Abstract
Neural precursor cell expressed and developmentally downregulated 4-2 protein (Nedd4-2) facilitates the endocytosis of epithelial Na channels (ENaC). Both mice and humans with a loss of regulation of ENaC by Nedd4-2 have salt-induced hypertension. ENaC is also expressed in the brain, where it is critical for hypertension on high salt diet in salt-sensitive rats. In the present studies we assessed whether Nedd4-2 knockout (−/−) mice have: 1) increased brain ENaC; 2) elevated CSF sodium on high salt diet; and 3) enhanced pressor responses to CSF sodium and hypertension on high salt diet, both mediated by brain ENaC.
Prominent choroid plexus and neuronal ENaC staining was present in −/− but not in wild-type (W/T) mice. In chronically instrumented mice, intracerebroventricular (icv) infusion of Na-rich aCSF increased MAP 3-fold higher in −/− than W/T. Icv infusion of the ENaC blocker benzamil abolished this enhancement. In telemetered −/− mice on high salt diet (8% NaCl), CSF [Na+], MAP and HR increased significantly, MAP by 30-35 mmHg. These MAP and HR responses were largely prevented by icv benzamil, but only to a minor extent by sc benzamil at the icv rate.
We conclude that increased ENaC expression in the brain of Nedd 4-2 −/− mice mediates their hypertensive response to high salt diet, by causing increased sodium levels in the CSF as well as hyper-responsiveness to CSF sodium. These findings highlight the possible causative contribution of CNS ENaC in the etiology of salt-induced hypertension.
Keywords: Salt-dependent hypertension, brain epithelial sodium channels, central nervous system, telemetry, benzamil
Introduction
Nedd4-2 is a ubiquitin ligase that polyubiquitinylates the epithelial Na channel (ENaC)1. The ubiquitinylation facilitates the endocytosis of ENaC from the plasma membrane, leading to its degradation in proteosomes1. Patients with mutations in the ENaC genes that cause a gain of function of ENaC have salt-sensitive hypertension (Liddle's syndrome). These mutations occur in regions of the ENaC genes encoding PY motifs that interact with WW domains of Nedd4-22,3. The gain of function of ENaC in this case is due to disruption of the ENaC-Nedd4-2 interaction, resulting in decreased removal of ENaC from the plasma membrane. The gene knockout of Nedd4-2 in mice produces overexpression of all 3 ENaC subunits in the kidney, and results in salt-dependent hypertension4. The Nedd4-2 −/− mouse thus serves as a model of Liddle's syndrome.
ENaC is also expressed in the brain, both in the choroid plexus and in neurons5-7. Chronic intracerebroventricular (icv) infusion of the ENaC blocker benzamil abolishes the sympathetic hyperactivity and hypertension caused by chronic icv infusion of Na+-rich aCSF8 or by high salt diet in salt-sensitive rats such as Dahl salt-sensitive (S) rats9. Dahl S also exhibit an increase in CSF [Na] on high salt10, as well as enhanced sympathoexcitatory and pressor responses to CSF [Na]11.
Since Nedd4-2 is also expressed in the brain12, we hypothesized that ENaC expression is increased in the brains of Nedd4-2 −/− mice and contributes to the salt-induced hypertension in this model. Accordingly, we first assessed brain ENaC expression and evaluated whether Nedd4-2 −/− mice also have increased CSF [Na] on high salt diet and enhanced pressor responses to sodium in the CSF. We then assessed whether central ENaC blockade by icv infusion of benzamil can prevent both the enhanced pressor responses to sodium and the hypertension on high salt diet.
Methods
Mouse source and husbandry
Nedd4-2 −/− and W/T mice were obtained in-house from a breeding colony established from founders that were transferred from the University of Iowa. Mice were housed in group cages prior to surgery but were housed individually postoperatively. Housing was provided in a temperature controlled environment with a 12h:12h light:dark cycle. Water and standard chow (0.3% NaCl) were provided ad libitum, except when a high salt diet (8% NaCl, Harlan Laboratories, Madison, WI) was substituted for standard chow. The present studies were carried out in accordance with guidelines established by the NIH and the Canadian Council on Animal Care and were approved by the University of Ottawa Animal Care Committee. Studies were performed predominantly in males, due to their larger size. When females were used in an experimental group, an equivalent number was used in most control groups. Moreover, BP and HR responses to icv Na+-rich aCSF are similar in male and female mice 13.
