Abstract
Successful allogeneic hematopoietic stem cell transplantation (HSCT) and solid organ transplantation require development of a degree of immune tolerance against allogeneic antigens. T lymphocytes play a critical role in allograft rejection, graft failure, and graft versus host disease (GVHD). T cell tolerance occurs by two different mechanisms; i) depletion of self-reactive T cells during their maturation in the thymus (central tolerance) ii) suppression/elimination of self-reactive mature T cells in the periphery (peripheral tolerance). Induction of transplant tolerance improves transplantation outcomes. Adoptive immunotherapy with immune suppressor cells including regulatory T cells, NK-T cells, veto cells and facilitating cells are promising therapies for modulation of immune tolerance. Achieving mixed chimerism with the combination of thymic irradiation and T cell depleting antibodies, costimulatory molecule blockade with/without inhibitory signal activation and elimination of alloreactive T cells with varying methods including pre or post-transplant cyclophosphamide administration appear to be effective methods to induce transplant tolerance.
Immune Tolerance and Transplantation
Successful allogeneic hematopoietic stem cell transplantation (HSCT) and solid organ transplantation requires a certain degree of immune tolerance development against allogeneic antigens. Achievement of immune tolerance may prevent a host versus graft reaction, which leads to graft rejection and failure, as well as preventing a graft versus host reaction, which results in graft versus host disease (GVHD) in recipients of HSCT. Induction of immune tolerance decreases the risk of acute and chronic graft rejection after solid organ transplantation and can improve transplanted organ survival. Lymphocytes, specifically T lymphocytes, play a critical role in allograft rejection, graft failure, and GVHD. Therefore, in this review we will focus on T cell tolerance.
T Cell Tolerance and Thymopoiesis
Our immune system is very adaptive, able to mount an immune response to varying immunological targets. In some conditions the immune system becomes unresponsive to certain antigens 1. T cell tolerance occurs by two different mechanisms. The first is the depletion of self-reactive T cells during their maturation in the thymus; only 1-2 % of thymocytes are able to reach a mature T cell status before they are released from the thymus. T cell clonal deletion is defined as the death of T cells with TCRs recognizing host antigens, which is the main tolerance mechanism during T cell development in the thymus 1,2.
The second mechanism is the suppression/elimination of self-reactive mature T cells in the periphery; which involves either immunologically active suppressive cells such as regulatory T cells or the inactivation of autoreactive T cell clones by inhibitory molecules3. If T cells are unable to proliferate and produce cytokines such as IL-2 or IL-4 in response to antigens, they may become anergic (non-responsive) to those antigens. Sometimes suppressor/regulatory cells cause anergy or clonal deletion of T cells by secreting inhibitory cytokines or inducing T cell apoptosis in the periphery.
The central tolerance mechanism of thymic maturation will be discussed first, followed by the mechanisms of peripheral tolerance.
T cell development -Thymic Education/Maturation
T cell development begins after the entry of T cell precursors into the thymus. In mice, cell surface markers define multiple types of lymphoid precursors with T cell lineage potential; these include common lymphoid progenitors (CLP1 and CLP2), circulating T cell precursors (CTPs), early thymic precursors (ETPs) and early lymphoid precursors (ELPs) 4-9. The ability of circulating progenitors to migrate within the thymus is tightly regulated and requires the CC chemokine receptors CCR7 and CCR9, CD44, α4 and β2 integrins, and P-selectin glycoprotein ligand-1 (PSGL1) (reviewed in 8). Lymphoid precursors from the BM migrate to the thymus and then will undergo subsequent differentiation under the control of factors including Interleukin-7 (IL-7) and Notch-1 10. A small group of ETPs demonstrate both T and B cell developmental potential in the thymus. However, most of the ETPs lose their B potential and require Notch signaling for T cell development 11,12. Progression beyond the DN3 stage depends on Notch-1 activation, which controls survival and proliferation through the DN3 transition to double positive (DP) cells in cooperation with pre-TCR signaling 13-15. DN thymocytes develop T-cell receptor (TCR) β- and α- locus rearrangements in the DN3 and DN4 stages. They then, start to express CD4 and CD8 as characterized by CD4+/8+ double-positive (DP) expression along with modest αβ TCR expression. Petrie et al. demonstrated that cultured immature thymocytes originally expressing specific TCR alpha and beta chains may lose surface expression of the original TCR alpha, but not beta chains 16. These data provide evidence that TCR alpha gene rearrangement continues even after surface expression of a TCR alpha/beta heterodimer, up until positive selection 16-18. This process is controlled by recombinant activator gene (RAG) expression 19, that starts in CD25+ double negative thymocytes through DP thymocytes. Interestingly, RAG reinduction occurs in the DP stage which is required for mature T cell development 19 (Figure 1).
