Abstract
There are more than 3.17 million people coping with long-term disabilities due to traumatic brain injury (TBI) in the United States. The majority of TBI research is focused on developing acute neuroprotective treatments to prevent or minimize these long-term disabilities. Therefore, chronic TBI survivors represent a large, underserved population that could significantly benefit from a therapy that capitalizes on the endogenous recovery mechanisms occurring during the weeks to months following brain trauma. Previous studies have found that the hippocampus is highly vulnerable to brain injury, in both experimental models of TBI and during human TBI. Although often not directly mechanically injured by the head injury, in the weeks to months following TBI, the hippocampus undergoes atrophy and exhibits deficits in long-term potentiation (LTP), a persistent increase in synaptic strength that is considered to be a model of learning and memory. Decoding the chronic hippocampal LTP and cell signaling deficits after brain trauma will provide new insights into the molecular mechanisms of hippocampal-dependent learning impairments caused by TBI and facilitate the development of effective therapeutic strategies to improve hippocampal-dependent learning for chronic survivors of TBI.
Keywords: Learning, Long-term potentiation, Memory, Protein kinase, Synapse, Traumatic brain injury
Introduction
Over 1.7 million people sustain a traumatic brain injury (TBI) every year in the United States and 124,000 remain permanently disabled [1]. The resulting economic burden is approximately $56.3 billion per year [2]. An incident that takes only a second to occur can result in lifelong devastating consequences. People who have had a TBI are 1.8 times likely to report subsequent binge drinking, 1.5 times likely to be depressed, 2.3–4.5 times likely to develop Alzheimer’s, 7.5 times likely to die, and 29 times more likely to develop epilepsy [3–7]. Not only are there great personal costs, but there are also profound negative societal consequences; for example, an estimated 87% of prison inmates have incurred a head injury in their lifetime [8].
The neurological and behavioral sequelae of TBI develop over the course of days to months after the initial trauma. Most behavioral recovery is observed during the first 6 months after injury [9]. However, this recovery often plateaus or declines between 6–12 months after the TBI [10]. Although there are endogenous reparative mechanisms, these are simply not sufficient to achieve full recovery for most people with moderate to severe TBI. The majority of TBI research is focused on preventing or reducing the early devastating consequences of TBI by developing acute neuroprotective strategies. However, there are an estimated 3.17 million people in the US who are coping with long-term disabilities from a TBI [11]. This underserved population would benefit greatly from rehabilitative therapies that capitalize upon ongoing endogenous plasticity mechanisms.
Structural and Functional Changes in the Hippocampus
Memory problems are particularly common in human TBI patients, either as a consequence of direct effects on memory encoding or via secondary effects on concentration and attention [12–15]. Several memory modalities are affected by TBI; for the purposes of this review, we will limit our focus to declarative memory which is affected in a significant percentage of TBI patients [12, 13]. The commonality of declarative memory deficits is due, in part, to the unique vulnerability of the hippocampus to TBI. The hippocampus is critical for the formation of declarative memories and in vivo magnetic resonance imaging has revealed a high prevalence of hippocampal atrophy among both moderate and severe TBI patients [12, 16–19]. This has been thought to contribute to the significant percentage of TBI patients reporting long-term hippocampal-dependent memory impairments, significantly interfering with patient recovery and quality of life [12, 13]. Using experimental models of brain injury, many studies have reported hippocampal-dependent learning deficits lasting for weeks to months [20–26].
One of the first mechanisms hypothesized to cause hippocampal-dependent learning deficits after TBI was hippocampal atrophy due to cellular and synaptic loss. Hippocampal atrophy is a common finding among chronic TBI survivors and is replicated in various experimental models of TBI [27, 28]. The atrophy of the hippocampus is not limited to the ipsilateral side, and is often bilateral and encompassing the fornix, resulting in deefferentation [12, 16–18, 29]. The cellular and synaptic loss is progressive, although there is some recovery of synaptic loss [30–34]. Particular areas of the hippocampus are more vulnerable than others; dentate hilar neurons, CA3 pyramidal cells, and newborn neurons in the inner granule layer of the dentate gyrus are selectively lost after TBI [32, 33, 35–39]. These lost cells are not likely replaced. TBI induces neurogenesis within the dentate gyrus which peaks within 1–2 weeks after TBI [40, 41]. It is uncertain whether this increase is sustained since the numbers of doublecortin-positive cells, a marker of immature neurons, are decreased from 14 days to 12 weeks post-injury [37, 39]. Axonal sprouting in both the dentate gyrus and stratum radiatum of area CA1 has also been observed and may be a potential regenerative response of the hippocampus [31, 34, 35, 42, 43]. The cellular and synaptic loss, although significant, is not profound. Atrophy of the hippocampus averages approximately only 10–15% in chronic TBI survivors [12, 17, 19]. However, lesion studies have found that a loss of at least 20–30% of the dorsal hippocampus and at least 39–52% of the ventral hippocampus is required to begin to observe impairments in hippocampal-dependent learning tasks [44].
There is a correlation between the amount of hippocampal atrophy due to cellular loss and the degree of hippocampal-dependent learning impairment during CNS injury [16, 44, 45]. There are exceptions though; during aging there are significant memory impairments resulting from altered synaptic function and loss of select neuronal populations [46–50]. Conversely, during epilepsy, significant hippocampal cell loss does not always result in measurable, significant hippocampal-dependent learning impairments [51]. In fact, lesion studies have found that just 26% of the hippocampus is capable of supporting hippocampal-dependent water maze spatial learning [44]. Together, these studies suggest that other mechanisms may also contribute to the deficits in hippocampal learning after TBI.