Genotyping
The Nedd4-2 gene knockout involves a deletion of exons 6–84. Previously published methods were used for genotyping4, using DNA extracted from the tail. Dual PCR reactions were run in parallel for each sample, each using a separate set of primers in which the reverse primer was specific for the W/T or −/− allele. A forward primer (P1, 5′TGAGCTCATTGCTTCACTTCC 3′) was common to both reactions. The reverse primer P2 (5′TTCATGCTCGAAGCCTTAGCCATCTCATGAA 3′) was the reverse complement of a sequence located within the region deleted by the knockout. Thus the 150 bp amplicon for the primers P1 and P2 identified the W/T allele. For the 2nd PCR reaction the reverse primer P3 (5′ GCTAGAGGCTGTCC TCACAAA 3′) was positioned just downstream of the deleted sequence and the P1-P3 primer combination only produced a product of amplifiable size (163 bp) for the knockout allele. For both reactions, 35 PCR cycles were run, at 94, 62 and 72°C (30 s each) in succession for denaturing, annealing and extension, respectively. These cycles were preceded by a 94°C denaturation period (5 min) and were followed by extension at 72°C (7 min).
Immunohistochemistry for ENaC expression in the brain
ENaC expression was studied in the choroid plexus and neurons of the pyramidal cortex, SFO, SON and PVN. Mice were perfused with ice cold PBS containing 4% paraformaldehyde (pH 7.4) following a lethal dose of pentobarbital and the brains were immediately removed and stored in the same solution at 4°C until the time of immunohistochemistry for ENaC. Tissue microarrays (TMA) were prepared to include areas of the brain containing the lateral ventricles and hypothalamus. Immunohistochemistry with anti α, β and γENaC antibodies was done on at least three TMA slides, to include the major areas of interest from all samples, and evaluated at multiple levels5,14. Details regarding the quality and specificity of the ENaC antibodies were previously published5,6,14. Distribution and intensity of staining in the cytoplasm and membrane were scored blindly in each of these areas. The score for distribution was represented as the percentage of cells that stained positive from 0 to 3 (0: no staining, 1: <30%, 2: 30-70% and 3: >70% of cells stained). Staining intensity was scored as 0-3 (0: no staining, 1: mild, 2: moderate and 3: strong). The two scores for each area were multiplied to yield a composite value [range: 0(0,0) to 9(3,3)].
Dosages and formulations
Benzamil for icv or sc infusion was dissolved into a mixture of 85% aCSF-15% polypropylene glycol (aCSF, 148 mM Na) or 85% Na-rich aCSF-15% polypropylene glycol (235 mM Na final concentration). The dose of benzamil for acute studies (7.4 ng/min) was established empirically, as to our knowledge a dose for acute icv benzamil in mice has not been published previously. The benzamil dose (2.64 ug/day) used for chronic icv infusion markedly decreases sympathetic activity in aortic banded mice on high salt15. aCSF contained (mM): NaCl (117), KCl (2.5), NaH2PO4.2H2O (0.65), NaH2PO4.7H2O (2.27), Na2SO4 (0.5), MgCl2.6H2O (2.14), CaCl2 (1.0) and NaHCO3 (27), and was adjusted to pH 7.0.
Acute responses to icv Na-rich aCSF
W/T and −/− mice received a guide cannula that was implanted into a lateral brain ventricle 6–8 days before study. One day before study, under isoflurane anesthesia each mouse received a carotid arterial catheter. The next day, in conscious W/T and −/− mice baseline BP and HR were recorded for 10 min prior to the start of an icv infusion of aCSF (148 mM Na) for 30 min at 0.4 μl/min. The aCSF infusion was immediately followed by an icv infusion of Na-rich aCSF (235 mM Na) for 90 min, also at 0.4 μl/min. This rate of infusion causes modest increases in BP and HR in W/T mice13. To assess possible osmolar effects, a separate group of −/− mice was infused with mannitol in aCSF, at equivalent osmolality to the Na-rich aCSF (ie. 491 versus 337 Osm/L for aCSF).
To determine whether the enhanced BP and HR responses to icv Na-rich aCSF in −/− mice can be blocked by icv benzamil, a similar protocol was used, except that during the first icv infusion period, benzamil (7.4 ng/min) was infused for 30 min. This was followed by an icv infusion for 90 min of the same rate of benzamil combined with Na-rich aCSF. A control group of −/− mice received the same two consecutive icv infusions, combined with vehicle. The effects of benzamil were also evaluated in W/T mice using the same protocol.