Figure 1.

Thymopoiesis; T cell development in the thymus includes negative and positive selection of thymocytes. DN; double negative thymocytes, TEC; thymic epithelial cells, cTEC; cortical TEC, mTEC; medullary TEC, DC; dendritic cells, DP; double positive thymocytes, RAG; recombination activating genes, TCR; T cell receptor.
Human T cell development in the thymus
In humans, the phenotype of lymphoid precursors is different from that detected in mice. Human stem cells express CD34, whereas in mice, lineage negative, SCA-1 and c-Kit positive cells contain true stem cells. These CD34+ precursors seed the thymus and differentiate into multiple lineages, including T, B and NK cells 20,21. Lin- CD34+ CD10+ CD24- progenitors with a low myeloid potential have been reported to generate B, T, and natural killer lymphocytes and co-express recombination activating gene 1 (RAG-1), terminal deoxynucleotide transferase (tdt), PAX5, interleukin 7 receptor alpha, and CD3ε. These progenitors are present in both cord blood and BM, but can also be found in the peripheral blood and thymus throughout life 22.
Positive Selection
All thymocytes undergo positive and negative selection during their maturation in the thymus 2. The average DP cell life span is 3-4 days. The fate of each DP cell depends upon the ability of its newly rearranged TCR to appropriately interact with MHC molecules. Most MHC molecules display self-peptides and positive selection involves the recognition of the MHC-self peptide complex 23. More than 95% of DP cells do not have any specificity to an MHC ligand, and these thymocytes die by neglect instead of positive selection 24,25. A small proportion of DP cells are able to bind an MHC ligand with mild avidity; this induces DP maturation to the CD4+ or CD8+ single-positive (SP) stage 24.
T cell clonal deletion in the thymus; Negative Selection
Intra-thymic T cell tolerance occurs via deletion of T cell clones autoreactive to self-antigens presented by hematopoietic cells and thymic epithelial cells 26,27. If the T cell receptor has high affinity to self-antigens (self MHC and peptide complex), this leads to the deletion of thymocytes by apoptosis 28,29. Thymocytes that express a low affinity TCR survive and continue to the next maturation step. The difference between low affinity and high affinity TCRs is critical for thymocyte fate but the mechanisms of this discrimination are not clearly documented 2,24. Fas is a member of the TNF receptor family and plays a role in the development of apoptosis in semi-mature medullary thymocytes 30, which is dependent upon specific antigen concentration in in vivo murine models 31. Strong TCR ligation stimulates rapid onset of Fas-dependent apoptosis of naïve T cells 32. The second required signal of negative selection is costimulation, especially interaction between antigen presenting cells (APC) and CD28 33,34. In the absence of costimulation, anti-TCR antibody stimulation results in significant apoptosis of thymocytes 33. Further studies in mice lacking B7-1 (CD80) and/or B7-2 (CD86) demonstrated a role for both B7-1 and B7-2 in negative selection. CD28 mediates these B7-dependent signals that promote negative selection 35. CTLA-4 delivers signals that inhibit selection, suggesting that CTLA-4 and CD28 have opposing functions in negative selection 35. Thymic medullary tissue is an APC-rich area in which thymocytes interact with varying costimulatory molecules (such as CD80 and CD86) that are expressed on thymic epithelial cells (TEC) and thymic dendritic cells, suggesting their involvement in negative selection. On the other hand, high-level TCR signaling results in apoptosis through the Fas/FasL pathway 32,36, which occurs in the thymic cortex (Figure 1). In the last decade a new transcriptional regulator was discovered in the thymus, named Autoimmune Regulator (AIRE) 37. Aire is primarily expressed in medullary thymic epithelial cells. It promotes self-tolerance by inducing transcription of a wide array of tissue-specific antigens (TSAs) in the thymus and plays a critical role in negative selection (reviewed in 38). In the absence of functional AIRE, medullary TECs express a severely restricted array of self-antigens which results in severe autoimmune disease 37,38. The role of Aire has not been defined in post-transplant tolerance.
