Abstract
Myb, a cellular progenitor of v-Myb oncogenes, is amplified in prostate cancer and exhibits greater amplification frequency in hormone-refractory disease. Here, we have investigated the functional significance of Myb in prostate cancer. Our studies demonstrate Myb expression in all prostate cancer cell lines (LNCaP, C4-2, PC3 and DU145) examined, whereas it is negligibly expressed in normal/benign prostate epithelial cells (RWPE1 and RWPE2). Notably, Myb is significantly upregulated, both at transcript (>60-fold) and protein (>15-fold) levels, in castration-resistant (C4-2) cells as compared with androgen-dependent (LNCaP) prostate cancer cells of the same genotypic lineage. Using loss and gain of function approaches, we demonstrate that Myb promotes and sustains cell cycle progression and survival under androgen-supplemented and -deprived conditions, respectively, through induction of cyclins (A1, D1 and E1), Bcl-xL and Bcl2 and downregulation of p27 and Bax. Interestingly, Myb overexpression is also associated with enhanced prostate-specific antigen expression. Furthermore, our data show a role of Myb in enhanced motility and invasion and decreased homotypic interactions of prostate cancer cells. Myb overexpression is also associated with actin reorganization leading to the formation of filopodia-like cellular protrusions. Immunoblot analyses demonstrate gain of mesenchymal and loss of epithelial markers and vice versa, in Myb-overexpressing LNCaP and -silenced C4-2 cells, respectively, indicating a role of Myb in epithelial to mesenchymal transition. Altogether, our studies provide first experimental evidence for a functional role of Myb in growth and malignant behavior of prostate cancer cells and suggest a novel mechanism for castration resistance.
Introduction
Prostate cancer is the most commonly diagnosed malignancy and second leading cause of cancer-related deaths in men in the USA (1). Effective treatment of early-stage localized disease involves surgery (radical prostatectomy) or radiation therapy, whereas androgen deprivation therapy is the first line of intervention for advanced metastatic disease. However, after an initial clinical response, prostate tumors relapse in majority of cases as androgen deprivation- or castration-resistant tumors, resulting in poor prognosis (2). Such a transition has been attributed to a variety of mechanisms that include AR overexpression, ligand-independent activation and other AR-independent mechanisms (3–6). Indeed, the development of prostate cancer and subsequent progression to castration resistance is a complex process that may involve multiple genetic and epigenetic changes promoting proliferation, survival and aggressive behavior of prostate cancer cells (7).
In an earlier study, Myb was identified among the genes that are amplified at higher frequency in hormone-refractory prostate cancer (8). Myb, also referred as c-Myb, is the cellular progenitor of the v-Myb oncogenes carried by the chicken retroviruses AMV and E26 that cause acute myeloblastic leukemia or erythroblastosis (9). Myb encodes for a transcription factor, which activates gene expression in most cases by binding to the responsive promoter regions, the Myb binding sites. In some cases, activation by Myb can also occur independent of its DNA binding (10). Earlier reports suggested a restricted expression of Myb in the immature hematopoietic cells of all lineages, which decreased as the cells differentiated (11). Later on, Myb expression was also reported in other tissues as well as in hematological and other solid malignancies (12–15). Functional studies in hematopoietic cells have suggested that Myb plays a role in maintaining the undifferentiated proliferative state of immature cells (16). Myb-knockout mice died at embryonic stage and exhibited an essential loss of most blood cell lineages (17). Studies have shown that Myb activity is essential for continued proliferation and survival of acute and chronic myeloid leukemias (AML and CML) (18,19) and reduced Myb levels can, in fact, impair the transformation by other leukaemogenic oncogenes (18,20). Myb confers its oncogenic activity by regulating the expression of a wide array of target genes and a pathogenic role of Myb has also been suggested in melanoma, head and neck, breast and colon cancers (21–23).
Here, we report an extensive study demonstrating the functional significance of Myb in prostate cancer. Using both gain and loss of function approaches, we show that Myb promotes growth and androgen deprivation–resistance of prostate cancer cells and confers aggressive phenotype by facilitating epithelial to mesenchymal transition (EMT).
Materials and methods
Cell culture
LNCaP, DU145, PC3 (ATCC, Rockville, MD) and C4-2 (UroCor, Oklahoma City, OK) cell lines were maintained in RPMI 1640 media (Invitrogen, Carlsbad, CA) supplemented with 5.0% fetal bovine serum and 100 μM each of penicillin and streptomycin (Invitrogen). RWPE1 and RWPE2 (ATCC) were maintained in keratinocyte serum free medium (Gelantis, San Diego, CA) containing 50 mg/ml gentamycin, 0.05 mg/ml bovine pituitary extract and 5 ng/ml epidermal growth factor. All cell lines were cultured in humidified atmosphere at 37°C with 5% CO2 and media was replaced every alternate day. Short tandem repeats genotyping and intermittent testing for androgen responsiveness (growth and androgen receptor activity) was used as a way to authenticate the cell lines.
Constructs, transfections and treatments
Short hairpin RNA expression constructs for Myb (pGFP-V-RS-shMyb) and scrambled control (pGFP-V-RS-Scr) were purchased from Origene (Rockville, MD), while a Myb overexpression construct was generated through subcloning of Myb insert from pCMV6-XL5-Myb plasmid (Origene) into pCMV6-NEO vector (Origene). For ectopic Myb overexpression and knockdown, LNCaP and C4-2 cell lines were transfected with pCMV6-Myb and pGFP-V-RS-shMyb, respectively, along with their respective control plasmids, using FuGENE as a transfection reagent as per the manufacturer's instructions. Stable pooled population of transfected cells were selected in RPMI-media containing G418 (200 μg/ml; for overexpression) or Puromycin (2 μg/ml; for short hairpin RNA), expanded and examined for stable Myb overexpression or silencing. To assess androgen deprivation–resistance, cells were grown in culture media supplemented with 5% charcoal-stripped serum (steroid-reduced) (Gemini Bio-Products, West Sacramento, CA).
RNA isolation and reverse transcription polymerase chain reaction
Total RNA was isolated using RNeasy Purification Kit (Qiagen, Maryland) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) following manufacturer’s instructions. Quantitative real-time PCR was performed in 96-well plates using SYBRGreen Master Mix (Applied Biosystems, Warrington, UK) on an iCycler system (Bio-Rad, Hercules, CA). The following PCR primer pairs were used: Myb forward (5′-TCAGGAAACTTCTTCTGCTCACA-3′); Myb reverse (5′-AGGTTCCCAGGTACT-gct-3′) and Glyceraldehyde 3-phosphate dehydrogenase forward (5′-GCTGTGTGGCAAAGTCCAAG) and Glyceraldehyde 3-phosphate dehydrogenase reverse (5′-GGTCAGGCTCCTGGAAGATA-3′). The thermal conditions for real-time PCR assays were as follows: cycle 1: 95°C for 10 min, cycle 2 (x40): 95°C for 10 s and 58°C for 45 s.
