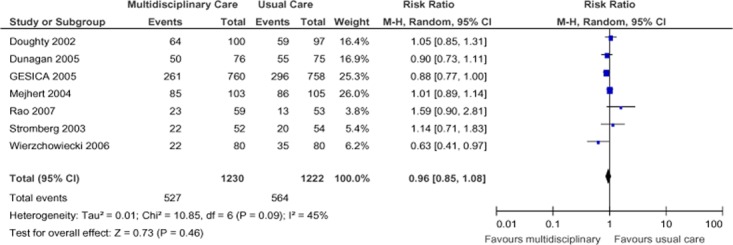
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Chronic Obstructive Pulmonary Disease
Four studies reported results of all-cause hospitalizations in terms of number of people with at least 1 admission during the follow-up period (Figure 5), (27-30) and estimates from these studies were pooled to determine a summary estimate. There was a statistically significant 25% RRR in all-cause hospitalizations in the SCBC group compared with the usual care group. There was no statistical heterogeneity between studies (I2 = 0). The GRADE quality of evidence was assessed as moderate for this outcome, indicating that further research may change the estimate of effect.
Figure 5: Meta-Analysis of Outcomes for All-Cause Hospitalization for COPD*.
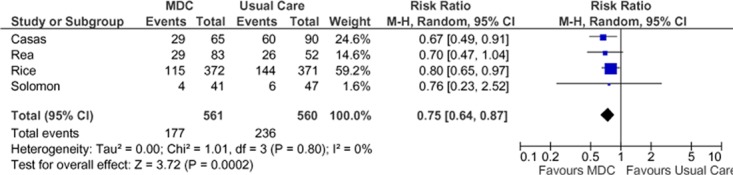
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; MDC, multidisciplinary care; M-H, Mantel Haenszel.
Disease-Specific Hospitalization
Heart Failure
Six studies reported heart failure–specific hospitalization at 1-year follow-up (Figure 6). (19-21;23;25;26) When pooled, the results of these studies showed a nonsignificant (14%) RRR in the SCBC group compared with the usual care group, with high statistical heterogeneity between studies (I2 = 60%). The quality of evidence for the pooled analysis was found to be low.
Figure 6: Meta-Analysis of Outcomes for Heart Failure–Specific Hospitalizations*.
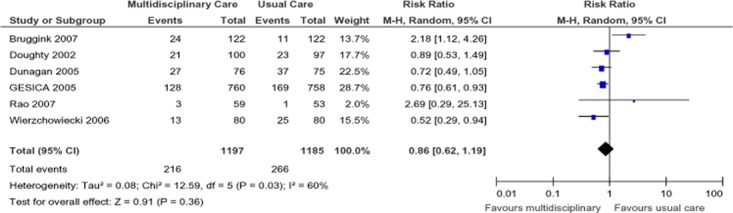
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Chronic Obstructive Pulmonary Disease
Three studies reported results of COPD-specific hospitalizations in terms of number of people with at least 1 admission during the follow-up period (Figure 7), (27;28;31) and estimates from these studies were pooled to determine a summary estimate. There was a statistically significant 33% RRR in all-cause hospitalizations in the SCBC group compared with the usual care group and no statistical heterogeneity between studies (I2 = 0). The GRADE quality of evidence was assessed as moderate for this outcome, indicating that further research may change the estimate of effect.
Figure 7: Meta-Analysis of Outcomes for COPD-Specific Hospitalizations*.
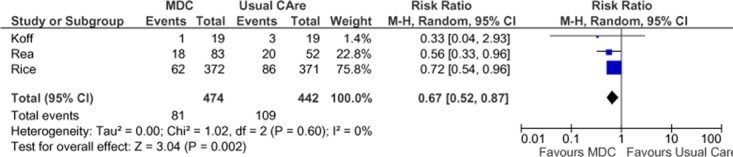
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; SCBC, specialized community-based care; M-H, Mantel Haenszel.
Emergency Department Visits
The EBAs on heart failure and COPD reported all-cause ED visits as an outcome. Only the EBA on COPD reported disease-specific ED visits. The EBAs on diabetes and chronic wounds did not identify ED visits as an outcome.
All-Cause Emergency Department Visits
Heart Failure
Only 1 study on SCBC for heart failure reported ED visits. (19) The data were presented as a composite of readmissions and ED visits, and the authors reported that 77% (59 out of 76) of the SCBC group and 84% (63 out of 75) of the usual care group had this composite outcome within the first year of follow-up (P = 0.029).
Chronic Obstructive Pulmonary Disease
Two studies reported results of all-cause ED visits in terms of number of people with at least 1 visit during the follow-up period (Figure 8). (27;30) Pooling the data from the studies yielded a statistically nonsignificant 36% reduction in all-cause ED visits in the SCBC group compared with the usual care group. There was inconsistency in the RR estimates between studies, as well as wide confidence intervals, denoting a lack of precision. The relatively low event rates could be contributing to a type II error and imprecision. Of note, the study by Rice et al (27;28) reported a statistically significant reduction in all-cause ED visits (P < 0.05). However, this study did not contain data that could be included in this meta-analysis. The GRADE quality of evidence was assessed as very low for this outcome, indicating that the estimate of effect is very uncertain.
Figure 8: Meta-Analysis of All-Cause Emergency Department Visits (Number of People With at Least 1 Visit During the Follow-Up Period)*.
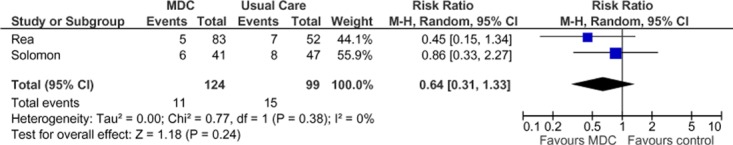
Abbreviations: CI, confidence interval; MDC, multidisciplinary care; M-H, Mantel Haenszel.
Disease-Specific Emergency Department Visits
Chronic Obstructive Pulmonary Disease
Two studies reported results of COPD-specific ED visits in terms of number of people with at least 1 visit during the follow-up period (Figure 9). (28;31) Pooling the data from the studies yielded a statistically significant 41% reduction in COPD-specific ED visits in the SCBC group compared to the non-SCBC group, and there was no statistical heterogeneity between studies. Inconsistency in the point estimates between studies may reflect the low event rates in the study by Koff et al. (31) The GRADE quality of evidence was assessed as moderate for this outcome, indicating that further research may change the estimate of effect.
Figure 9: Meta-Analysis of COPD-Specific Emergency Department Visits (Number of People With at Least 1 Visit During the Follow-Up Period)*.
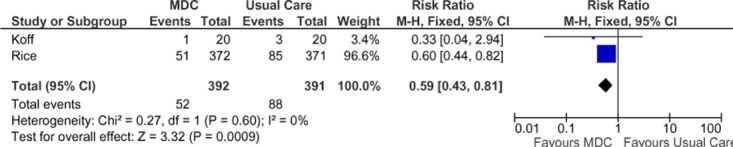
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; MDC, multidisciplinary care; M-H, Mantel Haenszel.
Length of Stay
Only the EBA on heart failure had studies reporting length of hospital stay as an outcome.
Seven studies reported duration of hospital stay, 4 in terms of mean days of stay (19;22;23;25) and 3 in terms of total hospital bed days. (20;24;26) Most studies reported all-cause duration of hospital stay, and 2 (25;26) also reported heart failure–specific duration of hospital stay. These data were not amenable to meta-analysis, as standard deviations were not provided. However, in all but 1 study (23), patients receiving SCBC had shorter hospital stays, whether measured as mean days in hospital or total hospital bed days.
Disease-Specific Patient Outcomes
The EBAs on COPD, diabetes, and chronic wounds reported disease-specific patient outcomes. Specifically, the EBA on COPD reported the change in lung function, the EBA on diabetes reported the change in hemoglobin A1c (HbA1c) and systolic blood pressure (SBP), and the EBA on chronic wounds reported pain and rates of wound healing.
Chronic Obstructive Pulmonary Disease: Lung Function
Two studies reported results of “percent of predicted forced expiratory volume in 1 second” (FEV1 % predicted) as a measure of lung function. (27;32) A negative change from baseline denotes deterioration in lung function, and a positive change from baseline denotes improvement in lung function. The SCBC group showed a statistically significant improvement in lung function for up to 12 months compared with the usual care group, but this effect was not maintained at the 2-year follow-up. The pooled weighted mean difference in FEV1 % predicted was 2.78 (−1.82, 7.37). The GRADE quality of evidence was assessed as very low for this outcome, indicating that the estimate of effect is uncertain.
Figure 10: Meta-Analysis of Mean Change From Baseline in FEV1 % Predicted*†.
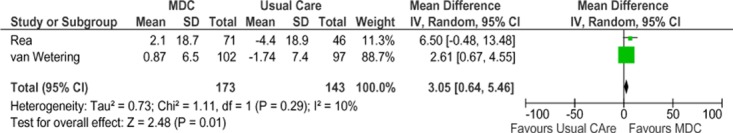
Abbreviations: CI, confidence interval; FEV1 % predicted, percent of predicted forced expiratory volume in 1 second; IV, inverse variance; MDC, multidisciplinary care; M-H, Mantel Haenszel; SD, standard deviation.
†Rea et al at 12 months, van Wetering et al at 4 months.
Figure 11: Meta-Analysis of Mean Change From Baseline in FEV1 % Predicted*†.
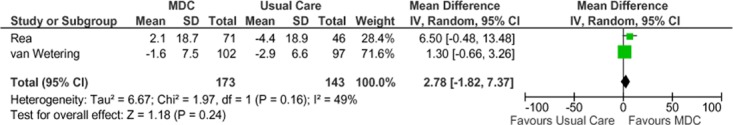
Abbreviations: CI, confidence interval; FEV1 % predicted, percent of predicted forced expiratory volume in 1 second; IV; MDC, multidisciplinary care; M-H, Mantel Haenszel; SD, standard deviation.
†Rea et al at 12 months, van Wetering et al at 24 months.
Diabetes: HbA1c
Model 1 (Registered Nurse, Registered Dietitian, and Physician Team)
Four of the 5 studies were eligible for inclusion in the meta-analysis of HbA1c results for Model 1 (Figure 12). (33-36) Overall, SCBC Model 1 resulted in a HbA1c reduction of 1.0% (95% CI, −1.27 to −0.73) compared with usual care, which is considered to be both statistically and clinically significant. The statistical heterogeneity associated with this comparison was minimal (I2 = 4%). The GRADE quality of evidence was assessed as moderate for this outcome.
Figure 12: Specialized Community-Based Care Model 1: Mean Change in HbA1c From Baseline to Follow-Up Compared With Usual Care (%)*.
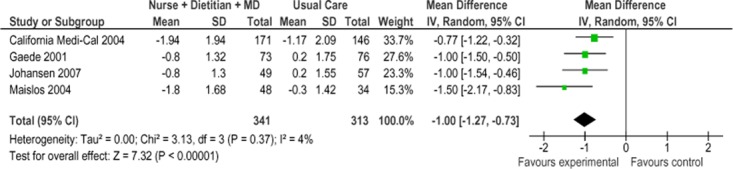
Abbreviations: CI, confidence interval; HbA1c, hemoglobin A1c; IV, inverse variance; MD, medical doctor; M-H, Mantel Haenszel; SD, standard deviation.