Synaptic Plasticity Changes after TBI
To identify other functional changes in the hippocampus that may contribute to memory loss, studies began to investigate if neurotransmission and synaptic plasticity mechanisms such as long-term potentiation (LTP), a persistent increase in synaptic strength and considered to be a model for learning and memory, are altered after TBI. In particular, laboratories have consistently found that there are significant TBI-induced electrophysiological changes that vary across different regions of the hippocampus. In area CA1 of the hippocampus, most studies have reported that basal excitatory synaptic transmission, as assessed by measuring the excitatory postsynaptic potential (EPSP) in response to varying stimulus intensities, is depressed from hours to days after TBI [33, 36, 52–56]. An increase in the population spike amplitude and a decrease in the population spike threshold have also been reported, suggesting that the balance of inhibition and excitation is altered [33, 52, 57–59]. The depression in basal EPSPs in area CA1 resolves within 1–2 weeks after TBI [53, 56] and future studies remain to determine if either inhibitory or excitatory basal synaptic transmission is altered in the months to years after TBI [60]. Conversely, in the perforant path pathway to the dentate gyrus, an enhancement of basal EPSPs [33, 36, 42, 54, 61] and a depression of basal inhibitory postsynaptic currents have typically been observed [33, 35, 38, 60, 62–64].
These regional-specific changes in basal synaptic transmission correspond to alterations in LTP. In area CA1 of the hippocampus, both post-tetanic potentiation, the initial potentiation observed within seconds after the tetanus, and the expression of LTP are impaired from hours to 8 weeks post-injury [25, 52, 53, 55, 57, 65–68]. In contrast, in the dentate gyrus, the expression of LTP lasting for 60 min is impaired for at least 7 days after TBI [36, 54].
The decrease in basal EPSPs in area CA1 of the hippocampus has confounded the interpretation of the impairments in LTP. Since most studies tetanized the hippocampal slices at currents that elicited an EPSP that was 40–50% of the maximum elicited EPSP, fewer afferent fibers were stimulated in hippocampal slices from TBI animals since the maximum elicited EPSP is lower in TBI hippocampal slices [33, 36, 52–56]. To compensate for this, Norris and colleagues adjusted the stimulus strength to give comparable EPSPs from sham and TBI hippocampal slices prior to the tetanus. They found that there were still deficits in hippocampal LTP at the Schaffer collater-CA1 pathway at 2 days, but not at 7 and 14 days post-injury [53]. The authors interpreted this result to indicate that there is recovery of afferentation of the CA1 region of the hippocampus in the days following injury, which corresponds to the partial recovery in synaptic density observed using anatomical techniques [24, 34]. These results suggest that the molecular mechanisms that contribute to basal synaptic transmission and LTP are impaired after TBI and that endogenous reparative or compensatory mechanisms, while certainly active in the hippocampus after mild to moderate trauma, are not sufficient to fully reverse the TBI-induced deficits in synaptic transmission and plasticity.
While LTP has been extensively investigated after TBI, long-term depression (LTD), a candidate mechanism of forgetting, has been studied by only a few groups with differing results. In area CA1 of the hippocampus, it has been reported that LTD is unaffected [55, 68] or enhanced by TBI [65]. These results may be due to the injury models used, fluid-percussion versus controlled cortical impact, which yield some differences in the patterns of hippocampal pathology [32, 33, 69]. Further studies are needed to investigate if LTD is altered in other regions of the hippocampus.
Biochemistry of Synaptic Plasticity and Learning
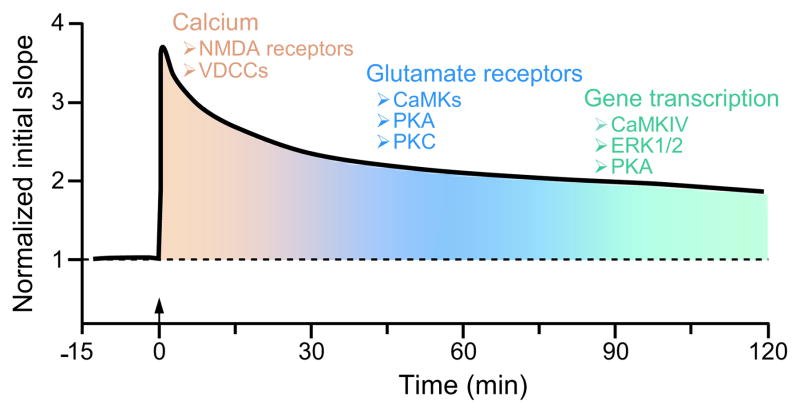
The molecular basis for the chronic deficits in hippocampal LTP remains unclear, and understanding how these biochemical mechanisms are affected by TBI will foster the development of pharmacological therapies to improve learning and memory after TBI. The molecular mechanisms that underlie LTP are well known and parallel to some extent the molecular mechanisms that underlie memory formation in the hippocampus. They consist of a series of interacting, overlapping biochemical events. Induction of LTP, as well as the initial formation of hippocampal memories, requires the activation of L-α-amino-3hydroxy-5-methyl-4-isoxazelopropionate (AMPA) and N-methyl-D-aspartate (NMDA) type glutamate receptors as well as voltage-dependent calcium channels (VDCCs), which influx calcium into the postsynaptic neuron. Intracellular calcium activates multiple protein kinases that subserve memory formation (Fig. 1). Some of these include extracellular signal-regulated kinase (ERK) 1/2, calcium/calmodulin-dependent protein kinase (CaMK) II, CaMKI, CaMKIV, protein kinase (PK) C and PKA [70–74]. Administration of ERK1/2, PKC, PKA, or general CaMK inhibitors impairs performance on several hippocampal-dependent learning tasks [70, 71, 75–77]. Consistent with this, genetic studies have confirmed the necessity of these protein kinases for hippocampal learning. Knockout mice for ERK2, CaMKII, CaMKIV, PKC, or PKA exhibit deficits in water maze learning and contextual fear conditioning – hippocampal-dependent learning tasks [72, 74, 78–81]. Thus, calcium influx initiates a cascade of biochemical events to increase synaptic strength via activation of ERK1/2, CaMKs, PKC, and PKA.