Western blot analysis
Cells were processed for protein extraction and western blotting as described earlier (24). Immunodetection was carried out using specific antibodies against: Myb, prostate-specific antigen (PSA), AR and Vimentin (all rabbit monoclonal) (Epitomics, Burlingame, CA), Slug, Snail, BAD, Bcl-xL (all rabbit monoclonal), Bax (rabbit polyclonal) (Cell Signaling Technology, Beverly, MA), E-cadherin and N-cadherin (mouse monoclonal) (BD transduction laboratories, Bedford, MA), p21 (mouse monoclonal), p27, Cyclin A1, Cyclin D1, Cyclin E1, Twist (all rabbit polyclonal) (Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (mouse monoclonal) (Sigma–Aldrich, St Louis MO). All secondary antibodies (Santa Cruz) were used at 1:2500 dilutions. Blots were processed with ECL plus Western Blotting detection kit (Thermo Scientific, Logan, UT) and the signal detected using an LAS-3000 image analyzer (Fuji Photo Film Co., Tokyo, Japan).
Immunofluorescence assay
Cells were grown at low density on sterilized coverslips, washed with 0.1 mol/l HEPES containing Hanks’ buffer and fixed in ice-cold methanol at −20°C for 2 min. After nonspecific blocking with 10% goat serum containing 0.05% Tween 20 for at least 30 min, cells were incubated with anti-Myb rabbit monoclonal antibody in PBS (1:100) for 90 min at room temperature followed by washing. Cells were then incubated with TRITC-conjugated goat anti-rabbit secondary antibodies (Santa Cruz) for 60 min and after washing, the coverslips were mounted on glass slides in antifade Vectashield mounting medium (Vector Laboratories, Burlingame, CA). For actin filament staining, cells grown on glass coverslips were fixed with 4% formaldehyde in PBS for 10 min at room temperature. The fixed cells were washed with PBS and permeabilized with 0.2% Triton X-100 in PBS for 5 min. After washing, the cells were stained with Alexafluor 488 phalloidin (Molecular Probes, Invitrogen, Eugene, OR) for 20 min, washed twice with PBS-Tween 20 and mounted on glass slides in antifade Vectashield mounting medium. Immunostaining was observed under Nikon Eclipse TE2000-U fluorescent microscope (Nikon Instruments, Melville, NY).
Growth kinetics assay
Cells (1 × 104/well) were seeded in triplicate in 6-well plates and allowed to grow for different time intervals. The growth rate was determined by counting the number of cells on a hemocytometer, every day for 8 days. Cell population doubling time (Td) was calculated during exponential growth phase (96–144 h) using the following formula: Td = 0.693 t/ln (Nt/N0), where t is time (in h), Nt is the cell number at time t, and N0 is the cell number at initial time (25).
Soft-agar colony formation and plating efficiency assay
Equal volumes of agarose (1.6%) and growth medium were mixed and plated to form bottom layer (0.8% agar growth medium) in 6-well plates. Cells (2.5 × 103cells/ml) were suspended in regular media, mixed with equal volume of 0.6% agarose and cell suspension-agar mix (2 ml) seeded as top layer in each well. Plates were incubated under normal culture conditions for 3 weeks for colony formation. Colonies were stained with 0.005% crystal violet (Sigma–Aldrich) in PBS, observed using Nikon Eclipse microscope (Nikon Instruments) and counted in ten randomly selected fields (×100 magnification). For plating efficiency, single cell suspensions were plated in 6-well plates at a density of 2.5 × 103cells/well in complete or steroid-reduced media for colony formation. After 2 weeks, colonies were fixed with methanol, stained with crystal violet, photographed and counted using Image analysis software (Gene Tools, Syngene, Frederick, MD).
Cell cycle analysis
Cells were synchronized by culturing them in serum-free media for 72 h and then incubated in either regular or steroid-reduced media for 24 h. After washing and trypsinization, cells were fixed with 70% ethanol overnight at 4°C, washed with cold PBS and stained with propidium iodide using PI/RNase staining buffer for 1 h at 37°C. Stained cells were analyzed by flow-cytometry on a BD-FACS Canto™ II (Becton–Dickinson, San Jose, CA). The percentage of cell population in various phases of cell cycle was calculated using Mod Fit LT software (Verity Software House, Topsham, ME).
Apoptosis assay
Apoptosis was measured by using the PE Annexin V apoptosis detection kit (BD Biosciences, San Diego, CA). The cells were grown in steroid-supplemented (fetal bovine serum) or -reduced (charcoal-stripped serum) condition for 96 h. Apoptosis was detected by staining the cells with PE Annexin V and 7AAD solution followed by flow cytometry.
Motility and invasion assays
For motility assay, cells (2 × 105) were plated in the top chamber of non-coated polyethylene teraphthalate membrane (6-well inserts, pore size 8 μM; BD Biosciences). For the invasion assay, 5 × 104 cells were plated in the top chamber of the transwell with a Matrigel-coated polycarbonate membrane (24-well inserts 0.8 μM, BD Biosciences). RPMI-1640 medium with 10% fetal bovine serum was added to the lower chamber as a chemoattractant. After 16 h of incubation, cells remaining on the upper surface of the insert membrane were removed by cotton swab. Cells that had migrated or invaded through the membrane/Matrigel to the bottom of the insert were fixed and stained with Diff-Quick cell staining kit (Dade Behring, Newark, DE) and mounted on slide.
Aggregation assay
Cells were tested for their ability to aggregate in hanging drop suspension cultures as previously demonstrated (25). In brief, drops of cell suspension (20 μl each containing 20 000 cells) were placed onto the inner surface of the lid of a Petri dish. The lid was then placed on the Petri dish so that the drops were hanging from the lid with the cells suspended within them. After overnight incubation at 37°C, the lid of the Petri dish was inverted and photographed using Nikon Eclipse microscope (Nikon Instruments).