Model 2 (Primary Care Physician and Pharmacist Team)
Two of the 3 studies were eligible for inclusion in the meta-analysis of HbA1c for Model 2. (37;38) Figure 13 presents the mean change in HbA1c from baseline to follow-up between groups for studies comparing SCBC Model 2 to usual care. Overall, the model resulted in a mean HbA1c reduction of 1.05% (95% CI, −1.57 to −0.52) compared with usual care, which is considered to be both statistically and clinically significant. No statistical heterogeneity was associated with this comparison (I2 = 0%). The GRADE quality of evidenced was assessed as high for this outcome.
Figure 13: Specialized Community-Based Care Model 2: Mean Change in HbA1c From Baseline to Follow-Up Compared With Usual Care (%)*.
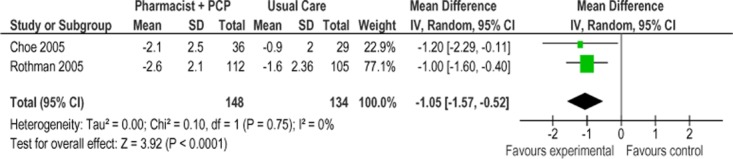
Abbreviations: CI, confidence interval; HbA1c, hemoglobin A1c; IV, inverse variance; M-H, Mantel Haenszel; PCP, primary care physician; SD, standard deviation.
Diabetes: Systolic Blood Pressure
Model 1 (Registered Nurse, Registered Dietitian, and Physician Team)
Two of the 5 studies were eligible for inclusion in the meta-analyses of systolic blood pressure for Model 1. (35;39) Figure 14 presents the mean change in SBP from baseline to follow-up between Model 1 and usual care. Overall, the model had no effect on the mean change in SBP between groups (−2.04 mm Hg; 95% CI, −13.80 to 9.72). However, because the evidence was assessed as very low quality evidence for this outcome, the estimate of effect is uncertain; further, there was high statistical heterogeneity associated with this comparison (I2 = 89%).
Figure 14: Specialized Community-Based Care Model 1: Mean Change in Systolic Blood Pressure From Baseline to Follow-up Compared With Usual Care (mm Hg)*.
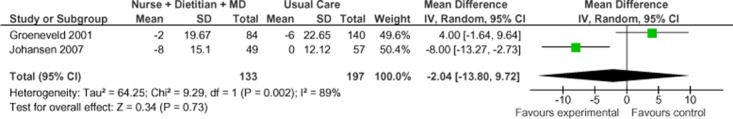
Abbreviations: CI, confidence interval; IV, inverse variance; MD, medical doctor; M-H, Mantel Haenszel; SD, standard deviation.
Model 2 (Primary Care Physician and Pharmacist Team)
Two of the 3 studies examined were eligible for inclusion in the meta-analysis of SBP for Model 2. (38;40) Figure 15 presents the mean change in SBP from baseline to follow-up between Model 2 and usual care. Overall, the model resulted in a statistically and clinically significant mean SBP reduction of 7.13 mm Hg (95% CI, −11.78 to 2.48). There was moderate statistical heterogeneity associated with this comparison (I2 = 46%). The GRADE quality of evidence was assessed as moderate for this outcome.
Figure 15: Specialized Community-based Care Model 2: Mean Change in Systolic Blood Pressure From Baseline to Follow-up Compared With Usual Care (mm Hg)*.

Abbreviations: CI, confidence interval; IV, inverse variance; PCP, primary care physician; SD, standard deviation.
Chronic Wounds: Wound Healing
Two studies that met the inclusion criteria for wound healing were identified: a randomized controlled trial (RCT) (41;42) and a controlled trial using a before-and-after study design. The studies varied in setting, composition of the wound care team, outcome measures, and follow-up periods. In both studies, the wound care team members received training in wound care management and followed a wound care management protocol.
In the RCT, Vu et al (41) reported a nonsignificant difference in the proportion of wounds healed in 6 months between the treatment and control group using univariate analysis (61.7% for treatment vs.52.5% for control; P = 0.074, RR = 1.19) There was also a nonsignificant difference in mean days to healing (82 for treatment vs. 101 for control, P = 0.095). More people in the treatment group than in the control group had a Brief Pain Inventory score of 0 (better pain control) at 6 months (38.6% for treatment vs. 24.4% for control; P = 0.017, RR = 1.58). Multivariate analysis yielded a statistically significant hazard ratio of 1.73 (95% CI, 1.20–1.50; P = 0.003) in the treatment group.
In the controlled trial, Harrison et al (42) reported a statistically significant difference in healing rates between the pre (control) and post (intervention) phases of the study. Of patients in the pre phase, 23% had healed ulcers 3 months after study enrollment, whereas 56% were healed in the post phase (P < 0.001, odds ratio = 4.17) (Figure 3). Furthermore, 27% of patients were treated daily or more often in the pre phase, whereas only 6% were treated at this frequency in the post phase (P < 0.001), corresponding to a 34% RRR in the frequency of daily treatments. The authors did not report the results of pain relief assessments.
The body of evidence was assessed using the GRADE methodology for 4 outcomes: proportion of wounds healed, proportion of patients with healed wounds, wound-associated pain relief, and proportion of patients needing daily wound treatments. The evidence was found to be of very low quality.
Figure 16: Proportion of Healed Wounds*.
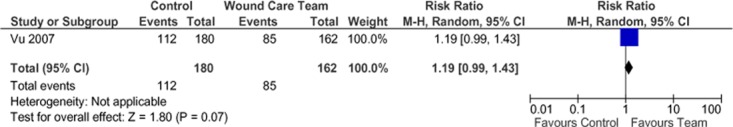
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Figure 17: Proportion of Patients With a Brief Pain Inventory Score of 0*.

Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Figure 18: Proportion of Patients With Healed Wounds*.
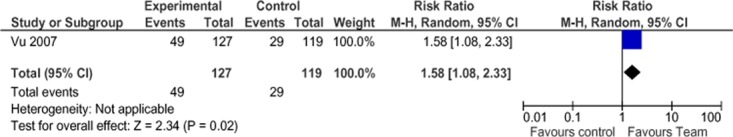
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Figure 19: Proportion of Patients Needing Daily Wound Treatments*.
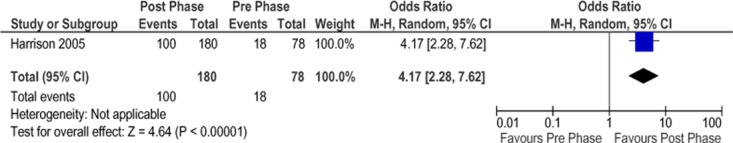
Abbreviations: CI, confidence interval; M-H, Mantel Haenszel.
Quality of Life
Quality of life was reported in the EBAs on heart failure and COPD. There were various scales used to assess quality of life in the heart failure studies, thus no pooling of results was attempted. The 2 studies that reported quality of life in the COPD studies used the same scale, so the results were pooled.
Heart Failure
Quality of life was reported in 5 studies using the Minnesota Living With Heart Failure Questionnaire (MLHFQ) (19-21;25;26) and in 1 study using the Nottingham Health Profile Questionnaire (22). The MLHFQ results are reported in our analysis. Two studies (20;21) reported the mean score at 1-year follow-up, although they did not provide the standard deviation. One study (25) reported the median and range scores at 1-year follow-up in each group. Two studies (19;26) reported the change scores of the physical and emotional subscales of the MLHFQ; 1 of them reported a statistically significant difference in the change in physical subscale from baseline to 1-year follow-up in favour of the SCBC group. A significant change in emotional subscale scores from baseline to 1-year follow-up was not reported in either of these studies.
Chronic Obstructive Pulmonary Disease
Two studies reported results of quality-of-life assessment based on St. George’s Respiratory Questionnaire (SGRQ). (28;32) A mean decrease in SGRQ score indicates an improvement in quality of life while a mean increase indicates deterioration in quality of life. In all studies the mean change score from baseline to the final time point showed either an improvement or less deterioration in the SCBC treatment group compared with the control group. The mean difference in change scores between the SCBC and control groups was statistically significant in both studies. The pooled weighted mean difference in total SGRQ score was −4.05 (95% CI, −6.47 to 1.63). The GRADE quality of evidence was assessed as moderate for this outcome, indicating that further research may change the estimate of effect.
Figure 20: Meta-Analysis of Mean Change in SGRQ Scores From Baseline to Final Time Point*.
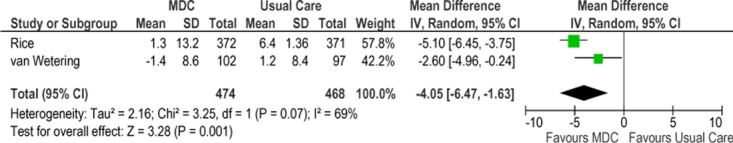
Abbreviations: CI, confidence interval; IV, inverse variance; MDC, multidisciplinary care; M-H, Mantel Haenszel; SGRQ, St. George’s Respiratory Questionnaire; SD, standard deviation.
Summary of SCBC Results for Heart Failure, COPD, and Diabetes
Components of SCBC Interventions for Heart Failure
The programs described in the studies assessing the effectiveness of SCBC for heart failure were quite heterogeneous. Table 4 outlines the results of the studies and components of the interventions. In terms of team composition, all the studies included a heart failure specialist (either a nurse or physician) and at least 1 other health care professional. Visits occurred at a frequency of once every 1 to 2 months. In most of the interventions, the first post-discharge visit occurred within 1 to 3 weeks of discharge, though the study by GESICA (21) did not limit patients to those being discharged from hospital. Four of the studies reported the health care professionals having availability outside of scheduled visits.
Table 2: Results of the Heart Failure Studies and Components of the Heart Failure Programs*.
| Study | All-Cause Mortality RR (95% CI) | HF-Specific Mortality RR (95% CI) | All-Cause Hosp RR (95% CI) | HF-Specific Hosp RR (95% CI) | Primary Outcome | Team | Time to 1st Visit After Discharge | Availability Outside of Appointment | Nurse Change Meds? | Involvement of GP | Group Sessions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruggink et al, 2005 (20) |
NS | — | — | NS | Mortality, readmit | HF physician, RN, RD | 1 week | Yes | No | None | No |
| Wierzchowiecki et al, 2005 (25) |
NS | NS | 0.63 (0.41–0.97) | 0.52 (0.29–0.94) | Unclear | Cardiologist, HF nurse, PT, psych | 2 weeks | Yes | No | GPs could call clinic | No |
| Mejhert et al, 2004 (22) |
0.59 (0.40–0.88) | — | NS | — | Quality of life | Cardiologist, RN | NR | NR | Yes | None | No |
| Stromberg et al, 2003 (24) |
0.36 (0.17–0.79) | 0.29 (0.12–0.72) | NS | — | Mortality, readmit | Cardiologist, HF nurse | 2–3 weeks | Yes | Yes | None | No |
| Doughty et al, 2002 (26) |
NS | — | NS | NS | Mortality, readmit | Cardiologist, HF nurse | 2 weeks | Yes | No | Every other visit was with GP | Yes |
| Dunagan et al, 2005 (19) |
NS | — | NS | NS | Readmit | RN, HF physician | 3 days | No | No | None | No |
| GESICA, 2005 (21) |
NS | 0.80 (0.68–0.95) | NS | 0.76 (0.61–0.93) | Mortality, hosp | RN | N/A | No | Yes, for diuretics | None | No |
Abbreviations: CI, confidence interval; GP, general practitioner; HF, heart failure; hosp, hospitalization; meds, medications; NR, not reported; NS, not significant; psych, psychologist; PT, physiotherapist; RD, registered dietitian; RN, registered nurse; RR, relative risk.
Of note, 3 studies reported that nurses could alter the dosages of medications within defined protocols. (21;22;24) These 3 studies were also the only ones to report a survival benefit in the intervention group compared with the control group. Offering group sessions or involving the general practitioner did not seem to impact mortality or hospitalization rates. Only the study by Dunagan et al (19) reported that patients were screened for depression, while the study by Stromberg et al (24) highlighted caregiver support as a component of the intervention.