Fig. 1.
The biochemical mechanisms required for hippocampal LTP and memory formation share many similar pathways. During induction of LTP, i.e. the first initial minutes after tetanization (arrow), there is a supralinear entry of calcium via VDCCs and NMDA receptors. This calcium influx activates several protein kinases including the CaMKs, PKA, and PKC. They either directly phosphorylate glutamate receptors to increase conductance through the receptors or phosphorylate proteins involved in the insertion of the receptors in the postsynaptic membrane. This leads to the potentiation of synaptic transmission. During the maintenance of LTP and long-term memory formation, several protein kinases including CaMKIV, ERK1/2, and PKA regulate transcription factors which increase gene transcription to enact structural changes at the synapse to maintain this potentiation.
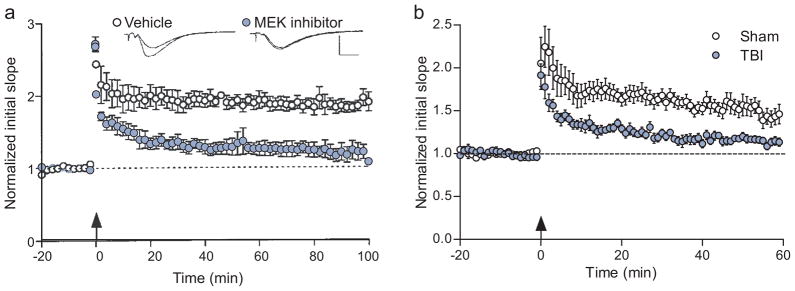
These studies have revealed differential requirements for protein kinases during short-term versus long-term memory formation, depending upon the learning task. Inhibition of ERK1/2 or PKA selectively interferes with long-term, but not short-term memory formation [77, 78, 81]. This is similar to the temporal requirements for hippocampal LTP, where inhibition of ERK1/2 activation blocks the maintenance phase, but not the induction phase of LTP [71]. ERK1/2 is required for long-term memory formation and maintenance of LTP by stimulating gene transcription through the transcription factor cAMP response-element binding protein (CREB) [82]. A comparison of the impairments in LTP with an ERK1/2 inhibitor versus the impairments in LTP after TBI reveals some similarities; for both maintenance is impaired (Fig. 2).
Fig. 2.
A comparison of the deficits in hippocampal LTP elicited by an ERK1/2 inhibitor versus induced by experimental TBI. A MAP kinase kinase (MEK) inhibitor to selectively inhibit ERK1/2 has no effect on the initial potentiation after tetanic stimulation to induce LTP, but blocks maintenance of LTP (a). Adapted from [71]. Moderate TBI results in similar deficits in hippocampal LTP (b). At 2 weeks after TBI, LTP was induced by tetanization of the Schaffer collateral pathway. Although the synaptic response is potentiated in the first few minutes after tetanization, the degree of potentiation in TBI hippocampal slices is not maintained as compared to sham hippocampal slices (C.M.A. and J.D.A. unpublished observations) [25, 52, 53, 55, 57, 65–68].
Biochemical Changes within the Hippocampus after TBI
The studies above suggest that activation of protein kinases required for the maintenance of LTP and their downstream effectors may be altered chronically after TBI. While many studies have contributed to our understanding of TBI-induced biochemical changes in the hippocampus in the acute stages after injury, few have been completed for the chronic stages of TBI.
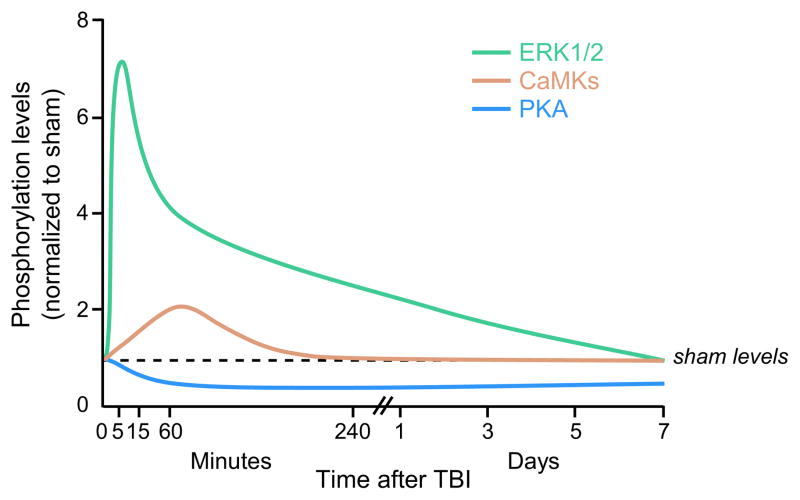
Acutely after TBI, and in particular for the fluid-percussion brain injury model, there is a large increase in intracellular calcium in the cortex and hippocampus that lasts for up to 4 days [83]. These global changes in calcium signaling through glutamate excitotoxicity and potassium depolarization waves stimulate several calcium-dependent cell signaling pathways that are also activated during hippocampal-dependent memory formation. We and others have found that the protein kinases CaMKI, II and IV, as well as ERK1/2 and PKC are activated within hours after TBI, and then return to basal levels by 24 hr (Fig. 3) [23, 84–90]. In contrast, cAMP levels and PKA activation are decreased from 4–24 hr after TBI and then return to basal levels by 3 days [91]. These changes are rapid and transient, and return to noninjured levels over the course of hours to days.
Fig. 3.