Results
Myb is overexpressed and associated with enhanced growth and clonogenicity in prostate cancer cells
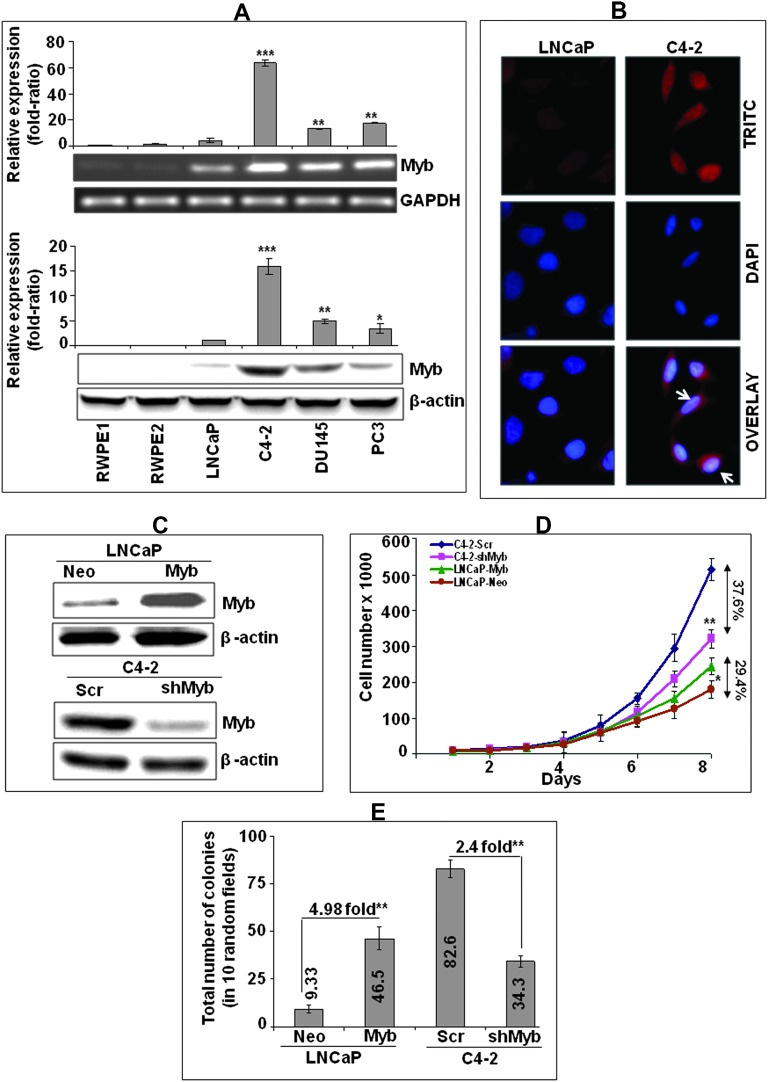
We observed an aberrant expression of Myb in all the prostate cancer cell lines (LNCaP, C4-2, DU145 and PC3), whereas no or negligible expression was noted in prostate epithelial cell lines (RWPE1 and RWPE2) (Figure 1A). Notably, Myb expression was significantly greater (p < 0.05) in all the castration-resistant (CR: C4-2, PC3 and DU145) cells as compared with androgen-dependent (AD: LNCaP) prostate cancer cells. Highest level of Myb expression was observed in CR C4-2 cells, which exhibited more than 60- and 15-folds increase at mRNA (Figure 1A, upper panel) and protein levels (Figure 1A, lower panel), respectively, as compared with its parental AD LNCaP cells. Immunofluorescence analysis demonstrated an intense staining of Myb in C4-2 cells, which was predominantly localized in the nucleus with some low diffuse staining in the cytoplasm (Figure 1B).
Fig. 1.
Myb expression and its association with growth characteristics of prostate cancer cells. (A) Quantitative analyses of Myb transcripts (upper panel) and protein (lower panel) levels in normal/benign human prostate epithelial (RWPE1 and RWPE2) and cancer (LNCaP, C4-2, DU145 and PC3) cell lines were performed by real-time reverse transcription polymerase chain reaction and immunoblot assays, respectively. Glyceraldehyde 3-phosphate dehydrogenase (real-time PCR) and β-actin (immunoblot) were used as internal controls. Relative quantities of Myb-specific PCR product was determined using the 2−ΔΔCT method, whereas Myb protein levels were estimated by densitometry. Bars represent the mean of fold ratio ± standard deviation (n = 3), *, p < 0.05; **, p < 0.005; ***, p < 0.001. (B) Immunofluorescence analysis of Myb expression and sub-cellular localization in lineage associated LNCaP (AD) and C4-2 (AI) cells was performed using rabbit anti-Myb monoclonal and TRITC-conjugated goat anti-rabbit secondary antibodies. (C) Immunoblot analysis of Myb expression in stable pooled populations of Myb-overexpressing LNCaP (LNCaP-Myb), Myb-silenced C4-2 (C4-2-shMyb) and their respective empty vector (LNCaP-Neo)- and scrambled-short hairpin RNA (C4-2-Scr)-transfected control lines. (D) Growth kinetics measurement of LNCaP-Myb and C4-2-shMyb cells along with their respective controls. Growth curve represents the data from triplicate experiments (mean ± standard deviation), *, p < 0.05; **, p < 0.005. (E) Soft agar colony forming assay. Bars represent the mean of total number of colonies in 10 random view fields ± standard deviation (n = 3), **, p < 0.005.
For functional analysis, Myb-overexpressing (LNCaP-Myb) or knockdown (C4-2-shMyb) stable sublines were generated along with their control transfectants (LNCaP-Neo and C4-2-Scr) and characterized for Myb overexpression or silencing (Figure 1C). We next examined the effect of Myb modulation on growth and clonogenicity of LNCaP and C4-2 cells, respectively. Our data demonstrated that overexpression of Myb in LNCaP cells significantly enhanced their growth rate, whereas it decreased in Myb-silenced C4-2 cells as compared with their respective control cells (Figure 1D). The total number of LNCaP-Myb cells on 8th day of culture indicated 29.4% increase in growth as compared with LNCaP-Neo cells, whereas 37.6% growth inhibition was observed in Myb-silenced C4-2-shMyb cells relative to C4-2-Scr cells (Figure 1D). Growth analysis during exponential phase (96–144 h) demonstrated a decrease in population doubling time of LNCaP-Myb (37.6 h) cells as compared with LNCaP-Neo (45.5 h) cells, whereas C4-2-shMyb cells exhibited an increase (30.2 h) compared with C4-2-Scr (26.4 h) cells. In an anchorage-independent clonogenicity assay, LNCaP-Myb cells showed ∼4.98-fold enhanced clonogenic ability as compared with LNCaP-Neo cells. In accordance with this data, clonogenicity was decreased by ∼2.4-fold in C4-2-shMyb cells as compared with the C4-2-Scr cells (Figure 1E). Altogether, our findings demonstrate a role of Myb in potentiating growth and clonogenicity of prostate cancer cells.