Effects of chronic icv benzamil on hypertension induced by high salt diet
A telemetry transducer/transmitter (model TA11PA-C10, Data Sciences International, St. Paul, MN) was implanted into 9–12 week old mice 16–18 days before the start of a high salt diet, with the tubing inserted into the abdominal aorta and glued into place with Vetbond™. The transmitter/battery was placed in the peritoneal cavity. In order to conserve the telemeter battery, the transmitter was turned off for part of days –2, 4 and 6 and the entire nights –2, 4 and 6. A high salt diet started on day 0 and continued for 10 days. BP recordings began the morning of day –3. Following 24 h of baseline BP monitoring, an icv infusion of benzamil (2.64 μg/day) was given from day –2 to the end of study on day 10 via a lateral ventricular guide cannula that was connected to a subcutaneously implanted Alzet model 1002 osmotic minipump (flow rate 0.25 μl/hr). Control groups received icv vehicle or sc benzamil at the same rate as icv.
Measurement of CSF [Na]
Groups of W/T and Nedd4-2 −/− mice remained on regular diet or were placed on a high salt diet for 8–10 days, and some −/− mice for 4 days. On the last day, under isoflurane anesthesia 2–5 μl of CSF was collected from the cisterna magna of each animal and in most cases pairs of CSF samples from the same genotype were pooled. The combined samples were diluted 1:8 with distilled water prior to assay. A sodium-sensitive microelectrode (Microelectrodes Inc., Bedford NH) was used to measure [Na] in the diluted sample. A set of standards ranging from 128–174 mM [Na] in aCSF was diluted and assessed similarly and served as a reference. Values of some samples were > 200 mM, which was considered due to contamination and these values were not included in the analysis.
Statistics
Comparisons of serial BP and HR data were carried out using a 1-way ANOVA for repeated measures (only genotype or treatment, not both, varied within each study). When ANOVA detected significant differences between groups, post-hoc comparisons were made by group t-tests. For comparisons of the AUC among treatments and strains (data in Fig 4), a 2-way ANOVA was used followed by a Student-Newman-Keuls post hoc multiple comparison.
Figure 4.
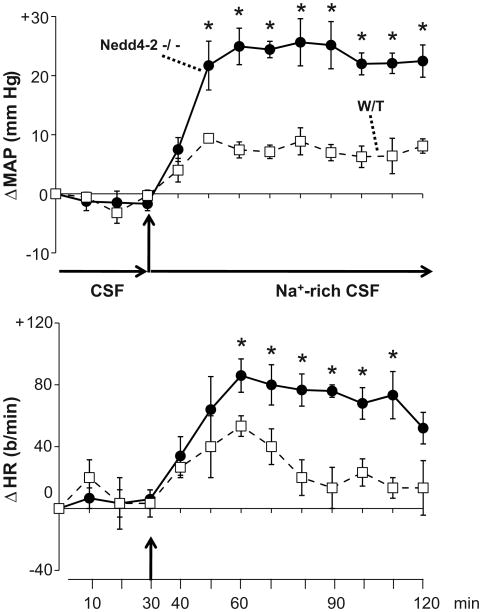
Enhanced responses of mean arterial pressure (MAP) and heart rate (HR) to icv infusion of Na-rich aCSF (235 mM Na) in Nedd4-2 −/− mice are prevented by icv benzamil. Groups of W/T or −/− mice (n=5-6) received icv vehicle in aCSF and Na-rich aCSF, while other groups (n=5-6) received the same two solutions containing benzamil (7.4 ng/min). Values represent the mean ± SEM of the change from baseline. Baseline MAP and HR prior to infusion of aCSF were: 124 ± 5 mmHg, 490 ± 39 b/min and 119 ± 7 mmHg, 507 ± 7 b/min for −/ − and W/T mice receiving icv vehicle vs. 127 ± 6 mmHg, 440 ± 20 b/min and 117 ± 6 mmHg, 480 ± 236 b/min for the respective benzamil groups.