Many cellular signaling pathways are acutely activated after TBI, but rapidly return to non-injured levels. CaMKI, CaMKII, CaMKIV, and ERK1/2 are activated after TBI, whereas PKA activation is reduced. Nearly all of these biochemical changes return to sham, non-injured levels within 3–7 days after brain trauma. Adapted from [87, 88, 91].
Similarly, expression of the neurotrophins brain-derived neurotrophic factor and nerve-growth factor are rapidly and markedly increased after TBI [92, 93]. In addition, the trkB receptor phosphorylation and mRNA levels increase from 3–6 hr post-injury [84, 92]. However, this stimulation of neurotrophin signaling is not sustained beyond 2 weeks after injury.
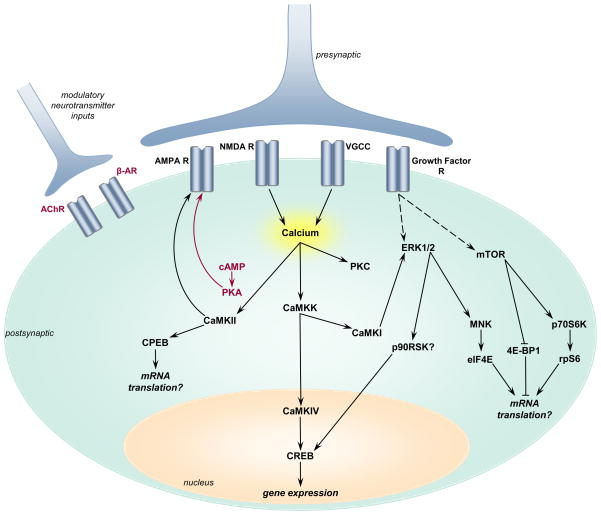
Correspondingly, the downstream effectors of these biochemical signaling pathways are typically transiently activated as well (Fig. 4). Several groups have reported that downstream of ERK1/2 and CAMKIV, the transcription factor CREB is activated within 30 min of TBI in the CA3 region of the hippocampus [22, 84, 88, 94]. There are intriguing suggestions that dendritic protein translation is misregulated since cytoplasmic element binding protein, a dendritic translation factor, is transiently activated within hours after trauma, yet activity-regulated cytoskeleton-associated protein, whose mRNA is dendritically localized, is down-regulated at 2 weeks post-injury [87, 95]. In addition, trauma induces a rapid activation of mammalian target of rapamycin and subsequent phosphorylation of the mRNA transcription regulators: eukaryotic initiation factor 4E (eIF4E), eukaryotic initiation factor 4E binding protein-1 (4E-BP-1), p70 ribosomal S6 kinase (p70S6K), and ribosomal S6 protein (rpS6) [96]. Non-specific activation of these protein kinases and their downstream effectors throughout the dendritic tree may disrupt the machinery in place for synapse-specific potentiation of individual synapses during learning and memory formation. Thus, activation of the protein kinases that underlie memory formation during TBI throughout the neuron may be a potential mechanism underlying retrograde amnesia after head injury [26, 33, 89].
Fig. 4.
Schematic of how the biochemical signaling pathways that underlie hippocampal LTP are altered after TBI. Pathways that are stimulated are illustrated in black, and pathways that are decreased are denoted in red. TBI induces depolarization of neurons and postsynaptic calcium entry which subsequently activates CaMKK and its downstream substrates CaMKI and CaMKIV. PKC and CaMKII are also activated by the increase in postsynaptic calcium. Downstream of CaMKII is the dendritic mRNA translation factor, CPEB, which is transiently phosphorylated. Whether this leads to an increase in dendritic mRNA translation after TBI is still unknown. In contrast to most pathways, cAMP levels and PKA activation are decreased immediately after TBI. This results in differential effects on the AMPA-type glutamate receptor subunit 1: increased phosphorylation at the CaMKII site (Ser831) and decreased phosphorylation at the PKA site (Ser845). TBI also induces an increase in the neurotrophins BDNF and NGF, which activate ERK1/2 and mTOR. Downstream of ERK1/2 and mTOR are multiple translation and transcription factor signaling pathways, including mitogen-activated protein kinase-interacting kinase 1 (Mnk1), eIF4E, 4E-BP1, p70S6K, and rpS6. CREB phosphorylation increases after TBI, although the exact protein kinase that regulates CREB during TBI is still unknown and likely candidates include p90 ribosomal S6 kinase (p90RSK) and CaMKIV. Modulatory neurotransmitter release and their receptors are depressed after TBI; these include a transient decrease in β-adrenergic receptor levels (β-AR) and acetylcholine receptor binding and levels. Although all of these molecules change rapidly after TBI, most return to basal levels within 2–4 weeks after brain trauma.
Upstream of these protein kinase signaling pathways are changes in several neurotransmitter receptor systems. The AMPA-type glutamate receptor subunit 1 (GluR-A) is phosphorylated at the CaMKII site (Ser831) and dephosphorylated at the PKA site (Ser845) at 1 hr after TBI [87]. There is a small, but significant increase in GluR-A levels in crude synaptosome fractions at 48 hr after TBI [87, 97]. NMDA receptor levels and phosphorylation change in a biphasic manner, with a transient increase in the minutes to hours following trauma, and then decreasing below sham levels at 4–7 days, with a restoration to basal, non-injured levels by 2 weeks following injury [97–100]. Trafficking and tethering of these receptors at the synapse may also be altered since there are significant decreases in postsynaptic density protein-95 and synapse-associated protein 97 between 1–7 days post-injury [101–103]. These biochemical findings are consistent with electrophysiological findings demonstrating that NMDA and AMPA currents are decreased at 7 days post-injury [68]. Similarly, metabotropic glutamate receptors decrease at 7 days post-injury and then recover back to non-injured levels by 15 days [104]. Further studies are needed to assess changes in these receptors in specific subregions of the hippocampus given the opposing shifts in excitation/inhibition observed in area CA1 versus the dentate gyrus region [33, 36, 38, 42, 52–55, 58, 61, 62, 68].