Overexpression of Myb supports androgen deprivation–resistant growth of prostate cancer cells and upregulates PSA expression
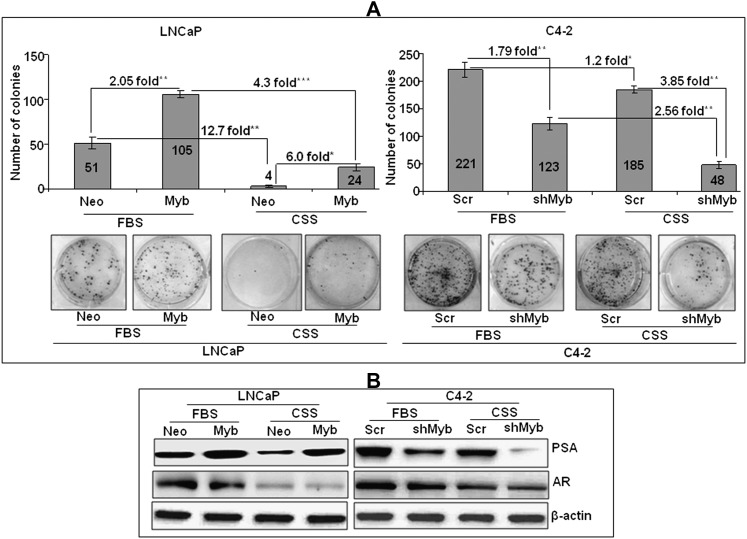
Considering enhanced amplification frequency (8) and observed overexpression of Myb in CR prostate cancer cells, we sought out to investigate its role in androgen deprivation–resistance. For this, we analyzed the plating efficiency (an ideal test to monitor growth in long-term) of Myb-overexpressing and -knockdown cells under steroid-supplemented and -reduced conditions. Our data showed an increased (∼2.05-fold) plating efficiency in LNCaP-Myb cells as compared with LNCaP-Neo cells under steroid-supplemented condition (Figure 2A). Similarly, C4-2-Scr cells also exhibited greater (∼1.79-fold) plating efficiency as compared with Myb-silenced C4-2 cells. Notably, when the plating efficiency was examined under steroid-deprived condition, a >12-fold reduction was observed in LNCaP-Neo cells, whereas it only decreased to ∼4.0-fold in LNCaP-Myb cells as compared with that in steroid-supplemented condition (Figure 2A). Likewise, C4-2-Scr cells exhibited a 1.2-fold decrease under steroid-reduced condition, whereas it was reduced by 2.56-fold in C4-2-shMyb cells as compared with the plating efficiency in steroid-supplemented condition.
Fig. 2.
Overexpression of Myb favors androgen deprivation–resistant growth and upregulates PSA expression. (A) Cells were seeded at low density (2.5 × 103cells/well) in steroid-supplemented (fetal bovine serum) and -reduced (charcoal-stripped serum) media. After 2 weeks, colonies were stained with crystal violet and visualized and photographed using imaging system. Bars represented mean ± standard deviation (n = 3); *, p < 0.05 **, p < 0.005; ***, p < 0.001. (B) PSA and AR expression under steroid-supplemented and -reduced condition in Myb-overexpressing or -silenced prostate cancer cells. Cells were grown in regular (fetal bovine serum) or steroid-reduced (charcoal-stripped serum) media for 24 h and the expression of PSA and AR was examined by immunoblot analysis.
Next, we examined if Myb-induced androgen deprivation–resistance correlated with changes in PSA expression. PSA is elevated in majority of prostate cancers and its expression is decreased (being an androgen-regulated gene) following androgen-deprivation therapy (26). However, a rebound of PSA is generally observed as the prostate cancer progresses to androgen deprivation–resistance (27,28). Interestingly, we also observed an elevated expression of PSA in Myb-overexpressing LNCaP cells, whereas it was reduced in Myb-silenced C4-2 cells as compared with their respective controls (Figure 2B). Furthermore, under steroid-reduced condition, PSA expression reduced considerably in low Myb-expressing (LNCaP-Neo and C4-2-shMyb) cells, whereas it was fairly sustained in Myb-overexpressing (LNCaP-Myb and C4-2-Scr) prostate cancer cells. Notably, while AR expression reduced considerably upon steroid deprivation, no change was observed in Myb-overexpressing or -silenced cells as compared with their respective controls (Figure 2B). Altogether, our data suggest that Myb overexpression supports androgen deprivation–resistant growth and is associated with elevated expression of PSA in prostate cancer cells.
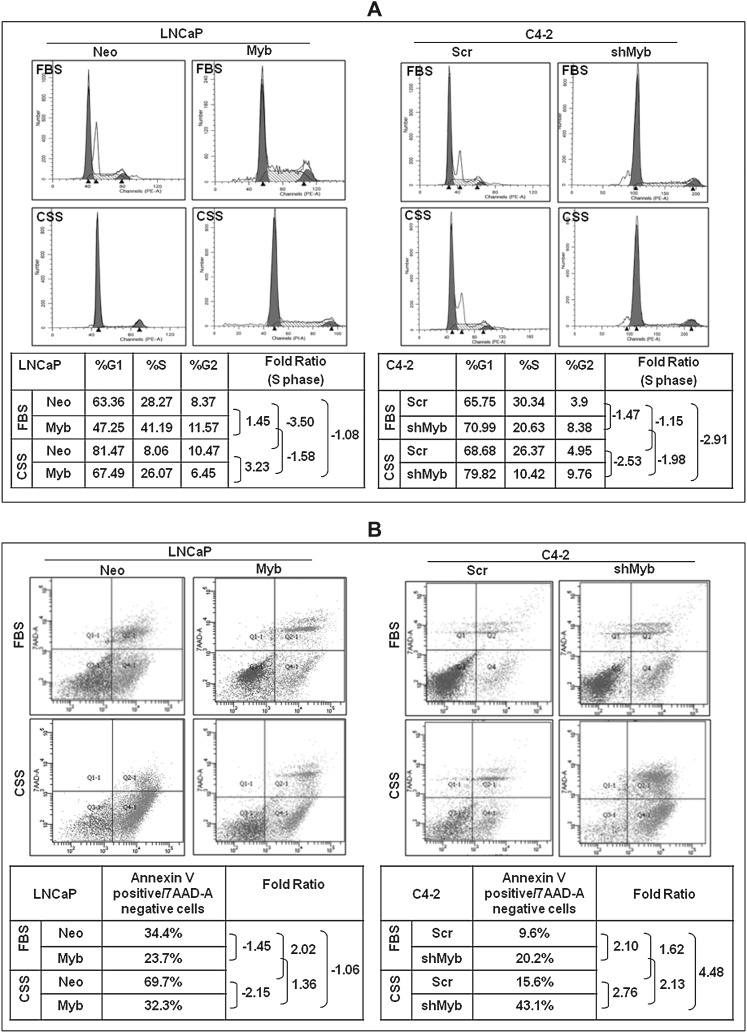
Myb promotes cell cycle progression and confers apoptosis resistance to prostate cancer cells
Growth suppression in androgen-dependent prostate cancer cells upon androgen ablation is associated with cell cycle arrest and induction of apoptosis, whereas castation-resistant cancer cells have developed mechanisms to sustain their growth under steroid-reduced condition (29). Therefore, we examined the effect of Myb expression on cell cycle progression and apoptosis of prostate cancer cells under both steroid-supplemented and -depleted conditions. Our data on cell cycle showed an enhanced fraction of cells in S-phase in Myb-overexpressing (LNCaP-Myb, 41.19%; C4-2-Scr, 30.34%) cells as compared with low Myb-expressing (LNCaP-Neo, 28.27%; C4-2-shMyb, 20.63%) cells (Figure 3A). Upon steroid depletion, LNCaP-Neo cells exhibited a 3.5-fold decrease in the number of cells in S-phase, whereas only 1.58-fold decrease was observed in LNCaP-Myb cells. Similarly, about 1.98-fold decrease in the number of cells in S-phase was observed in low Myb-expressing C4-2-shMyb cells, whereas it only decreased to 1.15-fold in C4-2-Scr cells upon steroid deprivation (Figure 3A).