* p = 0.0015 vs other 3 groups
a p < 0.05 for treatment effect (vehicle vs benzamil)
Results
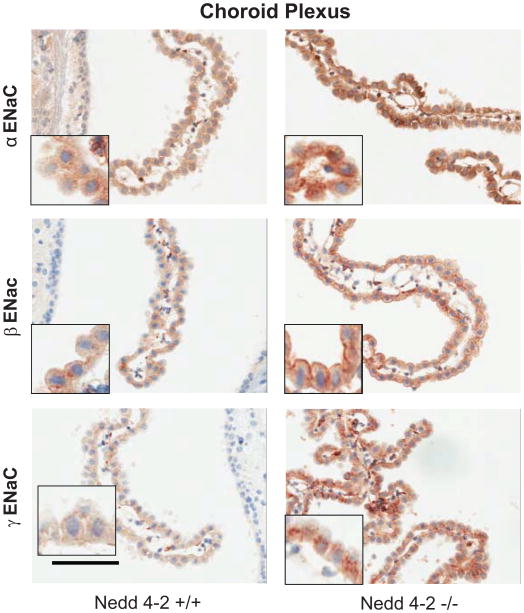
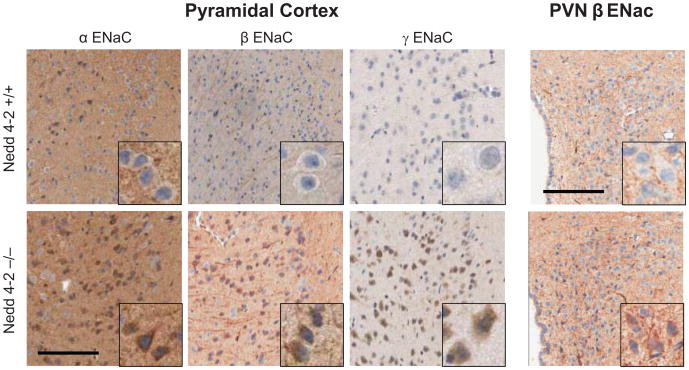
As shown in Fig. 1 and quantified in Table 1, W/T mice show modest, mainly cytoplasmic immunostaining for the 3 ENaC subunits in the choroid plexus, whereas prominent staining at the plasma membrane is present in the −/− mice for all 3 subunits and in the cytoplasm for only γENaC. Similarly, the W/T mice have only modest staining in neurons, In contrast, the −/− mice show enhanced staining of the neuronal cell bodies in the cortex for all 3 subunits, and in the SON and PVN for β ENaC, but not significantly in the SFO (Fig 2, Table 1).
Figure 1.
Immunoreactivity to α, β and γ ENaC subunits in the choroid plexus of Nedd4-2 −/− mice compared to W/T (+/+) controls. Diffuse and mostly cytoplasmic staining is present in the CP cells of W/T (+/+) controls as compared to clear staining on the plasma membrane in the Nedd4-2 −/− mice. Sections were counterstained with hematoxylin. bar: 100 μm
Table 1. Cellular distribution of ENaC subunits in the choroid plexus, cortex, SON, PVN and SFO of Nedd 4-2 −/− mice and W/T controls.
| Choroid Plexus | ||||
|---|---|---|---|---|
| ENaC Subunits | Cytoplasm | Plasma Membrane | ||
| W/T | −/− | W/T | −/− | |
| α ENaC | 3.9 ± 0.4 | 4.7 ± 0.6 | 2.3 ± 0.2 | 2.9 ± 0.2* |
| β | 1.3 ± 0.4 | 1.5 ± 0.2 | 2.6 ± 0.4 | 5.4 ± 0.9** |
| γ | 2.1 ± 0.4 | 4.3 ± 0.7* | 1.4 ± 0.4 | 2.8 ± 0.2** |
| Pyramidal Cortex | ||||
| α | 4.0 ± 0.2 | 6.1 ± 0.2* | 2.7 ± 0.4 | 5.4 ± 0.9** |
| β | 2.6 ± 0.4 | 4.9 ± 0.9* | 2.7 ± 0.6 | 4.4 ± 0.6* |
| γ | 4.0 ± 0.6 | 5.1 ± 0.5* | 2.6 ± 0.4 | 4.3 ± 0.5** |
| SON | ||||
| α | 2.2 ± 0.6 | 3.0 ± 1.2 | 1.8 ± 0.4 | 2.1 ± 0.7 |
| β | 2.4 ± 0.4 | 3.4 ± 0.5 | 2.4 ± 0.5 | 4.6 ± 0.6** |
| γ | 1.2 ± 0.2 | 1.6 ± 0.4 | 1.6 ± 0.2 | 1.5 ± 0.3 |
| PVN | ||||
| α | 1.2 ± 0.2 | 1.6 ± 0.4 | 1.4 ± 0.2 | 2.0 ± 0.4 |
| β | 1.2 ± 0.2 | 2.7 ± 0.5* | 2.3 ± 0.2 | 2.9 ± 0.5 |
| γ | 1.0 ± 0.04 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.04 |
| SFO | ||||
| α | 4.9 ± 1.2 | 4.7 ± 1.1 | 4.2 ± 0.9 | 3.3 ± 0.9 |
| β | 3.8 ± 0.7 | 4.7 ± 0.8 | 3.4 ± 0.8 | 4.3 ± 0.8 |
| γ | 2.9 ± 0.6 | 2.5 ± 0.6 | 2.2 ± 0.4 | 2.5 ± 0.6 |
Values are mean ± SEM (n = 7/group for choroid plexus and cortex; n = 5/group for the 3 nuclei).
Units for cytoplasmic and membranous distribution are arbitrary (see Methods).
p < 0.05;
p < 0.01 −/− versus W/T
Figure 2.