Besides glutamate receptors, significant depression in modulatory neurotransmitter systems has been reported; these changes are typically more persistent than the alterations observed with glutamate receptors. Binding to the α7 nicotinic acetylcholine receptor and levels of muscarinic acetylcholine receptors are persistently depressed for at least 3 weeks after trauma [105, 106]. Both evoked release of acetylcholine and dopamine are depressed for at least 2 weeks after trauma [107, 108]. β-adrenergic receptors are also depressed, but more transiently with a recovery to baseline by 24 hr post-injury [109]. Modulatory neurotransmitters are very important for attention and concentration during learning, and depression in the dopaminergic and cholinergic systems may underlie this commonly reported neurological sequelae after TBI [14, 15]. Thus, nearly all of these synaptic plasticity molecules are transiently altered in the hippocampus after TBI, but how they are regulated during the rehabilitative stages in a person coping with a TBI is unknown.
Experimentally, there are intriguing indications that cellular signaling pathways after TBI may be chronically misregulated. At 30 days after FPI, studies have found that calcium homeostasis in cultured hippocampal neurons from the CA3 region is misregulated [110, 111]. Although basal calcium levels and peak calcium entry during glutamate stimulation are similar between non-injured and injured CA3 hippocampal neurons, restoration of basal calcium levels is impaired. These findings indicate that activation of calcium-dependent signaling pathways is likely to be misregulated [83, 98]. Indeed, there is support for an increase in calcium-dependent phosphatase activity. Protein phosphatase 2b (i.e. calcineurin) activity is increased for up to 2–3 weeks after TBI, but both activity and protein levels return to non-injured levels after 3 weeks [102, 112].
Rehabilitation Strategies for Restoring Hippocampal Function
Rehabilitative strategies for restoring hippocampal memory functioning have had some success in the clinic [113]. There are significant changes in basal levels of neurotransmitters after TBI, and evoked release of dopamine and acetylcholine are depressed after TBI [107, 108]. This has provided experimental support for the use of a dopamine agonists and acetylcholinesterase inhibitors. Dopaminergic augmentation using bromocriptine and amantidine improve working memory and likely act on the prefrontal cortex [114, 115]. Methylphenidate and dextroamphetamine, which increase both dopamine and norepinephrine release, improve arousal, speed of processing and attention, but do not directly improve hippocampal cognitive impairments [116, 117]. Cholinesterase inhibitors, such as donepezil, rivastigmine, and less commonly prescribed physostigmine, have been tested as cognitive enhancers. These have had mixed success, with studies demonstrating only a modest improvement in cognitive impairments [113, 118–120]. Although NMDA receptor antagonists have been tested extensively to reduce glutamate excitotoxicity acutely after TBI, NMDA receptor agonists demonstrate efficacy at improving hippocampal synaptic plasticity and learning deficits after experimental TBI [67, 121]. This alternative pharmacotherapy has yet to be translated to the clinic.
Due to a lack of FDA-approved standard of care for pharmacological therapies available to chronic TBI patients, focus has been on physical and cognitive therapy [113]. There is substantial clinical evidence demonstrating the benefits of physical and cognitive therapy after TBI [122, 123]. Similarly, environmental enrichment and physical exercise improve outcome in experimental TBI by stimulating expression of synaptic plasticity molecules [23, 124].
Although these studies indicate that there is promise in developing a pharmacological treatment to improve learning deficits after TBI, the lack of an understanding of the underlying biochemical mechanisms that cause impairments in hippocampal synaptic plasticity and learning after TBI impedes substantial progress in the field. To identify the relevant therapeutic targets to restore hippocampal synaptic plasticity and learning, we determined if the molecular mechanisms of synaptic plasticity are misregulated in the hippocampus at chronic time points after brain trauma.
To investigate chronic impairments in protein kinase signaling after TBI, adult Sprague Dawley rats received sham surgery or parasagittal FPI. At 2, 8, or 12 weeks after sham surgery or FPI, we dissected the ipsilateral, injured hippocampus and generated acute hippocampal slices to study neuronal responses to stimulation. The hippocampal slices were stimulated with a potassium pulse or glutamate to trigger a transient depolarization and brief calcium influx into neurons. We found that there was a significant increase in ERK1/2 and CREB activation in hippocampal slices from sham surgery animals, but not from TBI animals [125]. Total and basal phosphorylation levels of ERK1/2 and CREB were not significantly altered from sham, non-injured levels at 2 and 8 weeks post-injury, suggesting that this was a deficit in activation of these signaling molecules, not a persistent decrease in basal levels. These data demonstrate that the remaining hippocampal neurons have impairments in the ability to activate cell signaling pathways critical for hippocampal synaptic plasticity and memory formation, and in particular, the ERK1/2 and CREB signaling pathways are vulnerable to disruption from brain trauma. Further work remains to determine if activation of other critical protein kinase pathways such as the CaMKs, PKA, or PKC are similarly affected by TBI.
Concluding Remarks
The majority of research in TBI is geared toward preventing disabilities from ever occurring, by intervening within the first hours to days after injury. Even though we will likely achieve successful acute neuroprotective strategies that will pass Phase III clinical trials and become standard of care, we still have a very large, underserved population of patients who are coping with chronic disabilities from TBI [11]. There are profound effects of the chronic disabilities from a TBI, not only for the patient, but also for their family and community. The deficits in learning and memory, communication skills, walking ability, self-care, and ability to return to the community as functional members are devastating, resulting in substantial numbers of TBI patients exhibiting depression and substance abuse [4, 7]. With the development of cognitive enhancers, this underserved population could regain significant functioning, by enhancing endogenous synaptic plasticity mechanisms. By decoding the molecular basis for chronic deficits in hippocampal synaptic transmission and plasticity after TBI, we will be able to use this knowledge to develop rationally-derived therapeutic strategies that will improve learning and memory in chronic TBI survivors.