Fig. 3.
Myb overexpression facilitates cell cycle progression and confers apoptotic resistance. (A) Synchronized cultures of high (LNCaP-Myb and C4-2-Scr) or low Myb (LnCaP-Neo and C4-2-shMyb)-expressing prostate cancer cells were incubated with steroid-supplemented (fetal bovine serum) or -reduced (charcoal-stripped serum) media for 24 h. Subsequently, distribution of cells in different phases of cell cycle was analyzed by propidium iodide (PI) staining followed by flow cytometry. (B) Myb-overexpressing or -silenced prostate cancer cells along with their respective controls were assessed for apoptosis, when cultured under steroid-supplemented and -reduced conditions for 96 h. Percentage of apoptotic cells were analyzed by flow cytometry using PE Annexin V.
We next examined the effect of Myb on apoptosis resistance of prostate cancer cells (Figure 3B). Our data indicated a lower apoptotic index (Annexin V positive/7AAD negative cells) in Myb-overexpressing LNCaP-Myb (23.7%) and C4-2-Scr (9.6%) cells as compared with low Myb-expressing LNCaP-Neo (34.4%) and C4-2-shMyb (20.2%) cells, respectively. Upon steroid deprivation, apoptotic indices increased considerably in both low and high Myb-expressing cells. However, greater increases (2.03- and 2.13-folds, respectively) were observed in low Myb-expressing LNCaP-Neo and C4-2-shMyb cells as compared with Myb-overexpressing LNCaP-Myb and C4-2-Scr cells (1.36- and 1.62-folds, respectively) (Figure 3B). Together, these findings indicate that Myb is able to suppress steroid depletion–induced cell cycle arrest and apoptosis to support androgen deprivation–resistant growth of prostate cancer cells.
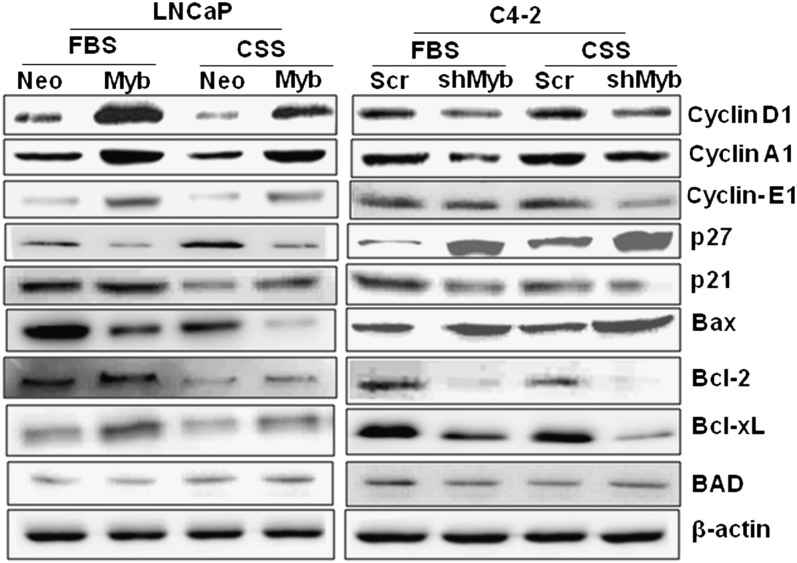
Modulation of myb expression alters the expression of cell cycle- and survival-associated proteins
Having observed a role of Myb in cell cycle progression and resistance to apoptosis, we next examined the effect of altered Myb expression on key proteins involved in cell proliferation and survival. Our data demonstrated an induced expression of cyclins (A1, D1 and E1) upon Myb overexpression in LNCaP cells, whereas it was decreased upon Myb silencing in C4-2 cells under both steroid-supplemented and -reduced conditions (Figure 4). In contrast, we observed a downregulation of p27/KIP1 (cyclin-dependent kinase inhibitor 1B) in Myb-overexpressing LNCaP, whereas it was upregulated in Myb-silenced C4-2 cells. Interestingly, a slight increase in the expression of another cyclin-dependent kinase inhibitor, p21/WAF1, was observed upon Myb overexpression in LNCaP cells, whereas it was decreased in Myb-silenced C4-2 cells. Among the survival proteins, the expression of both Bcl-xL and Bcl-2 was upregulated upon Myb overexpression in LNCaP cells, whereas it was downregulated in Myb-knockdown C4-2 cells. Likewise, we observed a decrease in the expression of pro-apoptotic Bax protein in Myb-overexpressing LNCaP cells and it was upregulated in Myb-silenced C4-2 cells. No change, however, was observed in the expression of another pro-apoptotic protein, BAD, in either Myb-overexpressing or -silenced cells (Figure 4).
Fig. 4.
Myb alters the expression of proteins associated with cell cycle and apoptosis. Myb-overexpressing (LNCaP-Myb) or -silenced (C4-2-shMyb) cells along with their control (LNCaP-Neo and C4-2-Scr, respectively) cells were examined for the expression of various cell cycle and survival-associated proteins after 24 h incubation under steroid-supplemented and -reduced condition. β-actin was used as an internal control.