Table 5 applies the components of Wagner’s Chronic Care Model to the studies. It appears that the titration of medications and lifestyle counselling are the 2 components that impact mortality and hospitalization rates.
Table 3: Components of Heart Failure Programs Applying Wagner’s Chronic Care Model*.
| Study | All-Cause Mortality RR (95% CI) | HF-Specific Mortality RR (95% CI) | All-Cause Hosp RR (95% CI) | HF-Specific Hosp RR (95% CI) | Disease Education | Medication Education | Titration of Meds | Diet Counsel | Exercise Counsel | Lifestyle Counsel | Self-Care Support | Regular Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruggink et al, 2005 (20) |
NS | — | — | NS | ✓ | ✓ | X | ✓ | ✓ | X | X | ✓ |
| Wierzchowiecki et al, 2005 (25) |
NS | NS | 0.63 (0.41–0.97) | 0.52 (0.29–0.94) | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ |
| Mejhert et al, 2004 (22) |
0.59 (0.40–0.88) | — | NS | — | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ |
| Stromberg et al, 2003 (24) |
0.36 (0.17–0.79) | 0.29 (0.12–0.72) | NS | — | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| Doughty et al, 2002 (26) |
NS | — | NS | NS | ✓ | ✓ | X | ✓ | ✓ | X | ✓ | ✓ |
| Dunagan et al, 2005 (19) |
NS | — | NS | NS | ✓ | X | ✓ | ✓ | X | X | ✓ | ✓ |
| GESICA, 2005 (21) |
NS | 0.80 (0.68–0.95) | NS | 0.76 (0.61–0.93) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X |
Abbreviations: CI, confidence interval; HF, heart failure; hosp, hospitalization; meds, medications; NS, not significant; RR, relative risk.
Components of Specialized Community-Based Care Interventions for COPD
The study by Koff et al (31) was excluded from the analysis of components of specialized community-based care because it was primarily a telehealth study. In the 5 studies analyzed, the teams consisted of a variety of health care providers including physiotherapists, nurses, physicians, and respiratory therapists (Table 6). (27-29;32;43) In all studies, visits occurred at a frequency of once every 4 to 6 weeks. The study by van Wetering et al (32) focused on exercise and physiotherapy. The study by Rice et al, (28) which reported that the intervention was effective in reducing hospitalizations and ED visits, consisted of an initial group session followed by monthly phone calls from a case manager. The primary outcomes of this study (and the ones reported by MAS) were hospitalizations and ED visits. The other studies reported different primary outcomes; as such, their ability to detect significant differences in health resource utilization outcomes may be limited because they were not powered to detect these differences. The interventions described by Casas et al (29) and Rea et al (27) involved a specialist team working with the primary care team, while the intervention in Solomon et al (30) was a pharmacist-led program. Of interest, both Rice et al (28) and Rea et al (27) reported that an individual action plan was created through consultation with the specialized community-based team and the patient. The other studies all reported providing individualized plans, without explicitly stating that the patient was consulted during the development of the plan. None of the studies identified reported screening for depression or caregiver support as an explicit component of the program.
Table 4: Results of the COPD Studies and Components of the COPD Programs*.
| Study | All-Cause Mortality RR (95% CI) | All-Cause Hosp RR (95% CI) | COPD-Specific Hosp RR (95% CI) | ED Visits RR (95% CI) | Lung Function RR (95% CI) | Primary Outcome | Team | Individual Action Plan Created With Patient | Availability Outside of Appointment | Nurse Could Change Medication | Involvement of GP | Group Sessions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van Wetering et al, 2010 (32) |
— | — | — | — | NS | Quality of life | PT, RN, RD | No | No | No | No | No |
| Rice et al, 2010 (28) |
NS | 0.80 (0.65–0.97) |
0.72 (0.54–0.96) |
0.60 (0.44–0.82) |
— | COPD hosp, ED visits | Respiratory therapist | Yes | Yes | No | No | Yes |
| Casas et al, 2006 (29) |
NS | 0.67 (0.49–0.91) |
— | — | — | Readmit | Specialized RN, PC team | No | Yes | No | Yes | Yes |
| Rea et al, 2004 (27) |
NS | NS | 0.56 (0.33–0.96) |
NS | NS | Change in hosp days | Respiratory physician, resp RN, GP | Yes | No | No | Yes | No |
| Solomon et al, 1998 (30) |
— | NS | — | NS | — | Unclear | Pharmacist | No | No | No | Yes | No |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; GP, general practitioner; hosp, hospitalization; NS, not significant; PC, primary care; PT, physiotherapist; RD, registered dietitian; RN, registered nurse; RR, relative risk.
Table 7 applies the components of Wagner’s Chronic Care Model to the studies.
Table 5: Components of COPD Programs Applying Wagner’s Chronic Care Model*.
| Study | All-Cause Mortality RR (95% CI) | All-Cause Hosp RR (95% CI) | COPD Specific Hosp RR (95% CI) | ED Visits RR (95% CI) | Lung Function RR (95% CI) | Disease Education | Medication Education | Diet Counsel | Exercise Counsel | Lifestyle Counsel | Self-Care Support | Regular Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van Wetering et al, 2010 (32) |
— | — | — | — | NS | ✓ | X | X | ✓ | ✓ | ✓ | ✓ |
| Rice et al, 2010 (28) |
NS | 0.80 (0.65–0.97) |
0.72 (0.54–0.96) |
0.60 (0.44–0.82) |
— | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ |
| Casas et al, 2006 (29) |
NS | 0.67 (0.49–0.91) |
— | — | — | ✓ | ✓ | X | X | X | ✓ | ✓ |
| Rea et al, 2004 (27) |
NS | NS | 0.56 (0.33–0.96) |
NS | NS | ✓ | ✓ | X | X | ✓ | ✓ | ✓ |
| Solomon et al, 1998 (30) |
— | NS | — | NS | — | ✓ | ✓ | X | X | X | ✓ | X |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; hosp, hospitalization; NS, not significant; RR, relative risk.
Components of SCBC Interventions for Diabetes
All 8 studies that measured change in HbA1c reported that HbA1c values were significantly improved in the SCBC group compared with the control group (Table 8). (33-40) In all but 1 of the 4 studies that reported a change in SBP, (39) SBP was significantly lower in the intervention group than in the control group at follow-up. Only the study by Groeneveld et al (39) did not report a significant improvement in SBP in the intervention group. Of note, patients were seen every 3 months in this study, while patients in the other studies were seen at more frequent intervals (every 2 to 6 weeks). None of the studies reported screening for depression.
Table 6: Results of the Diabetes Studies and Components of the Diabetes Programs*.
| Study | HbA1c WMD (95% CI) | SBP WMD (95% CI) | Primary Outcome | Team | Frequency of Visits | Caregiver Support | Availability Outside of Appointment | Nurse Could Change Medication | Involvement of GP | Group Sessions |
|---|---|---|---|---|---|---|---|---|---|---|
| Medi-Cal, 2004 (33) |
–0.77 (–1.22 to –0.32) | — | HbA1c | Diabetes RN, RD, endocrinologist | Unclear | No | Yes | No | Yes | No |
| Gaede et al, 2001 (34) | –1.00 (–1.50 to –0.50) | — | Unclear | RN, RD, GP | Frequent (> 10 within 1 year) | Yes | No | No | No | Yes |
| Groeneveld et al, 2001 (39) |
— | NS | HbA1c | Diabetes RN, RD, GP | Every 3 months | No | No | No | Yes | No |
| Johansen et al, 2007 (35) |
–1.00 (–1.54 to –0.46) | –8.00 (–13.27 to –2.73) | Change in CHD risk | RN, RD, GP, PT | At least 4 times in 6 months | No | No | No | No | Yes |
| Maislos et al, 2004 (36) |
–1.50 (–2.17 to –0.83) | — | HbA1c | Diabetes RN, RD, GP | “Regular” | No | No | No | No | No |
| Choe et al, 2005 (37) |
–1.20 (–2.29 to –0.11) | — | HbA1c | Pharmacist, GP | Monthly | No | No | No | Yes | No |
| McLean et al, 2008 (40) |
— | –5.10 (–9.24 to –0.96) | SBP | Pharmacist, RN | Every 6 weeks | No | No | No | Yes | No |
| Rothman et al, 2005 (38) |
–1.00 (–1.60 to –0.40) | –9.90 (–15.44 to –4.36) | HbA1c, SBP | Pharmacist, GP | Every 2–4 weeks | No | No | No | Yes | No |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HbA1c, hemoglobin A1c; GP, general practitioner; NS, not significant; PT, physiotherapist, RD, registered dietitian; RN, registered nurse; SBP, systolic blood pressure; WMD, weighted mean difference.
Table 9 applies the components of Wagner’s Chronic Care Model to the studies.
Table 7: Components of Diabetes Programs Applying Wagner’s Chronic Care Model*.
| Study | HbA1c WMD (95% CI) | SBP WMD (95% CI) | Disease Education | Med Education | Titration of Meds | Diet Counsel | Exercise Counsel | Lifestyle Counsel | Self-Care Support | Regular Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Medi-Cal, 2004 (33) |
–0.77 (–1.22 to –0.32) | — | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gaede et al, 2001 (34) |
–1.00 (–1.50 to –0.50) | — | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Groeneveld et al, 2001 (39) |
— | NS | ✓ | X | ✓ | ✓ | X | X | X | ✓ |
| Johansen et al, 2007 (35) |
–1.00 (–1.54 to –0.46) | –8.00 (–13.27 to –2.73) | ✓ | X | ✓ | ✓ | ✓ | X | X | ✓ |
| Maislos et al, 2004 (36) |
–1.50 (–2.17 to –0.83) | — | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ |
| Choe et al, 2005 (37) |
–1.20 (–2.29 to –0.11) | — | ✓ | X | ✓ | X | X | X | ✓ | ✓ |
| McLean et al, 2008 (40) |
— | –5.10 (–9.24 to –0.96) | ✓ | X | ✓ | X | X | ✓ | ✓ | ✓ |
| Rothman et al, 2005 (38) |
–1.00 (–1.60 to –0.40) | –9.90 (–15.44 to –4.36) | ✓ | X | ✓ | X | X | X | ✓ | ✓ |
Abbreviations: CI, confidence interval; HbA1c, hemoglobin A1c; NS, not significant; SBP, systolic blood pressure; WMD, weighted mean difference.
Part 3: Intermediate Care in Family Practice—Evidence-Based Analysis
Research Question
Is intermediate care (also known as SCBC) provided within a family practice team effective at optimizing health resource utilization?
Research Methods
Literature Search
Search Strategy
A literature search was performed on May 11, 2011 (updated on October 11, 2011) using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) for studies published from January 1, 2000, to October 10, 2011. Abstracts were reviewed by a single reviewer and, for those studies meeting all of the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full reports
published between January 1, 2000, and October 10, 2011
systematic reviews, meta-analyses, RCTs
studies including patients with confirmed chronic conditions (not suspected)
studies of interventions aimed at managing multiple chronic conditions
multidisciplinary team (2 or more health care professionals)
intervention provided primarily in family practice setting
Exclusion Criteria
nonrandomized trials, observational studies, retrospective studies, case series
studies focusing on the prevention of chronic disease in those without confirmed disease
studies not published in English
Outcomes of Interest
health resource utilization, including hospitalization, readmission, family practice visits, specialist visits, and emergency department visits
quality of life
Statistical Analysis
Due to heterogeneity in the methods and outcomes reported in the RCTs, no statistical analyses were performed; this is a narrative review of the randomized controlled trials identified.