Peripheral Tolerance
After positive and negative selection, mature T cells are released from the thymus into the peripheral circulation and secondary lymphoid organs. Negative selection in the thymus effectively deletes thymocytes that have high affinity TCR to self-peptide-MHC complexes. Peripheral immune tolerance mechanisms are critical for controlling mature T cells with low/moderate affinity TCRs to self MHC/peptide complexes (reviewed in 3). Small amounts of T lymphocytes escape selection in the thymus; these can be eliminated in the periphery by deletion. Induced anergy and/or suppression by other immunologically active cells (regulatory cells/suppressor cells) also play roles in lessening the effects of these self-reactive T cell clones that escaped thymic selection.
Peripheral Deletion
Varying levels of antigenic stimulation of mature T cells in the periphery can result in T cell clonal deletion. A small amount of antigenic stimulation can induce T cell tolerance by partial down-regulation of T cell receptors (TCR) on self-reactive CD8+ cells 39. However, T cell apoptosis is generally required for the induction of peripheral transplant tolerance 40. Clonal deletion of autoreactive T cells occurs through apoptosis via activation of the Fas/FasL pathway and the Bim dependent mitochondrial pathway 3. Tissue-associated self-antigens can be cross-presented by APCs to naïve CD8+ T cells 41, which creates the potential for the development of autoimmunity. However, cross-presentation of self-antigen also leads to deletion of those autoreactive CD8+ T cells42. Davey et al. showed that self-tolerance can be maintained by the deletion of activated CD8+ T cells via Bim activation, which leads to BCL-2 inhibition and apoptosis of T cells 43. Bim deficient and Fas deficient (lpr/lpr) mice show defective peripheral tolerance induction, leading to massively increased size of their lymph nodes and spleen and development of autoimmunity, suggesting that both molecules play a role in peripheral deletion of T cells 44,45.
T cell Anergy and Costimulatory Signals
T cell activation requires two signals: i) TCR signal ii) costimulatory signal. T cells are not able to mount an immune response without a second costimulatory signal. CD28 is the main co-stimulatory receptor and has two ligands, B7.1 (CD80) and B7.2 (CD86), that are expressed on APCs (Figure 2). CD28 signals are critical for T cell activation, proliferation and survival after T cell interaction with APCs 46. CD28 activation results in increased expression of cyclins and cyclin dependent kinases (cdk), downregulation of cdk inhibitor cdk27kip1 and upregulation of glucose metabolism through phosphoinositol 3-kinase (PI3K) and Akt activation 47-50. CD28 controls T cell survival by enhancing BCLXL expression in T cells, which prevents T cell death from apoptotic signals such as Fas activation or IL-2 withdrawal 51. Activation of T cells in the absence of CD28 results in an anergic state (reviewed in 52) with defective phospholipase activation and intracellular calcium mobilization 53, which is also associated with increased levels of the cdk inhibitor p27kip1 50,54. Importantly, blockage of CD28/B7 interaction promotes transplant tolerance in animal models 55.
Figure 2.

T Cell-APC interaction with costimulatory molecules. CTLA-4; Cytotoxic T- Lymphocyte Antigen 4, TCR; T cell receptor, MHC; Major histocompatibility molecules, ICOS; Inducible T-cell costimulator, PD-1; Programmed death-1, PD1-L; Programmed death-1 ligand.
Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) and Inhibition of CD28 Costimulatory Signal
CTLA-4, a member of the immunoglobulin superfamily, was the first inhibitory molecule discovered, and has a similar structure to CD28. Both molecules bind to CD80 and CD86 on antigen-presenting cells. Lack of CTLA-4 results in severe lymphoproliferative disease and lethality in rodents 56. CTLA-4 inhibits CD28 dependent T cell activation, cell cycle progression and IL-2 production of T cells 57,58. Interestingly, CTLA-4 inhibition is more pronounced after initiation of T cell activation and anti-CTLA-4 antibody activity is optimized with continuous CD28 signaling, suggesting that upregulation of CTLA-4 is CD28 dependent 58.