Myb overexpression promotes cell motility and invasion and diminishes cell–cell interaction
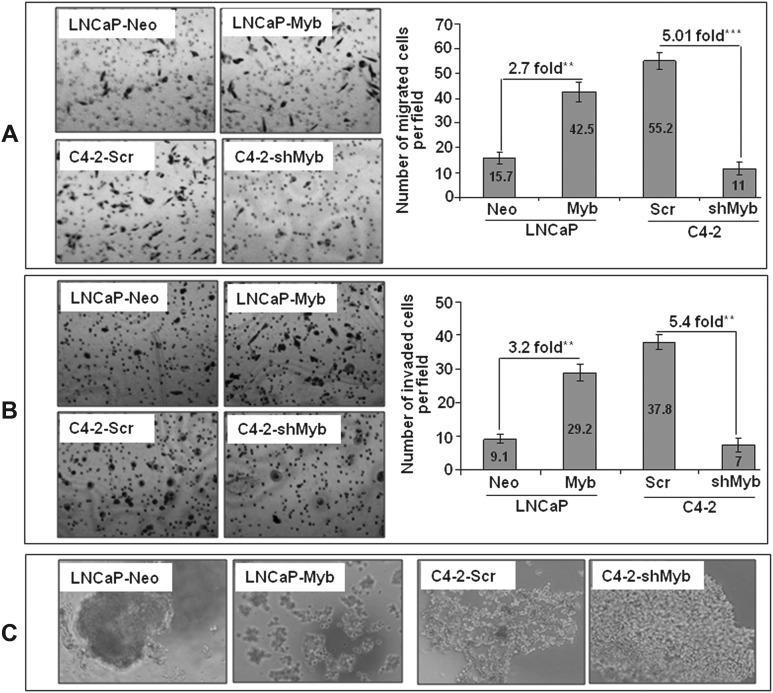
As progression to castration resistance is associated with increased aggressiveness (30), we investigated a role of Myb in promoting the malignant behavior of prostate cancer cells. First, we studied the effect of altered Myb expression on motility and invasiveness, which are important characteristics of the aggressive cancer cells. Our data showed that there was a 2.7-fold increase in the motility of LNCaP cells upon Myb overexpression, whereas a 5.0-fold decrease was observed in Myb-knockdown C4-2 cells as compared with their respective controls (Figure 5A). We also observed that LNCaP-Myb cells were more (3.2-fold) invasive as compared with the LNCaP-Neo cells, whereas C4-2-shMyb cells exhibited decreased (5.4-fold) invasiveness as compared with C4-2-Scr cells (Figure 5B). As malignant cells tend to lose cell–cell interaction during progression toward more aggressive and metastatic phenotype, we examined the effect of Myb on prostate cancer cells in a cell aggregation assay. Our data showed a decreased cell–cell interaction in Myb-overexpressing LNCaP cells, whereas it was increased in Myb-silenced C4-2 cells as compared with their respective controls (Figure 5C). Altogether, our data indicate that Myb overexpression is associated with aggressive behavior of the prostate cancer cells.
Fig. 5.
Myb overexpression leads to enhanced motility and invasion and diminishes cell–cell interaction. Cells were seeded on noncoated or Matrigel-coated membranes for motility (A) and invasion (B) assays, respectively and incubated for 16 h. Media containing 10% fetal bovine serum in the lower chamber was used as a chemoattractant. Cells that had migrated or invaded through the membrane/Matrigel to the bottom of the insert were fixed, stained and counted in 10 random view fields. Bars represent the mean ± standard deviation (n = 3) of number of migrated or invaded cells per field, **, p < 0.005; ***, p < 0.001. (C) Effect on cell–cell interaction was determined by hanging drop assay. Overexpression of Myb was associated with diminished cell–cell interaction in both LNCaP and C4-2 prostate cancer cells.
Myb overexpression favors epithelial to mesenchymal transition of prostate cancer cells
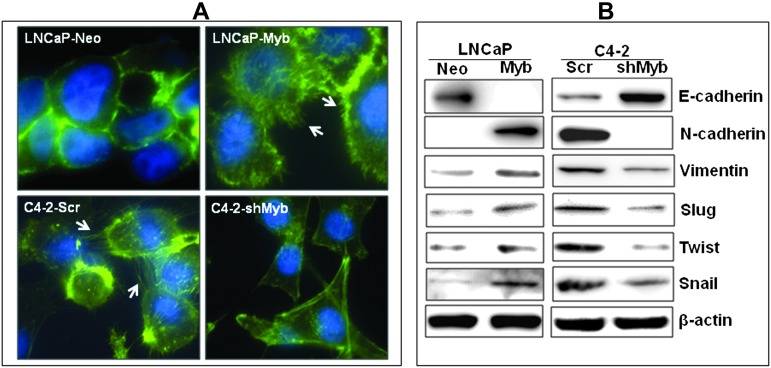
Cancer cells gain mesenchymal features during their progression, a process referred to as epithelial to mesenchymal transition (EMT) (31). Mesenchymal cells are relatively more motile and exhibit less cell–cell communication; therefore, we investigated whether Myb had a role in EMT of prostate cancer cells. Considering the fact that actin-dependent membrane protrusions serve as a critical determinant of EMT (32), we examined actin organization in Myb-overexpressing or -knockdown prostate cancer cells. Staining of filamentous-actin with FITC-conjugated phalloidin revealed the presence of many filopodial structures in Myb-overexpressing (LNCaP-Myb and C4-2-Scr) cells, whereas they were absent or less obvious in the low Myb-expressing (LNCaP-Neo and C4-2-shMyb) cells (Figure 6A). We next studied the expression of a series of EMT marker proteins in Myb-overexpressing or -silenced prostate cancer cells. Our data demonstrated decreased expression of epithelial (E-cadherin) and increased expression of mesenchymal markers (N-cadherin, Vimentin, Slug, Snail and Twist) in Myb-overexpressing (LNCaP-Myb and C4-2-Scr) cells as compared with low Myb-expressing (LNCaP-Neo and C4-2-shMyb) cells (Figure 6B). These findings support a role of Myb in favoring EMT of prostate cancer cells.
Fig. 6.
Myb overexpression induces EMT. (A) Actin organization was examined as a measure of EMT in Myb-overexpressing or -silenced prostate cancer cells. Cells were grown on glass coverslips, fixed and stained with Alexa Fluor 488–conjugated phalloidin. Cells were then analyzed and photographed using fluorescent microscope. Myb-overexpressing (LNCaP-Myb and C4-2-Scr) cells exhibited several filopodial and lamellipodia-like projections (white arrows) as compared with low Myb-expressing (LNCaP-Neo and C4-2-shMyb) cells. (B) Expression profiles of various epithelial (E-cadherin) and mesenchymal markers (N-cadherin, Vimentin, Slug, Snail and Twist) were examined in Myb-overexpressing or -silenced cells by immunoblot analyses. Myb overexpression was associated with loss of epithelial and gain of mesenchymal markers, indicating its role in EMT.