Quality of Evidence
The quality of evidence assigned to individual studies was determined using a modified CONSORT Statement Checklist for Randomized Controlled Trials. (44) The CONSORT Statement was adapted to include 3 additional quality measures: adequacy of control group description, significant differential loss to follow-up between groups, and 30% or higher study attrition. Individual study quality was defined based on total scores according to the CONSORT Statement checklist: very low (0 to < 40%), low (≥ 40% to < 60%), moderate (≥ 60% to < 80%), and high (≥ 80% to 100%).
The quality of the body of evidence for each outcome was examined according to the GRADE Working Group criteria. (44) The overall quality was determined to be very low, low, moderate, or high using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that randomized controlled trials are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, 3 main factors that may raise the quality of evidence were considered: large magnitude of effect, dose response gradient, and accounting for all residual confounding factors. (45) For more detailed information, please refer to the latest series of GRADE articles. (45)
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain. |
Results of Evidence-Based Analysis
Seven RCTs met the inclusion criteria. (46-52) Four of the studies were conducted in the United States and the other in the Ottawa region of Ontario. (51) While the studies were well conducted, there was a fair amount of inconsistency in their results. Two of the studies (47;49) were powered to detect a difference in health resource utilization, while the other 3 (46;51;52) were powered to detect a change in quality-of-life scores. The quality of evidence was assessed using the GRADE tool and found to be moderate for the following outcomes: hospitalization, readmission, emergency department visits, and quality of life.
Table 10 describes the study characteristics of the RCTs included. The number of subjects in the studies ranged from 241 to 951. All studies included at least 1 year of follow-up. In 2 of the studies patients were randomized to the intervention, while in the other studies the physician or physician group was randomized to the intervention. Every study included a home-based component with at least 1 at-home visit by a health care provider to assess the patient. All health care providers were generalists (i.e., without training in a specific chronic disease). With the exception of the study by Counsell et al (52), all studies included patients with moderate to high disease acuity. Disease acuity was measured differently across the studies, but generally assessed a patient’s likelihood for health resource utilization based on risk factors and/or prior health resource use.
Table 8: Randomized Controlled Trials Comparing Intermediate Care in Family Practice to Usual Care for Patients With Multiple Chronic Diseases*.
| Study | Patient Population | Design | # of Patients | Duration of Study | Intervention Team | Intervention | Control |
|---|---|---|---|---|---|---|---|
| Boult et al, 2011 and Marsteller et al, 2010 (USA) (46;50) |
≥ 65 years; high risk of high utilization of health services | Cluster randomized; primary care team randomized | 850 | 20 months | Nurse with training in guided care | 50–60 patient caseload; nurse performed comprehensive assessment at home and created a care guide for the patient | Usual care with PCP |
| Hogg et al, 2009 (Canada) (51) |
> 50 years; at risk of experiencing adverse health outcomes | Patients randomized | 241 | 12–18 months (mean 14.9 months) | PCP, NP, pharmacist | Care provided by nurse or pharmacist, usually in the home or by telephone; care plan established for each patient | Usual care with PCP |
| Counsell et al, 2007 (USA) (52) |
≥ 65 years; low income | PCPs randomized | 951 | 24 months | NP, social worker, PCP, geriatric interdisciplinary team | 2 years of home-based care management by NP and social worker; following 12 care protocols common to geriatric conditions | Usual care with PCP |
| Scott et al, 2004 and Coleman et al, 2001 (USA) (47;48) |
≥ 60 years; ≥ 11 outpatient visits in previous 18 months; ≥ 1 chronic condition | Patients randomized | 294 | 24 months | PCP, pharmacist, nurse | Monthly group meeting; one-on-one appointments after group session with PCP as needed | Usual care with PCP |
| Sommers et al, 2000 (USA) (49) |
≥ 65 years; ≥ 1 visit in 3 month period; unable to carry out at least 1 instrumental activity of daily living† | PCPs randomized | 543 | Mean 14 months (range 10–18 months) | PCP, nurse with geriatrics training, social worker | Nurse or social worker has an initial home visit to assess patient; team creates a risk reduction plan; nurse and social worker meet with patients at least once every 6 weeks; team meets monthly to discuss status of patients | Usual care with PCP |
Abbreviations: NP, nurse practitioner; PCP, primary care physician.
Instrumental activities of daily living include getting around outside the home, meal preparation, household chores, taking medication, money management.
Boult et al, (46) who published the most recent study identified comparing multidisciplinary care to usual care in a primary care setting, did not find a significant reduction in health resource utilization. However, this study was powered to detect differences in quality of life, not health resource utilization. Investigators did not report a difference in quality of life between the SCBC and usual care groups. The study randomized physician teams within practices, rather than patients, to be providers of either “guided care” or usual care. Nurses were trained to provide guided care to a caseload of 50 to 60 patients. Guided care involves a comprehensive home assessment in which a nurse creates a care guide and action plan, along with monthly patient monitoring. The nurse helps the patient with self-care and engagement, coordinates the patient’s care providers, provides caregiver support, and facilitates access to community resources. Patients were eligible for enrollment if they were 65 years or older and at high risk of using health care services (defined by the Hierarchical Condition Category predictive model).
The only Canadian study that met the inclusion criteria, published by Hogg et al in 2009, (51) randomized patients to receive either Anticipatory and Preventive Team Care (APTCare) or usual care. Patients were 50 years or older and at risk of experiencing adverse health outcomes based on their physician’s opinion. Initially the study was designed to detect a difference in ED visits between the APTCare and usual care groups, but this was modified when the investigators determined that the number of ED visits was too low to be an appropriate outcome. The primary outcome was thus changed to quality of care through a chronic disease management score. The study did report a significant improvement in the quality of care score in the APTCare versus the usual care group, but no significant differences in any of the secondary health resource utilization outcomes.
The RCT by Counsell et al (52) included 951 low-income patients aged 65 years or older. The study evaluated the GRACE (Geriatric Resources for Assessment and Care of Elders) model, which aims to improve geriatric care by integrating geriatric and primary care across the care continuum. The primary care physician was randomized to either the intervention group or the usual care group. In the intervention group the primary care physician collaborated with a nurse practitioner and social worker, who provided home-based care over a 2-year period. The team developed an individualized care plan based on an extensive home assessment of the patient. The nurse or social worker met with patients primarily in their homes, but also in the primary care– physician’s office, hospital, or nursing home. Telephone contact was also maintained with patients and caregivers. At minimum each patient had a face-to-face appointment after the initial visit, followed by monthly face-to-face or telephone appointments. An appointment was also scheduled after an ED visit or hospitalization. The primary outcomes were health and functional status (measured with the Short Form 36) and activities of daily living. Emergency department visits and hospitalizations were also reported, but the study was not powered to detect differences in health resource utilization.
An RCT conducted at a large Health Management Organization in the United States was reported in 2 articles, (47;48) Patients were eligible for inclusion if they were at least 60 years old with at least 1 self-reported chronic condition and had more than 11 outpatient visits within the past 18 months. The intervention included monthly group visits led by a primary care physician, nurse, and pharmacist (and occasionally other health care providers depending on the topic for discussion). Patients had the opportunity to visit their physician after the group session. The main outcome measures were health and functionality, activities of daily living, and health resource utilization. A major limitation of this study is the probable selection bias, given that only patients expressing an interest in the group care model (in a survey) were selected to participate. Thus, the study lacks generalizability. An expert consulted about the study suggested that this model of care may work in a trial setting, but would be difficult to implement because not all patients are interested in group care and reimbursement can be challenging for providers depending on payment structure.
Sommers et al (49) reported the results of a cluster randomized trial in which the physician offices were randomized to intervention with either SCBC or usual care. The offices in the intervention group received a social worker and nurse to work in the office, while the usual care group did not receive any additional staff. Patients were eligible for inclusion if they were 65 years or older, had at least 2 chronic conditions, and required assistance with at least 1 instrumental activity of daily living. Examples of instrumental activities of daily living include getting around outside the home, meal preparation, household chores, taking medications, and money management. The primary outcome of the study was health resource utilization. The study detected a statistically significant reduction in hospitalizations, readmission rates, and family physician visits in the intervention group compared with the usual care group. There was no significant difference in ED visits or home care visits between the intervention and usual care groups.
Table 11 describes the characteristics of the interventions provided by each of the RCTs. With the exception of the study by Scott et al (47), all the studies followed a relatively similar model in which patients received a home visit followed by a care plan developed by a multidisciplinary team based in the primary care physician’s office. The study by Scott et al (47) focused on 90-minute group sessions and did not provide home visits or care plans.
Table 9: Components of Specialized community-based Care Interventions in Family Practice*.
| Study | Team | Home Visit | Care Plan | Components of Care Plan | Types of Interaction | Role of Team Members | Frequency of Patient Interactions |
|---|---|---|---|---|---|---|---|
| Boult et al, 2011 and Marsteller et al, 2010 (USA) (46;50) |
PCP, nurse | Yes (nurse) | Yes (care guide and action plan) | Patient identifies priorities for optimizing health; care guide is for providers; action plan is for patient to set goals | Telephone; home visits; PCP office; group sessions to learn about designing action plan | Coordinating care; smoothing transitions between care sites; promoting self-management; educating and supporting caregivers; linking to community services | At least monthly |
| Hogg et al, 2009 (Canada) (51) |
PCP, NP, pharmacist | Yes (nurse and pharmacist) | Medication management review (pharmacist); care plan (NP) | Medication review identifies potential drug-related problems; care plan identifies active health issues and outlines management goals for patient and team | Telephone; home visits; PCP office (mostly PCP) | Pharmacist identifies potential drug-related issues; NP coordinates care | Not reported |
| Counsell et al, 2007 (USA) (52) |
PCP, NP, SW, geriatrician | Yes (nurse or SW) | Yes (care plan) | Discussed with PCP; - set targets and treatment strategies | Telephone; home visits; PCP office | NP and SW meet with patients and liaise with PCP and geriatrician | At least monthly telephone or in-person visit, and in-person visit after ED visit or hospitalization |
| Scott et al, 2004 and Coleman et al, 2001 (USA) (47;48) |
PCP, nurse, pharmacist | No | No | No care plan | Group session (education and caregiving component) (8–12 patients); one-on-one appointments | Provide education to the group and offer one-on-one support as needed | Monthly 90-minute group sessions with PCP and nurse |
| Sommers et al, 2000 (USA) (49) |
PCP, nurse, SW | Yes (nurse or SW) | Yes (risk reduction plan) | Set target objectives (e.g., reduce arthritis pain); treatment strategies (e.g., 4 short walks daily) | Telephone; home visit; small group session; PCP office | Nurse and/or SW assess new problems, check disease status, coach on self-management skills, and promote use of community-based services | At least every 6 weeks |
Abbreviations: ED, emergency department; NP, nurse practitioner; PCP, primary care physician; SW, social worker.