CTLA-4 has a significantly higher binding affinity for the B7 molecules than that of CD28 and acts as a competitive antagonist of CD28 52. The presence of inhibitory effects of CTLA-4 in CD28 deficient mice demonstrates that CTLA-4 can function independently from CD28 59. This inhibitory function of CTLA-4 is mediated by its cytoplasmic tail which interacts with other T cell signaling molecules including tyrosine kinases, MAP kinases and the PKB/AKT pathway (reviewed in 52). CTLA-4 Ig is a soluble form of the extracellular domain of CTLA-4, which binds B7 with high avidity, and treatment with CTLA- 4 Ig results in immunosuppression with decreased T cell dependent antibody secretion 60. Treatment with CTLA-4 Ig in conjunction with BM transplantation induces long-term allograft survival and donor unresponsiveness in murine models 61.
Programmed Death-1 (PD-1)/PD-1 Ligands
PD-1 (CD279) is a member of the CD28 family that is expressed on activated T, B, and myeloid cells. PD-1 ligands (PD-L1 and PD-L2) are expressed on T cells, B cells, antigen-presenting cells, endothelial cells, and tumor tissues. PD-L1 (CD274, B7-H1), a transmembrane glycoprotein belonging to the immunoglobulin (Ig) superfamily plays an integral role in the regulation of immune tolerance and homeostasis. PD-1/PD-L1 interactions lead to inhibitory signals, and ligation of PD-1 occurs during autoimmunity, allergy, allograft rejection, antitumor immunity, and chronic virus infection 62-64. PD-1 KO mice develop autoimmunity suggesting that PD-1 has a role in immune tolerance 65,66. PD-1 is expressed on T cells after activation and delivers co-inhibitory signals via an immunoreceptor tyrosine-based switch motif in the cytoplasmic domain 67,68. The ability of PD-1 to block T cell activation requires receptor ligation, suggesting that co-localization of PD-1 with CD3 and/or CD28 may be necessary for inhibition of T cell activation 68. PD-1 signals interfere with CD28 mediated activation of phosphatidylinositol-3-kinase (PI3K) and subsequently inhibit interleukin-2 production resulting in an anergic state in T lymphocytes 69. PD-1 has been recently shown to play a role in control of homeostatic proliferation of CD8+ T cells in lymphopenic hosts by inducing apoptosis of T cells 70. Interestingly, peripheral deletion of alloreactive CD8+ T cells is related to the PD-1/PD-L1 pathway, but tolerization of CD4+ T cells occurs independently from this pathway in murine transplant models 71. PD-L1 also interacts with CD80 to inhibit T cell activation 72 and this interaction is required for maintenance of peripheral tolerance 73. Mice deficient for the PD-L1 gene or wild-type mice treated with anti–B7-H1 blocking monoclonal antibody (mAb) exhibit exacerbated autoimmuninty associated with activation of self-reactive CD4+ and CD8+ T cells 74,75. PD-L2 is a second ligand for PD-1 which inhibits activation of and cytokine production by T cells 76. The role of PD-L2 in the development of post transplant tolerance is not clearly documented. PD-L2 may limit CD8+ T cell proliferation in recipients of HSCT 77.
CD40/CD40L Pathway
CD40 and its ligand CD154 (CD40L) are members of the tumor necrosis factor (TNF)/TNF-receptor family. CD40 is constitutively expressed on APCs such as B cells, macrophages, dendritic cells, and thymic epithelium, but can also be found on endothelial cells and fibroblasts. CD154 (CD40L) is expressed on activated T cells and NK cells 78,79. Signaling through CD40/CD154 is critical for activation of B and dendritic cells. CD40 ligation leads to secretion of IL-1, TNF-α, and IL-12, and to endothelial cell secretion of monocyte chemotactic factors 80,81. Signaling downstream of the CD40 molecules is mediated by TRAFs and leads to the activation of NFκB, which results in the upregulation of MHC molecules and co-stimulatory molecules in B cells (reviewed in 82). CD154 is rapidly induced on CD4+ and some CD8+ T cells following T cell activation after stimulation with cognate antigen. CD40/CD40L activation primarily provides a costimulatory signal to the APC rather than to the T cell, but eventually T cells are indirectly stimulated after APC activation 83. The blockage of the CD40/CD40L pathway alone or in combination with CTLA-Ig results in tolerance to MHC-mismatched skin and cardiac grafts in murine models 84,85. Moreover, treatment with anti-CD154 antibody with or without CTLA-Ig prevents acute allograft rejection in non-human primates 86,87.
Suppression of immune responses; regulatory cells
The suppressive functions of T cells on the immune system has been known since the 1970s 88, but a clear definition of T regulatory cells was not achieved until the mid 90s. Regulatory T cells (Treg) play a major role in the development of tolerance by suppression of immune responses (reviewed in 89). Other immunologically active cells can also suppress immune responses including NK-T cells, double negative (CD4-CD8-), CD8+CD28- cells, and veto cells.