Immunoreactivity to α, β and γ ENaC subunits in pyramidal neurons of the cingulate cortex and to β ENaC in the PVN in Nedd4-2 −/− mice compared to W/T (+/+) controls. In −/− mice distinct immunoreactivity for all 3 subunits is present in the cell-bodies of cortical neurons as well as for β ENaC in axons. The PVN shows diffuse staining for β ENaC in −/− mice, compared to modest staining in the W/T mice. Sections were counterstained with hematoxylin. bar: 100 μm
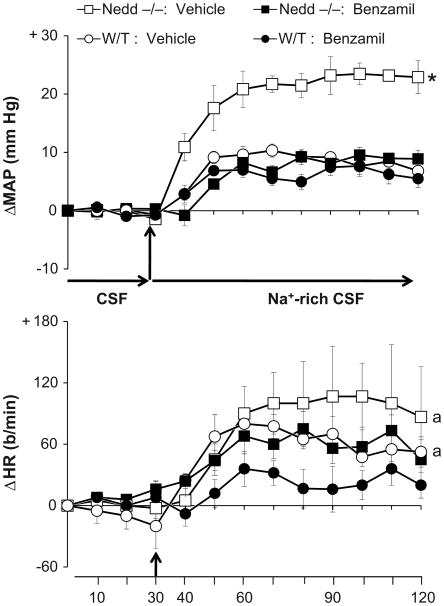
Baseline BP and HR prior to icv infusions of aCSF and Na-rich aCSF were similar in the two genotypes (Legend for Fig 3). Infusion of aCSF (148 mM Na) for 30 min did not change BP or HR (Fig 3). Na-rich aCSF (235 mM Na) increased BP significantly in both genotypes, but the responses of the −/− mice were nearly three-fold greater than those of W/T (p < 0.05, −/− vs. W/T, Fig. 3). Na-rich aCSF increased HR significantly in both −/− and W/T genotypes, but the HR response was ∼2-fold greater in the −/− vs. W/T mice (p < 0.05 vs. W/T, Fig. 3). Icv infusion of mannitol for 45 min at the same rate or twice the rate did not change BP or HR in −/− mice (for MAP: +2 ± 0.4 (n=5), and + 3 ± 0.7 mmHg (n=3), respectively).
Figure 3.
Enhanced responses of mean arterial pressure (MAP) and heart rate (HR) to icv infusion of Na-rich aCSF (235 mM Na) at 0.4 μl/min in Nedd4-2 −/− mice versus W/T controls (n=5/group). Blood pressures were averaged over 10-min intervals. Values represent the mean ± SEM of the change from baseline. Baseline MAP and HR preceding the start of aCSF were: 114 ± 3 mmHg, 513 ± 35 b/min for W/T vs. 114 ± 2 mmHg, 516 ± 27 b/min for −/− mice.
* p < 0.05 vs W/T
To determine whether the exaggerated BP and HR responses to acute increases in CSF [Na] in −/− mice are mediated by ENaC in the brain, benzamil was included in the protocol. There was no significant change in BP or HR in −/− or W/T mice treated with aCSF, whether or not benzamil was present (Fig 4). In −/− mice treated with icv Na-rich aCSF containing vehicle, BP increased to a similar extent as that seen for −/− mice given Na-rich aCSF in Fig 3 (∼20–25 mm Hg, p < 0.05 vs. baseline and vs BP response in W/T mice, Fig 4). Icv benzamil markedly (p < 0.05 vs. vehicle) reduced the BP response to icv Na-rich aCSF in −/− mice to levels that were not different from the W/T response, but did not affect the response in W/T mice (Fig 4). Icv benzamil inhibited (p < 0.05) the increase in HR in both strains (Fig 4).