Table 12 lists the health resource utilization and quality-of-life outcomes reported in the studies. The results of the analysis are mixed. Studies had very heterogeneous results for each of the 5 health resource utilization outcomes reported, making it impossible to pool the results for any of the outcomes. Of note, the 2 RCTs powered to detect differences in health resource utilization found the most significant differences in health resource utilization. (47;49)
Table 10: Health Resource Utilization Outcomes of RCTs Comparing Intermediate Care in Family Practice Compared to Usual Care for Older Patients With Multiple Chronic Conditions*.
| Study | Study Arms | # of Patients (Mean Age) | Hospital Admission | Readmission | ED Visits | Quality of Life | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Boult et al, 2011; Marsteller et al, 2010 (USA) (46;50) |
SCBC | 446 (77.1 y) |
Mean admissions per patient per year | 0.70 | Mean readmissions within 30 days per patient per year | 0.13 | Mean ED visits per patient per year | 0.44 | NR | |
| Usual care | 404 (77.8 y) |
0.72 P = NS |
0.17 P = NS |
0.44 P = NS |
||||||
| Hogg et al, 2009 (Canada) (51) |
SCBC | 120 (69.6 y) |
Difference in mean admissions per patient† | 0.40 | NR | Mean ED visits per patient† | 0.63 | SF-36 physical SF-36 mental |
No significant difference on either | |
| Usual care | 121 (72.8 y) |
0.46 P = 0.67 |
0.73 P = 0.48 |
|||||||
| Counsell et al, 2007 (USA) (52) |
SCBC | 474 (71.8 y) |
Cumulative mean admissions per patient within 2 years | 0.70 | Proportion of patients readmitted within 30 days | 26% | Cumulative 2-year ED visit rate per 1000 | 1445 | SF-36 physical SF-36 mental |
SF-36 physical no significant difference SF-36 mental P < 0.001 |
| Usual care | 477 (71.6 y) |
0.74 P = 0.66 |
32% P = 0.24 |
1748 P = 0.03 |
||||||
| Scott et al, 2004; Coleman et al, 2001 (USA) (47;48) |
SCBC | 145 (74.2 y) |
Mean admissions per patient within 2 years | 0.44 SD 0.89 |
NR | Mean 2-year ED visits per patient | 0.66 SD 1.3 | Mean quality of life score | 7.2 SD 1.8 | |
| Usual care | 149 (74.1 y) |
0.82 SD 1.7 P = 0.013 |
1.1 SD 1.5 P = 0.008 |
6.3 SD 2.0 P = 0.002 |
||||||
| Sommers et al, 2000 (USA) (49) |
SCBC | 280 (78 y) |
Difference at 1 year in rate of hospital admissions per patient | –0.02 | Difference at 1 year in rate of ≥ 1 readmission within 60 days of discharge per patient | –2.0 | Difference at 1 year in rate of ≥ 1 ED visit(s) per patient | 1.2 | Mean difference SF-36 | 0.1 |
| Usual care | 263 (77 y) |
0.18 P = 0.03 |
5.4 P = 0.03 |
–0.66 P = 0.77 |
0 P = 0.08 |
|||||
Abbreviations: ED, emergency department; SCBC, specialized community-based care; NR, not reported; NS, not significant; RCT, randomized controlled trial; SF-36, Short Form 36; SD, standard deviation.
Mean number of events over study period of 12–18 months (mean, 14.9 months).
Limitations
There are a few limitations worth noting. First, while the studies all reported measures of health resource utilization, the outcomes were not reported consistently enough to enable meta-analyses to be conducted. The studies each offered programs that differed from each other, making it difficult to determine which aspects of the programs were effective. The 2 studies powered to detect a difference in health resource utilization were smaller than all but 1 of the other studies. Both these studies detected a difference in hospitalization, while the other 3 did not. The nature of the program or inadequate power calculations may or may not account for this inconsistency.
Intermediate Care in Family Practice Compared to Disease-Specific Clinics: Previous EBAs
Due to the heterogeneity of the studies it is not possible to quantitatively compare the studies of intermediate care (also known as SCBC) within family practice to those within disease-specific clinics. It is possible, however, to describe the characteristics and outcomes of these studies to identify any consistencies or differences across the studies. The original EBAs on heart failure, COPD, and diabetes included 8, 6, and 8 RCTs, respectively. (15-17) Table 13 lists the characteristics of these studies, including primary location of service, disease severity of patients, and health care providers offering the intervention.
Table 11: Comparison of Disease Severity and Health Care Provider Across RCTs of SCBC*.
| Study | Disease Severity | Team† |
|---|---|---|
| Heart Failure | ||
| Rao et al, 2007 (23) | Low (suspected HF) | Specialist |
| Bruggink et al, 2007 (20) | High (discharge) | Specialist |
| Wierzchowiecki et al, 2006 (25) | High (discharge) | Specialist |
| Dunagan et al, 2005 (19) | High (discharge) | Specialist |
| GESICA, 2005 (21) | Low (stable chronic HF) | Specialist |
| Mejhert et al, 2004 (22) | Moderate-high (NYHA II-IV) | Specialist |
| Stromberg et al, 2003 (24) | Moderate-high (NYHA II-IV) | Specialist |
| Doughty et al, 2002 (26) | High (discharge) | Specialist |
| COPD | ||
| Rice et al, 2010 (28) | High (severe) | Specialist |
| van Wetering et al, 2010 (32) | Moderate-high (stage 2–3) | Specialist |
| Koff et al, 2009 (31) | High (stage 3–4) | Specialist |
| Casas et al, 2006 (29) | High (discharge) | Specialist |
| Rea et al, 2004 (27) | Moderate-high (moderate-severe) | Specialist |
| Solomon et al, 1998 (30) | Moderate-high (moderate-severe) | Specialist |
| Diabetes | ||
| McLean et al, 2008 (40) | All (with HBP) | Generalist |
| Johansen et al, 2007 (35) | All (≥ 1 CV risk factor) | Generalist |
| Choe et al, 2005 (37) | Moderate-high (poor glycemic control) | Generalist |
| Rothman et al, 2005 (38) | Moderate-high (poor glycemic control) | Generalist |
| Maislos et al, 2004 (36) | All | Specialist |
| Medi-Cal, 2004 (33) | All | Specialist |
| Gaede et al, 2001 (34) | All | Generalist |
| Groeneveld et al, 2001 (39) | Moderate-high (poor glycemic control) | Specialist |
| Multiple Chronic Conditions | ||
| Boult et al, 2011 and Marsteller et al, 2010 (USA) (46;50) | Moderate-high (high risk of using health services heavily) | Generalist |
| Hogg et al, 2009 (51) | Moderate (at risk of experiencing adverse health outcomes) | Generalist |
| Counsell et al, 2007 (52) | All | Generalist |
| Coleman et al, 2001 Scott et al, 2004 (47;48) | Moderate (high outpatient visits) | Generalist |
| Sommers et al, 2000 (49) | Moderate-High (high outpatient visits, limitations with iADL) | Generalist |
Abbreviations: CV, cardiovascular; HBP, high blood pressure; HF, heart failure, iADL, instrumental activities of daily living; SCBC, specialized community-based care; NYHA, New York Heart Association Classification.
Team refers to either a specialist team (with at least 1 health care provider with disease-specific training) or to a generalist team (with health care providers without disease-specific training).
The heart failure studies all included at least 1 specialist per team, and 6 out of the 8 studies had patients with moderate to high disease severity. In the pooled analysis of all-cause mortality, the benefit of SCBC was higher when all studies were included than when only the 6 studies noted above were included. The I2 was also lower when all studies were included, suggesting lower heterogeneity among the studies.
All the COPD studies included at least 1 specialist per team and only had patients with moderate to high disease severity.
The studies included in the original EBA on SCBC for diabetes were more variable in terms of disease severity and providers of care. Three of the 8 studies included patients with moderate to high disease severity, and 3 of the 8 studies had a diabetes specialist on the team.
Four of the 5 studies that investigated SCBC for multiple chronic diseases in a family practice had patients with moderate to high disease severity, and none of these studies included a specialist in any particular chronic disease area
Economic Analyses
Summary of Economic Literature on Intermediate Care
There have been a few reports of the costs of intermediate care. The results suggest that the decision to fund intermediate care programs warrants caution. Economic literature reviews by Holmes et al (53) and Goetzel et al (54) concluded that there is sufficient evidence to substantiate the cost-effectiveness of heart failure programs, but that the evidence for other chronic disease programs is not as rigorous. Holmes et al did not find diabetes programs to be cost-effective, while Goetzel et al (54) concluded that more research was needed to determine if they were cost-effective. Goetzel et al (54) also concluded that asthma and depression programs do not appear to be cost-effective, but their conclusions were based on limited evidence.
EBAs: Economic Analyses
The results of the economic analyses for each of the evidence-based analyses (EBAs) on intermediate care (also known as specialized community-based care [SCBC]) are described below. An Ontario-based economic analysis and budget impact could not be completed for the EBA on specialized community-based care for chronic wounds because of the low quality of evidence supporting the effectiveness of a wound care team.
Heart Failure
A cost-effectiveness analysis of heart failure clinics in Ontario was performed from the perspective of the Ministry of Health and Long-Term Care. (15) “Standard care” was defined as care provided by a single practitioner and “heart failure clinic” care as care involving at least 1 physician and nurse, 1 of whom has specialized training in heart failure. A standard care cohort in fiscal year 2005 was identified using the Canadian Health Institute for Health Information (CIHI) Discharge Abstract Database (DAD) as patients above the age of 25 years who were discharged from hospital with a diagnosis of heart failure. The comparator heart failure clinic cohort was modeled after the standard care cohort with an improvement in life expectancy applied according to the estimate of benefit (i.e., survival curve benefit) derived from a systematic review of RCTs comparing heart failure clinics to standard care. Incremental costs of providing care in an heart failure clinic were determined from an existing heart failure clinic in Ontario and added to the aggregated costs of standard care. The time horizon of the analysis was 12 years.
Overall, heart failure clinics were associated with a 29% reduction in all-cause mortality, but a 12% increase in hospitalizations. Heart failure clinic care was found to be cost-effective, with an incremental cost-effectiveness ratio (ICER) of $18,259 (Cdn) per life-year gained. The 12-year cumulative cost per patient in the heart failure clinic cohort was $66,532 (Cdn), compared with $53,638 (Cdn) in the standard care group. The positive results of the cost-effectiveness analysis were robust to increased diagnostic test costs in heart failure clinics, increased or decreased life-expectancy benefit (i.e., with ranges from the systematic review), and patient dropout rate.
Chronic Obstructive Pulmonary Disease
Objective
The Program for Assessment of Technology in Health (PATH) was commissioned by the Ontario MAS to predict the long-term costs and effects along with the cost-effectiveness of strategies for the management and treatment of COPD. (55) This report summarizes the structure and inputs for the COPD economic model used to estimate the cost-effectiveness of multidisciplinary teams to manage COPD. Additionally, an estimate of the budget impact of implementing this COPD intervention is reported.
Comparators and Effect Estimates
The following summary estimates from the MAS systematic review comparing multidisciplinary teams to usual care were used in the model to predict long-term outcomes:
COPD-specific hospitalizations: RR, 0.67; 95% CI, 0.52–0.87.
Mortality: RR, 0.81; 95% CI, 0.52–1.27.
Effects were assumed to last 1 year.
Resource Use and Costs
COPD Model
Because COPD is a chronic progressive disease, a Markov model was used for the analyses. The model comprised different health states based upon the Global Initiative for Chronic Obstructive Lung Disease COPD severity classification. Severity is defined as a percentage of predicted FEV1. The 4 severity-based health states in the model are mild (80% > FEV1), moderate (50% ≤ FEV1 < 80%), severe (30% ≤ FEV1 < 50%), and very severe (FEV1 < 30%). Patients were assigned different costs and utilities depending on the severity of their health state during each model cycle and they could transition between these states.
In addition to moving between health states, patients are at risk of acute exacerbations of COPD. In each model cycle, patients could have no acute exacerbation, a minor acute exacerbation, or a major exacerbation. For the purpose of the model, a major COPD exacerbation was defined as one requiring hospitalization. Patients suffering a severe exacerbation are at risk of inpatient death. Patients were assigned different costs and utilities in each model cycle depending on whether they experience an exacerbation and the severity of the exacerbation.