Acknowledgments
This study was supported by grants from the National Institutes of Health: NS069721, AG033266 and NS056072 and the United States Army Medical Research and Materiel Command: PR054538.
Footnotes
Conflict of Interest The author declares no conflict of interest pertaining to the submitted work.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 2.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 3.Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–6. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 4.Holsinger T, Steffens DC, Phillips C, Helms MJ, Havlik RJ, Breitner JC, et al. Head injury in early adulthood and the lifetime risk of depression. Arch Gen Psychiatry. 2002;59:17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 6.Selassie AW, McCarthy ML, Ferguson PL, Tian J, Langlois JA. Risk of posthospitalization mortality among persons with traumatic brain injury, South Carolina 1999–2001. J Head Trauma Rehabil. 2005;20:257–69. doi: 10.1097/00001199-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Horner MD, Ferguson PL, Selassie AW, Labbate LA, Kniele K, Corrigan JD. Patterns of alcohol use 1 year after traumatic brain injury: a population-based, epidemiological study. J Int Neuropsychol Soc. 2005;11:322–30. doi: 10.1017/S135561770505037X. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter B, Fann JR, Ehde D. Traumatic brain injury in a county jail population: prevalence, neuropsychological functioning and psychiatric disorders. Brain Inj. 2003;17:731–41. doi: 10.1080/0269905031000088649. [DOI] [PubMed] [Google Scholar]
- 9.Christensen BK, Colella B, Inness E, Hebert D, Monette G, Bayley M, et al. Recovery of cognitive function after traumatic brain injury: a multilevel modeling analysis of Canadian outcomes. Arch Phys Med Rehabil. 2008;89:S3–15. doi: 10.1016/j.apmr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Inj. 1996;10:841–8. doi: 10.1080/026990596123945. [DOI] [PubMed] [Google Scholar]
- 11.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 12.Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76:129–31. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 2005;19:271–82. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- 14.Whyte J, Polansky M, Cavallucci C, Fleming M, Lhulier J, Coslett HB. Inattentive behavior after traumatic brain injury. J Int Neuropsychol Soc. 1996;2:274–81. doi: 10.1017/s1355617700001284. [DOI] [PubMed] [Google Scholar]
- 15.McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment - a review. Brain Inj. 2004;18:331–50. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- 16.Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–22. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tate DF, Bigler ED. Fornix and hippocampal atrophy in traumatic brain injury. Learn Mem. 2000;7:442–6. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- 18.Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal non-missile human head injury. Acta Neuropathol (Berl) 1992;83:530–4. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- 19.Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. Am J Neuroradiol. 2002;23:255–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–69. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 21.Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–69. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 22.Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J Neurosci. 1995;15:2030–9. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–39. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–27. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- 25.Sanders MJ, Dietrich WD, Green EJ. Cognitive function following traumatic brain injury: Effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J Neurotrauma. 1999;16:915–25. doi: 10.1089/neu.1999.16.915. [DOI] [PubMed] [Google Scholar]
- 26.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 27.Smith DH, Chen XH, Xu BN, McIntosh TK, Gennarelli TA, Meaney DF. Characterization of diffuse axonal pathology and selective hippocampal damage following inertial brain trauma in the pig. J Neuropathol Exp Neurol. 1997;56:822–34. [PubMed] [Google Scholar]
- 28.Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol (Berl) 2002;103:607–14. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- 29.Tran LD, Lifshitz J, Witgen BM, Schwarzbach E, Cohen AS, Grady MS. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J Neurotrauma. 2006;23:1330–42. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell WL, Dhillon K, Harper L, Espin J, MacIntosh TK, Smith DH, et al. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol. 2003;62:272–9. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- 31.Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–32. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 32.Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J Neurotrauma. 2003;20:929–41. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- 33.Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, et al. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 34.Campbell JN, Churn SB, Register D. Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1761. In press. [DOI] [PubMed] [Google Scholar]
- 35.Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21:8523–37. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang BL, Chen X, Tan T, Yang Z, Carlos D, Jiang RC, et al. Traumatic brain injury impairs synaptic plasticity in hippocampus in rats. Chin Med J (Engl) 2011;124:740–5. [PubMed] [Google Scholar]
- 37.Atkins CM, Truettner JS, Lotocki L, Sanchez-Molano J, Kang Y, Alonso OF, et al. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;28:35–42. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–53. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–70. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, et al. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- 41.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–9. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, et al. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524(Pt 1):117–34. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–97. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–25. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66:187–92. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 46.Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–65. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, et al. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107:1624–9. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–65. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 49.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–30. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–31. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- 51.Mohajeri MH, Saini K, Li H, Crameri A, Lipp HP, Wolfer DP, et al. Intact spatial memory in mice with seizure-induced partial loss of hippocampal pyramidal neurons. Neurobiol Dis. 2003;12:174–81. doi: 10.1016/s0969-9961(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 52.Miyazaki S, Katayama Y, Lyeth BG, Jenkins LW, DeWitt DS, Goldberg SJ, et al. Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res. 1992;585:335–9. doi: 10.1016/0006-8993(92)91232-4. [DOI] [PubMed] [Google Scholar]