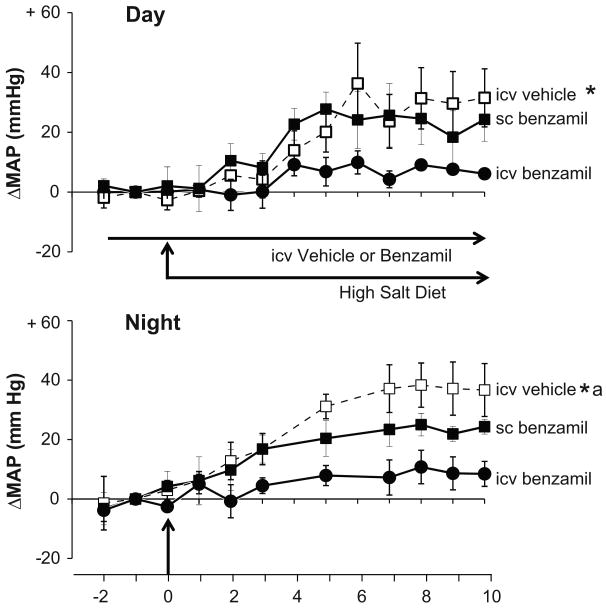
Next, we determined whether in −/− mice ENaC in the brain regulates CSF [Na]. CSF [Na] in mM was: 160 ± 2 (n = 7) in W/T on regular salt, 157 ± 7 (n = 8) in W/T on high salt, 156 ± 3 (n=9) in −/− on regular Na and increased to 166±1 (n = 3) after 4 days and to 167±4 (n = 10) in −/− after 8 days on high salt (p <0.05 vs. −/− on regular salt). Within 4 days on high salt, Nedd4-2 −/− mice treated with icv vehicle developed increases in daytime and nighttime BP, up to 30–35 mm Hg after 6-8 days (p <0.01 vs. baseline, Fig 5). In contrast, in −/− mice receiving an icv infusion of benzamil, the salt-induced BP response was < 10 mm Hg (p <0.05 vs baseline) during the day and night (p <0.01 vs. vehicle group for both day and night BP responses). Sc infusion of benzamil at the same rate did not affect the increase in BP on high salt diet during the day, but blunted the response by ∼1/3 (p <0.05) during the night (Fig 5). The latter effect was significantly less than that of icv benzamil (30 vs 85% reduction, p<0.05). After the start of high salt diet, HR increased significantly by 30–40 bpm in icv vehicle treated −/− mice, but not in those treated with icv or sc benzamil (data not shown).
Figure 5.
Central infusion of the ENaC blocker, benzamil largely prevents the salt-induced hypertension in Nedd 4-2 −/− mice. Panels show the day (7 am - 6 pm) and night (7 pm - 6 am) BP responses to high salt diet in −/− mice receiving either vehicle (n=4) or benzamil icv (n=5) or sc (n=5). Infusions of vehicle or benzamil (2.64 μg/day) started on day -2 and continued until the end of the study on day 10. High salt diet (8% NaCl) was given on days 0-10. Values represent the means ± SEM of the changes from baseline (average from day -1). The daytime baseline mean MAP and HR (average of day -1, in mm Hg and b/min) were: 115 ± 3 and 544 ± 28, for the icv vehicle group; 115 ± 7 and 509 ± 25, for the icv benzamil group and 108 ± 3 and 509 ± 36 for the sc benzamil group. The nighttime baseline mean MAP and HR (night -1 values, in mm Hg and b/min) were: 115 ± 2 and 543 ± 26, for the icv vehicle group; 125 ± 5 and 515 ± 30, for the icv benzamil group and 114 ± 2 and 534 ± 36 for the sc benzamil group.
* p < 0.001 vs icv benzamil group; a p < 0.05 vs sc benzamil group
Discussion
The present study demonstrates the major new finding that hypertension induced by a high salt diet in mice with a homozygous knockout of the Nedd4-2 gene is largely prevented by icv infusion of benzamil. The −/− mice develop increased CSF [Na] on high salt diet and show markedly enhanced BP and HR responses to sodium in the CSF, which can be blocked by icv benzamil. Altogether, these findings strongly suggest that the salt-induced hypertension in this mouse model of Liddle's syndrome depends critically on increased expression of ENaC in the brain.
In rat models of salt-induced hypertension, an increase in CSF [Na] occurs on high salt diet and appears to precede the increase in BP10. In rats, chronic elevations in CSF [Na] by icv infusion of Na-rich aCSF increase BP via activation of the same systems in the brain (including ENaC) that produce the hypertensive response to high salt diet in salt-sensitive strains7,8,16. Taken together, these findings suggest a role for increased CSF [Na] in the etiology of the salt-induced hypertension. The mechanisms responsible for the increase in CSF [Na] in Dahl S and SHR on high salt have not yet been elucidated14. In rats, ENaC is present on both the basolateral and apical membranes of choroid plexus (CP) cells6,14, whereas the Na+, K+-ATPase is located only on the apical (CSF facing) side. In normotensive rats, apical ENaC expression predominates and icv benzamil increases CSF [Na]6. In the present study, the −/− mice show increased expression of all 3 ENaC subunits on both the basolateral and apical membranes of CP cells. In −/− mice after 8-10 days on high salt diet both plasma [Na+]4 and CSF [Na] show clear increases. It appears that in −/− mice on high salt diet the increase in plasma [Na]4 and the overexpression of ENaC on the basolateral membrane of CP cells lead to increased Na entry into the cells and elevated [Na]i in the CP, which presumably causes apical Na+, K+-ATPase to transport the excess sodium into the CSF. Increased apical ENaC expression may enhance Na+ re-absorption from the CSF, which may contribute to the normal CSF [Na] in −/− mice on regular salt and may attenuate the increase in CSF [Na] on high salt diet. Further studies are needed to assess whether or not increases in both plasma and CSF [Na] precede the increases in BP on high salt diet.