Program Costs
Resources reported in the trials were costed and totalled for each trial. Averaging the total costs yielded a cost per patient over a program duration range of 6 to 12 months. Health professionals/services accessed during visits included general physicians, dietitians, social workers, physiotherapists, respiratory nurses, and pharmacy consultations. Costs were obtained from the Ontario Schedule of Physician Benefits and the Ministry’s Guide to Interdisciplinary Provider Compensation for health care staff. The cost per program per patient was calculated to be $1,041.03 (Cdn).
Cost-Effectiveness Analysis Results
Assuming a base case cost of $1,041 (Cdn) per specialized community-based care program per patient, the ICER was calculated to be $14,123 (Cdn) per quality-adjusted life-year. Varying the cost of the program in a 1-way sensitivity analysis to reflect variation in resource utilization reported in the literature increased the ICER to $55,322 (Cdn) per quality-adjusted life-year. Using confidence intervals from the systematic review, distributions were assigned to the summary point estimates and probabilistic sensitivity analyses were run. The probability of specialized community-based care being cost-effective increased as the willingness to pay increased.
Budget Impact Analysis Results
Family health teams (FHTs) often have chronic disease management programs, including those for COPD. Data collected by the Ministry from about half the FHTs in fiscal year 2010 suggest that 81,289 patients with COPD were accessing COPD management programs within these FHTs. This translates to a potential cost of $85 million (Cdn) in fiscal year 2010. This is an underestimate, however, as not all FHTs reported data, and the figure does not incorporate data from non-FHT sources in the province. As such, specialized community-based care resources used in Ontario are unknown and difficult to measure.
Diabetes
Objective
PATH was commissioned by MAS to predict the long-term costs and effects of strategies for successful management and treatment of type 2 diabetes, as well as their cost-effectiveness. (17) The objective of this economic analysis was to compare the lifetime costs, effects, health events, and cost-effectiveness of a specialized SCBC program versus no program in adults with type 2 diabetes using the Ontario Diabetes Economic Model (ODEM).
An assessment of type 2 diabetes interventions requires an evaluation of both short- and long-term cost and effectiveness. Early management of diabetes can help delay and even prevent complications that can have a large impact on patients’ quality of life and health care costs. Reductions in future complications may also offset “up-front” medical resources invested in intensive disease management.
Comparators and Effect Estimates
Table 14 describes the effectiveness of the Sault Ste Marie primary care multidisciplinary management program in adults with type 2 diabetes. (A more detailed description of the effectiveness analysis appears in the O’Reilly et al 2007 (56) article in the Canadian Journal of Diabetes.) MAS did not conduct the systematic review on multidisciplinary diabetes programs. The effect of a current model of care for diabetes management in the Sault Ste Marie Health Centre was measured by PATH and analyzed through ODEM. The study specifics have been described elsewhere. (56) Effects were assumed to last for a period of 1 year.
Table 12: Effectiveness of Sault Ste Marie Multidisciplinary Diabetes Management Program in Adults With Type 2 Diabetes*.
| Time-Varying Risk Factor | Before (SE) | After (SE) | Change (95% CI) | P value |
|---|---|---|---|---|
| HbA1c | 8.14% (0.10) | 7.12% (0.07) | –1.02% (–1.25 to –0.79) |
< 0.001 |
| SBP (mm Hg) | 138.68 (0.98) | 137.36 (0.95) | –1.32 (–3.42 to 0.78) |
0.219 |
| Total cholesterol | 5.43 (0.06) | 4.97 (0.05) | –0.47 (–0.58 to –0.35) |
< 0.001 |
| HDL cholesterol | 1.14 (0.02) | 1.20 (0.02) | 0.06 (0.03 0.09) |
< 0.001 |
| Smokers | 19.4% (2.6) | 13.8% (1.8) | –5.6% (–11.6 to 0.01) |
0.070 |
Abbreviations: SBP, systolic blood pressure; CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein, SE; standard error.
Resource Use and Costs
Ontario Diabetes Economic Model
The recently developed United Kingdom Prospective Diabetes Study Outcomes Model uses a system of equations in a computer simulation to predict the occurrence and timing of 7 diabetes-related complications (fatal or nonfatal myocardial infarction, other ischemic heart disease, stroke, heart failure, amputation, renal failure, and blindness) and death to calculate life expectancy and quality-adjusted life expectancy for type 2 diabetes patients. To account for event-related dependencies, the model makes use of time-varying risk factors (e.g., blood pressure and HbA1c), which also facilitates its application to patient groups at different stages of the disease. The model is based on data from over 5,000 patients with over 53,000 years of patient follow-up. To be applied to other geographic areas (such as Ontario), however, the model requires adapting. Specifically, cross-country differences may exist in the incidence and prevalence of diabetes, baseline demographics, diabetes risk factors, overall mortality or mortality from diabetes-related complications, costs (e.g. treatment and management of complications), and the cost and effects of treatment programs. Accordingly, the model was populated with Ontario-specific data for use in the province.
In brief, more than 734,000 patients with diabetes were identified in the Ontario Diabetes Database and followed for up to 10 years. Various administrative databases were linked to this population in order to measure the prevalence and incidence of complications, health care resource utilization (i.e., inpatient and outpatient hospitalizations, outpatient visits, prescription drugs, emergency department visits, and home care), and death. Unit costs were collected and assigned to each of the different health care sectors. Complication-specific costs were divided into 2 time periods:
immediate costs that accrue within the year in which a complication first occurs; and
long-term costs that reflect ongoing costs in subsequent years associated with the management of the complication (including subsequent events of the same type).
Hospital inpatient and noninpatient events and costs were estimated for each complication, using a Ministry perspective. All health care costs used in the model were based on direct costs; it was not possible to measure productivity or other indirect costs from the data available. The ODEM was then used to conduct the cost-effectiveness analysis. Further description of the ODEM has been published elsewhere. (57)
Program Costs
The total program costs were estimated at $240,000. The per-patient cost (n = 401) was $664, with diabetes-related medication costs averaging $230 per patient and program costs averaging $434 per patient. Costs were reported in 2007 Canadian dollars.
Cost-Effectiveness Analysis and Budget Impact Analysis Results
Table 15 summarizes the multidisciplinary diabetes program—including population, health system impact, and assumptions used to calculate the population eligible for such a program—based on the ODEM analysis over a 40-year time horizon. It was assumed that all type 2 diabetes patients in Ontario (n = 744,677) are eligible to access a multidisciplinary diabetes program. (58)
Table 13: Summary of Diabetes Programs Based on ODEM*.
| Incremental Costs, QALY, CE, and Events per 1,000 (Program vs. No Program) | Multidisciplinary Diabetes Program (Per Patient) | Multidisciplinary Diabetes Program (All Eligible Patients = 744,677) |
|---|---|---|
| Δ HbA1c | –1.02% | –1.02% |
| Δ Costs | $7,551 (Cdn) | $5.6 billion (Cdn) |
| Δ QALYs | 0.390 | 290,424 |
| $/QALY gained | $19,869/QALY | $19,869/QALY |
| Δ IHD | 20.5 | 15,265 |
| Δ MI | 54.9 | 40,882 |
| Δ Heart failure | 11.5 | 8,563 |
| Δ Stroke | 18.9 | 14,074 |
| Δ Amputation | 17.7 | 13,180 |
| Δ Blindness | 8.3 | 6,180 |
| Δ Renal Failure | 1.1 | 819 |
Abbreviations: CE, cost-effectiveness; HbA1c, hemoglobin A1c; IHD, ischemic heart disease; MI, myocardial infarction; ODEM, Ontario Diabetes Economic Model; QALY, quality-adjusted life year.
Based on an analysis of ODEM using clinical efficacy data obtained from the above MAS systematic review, multidisciplinary diabetes programs would be considered cost-effective for the treatment and management of adults with type 2 diabetes.
Conclusions
Part 1: Systematic Review of Intermediate Care
Overall, the literature on intermediate care is complex and marked by heterogeneity in study design, quality of interventions, and components. The interventions varied in terms of services provided, health care professionals involved, mode of delivery (telephone, in person, etc.), chronic diseases managed, and duration. It is thus difficult to draw definitive conclusions from the literature.
Part 2: Synthesis of Evidence on Intermediate Care
There appears to be an added benefit to offering SCBC to patients with heart failure, COPD, and diabetes. However, it is difficult to assess which components of the SCBC intervention play a critical role in improving outcomes. MAS attempted to tease out these components according to disease condition.
Part 3: Intermediate Care in Family Practice—Evidence-Based Analysis
Five RCTs comparing SCBC within family practice to usual care were identified. Both of the RCTs powered to detect a difference in health resource utilization found a significant reduction in hospitalizations in the SCBC group compared with the usual care group. The GRADE quality of evidence was assessed to be moderate across the studies that investigated SCBC in family practice.
Acknowledgements
Contributing Authors from MAS
Nicole Bradley
Alex Chambers
Nancy Sikich
Health Economists
Kiran Chandra, PATH
Luciano Ieraci, THETA
Medical Information Officer
Kellee Kaulback
Editorial Staff
Gabrielle Bauer
Irina Alecu
Appendices
Appendix 1: Literature Search Strategies
Initial Search date: May 11, 2011 and updated October 11, 2011
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Search strategy:
disease management program*.ti,ab. (2761)
exp Intermediate Care Facilities/ use mesz (606)
(intermedia* adj2 care).ti,ab. (2362)
exp ambulatory care/ (74541)
exp Ambulatory Care Facilities/ use mesz (40243)
exp ambulatory care nursing/ use emez (8)
exp Outpatients/ use mesz (7095)
exp Outpatient Department/ use emez (31690)
exp outpatient care/ use emez (17035)
((outpatient* or ambulatory) adj2 (care* or service* or clinic* or facility or facilities)).ti,ab. (75323)
exp Cooperative Behavior/ use mesz (21503)
exp Patient Care Team/ use mesz (48910)
exp Nursing, Team/ use mesz (1976)
exp TEAM NURSING/ use emez (20)
exp Cooperation/ use emez (31465)
exp TEAMWORK/ use emez (8964)
exp interdisciplinary communication/ (12130)
exp Interprofessional Relations/ use mesz (47523)
exp Interinstitutional Relations/ use mesz (8653)
exp “Delivery of Health Care, Integrated”/ use mesz (6849)
exp Integrated Health Care System/ use emez (4869)
((transitional or multidisciplin* or multifacet* or multi-disciplin* or multi-facet* or cooperat* or co-operat* or interdisciplin*or inter-disciplin* or collaborat* or multispecial* or multi-special* or share or sharing or shared or integrat* or joint or multi-modal or multimodal) adj2 (care or team*)).ti,ab. (40155)
(team* or liaison).ti,ab. (173636)
exp Community Health Services/ use mesz (443411)
exp community care/ use emez (86202)
exp Program Development/ (33443)
exp Program Evaluation/ use mesz (47688)
exp Health Program/ use emez (70942)
exp Case Management/ (13318)
exp “episode of care”/ use mesz (1302)
exp “Continuity of Patient Care”/ use mesz (12378)
exp Subacute Care/ use mesz (703)
*Disease Management/ (5737)
disease management program*.ti,ab. (2761)
(patient care adj2 manage$).ti,ab. (970)
(care adj2 model*).ti,ab. (12592)
exp Primary Health Care/ (150607)
exp Physicians, Family/ use mesz (14183)
exp general practice/ use emez (61307)
exp general practitioner/ use emez (45454)
exp “Referral and Consultation”/ use mesz (50519)
exp patient referral/ use emez (55714)
((heart failure or wound care or pressure ulcer* or chronic care or chronic disease or chronic illness or copd or chronic obstructive pulmonary disease or diabetes or DM) adj2 (program* or clinic* or center* or centre*)).ti,ab. (18501)
or/2-43 (1395765)
exp Chronic Disease/ (341893)
chronic.ti,ab. (1483743)
45 or 46 (1587163)
44 and 47 (70010)
limit 48 to (controlled clinical trial or meta analysis or randomized controlled trial) (4242)
exp Evidence-based Medicine/ (534394)
exp Technology Assessment, Biomedical/ use mesz (8536)
exp Biomedical Technology Assessment/ use emez (10865)
(health technology adj2 assess$).ti,ab. (2933)
(meta analy$ or metaanaly$ or pooled analysis or (systematic$ adj2 review$)).mp. or (published studies or published literature or medline or embase or data synthesis or data extraction or cochrane).ti,ab. (289008)
exp Random Allocation/ or random*.ti,ab. (1287900)
Randomized Controlled Trial/ or exp Randomization/ or exp RANDOM SAMPLE/ (715074)
exp Double-Blind Method/ (214344)
Double Blind Procedure/ or exp Triple Blind Procedure/ (101022)
exp Control Groups/ (28191)
exp Control Group/ use emez (26872)
exp Placebos/ (216015)
exp PLACEBO/ (185441)
(RCT or placebo? or sham?).mp. [mp=ps, rs, ti, ot, ab, nm, hw, ui, sh, tn, dm, mf, dv, kw] (529353)
or/49-62 (1982978)
48 and 64 (10709)
limit 65 to (english language and yr=″2008 -Current″) (3658)
Appendix 2: GRADE Tables
Table A1: GRADE Evidence Profile for Comparison of Multidisciplinary Teams in Family Practice and Usual Care.