Discussion
Myb is a transcription factor known to regulate the expression of several genes that play crucial roles during cellular proliferation, differentiation and survival (9). Its role has also been demonstrated in hematopoietic and some solid malignancies along with a recent report associating its amplification with hormone-refractory prostate cancer (8,12–14). However, the contribution of Myb in the pathogenesis of prostate cancer has remained unexplored. The present study demonstrates, for the first time, pathologically relevant functions of Myb in prostate cancer. Our findings strongly support that Myb overexpression in prostate cancer due to gene amplification (and/or other yet unidentified mechanisms) may be one of the mechanisms potentiating prostate cancer growth, androgen deprivation-resistant progression and aggressive tumor phenotypes. This notion is based on (i) overexpression of Myb in prostate cancer cells with relatively greater expression in hormone-refractory cells, (ii) Myb-induced promotion of growth and clonogenicity of prostate cancer cells, (iii) Myb-supported androgen deprivation–resistance and induction of PSA expression, (iv) enhanced tumor motility and invasion, and loss of homotypic interaction of Myb-overexpressing tumor cells and (v) Myb-induced EMT. These findings underscore the significance of Myb as a novel molecular target in prostate cancer potentially controlling the progression to hormone therapy–resistant aggressive phenotypes.
Emergence of hormone-independent disease following androgen-deprivation therapy is a major clinical problem for the reason that the relapsed disease also does not respond well to alternative therapies (29). Therefore, characterization of Myb as a novel target promoting androgen deprivation–resistance and aggressiveness of prostate cancer cells is highly significant. Multiple mechanisms have been proposed for castration-resistant progression of prostate cancer (7). Considering the highly heterogeneous nature of this malignancy, it is possible that more than one mechanisms with unique or overlapping functions are operative. The data presented herein provide a mechanism in which Myb overexpression promotes androgen deprivation–resistance by sustaining cell cycle progression and preventing apoptosis under androgen-deprived condition. Importantly, our data also show an induced expression of PSA in Myb-overexpressing prostate cancer cells. This is of great significance, as PSA is an androgen-responsive gene and its serum levels are being used for prostate cancer screening and monitoring of the disease after androgen ablation therapy (27,28). It is believed that relapse of PSA after androgen-deprivation therapy is due to reactivation of AR signaling through AR overexpression, mutations, altered expression of AR coregulators, intracrine signaling, etc. (5,6,33). Nonetheless, in other reports, additional AR-independent mechanisms of PSA upregulation have also been suggested (34,35). As our data showed no noticeable change in AR expression upon Myb modulation, we propose that Myb either induces PSA expression in an AR-independent manner or sustain AR signaling through yet unidentified mechanisms. PSA promoter contains multiple Myb-binding sites (Singh,A.P, unpublished data), therefore, it is possible that Myb induces PSA expression through direct transcriptional upregulation. Alternatively, Myb may cooperate with AR to promote and sustain PSA expression under androgen-supplemented and -depleted conditions, respectively. In fact, it has been shown earlier that Myb cooperates with other transcription factors, such as C/EBP, Ets, CBF and PU.1 to regulate gene expression (36–38). Therefore, it will be of great interest to examine the combinatorial actions of Myb and AR in prostate cancer.
Normal cell growth is maintained and modulated by both proliferative and apoptotic signals and disruption of their balance contributes to the oncogenic process. It has been reported previously that androgen supports survival and proliferation of prostate cancer cells, and its ablation leads to cell cycle arrest and induction of apoptosis (29,39,40). During the castration-resistant progression, prostate cancer cells are able to bypass these growth checkpoints due to overexpression of cyclins and/or anti-apoptotic proteins and/or loss of cell cycle inhibitors and/or pro-apoptotic proteins (2,7). Data presented herein demonstrate that Myb overexpression induces the expression of cell cycle- and survival-associated proteins and is sufficient to sustain their levels under androgen-depleted condition to support cell growth. In corroboration with these observations, it has been reported earlier that Myb upregulates the expression of cyclin A1 in myeloid leukemia cells (41). A reduced expression of cyclin E1 is also reported in Myb-mutant mouse strains causing a proliferation defect (42). A significant overexpression of Bcl-xL was also observed in Myb-overexpressing colon cancer cells correlating with enhanced tumorigenicity in mice xenograft model (43). Myb is also shown to promote the survival of CD4+CD8+ double-positive thymocytes through upregulation of Bcl-xL (44). In other studies, an association of Bcl-2 with Myb has also been reported in T-lymphocytes (45), colon tissue (16), and cancer cells (23,46).
Our data also demonstrate a role of Myb in potentiating malignant behavior of prostate cancer cells and favoring EMT. This is highly significant considering the fact that the relapsed hormone-refractory cancers are also highly aggressive and more metastatic than the hormone-dependent disease (30). Myb has been shown previously to promote migration and invasion by direct or indirect mechanisms in smooth muscle and hepatocellular carcinoma cells (47,48). Furthermore, in two recent reports, a role of Myb in inducing EMT has also been demonstrated (49,50). In one study, it was shown that Myb acted downstream of BMP4 signaling cascade and its elevated expression cooperated with BMP4 to trigger EMT and migration of neural crest cells (50). The other study showed that Myb regulated the expression of Slug in tumor cells of different origin and altered the expression of a variety of epithelial and mesenchymal markers (49). Importantly, it was also shown that Myb-dependent Slug expression was essential for the homing of chronic myeloid leukemia K562 cells to the bone marrow (49). These observations together with our findings strongly support a role of Myb in aggressive behavior and metastasis of the cancer cells.
In summary, we have provided evidence for a functional role of Myb in growth, androgen deprivation–resistance and malignant behavior of the prostate cancer cells. These are important observations suggesting that Myb could be developed as a marker for predicting the response to hormone therapy and should provide the impetus for future studies on prognostic and therapeutic assessments of Myb in prostate cancer.