In rats, ENaC not only plays a role in regulation of CSF [Na], but also mediates the sympathoexcitatory and pressor responses to a chronic increase in CSF [Na]8. The actual cellular, presumably neuronal, location of these channels still needs to be determined. In the −/− genotype, neuronal ENaC staining is significantly increased but – in contrast to the CP and cortex – only for the β subunit in the SON and PVN and not in the SFO. These findings suggest cell-type specific regulation of ENaC ubiquitination by Nedd 4-2. A 90 minutes icv infusion of Na-rich aCSF provokes a ∼3-fold greater pressor response in −/− than W/T mice, whereas icv mannitol does not change BP. The enhancement in the BP response to Na+-rich aCSF is abolished by icv benzamil, indicating that it is due to enhanced ENaC in the brain. Similar to Dahl S rats, the Nedd4-2 −/− mice show both an increase in CSF [Na] on high salt and hypersensitivity to sodium in the CSF. Whether this hypersensitivity persists during a chronic increase in CSF[Na+], as is the case in Dahl S rats11, still needs to be assessed.
The current telemetry data show that on high salt diet BP of the −/− mice starts to increase after 3–4 days, and after 7–10 days shows marked increases by 30–35 mm Hg, similar to the increase previously reported4. HR increases in parallel, consistent with sympathetic hyperactivity. Central infusion of benzamil prevents most of the increases in BP and HR, indicating that ENaC in the brain is critical for the salt-induced hypertension in this model. Peripheral infusion of benzamil at the icv rate does not affect the initial rise in BP or the further increase in daytime BP. It does attenuate the further rise in night BP, but this reduction is substantially less than achieved by icv benzamil (ie 30% for sc versus 85% for icv). This delayed and partial response to sc benzamil may reflect a slowly developing central blockade. Night (ie active phase) BPs may be more sensitive to such blockade, but peripheral effects cannot be excluded. Although brain ENaC appears to mediate (most of) the salt-induced hypertension, it cannot be deduced from the present data whether the brain effects of high salt in −/− mice are secondary to, enhanced by, or independent of renal sodium retention. In balance studies, no evidence for renal sodium retention was found when −/− mice were placed on high salt diet4. However, even if present, renal effects per se appear not sufficient to cause the hypertension, i.e., they appear to depend on increased brain ENaC.
Limitation of Present Study
Two connected limitations should be considered. ENaC is the best characterized target of Nedd4-2. However, as recently reviewed17, Nedd4-2 may also regulate neuronal voltage-gated sodium channels, and their up-regulation in the brain may potentially contribute to the salt-induced hypertension in the −/− mice. Benzamil is able to block these channels as well18, but at several fold higher IC50 than its IC50 for blockade of ENaC. Ito et al15 estimated that the rate of infusion of benzamil used in their and the present studies would result in a CSF concentration of <100 nM, and according to our calculations 10-15 nM. Both concentrations would be in the range required for ENaC blockade, whereas the concentrations required for blockade of voltage-gated sodium channels are several fold higher.
Perspectives
Since the Nedd4-2 −/− mouse is a model for Liddle's syndrome, the present findings raise the question whether similar aberrant regulation of CSF [Na] and/or hypersensitivity to sodium in the CSF contribute to the salt-induced hypertension in Liddle's patients or other forms of human salt-dependent hypertension. Sodium transport proteins such as ENaC and Na+, K+-ATPase that are expressed in parallel in the brain, vascular smooth muscle and kidneys are more the rule than the exception. Therefore, in cases of salt dependent hypertension where mutations occur in such genes, aberrant neural BP control and/or secretion of sodium into the CSF should also be considered.
Novelty and Significance.
What is New?
Disruption of ENaC ubiquitination by Nedd 4-2 knockout also increases ENaC expression in choroid plexus and neurons.
These −/− mice develop increased CSF [Na+] on high salt and exhibit markedly enhanced BP responses to CSF [Na+] ↑.
Central ENaC blockade by benzamil prevents most of the hypertension by high salt diet in these mice.
What is Relevant?
Sodium transport proteins such as ENaC and Na+,K+-ATPase are not only expressed in the kidneys but also in brain areas involved in cardiovascular regulation.
In salt dependent hypertension with enhanced activity of these proteins, brain mechanisms should be considered as well.