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Hospitalizations | |||||||
| 5 (RCTs) | No serious limitations | Serious limitations (–1)† | No serious limitations | No serious limitations | Undetected | No |
 Moderate Moderate |
| Readmissions | |||||||
| 3 (RCTs) | No serious limitations | Serious limitations (–1)‡ | No serious limitations | No serious limitations | Undetected | No |
 Moderate Moderate |
| ED Visits | |||||||
| 5 (RCTs) | No serious limitations | Serious limitations (–1)§ | No serious limitations | No serious limitations | Undetected | No |
 Moderate Moderate |
| Quality of Life | |||||||
| 4 (RCTs) | No serious limitations | Serious limitations (–1)‖ | No serious limitations | No serious limitations | Undetected | No |
 Moderate Moderate |
Abbreviations: ED, emergency department; No., number; RCT, randomized controlled trial.
Two of 5 studies reported a significant improvement in hospitalization rates. Both of the studies were powered to detect a difference in health resource utilization.
One of 3 studies reported a significant improvement in readmission rates. The study that found the improvement was the only study powered to detect a difference in health resource utilization.
Two of 5 studies reported a significant improvement in ED visits. One of the studies that reported the significant difference was powered to detect a difference.
Two of 4 studies reported a significant improvement in quality of life.
Table A2: Risk of Bias Among Randomized Controlled Trials for the Comparison of Multidisciplinary Teams in Family Practice and Usual Care.
| Author, Year | Allocation Concealment | Blinding | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Boult et al, 2011 and Marsteller et al, 2010 (46;50) | No limitations | Limitations* | No limitations | No limitations | No limitations |
| Hogg et al, 2009 (51) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Counsell et al, 2007 (52) | No limitations | No limitations | No limitations | No limitations | No limitations |
| Scott et al, 2004 and Coleman et al, 2001 (47;48) | No limitations | Limitations* | No limitations | No limitations | Limitations† |
| Sommers et al, 2000 (49) | No limitations | No limitations | No limitations | No limitations | No limitations |
It is unclear if blinding was used in this study.
Patients were selected to participate in this study if they chose that they were interested in the group care model on a survey. Thus, this study lacks generalizability.
Suggested Citation
This report should be cited as follows:
Health Quality Ontario. Specialized community-based care: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2012 November; 12(20):1-60. Available from: www.hqontario.ca/en/mas/tech/pdfs/2012/full-report-specialized-care.pdf
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/EMBASE, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
About Health Quality Ontario
Health Quality Ontario (HQO) is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. HQO works with clinical experts, scientific collaborators and field evaluation partners to develop and publish research that evaluates the effectiveness and cost-effectiveness of health technologies and services in Ontario.
Based on the research conducted by HQO and its partners, the Ontario Health Technology Advisory Committee (OHTAC) — a standing advisory sub-committee of the HQO Board — makes recommendations about the uptake, diffusion, distribution or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders and policy-makers.
This research is published as part of Ontario Health Technology Assessment Series, which is indexed in CINAHL, EMBASE, MEDLINE, and the Centre for Reviews and Dissemination. Corresponding OHTAC recommendations and other associated reports are also published on the HQO website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, HQO and/or its research partners reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, HQO collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social, and legal issues relating to the intervention assist in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals and organizations wishing to comment on reports and recommendations prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This report was prepared by HQO or one of its research partners for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to HQO. It is possible that relevant scientific findings may have been reported since completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the HQO website for a list of all publications: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4435-9535-3 (PDF)
List of Tables
| Table 1: Frequently Reported Components of Intermediate Care Interventions |
| Table 2: Summary of Existing Systematic Reviews and Meta-Analyses* |
| Table 3: Description of Studies in HSAC’s Systematic Review of Interventions to Reduce Ambulatory Care–Sensitive Hospitalizations* |
| Table 4: Results of the Heart Failure Studies and Components of the Heart Failure Programs* |
| Table 5: Components of Heart Failure Programs Applying Wagner’s Chronic Care Model* |
| Table 6: Results of the COPD Studies and Components of the COPD Programs* |
| Table 7: Components of COPD Programs Applying Wagner’s Chronic Care Model* |
| Table 8: Results of the Diabetes Studies and Components of the Diabetes Programs* |
| Table 9: Components of Diabetes Programs Applying Wagner’s Chronic Care Model* |
| Table 10: Randomized Controlled Trials Comparing Intermediate Care in Family Practice to Usual Care for Patients With Multiple Chronic Diseases* |
| Table 11: Components of Specialized community-based Care Interventions in Family Practice* |
| Table 12: Health Resource Utilization Outcomes of RCTs Comparing Intermediate Care in Family Practice Compared to Usual Care for Older Patients With Multiple Chronic Conditions* |
| Table 13: Comparison of Disease Severity and Health Care Provider Across RCTs of SCBC* |
| Table 14: Effectiveness of Sault Ste Marie Multidisciplinary Diabetes Management Program in Adults With Type 2 Diabetes* |
| Table 15: Summary of Diabetes Programs Based on ODEM* |
| Table A1: GRADE Evidence Profile for Comparison of Multidisciplinary Teams in Family Practice and Usual Care |
| Table A2: Risk of Bias Among Randomized Controlled Trials for the Comparison of Multidisciplinary Teams in Family Practice and Usual Care |
List of Figures
| Figure 1: Meta-Analysis of Outcomes for All-Cause Mortality for Heart Failure* |
| Figure 2: Meta-Analysis of Outcomes for All-Cause Mortality for COPD* |
| Figure 3: Meta-Analysis of Outcomes for Heart Failure–Specific Mortality* |
| Figure 4: Meta-Analysis of Outcomes for All-Cause Hospitalization for Heart Failure* |
| Figure 5: Meta-Analysis of Outcomes for All-Cause Hospitalization for COPD* |
| Figure 6: Meta-Analysis of Outcomes for Heart Failure–Specific Hospitalizations* |
| Figure 7: Meta-Analysis of Outcomes for COPD-Specific Hospitalizations* |
| Figure 8: Meta-Analysis of All-Cause Emergency Department Visits (Number of People With at Least 1 Visit During the Follow-Up Period)* |
| Figure 9: Meta-Analysis of COPD-Specific Emergency Department Visits (Number of People With at Least 1 Visit During the Follow-Up Period)* |
| Figure 10: Meta-Analysis of Mean Change From Baseline in FEV1 % Predicted*† |
| Figure 11: Meta-Analysis of Mean Change From Baseline in FEV1 % Predicted*† |
| Figure 12: Specialized Community-Based Care Model 1: Mean Change in HbA1c From Baseline to Follow-Up Compared With Usual Care (%)* |
| Figure 13: Specialized Community-Based Care Model 2: Mean Change in HbA1c From Baseline to Follow-Up Compared With Usual Care (%)* |
| Figure 14: Specialized Community-Based Care Model 1: Mean Change in Systolic Blood Pressure From Baseline to Follow-up Compared With Usual Care (mm Hg)* |
| Figure 15: Specialized Community-based Care Model 2: Mean Change in Systolic Blood Pressure From Baseline to Follow-up Compared With Usual Care (mm Hg)* |
| Figure 17: Proportion of Patients With a Brief Pain Inventory Score of 0* |
| Figure 18: Proportion of Patients With Healed Wounds* |
| Figure 19: Proportion of Patients Needing Daily Wound Treatments* |
| Figure 20: Meta-Analysis of Mean Change in SGRQ Scores From Baseline to Final Time Point* |
List of Abbreviations
- APTCare
Anticipatory and Preventive Team Care
- CI
Confidence interval(s)
- COPD
Chronic obstructive pulmonary disease
- DEP
Diabetes education program
- EBA
Evidence-based analysis
- ED
Emergency department
- FEV1
Forced expiratory volume in 1 second
- FHT
Family health team
- GRACE
Geriatric Resources for Assessment and Care of Elders
- HbA1c
Hemoglobin A1c
- HQO
Health Quality Ontario
- ICER
Incremental cost-effectiveness ratio
- MAS
Medical Advisory Secretariat
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- ODEM
Ontario Diabetes Economic Model
- PATH
Program for Assessment of Technologies in Health
- RCT
Randomized controlled trial
- RR
Relative risk
- RRR
Relative risk reduction
- SBP
Systolic blood pressure
- SD
Standard deviation
- SGRQ
St. George’s Respiratory Questionnaire
- THETA
Toronto Health Economics and Technology Assessment Collaborative
References
- 1.Ontario Health Quality Council Q Monitor: 2008 report on Ontario’s health system. Toronto: Ontario Health Quality Council. 2008.
- 2.Gilmour H, Park J. Dependency, chronic conditions and pain in seniors. [[cited 2011 Sep 11]]. [Internet]. Ottawa, ON: Statistics Canada; 2006. Available from: http://www.statcan.ca/english/freepub/82-003-SIE/2005000/pdf/82-003-SIE20050007443.pdf . [PubMed]
- 3.World Health Organization Innovative care for chronic conditions: building blocks for action. Geneva: World Health Organization. 2002.