- 53.Norris CM, Scheff SW. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J Neurotrauma. 2009;26:2269–78. doi: 10.1089/neu.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B, Chen X, Lin Y, Tan T, Yang Z, Dayao C, et al. Impairment of synaptic plasticity in hippocampus is exacerbated by methylprednisolone in a rat model of traumatic brain injury. Brain Res. 2011;1382:165–72. doi: 10.1016/j.brainres.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 55.D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- 56.Reeves TM, Kao CQ, Phillips LL, Bullock MR, Povlishock JT. Presynaptic excitability changes following traumatic brain injury in the rat. J Neurosci Res. 2000;60:370–9. doi: 10.1002/(SICI)1097-4547(20000501)60:3<370::AID-JNR12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 57.Reeves TM, Lyeth BG, Povlishock JT. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp Brain Res. 1995;106:248–56. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- 58.Akasu T, Muraoka N, Hasuo H. Hyperexcitability of hippocampal CA1 neurons after fluid percussion injury of the rat cerebral cortex. Neurosci Lett. 2002;329:305–8. doi: 10.1016/s0304-3940(02)00707-3. [DOI] [PubMed] [Google Scholar]
- 59.Reeves TM, Lyeth BG, Phillips LL, Hamm RJ, Povlishock JT. The effects of traumatic brain injury on inhibition in the hippocampus and dentate gyrus. Brain Res. 1997;757:119–32. doi: 10.1016/s0006-8993(97)00170-4. [DOI] [PubMed] [Google Scholar]
- 60.Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, Kelly KM. Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis. 2010;38:464–75. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50:708–17. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- 62.Bonislawski DP, Schwarzbach EP, Cohen AS. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol Dis. 2007;25:163–9. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt RF, Scheff SW, Smith BN. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol. 2010;103:1490–500. doi: 10.1152/jn.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunt RF, Scheff SW, Smith BN. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J Neurosci. 2011;31:6880–90. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albensi BC, Sullivan PG, Thompson MB, Scheff SW, Mattson MP. Cyclosporin ameliorates traumatic brain-injury-induced alterations of hippocampal synaptic plasticity. Exp Neurol. 2000;162:385–9. doi: 10.1006/exnr.1999.7338. [DOI] [PubMed] [Google Scholar]
- 66.Sick TJ, Perez-Pinzon MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–92. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- 67.Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21:2033–41. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- 68.Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–50. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–47. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- 70.Tan SE, Liang KC. Spatial learning alters hippocampal calcium/calmodulin-dependent protein kinase II activity in rats. Brain Res. 1996;711:234–40. doi: 10.1016/0006-8993(95)01411-x. [DOI] [PubMed] [Google Scholar]
- 71.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–9. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 72.Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, et al. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–14. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kasahara J, Fukunaga K, Miyamoto E. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J Biol Chem. 2001;276:24044–50. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- 74.Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–83. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 75.Jerusalinsky D, Quillfeldt JA, Walz R, Da Silva RC, Medina JH, Izquierdo I. Post-training intrahippocampal infusion of protein kinase C inhibitors causes amnesia in rats. Behav Neural Biol. 1994;61:107–9. doi: 10.1016/s0163-1047(05)80063-9. [DOI] [PubMed] [Google Scholar]
- 76.Wolfman C, Fin C, Dias M, Bianchin M, Da Silva RC, Schmitz PK, et al. Intrahippocampal or intraamygdala infusion of KN62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, causes retrograde amnesia in the rat. Behav Neural Biol. 1994;61:203–5. doi: 10.1016/s0163-1047(05)80001-9. [DOI] [PubMed] [Google Scholar]
- 77.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–74. [PMC free article] [PubMed] [Google Scholar]
- 78.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–76. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–11. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 80.Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–71. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- 81.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–26. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 82.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–55. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 83.Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: A 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- 84.Hu B, Liu C, Bramlett H, Sick TJ, Alonso OF, Chen S, et al. Changes in trkB-ERK1/2-CREB/Elk-1 pathways in hippocampal mossy fiber organization after traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:934–43. doi: 10.1097/01.WCB.0000125888.56462.A1. [DOI] [PubMed] [Google Scholar]
- 85.Yang K, Taft WC, Dixon CE, Todaro CA, Yu RK, Hayes RL. Alterations of protein kinase C in rat hippocampus following traumatic brain injury. J Neurotrauma. 1993;10:287–95. doi: 10.1089/neu.1993.10.287. [DOI] [PubMed] [Google Scholar]
- 86.Folkerts MM, Parks EA, Dedman JR, Kaetzel MA, Lyeth BG, Berman RF. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J Neurotrauma. 2007;24:638–50. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- 87.Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:1507–18. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- 88.Atkins CM, Oliva AA, Jr, Alonso OF, Chen S, Bramlett HM, Hu BR, et al. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007;26:810–9. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- 89.Dash PK, Mach SA, Moore AN. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–67. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 90.Otani N, Nawashiro H, Fukui S, Nomura N, Shima K. Temporal and spatial profile of phosphorylated mitogen-activated protein kinase pathways after lateral fluid percussion injury in the cortex of the rat brain. J Neurotrauma. 2002;19:1587–96. doi: 10.1089/089771502762300247. [DOI] [PubMed] [Google Scholar]