Summary
We demonstrate that Nedd 4-2 −/− mice also exhibit increased ENaC in the brain, and that brain ENaC is needed for the dietary salt-induced hypertension in this animal model of Liddle's Syndrome.
Acknowledgments
The authors thank Xiaohong Hou, Louise Pelletier, Hengwei Wu and Roselyn White for expert technical assistance, and Mrs. Danielle Oja for her excellent skills in assisting in the preparation and formatting of this article. We thank Dr. Mordecai Blaustein of the University of Maryland for critical discussions and review of the manuscript.
Sources of Funding: This work was supported by Operating Grants from the Canadian Institutes of Health Research (MOP 74432 to FHHL and JWV), the NIH (P50-DK52617 to BY), and the Heart and Stroke Foundation of Ontario (NA-6324 to JWV). Frans H. H. Leenen is the recipient of the Pfizer Chair in Hypertension Research, an endowed research chair funded jointly by Pfizer Canada, the University of Ottawa Heart Institute Foundation, and the Canadian Institutes of Health Research.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CP
choroid plexus
- Dahl S
Dahl salt sensitive rat
- ENaC
Epithelial Na channel
- icv
intracerebroventricular
- KO or −/−
Knockout genotype
- [Na]
sodium concentration, as in CSF [Na]
- Nedd4-2
neural precursor cell expressed and developmentally downregulated 4-2 gene or protein
- PBS
phosphate buffered saline
- PVN
paraventricular nucleus of the hypothalamus
- SFO
subfornical organ
- SON
supraoptic nucleus
- W/T or +/+
wild type genotype
Footnotes
Conflict(s) of Interest/Disclosure(s): The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raikwar NS, Thomas CP. Nedd4-2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel. Am J Physiol. 2008;294:F1157–F1165. doi: 10.1152/ajprenal.00339.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, Nelson-Williams C, Rossier BC, Lifton RP. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci USA. 1995;92:11495–1149. doi: 10.1073/pnas.92.25.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 4.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin MS, Wang HW, Reza E, Whitman S, Tuana B, Leenen FHH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers of the rat brain. Am J Physiol. 2005;289:R1787–R1797. doi: 10.1152/ajpregu.00063.2005. [DOI] [PubMed] [Google Scholar]
- 6.Wang HW, Amin MS, El-Shahat E, Huang BS, Tuana BS, Leenen FHH. Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol. 2010;299:R222–R233. doi: 10.1152/ajpregu.00834.2009. [DOI] [PubMed] [Google Scholar]
- 7.Teruyama R, Sakuraba M, Wilson LL, Wandrey NEJ, Armstrong WE. Epithelial Na+ sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol. 2012;302:E273–E285. doi: 10.1152/ajpendo.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Leenen FHH. Brain sodium channels and central sodium induced increases in brain ouabain-like compound and blood pressure. J Hypertens. 2003;21:1519–1524. doi: 10.1097/00004872-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Leenen FHH. Brain sodium channels mediate increases in brain “ouabain” and blood pressure in Dahl S rats. Hypertens. 2002;40:96–100. doi: 10.1161/01.hyp.0000022659.17774.e4. [DOI] [PubMed] [Google Scholar]
- 10.Huang BS, Van Vliet BN, Leenen FHH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high salt diet. Am J Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 11.Huang BS, Wang H, Leenen FHH. Enhanced sympatho-excitatory and pressor responses to central Na+ in Dahl salt-sensitive vs resistant rats. Am J Physiol. 2001;281:H1881–H1889. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Harvey KF, Kinoshita M, Copeland NG, Noda M, Jenkins NA. cDNA cloning, expression analysis, and mapping of the mouse Nedd4 gene. Genomic. 1997;40:435–443. doi: 10.1006/geno.1996.4582. [DOI] [PubMed] [Google Scholar]
- 13.Hou X, Theriault SF, Dostanic-Larson I, Moseley AE, Lingrel JB, Wu H, Dean S, Van Huysse JW. Enhanced pressor response to increased CSF sodium concentration and to central ANG I in heterozygous α2 Na+ -K+ -ATPase knockout mice. Am J Physiol. 2009;296:R1427–R1438. doi: 10.1152/ajpregu.00809.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin MS, Reza E, Wang H, Leenen FHH. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertens. 2009;54:860–867. doi: 10.1161/HYPERTENSIONAHA.108.125807. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Hirooka Y, Sunagawa K. Acquisition of brain Na sensitivity contributes to salt-induced sympathoexcitation and cardiac dysfunction in mice with pressure overload. Circ Res. 2009;104:1004–11. doi: 10.1161/CIRCRESAHA.108.188995. [DOI] [PubMed] [Google Scholar]
- 16.Leenen FHH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–1139. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]