- 4.Ontario Ministry of Health and Long-Term Care Health care options directory. [[cited 2012 Nov 9]]. [Internet]. Toronto, ON: Ontario Ministry of Health and Long-Term Care. Available from: http://www.hco-on.ca/English/search/
- 5.Foy R, Hempel S, Rubenstein L, Suttorp M, Seeling M, Shanman R, et al. Meta-analysis: Effect of intervatic communication between collaborating primary care physicians and specialists. Ann Intern Med. 2010;152(4):247–58. doi: 10.7326/0003-4819-152-4-201002160-00010. [DOI] [PubMed] [Google Scholar]
- 6.Boult C, Green AF, Boult LB, Pacala JT, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the institute of medicine’s “retooling for an Aging America” report. J Am Geriatr Soc. 2009;57:2328–37. doi: 10.1111/j.1532-5415.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman K, Mattke S, Perrault PJ, Wagner EH. Untangling practice redesign from disease management: how do we best care for the chronically ill? Annu Rev Public Health. 2009 Apr 29;30:385–408. doi: 10.1146/annurev.publhealth.031308.100249. [DOI] [PubMed] [Google Scholar]
- 8.Dennis SM, Zwar N, Griffiths R, Roland M, Hasan I, Powell DG, et al. Chronic disease management in primary care: from evidence to policy. Med J Aust. 2008;188(8):S53–S56. doi: 10.5694/j.1326-5377.2008.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 9.Basu A, Brinson D. The effectiveness of interventions for reducing ambulatory sensitive hospitalisations: a systematic review. HSAC Report. 2008;1(6) [Google Scholar]
- 10.Mitchell GK, Tieman JJ, Shelby-James TM. Multidisciplinary care planning and teamwork in primary care. Med J Aust. 2008;188(8):S61–S64. doi: 10.5694/j.1326-5377.2008.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Allwright S, O’Dowd T. Does sharing care across the primary-specialty interface improve outcomes in chronic disease? A systematic review. Am J Manag Care. 2008;14(4):213–24. [PubMed] [Google Scholar]
- 12.Smith SM, Allwright S, O’Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev. 2007 Jul;18(3):1–221. doi: 10.1002/14651858.CD004910.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Coster S, Norman I. Cochrane reviews of educational and self-management interventions to guide nursing practice: a review. Int J Nurs Stud. 2009 Apr;46(4):508–28. doi: 10.1016/j.ijnurstu.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 14.McDonald K. M, Sundaram V, Bravata D. M, Lewis R, Lin N, Kraft S, et al. Closing the quality gap: a critical analysis of quality improvement strategies. [[cited Nov 9, 2012]]. [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available from: http://www.ahrq.gov/clinic/tp/caregaptp.htm . [PubMed]
- 15.Medical Advisory Secretariat Community-based care for the specialized management of heart failure: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(17):1–42. [Internet] Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_smcc_20091019.html . [PMC free article] [PubMed] [Google Scholar]
- 16.Sikich N. Community-based multidisciplinary care for patients with stable chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(5):1–51. [Internet] Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_copd_mdc_20120313.html . [PMC free article] [PubMed] [Google Scholar]
- 17.Medical Advisory Secretariat Community-based care for the management of type 2 diabetes: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(23):1–58. [Internet] Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_diabetes_20091020.html . [PMC free article] [PubMed] [Google Scholar]
- 18.Medical Advisory Secretariat Community-based care for chronic wound management: an evidence-based analysis. Ontario Health Technology Assessment Series. 2009;9(18):1–24. [Internet] Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_smcc_20091019.html . [PMC free article] [PubMed] [Google Scholar]
- 19.Dunagan WC, Littenberg B, Ewald GA, Jones CA, Beckham Emery V, Waterman BM, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11(5):358–65. doi: 10.1016/j.cardfail.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Bruggink-Andre de la Porte PWF, Lok DJA, van Wijngaarden J, Cornel JH, Pruijsers-Lamers D, Van Veldhuisen DJ, et al. Heart failure programmes in countries with a primary care-based health care system. Are additional trials necessary? Design of the DEAL-HF study. Eur J Heart Fail. 2005;7:910–20. doi: 10.1016/j.ejheart.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.GESICA investigators Randomised trial of telephone intervention in chronic heart failure: DIAL trial. Br Med J. 2005;331(7514):425–30. doi: 10.1136/bmj.38516.398067.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mejhert M, Kahan T, Persson H, Edner M. Limited long term effects of a management programme for heart failure. Heart. 2004;90:1010–5. doi: 10.1136/hrt.2003.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao A, Walsh J. Impact of specialist care in patients with newly diagnosed heart failure: a randomised controlled study. Int J Cardiol. 2007;115:196–202. doi: 10.1016/j.ijcard.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Stromberg A, Martensson J, Fridlund B, Levin LA, Karlsson JE, Dahlstrom U. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure. Eur Heart J. 2003;24:1014–23. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 25.Wierzchowiecki M, Poprawski K, Nowicka A, Kandziora M, Piatkowska A, Jankowiak M, et al. A new programme of multidisciplinary care for patients with heart failure in Poznan: one year follow up. Kardiol Pol. 2006;64:1063–70. [PubMed] [Google Scholar]
- 26.Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncaster S, Whalley GA, et al. Randomized, controlled trial of integrated heart failure management. Eur Heart J. 2002;23(2):139–46. doi: 10.1053/euhj.2001.2712. [DOI] [PubMed] [Google Scholar]
- 27.Rea H, McAuley J, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34(11):608–14. doi: 10.1111/j.1445-5994.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 28.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease a randomized controlled trial. Am J Respir Crit Care Med. 2010;182:890–6. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 29.Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur J Respir. 2006;28:123–30. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]
- 30.Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, et al. Part 2. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pham Assoc. 1998;38(5):574–85. doi: 10.1016/s1086-5802(16)30371-0. [DOI] [PubMed] [Google Scholar]
- 31.Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33(5):1031–8. doi: 10.1183/09031936.00063108. [DOI] [PubMed] [Google Scholar]
- 32.Van Wetering CR, Hoogendoorn M, Mol SJM, Rutten-van Molken MPMH, Schols AM. Short and long term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. 2010;65:7–13. doi: 10.1136/thx.2009.118620. [DOI] [PubMed] [Google Scholar]
- 33.California Medi-Cal Type 2 Diabetes Study Group Closing the gap: effect of diabetes case management on glycemic control among low-income ethnic minority populations. Diabetes Care. 2004;27(1):95–103. doi: 10.2337/diacare.27.1.95. [DOI] [PubMed] [Google Scholar]
- 34.Gaede P, Beck M, Vedel P, Pedersen O. Limited impact of lifestyle education in patients with Type 2 diabetes mellitus and microalbuminuria: results from a randomized intervention study. Diabetic Med. 2001;18:104–8. doi: 10.1046/j.1464-5491.2001.00444.x. [DOI] [PubMed] [Google Scholar]
- 35.Johansen OE, Gullestad L, Blaasaas KG, Orvik E, Birkeland KI. Effects of structured hospital-based care compared with standard care for Type 2 diabetes--The Asker and Baerum Cardiovascular Diabetes Study, a randomized trial. Diabetic Med. 2007;24:1019–27. doi: 10.1111/j.1464-5491.2007.02198.x. [DOI] [PubMed] [Google Scholar]
- 36.Maislos M, Weisman D. Multidisciplinary approach to patients with poorly controlled type 2 diabetes mellitus: a prospective, randomized study. Acta Diabetol. 2004;41:44–8. doi: 10.1007/s00592-004-0143-1. [DOI] [PubMed] [Google Scholar]
- 37.Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S. Proactive case management of high-risk patients with Type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am J Manag Care. 2005;11:253–60. [PubMed] [Google Scholar]
- 38.Rothman RL, Malone R, Bryant B, Shintani AK, Crigler B, DeWalt DA, et al. A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118(6):276–84. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Groeneveld Y, Petri H, Hermans J, Springer M. An assessment of structured care assistance in the management of patients with type 2 diabetes in general practice. Scand J Prim Health Care. 2001;19:25–30. doi: 10.1080/028134301300034585. [DOI] [PubMed] [Google Scholar]
- 40.McLean DL, McAlister FA, Johnson JA, King KM, Makowsky MJ, Jones CA, et al. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus. Arch Intern Med. 2008;168(21):2355–61. doi: 10.1001/archinte.168.21.2355. [DOI] [PubMed] [Google Scholar]
- 41.Vu T, Harris A, Duncan G, Sussman G. Cost-effectiveness of multidisciplinary wound care in nursing homes: a pseudo-randomized pragmatic cluster trial. Fam Pract. 2007;24(4):372–9. doi: 10.1093/fampra/cmm024. [DOI] [PubMed] [Google Scholar]
- 42.Harrison MB, Graham ID, Lorimer K, Friedberg E, Pierscianowski T, Brandys T. Leg ulcer care in the community, before and after implementtaion of an evidence-based service. Can Med Assoc J. 2005;172(11):1447–52. doi: 10.1503/cmaj.1041441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon DH, Gleeson T, Iversen M, Avorn J, Brookhart MA, Lii J, et al. A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int. 2010 Jan;21(1):137–44. doi: 10.1007/s00198-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GRADE Working Group Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Boult C, Reider L, Leff B, Frick KD, Boyd CM, Wolff JL, et al. The effect of guided care teams on the use of health services: Results from a cluster-randomized controlled trial. Arch Intern Med. 2011;171(5):460–6. doi: 10.1001/archinternmed.2010.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott JC, Conner DA, Venohr I, Gade G, McKenzie M, Kramer AM, et al. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the cooperative health care clinic. J Am Geriatr Soc. 2004;52(9):1463–70. doi: 10.1111/j.1532-5415.2004.52408.x. [DOI] [PubMed] [Google Scholar]
- 48.Coleman EA, Eilersten TB, Kramer AM, Magid DJ, Beck A, Conner D. Reducing emergency visits in older adults with chronic illness: a randomized, controlled trial of group visits. Eff Clin Prac. 2001;4(2):49–57. [PubMed] [Google Scholar]
- 49.Sommers LS, Marton KI, Barbaccia JC, Randolph J. Physician, nurse and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med. 2000;160:1825–33. doi: 10.1001/archinte.160.12.1825. [DOI] [PubMed] [Google Scholar]
- 50.Marsteller JA, Hsu YJ, Reider L, Frey K, Wolff J, Boyd C, et al. Physician satisfaction with chronic care processes: a cluster-randomized trial of guided care. Ann Fam Med. 2010 Jul;8(4):308–15. doi: 10.1370/afm.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician. 2009;55(12):76–85. [PMC free article] [PubMed] [Google Scholar]
- 52.Counsell SR, Callhan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management of low income seniors. J Am Med Assoc. 2007;298(22):2623–33. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 53.Holmes AM, Ackermann RD, Zillich AJ, Katz BP, Downs SM, Inui TS. The net fiscal impact of a chronic disease management program: Indiana medicaid. Health Affairs. 2008;27(1):855–64. doi: 10.1377/hlthaff.27.3.855. [DOI] [PubMed] [Google Scholar]
- 54.Goetzel RZ, Ozminkowski RJ, Villagra VG, Duffy J. Return on investment in disease management: a review. Health Care Financ Rev. 2005;26(4):1–19. [PMC free article] [PubMed] [Google Scholar]
- 55.Chandra K, Blackhouse G, McCurdy BR, Bornstein M, Campbell K, Costa V, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser. 2012;12(12):1–61. [Internet] Available from: http://www.hqontario.ca/en/mas/mas_ohtas_tech_copd_economic_20120313.html . [PMC free article] [PubMed] [Google Scholar]
- 56.O’Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux J, Tarride JE, et al. Long-term cost-utility analysis of a multidisciplinary primary care diabetes management program in Ontario. Can J Diabetes. 2007;31(3):205–14. [Google Scholar]
- 57.O’Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux J, Guan J, et al. Development of an Ontario Diabetes Economic Model (ODEM) and application to a multidisciplinary primary care diabetes management program. Report prepared for the Ontario Ministry of Health and Long-Term Care. 2006. [[cited: 2011 Dec 1]]. [Internet]. Hamilton, ON: Program for Assessment of Technology in Health,St.Joseph’s Healthcare Hamilton/McMaster University. 24 p. Available from: http://www.path-hta.ca/Publications-Presentations/Publications/Al.aspx .
- 58.Lipscombe LL, Hux JE. Population based trends in diabetes prevalence, incidence and mortalityi n Ontario, Canada from 1995 to 2005. Lancet. 2007;369(9563):750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]