Funding
National Institute of Health (NIH)/National Cancer Institute (NCI) (CA137513); Department of Defense (DOD)/US Army (W81XWH-09-1-0137); University of South Alabama Mitchell Cancer Institute (USAMCI).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- EMT
epithelial to mesenchymal transition
- PSA
prostate-specific antigen
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J.Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BJ, et al. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Attard G, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 4.Chen T, et al. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–2135. [PubMed] [Google Scholar]
- 5.Culig Z, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol.Endocrinol. 1993;7:1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 6.Hobisch A, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 7.Schroder FH. Progress in understanding androgen-independent prostate cancer (AIPC): a review of potential endocrine-mediated mechanisms. Eur. Urol. 2008;53:1129–1137. doi: 10.1016/j.eururo.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J, et al. Gene amplifications associated with the development of hormone-resistant prostate cancer. Clin. Cancer Res. 2003;9:5271–5281. [PubMed] [Google Scholar]
- 9.Ramsay RG, et al. MYB function in normal and cancer cells. Nat. Rev. Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 10.Kanei-Ishii C, et al. c-Myb-induced trans-activation mediated by heat shock elements without sequence-specific DNA binding of c-Myb. J. Biol. Chem. 1994;269:15768–15775. [PubMed] [Google Scholar]
- 11.Gonda TJ, et al. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984;310:249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- 12.Kauraniemi P, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60:5323–5328. [PubMed] [Google Scholar]
- 13.Melani C, et al. Inhibition of proliferation by c-myb antisense oligodeoxynucleotides in colon adenocarcinoma cell lines that express c-myb. Cancer Res. 1991;51:2897–2901. [PubMed] [Google Scholar]
- 14.Park JG, et al. Large-scale molecular mapping of human c-myb locus: c-myb proto-oncogene is not involved in 6q- abnormalities of lymphoid tumors. Oncogene. 1992;7:1603–1609. [PubMed] [Google Scholar]
- 15.Torelli G, et al. Expression of c-myb protooncogene and other cell cycle-related genes in normal and neoplastic human colonic mucosa. Cancer Res. 1987;47:5266–5269. [PubMed] [Google Scholar]
- 16.Zorbas M, et al. c-Myb is critical for murine colon development. Oncogene. 1999;18:5821–5830. doi: 10.1038/sj.onc.1202971. [DOI] [PubMed] [Google Scholar]
- 17.Mucenski ML, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 18.Hess JL, et al. c-Myb is an essential downstream target for homeobox-mediated transformation of hematopoietic cells. Blood. 2006;108:297–304. doi: 10.1182/blood-2005-12-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabretta B, et al. Normal and leukemic hematopoietic cells manifest differential sensitivity to inhibitory effects of c-myb antisense oligodeoxynucleotides: an in vitro study relevant to bone marrow purging. Proc. Natl. Acad. Sci. USA. 1991;88:2351–2355. doi: 10.1073/pnas.88.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lidonnici MR, et al. Requirement of c-Myb for p.210(BCR/ABL)-dependent transformation of hematopoietic progenitors and leukemogenesis. Blood. 2008;111:4771–4779. doi: 10.1182/blood-2007-08-105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hijiya N, et al. Biologic and therapeutic significance of MYB expression in human melanoma. Proc. Natl. Acad. Sci. USA. 1994;91:4499–4503. doi: 10.1073/pnas.91.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson M, et al. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson MA, et al. c-Myb down-regulation is associated with human colon cell differentiation, apoptosis, and decreased Bcl-2 expression. Cancer Res. 1998;58:5168–5175. [PubMed] [Google Scholar]
- 24.Singh S, et al. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh AP, et al. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 26.Stamey TA, et al. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. IV. Anti-androgen treated patients. J. Urol. 1989;141:1088–1090. doi: 10.1016/s0022-5347(17)41177-3. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, et al. Short hairpin RNA knockdown of the androgen receptor attenuates ligand-independent activation and delays tumor progression. Cancer Res. 2006;66:10613–10620. doi: 10.1158/0008-5472.CAN-06-0028. [DOI] [PubMed] [Google Scholar]
- 28.Ellis WJ, et al. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin. Cancer Res. 1996;2:1039–1048. [PubMed] [Google Scholar]
- 29.Bhardwaj A, et al. Modulation of protein phosphatase 2A (PP2A) activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol. Cancer Ther. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennbacken K, et al. Prostate cancer progression into androgen independency is associated with alterations in cell adhesion and invasivity. Prostate. 2006;66:1631–1640. doi: 10.1002/pros.20469. [DOI] [PubMed] [Google Scholar]
- 31.Singh A, et al. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar J, et al. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010;70:3780–3790. doi: 10.1158/0008-5472.CAN-09-4439. [DOI] [PubMed] [Google Scholar]
- 33.Locke JA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 34.Sun D, et al. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71:1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung F, et al. Regions of prostate-specific antigen (PSA) promoter confer androgen-independent expression of PSA in prostate cancer cells. J. Biol. Chem. 2000;275:40846–40855. doi: 10.1074/jbc.M002755200. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Munain C, et al. c-Myb and core-binding factor/PEBP2 display functional synergy but bind independently to adjacent sites in the T-cell receptor delta enhancer. Mol. Cell Biol. 1995;15:3090–3099. doi: 10.1128/mcb.15.6.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oelgeschlager M, et al. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol. Cell Biol. 1996;16:4717–4725. doi: 10.1128/mcb.16.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro LH. Myb and Ets proteins cooperate to transactivate an early myeloid gene. J. Biol. Chem. 1995;270:8763–8771. doi: 10.1074/jbc.270.15.8763. [DOI] [PubMed] [Google Scholar]
- 39.Knudsen KE, et al. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 40.Eto M, et al. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003;57:66–79. doi: 10.1002/pros.10275. [DOI] [PubMed] [Google Scholar]
- 41.Muller C, et al. c-myb transactivates the human cyclin A1 promoter and induces cyclin A1 gene expression. Blood. 1999;94:4255–4262. [PubMed] [Google Scholar]
- 42.Malaterre J, et al. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc. Natl. Acad. Sci. USA. 2007;104:3829–3834. doi: 10.1073/pnas.0610055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biroccio A, et al. c-Myb and Bcl-x overexpression predicts poor prognosis in colorectal cancer: clinical and experimental findings. Am. J. Pathol. 2001;158:1289–1299. doi: 10.1016/S0002-9440(10)64080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan J, et al. c-Myb promotes the survival of CD4+CD8+ double-positive thymocytes through upregulation of Bcl-xL. J. Immunol. 2010;184:2793–2804. doi: 10.4049/jimmunol.0902846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomoni P, et al. Resistance to apoptosis in CTLL-2 cells constitutively expressing c-Myb is associated with induction of BCL-2 expression and Myb-dependent regulation of bcl-2 promoter activity. Proc. Natl. Acad. Sci. USA. 1997;94:3296–3301. doi: 10.1073/pnas.94.7.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkins HR, et al. Estrogen prevents sustained COLO-205 human colon cancer cell growth by inducing apoptosis, decreasing c-myb protein, and decreasing transcription of the anti-apoptotic protein bcl-2. Tumour. Biol. 2010;31:16–22. doi: 10.1007/s13277-009-0003-2. [DOI] [PubMed] [Google Scholar]
- 47.Pitsch RJ, et al. Inhibition of smooth muscle cell proliferation and migration in vitro by antisense oligonucleotide to c-myb. J. Vasc. Surg. 1996;23:783–791. doi: 10.1016/s0741-5214(96)70240-9. [DOI] [PubMed] [Google Scholar]
- 48.Chen RX, et al. Transcription factor c-Myb promotes the invasion of hepatocellular carcinoma cells via increasing osteopontin expression. J. Exp. Clin. Cancer Res. 2010;29:172. doi: 10.1186/1756-9966-29-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanno B, et al. Expression of Slug is regulated by c-Myb and is required for invasion and bone marrow homing of cancer cells of different origin. J. Biol. Chem. 2010;285:29434–29445. doi: 10.1074/jbc.M109.089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karafiat V, et al. Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cell Mol. Life Sci. 2005;62:2516–2525. doi: 10.1007/s00018-005-5297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]