- 91.Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–58. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hicks RR, Martin VB, Zhang L, Seroogy KB. Mild experimental brain injury differentially alters the expression of neurotrophin and neurotrophin receptor mRNAs in the hippocampus. Exp Neurol. 1999;160:469–78. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- 93.Truettner J, Schmidt-Kastner R, Busto R, Alonso OF, Loor JY, Dietrich WD, et al. Expression of brain-derived neurotrophic factor, nerve growth factor, and heat shock protein HSP70 following fluid percussion brain injury in rats. J Neurotrauma. 1999;16:471–86. doi: 10.1089/neu.1999.16.471. [DOI] [PubMed] [Google Scholar]
- 94.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016:154–62. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 95.Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, et al. Trauma-induced alterations in cognition and arc expression are reduced by previous exposure to (56)Fe irradiation. Hippocampus. 2010 doi: 10.1002/hipo.20920. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2006;27:939–49. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 97.Schumann J, Alexandrovich GA, Biegon A, Yaka R. Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J Neurotrauma. 2008;25:945–57. doi: 10.1089/neu.2008.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–22. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 99.Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J Neurosci Res. 2002;67:781–6. doi: 10.1002/jnr.10181. [DOI] [PubMed] [Google Scholar]
- 100.Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci U S A. 2004;101:5117–22. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wakade C, Sukumari-Ramesh S, Laird MD, Dhandapani KM, Vender JR. Delayed reduction in hippocampal postsynaptic density protein-95 expression temporally correlates with cognitive dysfunction following controlled cortical impact in mice. J Neurosurg. 2010;113:1195–201. doi: 10.3171/2010.3.JNS091212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campbell JN, Low B, Kurz JE, Patel SS, Young MT, Churn SB. Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J Neurotrauma. 2011;28:1–18. doi: 10.1089/neu.2011.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25:513–26. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 104.Gong QZ, Phillips LL, Lyeth BG. Metabotropic glutamate receptor protein alterations after traumatic brain injury in rats. J Neurotrauma. 1999;16:893–902. doi: 10.1089/neu.1999.16.893. [DOI] [PubMed] [Google Scholar]
- 105.Hoffmeister PG, Donat CK, Schuhmann MU, Voigt C, Walter B, Nieber K, et al. Traumatic brain injury elicits similar alterations in alpha7 nicotinic receptor density in two different experimental models. Neuromolecular Med. 2010 doi: 10.1007/s12017-010-8136-4. In press. [DOI] [PubMed] [Google Scholar]
- 106.Ciallella JR, Yan HQ, Ma X, Wolfson BM, Marion DW, DeKosky ST, et al. Chronic effects of traumatic brain injury on hippocampal vesicular acetylcholine transporter and M2 muscarinic receptor protein in rats. Exp Neurol. 1998;152:11–9. doi: 10.1006/exnr.1998.6831. [DOI] [PubMed] [Google Scholar]
- 107.Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, et al. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–65. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- 108.Dixon CE, Bao J, Johnson KM, Yang K, Whitson J, Clifton GL, et al. Basal and scopolamine-evoked release of hippocampal acetylcholine following traumatic brain injury in rats. Neurosci Lett. 1995;198:111–4. doi: 10.1016/0304-3940(95)11979-7. [DOI] [PubMed] [Google Scholar]
- 109.Prasad MR, Tzigaret CM, Smith D, Soares H, McIntosh TK. Decreased alpha 1-adrenergic receptors after experimental brain injury. J Neurotrauma. 1992;9:269–79. doi: 10.1089/neu.1992.9.269. [DOI] [PubMed] [Google Scholar]
- 110.Sun DA, Deshpande LS, Sombati S, Baranova A, Wilson MS, Hamm RJ, et al. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur J Neurosci. 2008;27:1659–72. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deshpande LS, Sun DA, Sombati S, Baranova A, Wilson MS, Attkisson E, et al. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci Lett. 2008;441:115–9. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurz JE, Parsons JT, Rana A, Gibson CJ, Hamm RJ, Churn SB. A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J Neurotrauma. 2005;22:476–90. doi: 10.1089/neu.2005.22.476. [DOI] [PubMed] [Google Scholar]
- 113.Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, Bruns J, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23:1468–501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 114.Whyte J, Vaccaro M, Grieb-Neff P, Hart T, Polansky M, Coslett HB. The effects of bromocriptine on attention deficits after traumatic brain injury: a placebo-controlled pilot study. Am J Phys Med Rehabil. 2008;87:85–99. doi: 10.1097/PHM.0b013e3181619609. [DOI] [PubMed] [Google Scholar]
- 115.Sawyer E, Mauro LS, Ohlinger MJ. Amantadine enhancement of arousal and cognition after traumatic brain injury. Ann Pharmacother. 2008;42:247–52. doi: 10.1345/aph.1K284. [DOI] [PubMed] [Google Scholar]
- 116.Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M, et al. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am J Phys Med Rehabil. 2004;83:401–20. doi: 10.1097/01.phm.0000128789.75375.d3. [DOI] [PubMed] [Google Scholar]
- 117.Hornstein A, Lennihan L, Seliger G, Lichtman S, Schroeder K. Amphetamine in recovery from brain injury. Brain Inj. 1996;10:145–8. doi: 10.1080/026990596124647. [DOI] [PubMed] [Google Scholar]
- 118.Silver JM, Koumaras B, Chen M, Mirski D, Potkin SG, Reyes P, et al. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology. 2006;67:748–55. doi: 10.1212/01.wnl.0000234062.98062.e9. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L, Plotkin RC, Wang G, Sandel ME, Lee S. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1050–5. doi: 10.1016/j.apmr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 120.Morey CE, Cilo M, Berry J, Cusick C. The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 2003;17:809–15. doi: 10.1080/0269905031000088586. [DOI] [PubMed] [Google Scholar]
- 121.Albensi BC, Igoechi C, Janigro D, Ilkanich E. Why do many NMDA antagonists fail, while others are safe and effective at blocking excitotoxicity associated with dementia and acute injury? Am J Alzheimers Dis Other Demen. 2004;19:269–74. doi: 10.1177/153331750401900502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marshall S, Teasell R, Bayona N, Lippert C, Chundamala J, Villamere J, et al. Motor impairment rehabilitation post acquired brain injury. Brain Inj. 2007;21:133–60. doi: 10.1080/02699050701201383. [DOI] [PubMed] [Google Scholar]
- 123.Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86:1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 124.Hamm RJ, Temple MD, O’Dell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–7. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- 125.Atkins CM, Falo MC, Alonso OF, Bramlett HM, Dietrich WD. Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci Lett. 2009;459:52–6. doi: 10.1016/j.neulet.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]