Regulatory T Cells
Regulatory T cells were first defined as CD4+CD25+ double positive cells with suppressive functions on immunological response in rodents 90. Approximately 5-10 % of peripheral CD4+ cells express IL-2 receptors on their surface. The depletion of CD4+CD25+ cells results in development of autoimmune disease 91,92. Normally, the thymus produces immunoregulatory CD4+CD25+ T cells that are anergic to TCR stimulation and are able to suppress proliferation of other T cells 93. They might have intermediate affinity to MHC/peptide resulting in an escape from negative selection in the thymus 94.
Naturally occurring Treg specifically express a transcription factor (Foxp3), which is the main inducer, regulator, and survival factor in Treg development and function 95,96. Only CD4+CD25+ single positive thymocytes exhibit expression of Foxp3 in the thymus. Foxp3 transduced CD4+CD25- cells are capable of suppressing other T cells and autoimmune colitis in murine models 95. Retroviral transduction of Foxp3 into CD25- T cells upregulates CD25 and CTLA-4 expression on their surface. CD4+CD25+ cells are increased in Foxp3 transgenic mice and other T cell subsets also gain suppressive properties in the presence of over-expression of Foxp3 97. Naïve T cells in the periphery can also acquire Foxp3 expression and suppressive function in conditions such as chronic antigenic stimulation in the presence of transforming growth factor (TGF)-β1 98-100. TGF-β1 signaling is required for the maintenance of immune suppressive activity and expansion of regulatory T cells 101,102. Interestingly TGF-β1 deficient mice have a reduced number of peripheral Treg while preserving normal thymic Treg development. A defect in TGF-beta-mediated signaling in Treg is associated with a decrease in Foxp3 expression and suppressor activity 102. Other types of T regulatory cells can be induced in the periphery in the presence of interleukin-10 (IL-10) 103, called Tr1 cells. Tr1 cells secrete IL-10 and TGF-β1 and their suppressive activities are independent of Foxp3 expression 104. Both naturally occurring Treg cells and Tr1 cells are hyporesponsive to stimulation of their TCR but can slowly proliferate in presence of IL-2. Recently, CD8+ Foxp3+ Treg have been shown to have a suppressive function in autoimmune disorders and after allergen immunotherapy as well as in GVHD 105-107.
Function of regulatory T cells
CD4+CD25+ T cells suppress the proliferation of CD4+ as well as CD8+ T cells, which requires direct cell contact with Treg 108. Survival and function of Treg is dependent on presence of IL-2; IL-2 deficient mice develop lymphoproliferation and severe autoimmune disease 109,110. Expression of IL-2R (CD25) on their surface and signaling through IL-2R is required for optimum T regulatory function 111. Treg are anergic after stimulation and therefore, IL-2 secretion from conventional T cells is critical for development of suppressive activity of Treg. 112,113. The crosstalk between T effector cells and T regulatory cells is shown in Figure 3. As mentioned above, IL-10 is required for Tr1 development in the periphery. Interestingly, CD4+CD25+Treg suppressive function in vitro does not require IL-10 and TGF-β1 secretion, as has been shown with suppressor T cells from IL-10-deficient and TGF-β1 deficient mice, which seem to suppress effectively 108,114,115. On the other hand CD4+CD25+ Treg can secrete IL-10 in vivo and autoimmunity can be suppressed by regulatory cells through IL-10 secretion 116. Treg interaction with CD80/CD86 through the CTLA-4 molecule may lead to suppression of effector T cells 117. Interaction of CTLA-4 expressing Treg with APCs induces the activation of indoleamine 2,3-dioxygenase (IDO), which results in both a local deprivation of tryptophan and the production of inhibitory molecules known as kynurenines 118,119 (Figure 3). CTLA-4 expressing CD4+CD25+ regulatory cells show a stronger suppressive activity than their CTLA-4- counterparts 120. Moreover, blocking CTLA-4 on Treg can abrogate the suppression of T effectors 121. CD28-deficient CD25+ CD4+ T cells can also suppress activation of normal T cells, indicating that CD28 is not required as a costimulatory molecule for activation of the regulatory T cells. Treg may also act as cytotoxic T cells that expresses granzyme A after activation and are able to kill activated CD4+ and CD8+ T cells by a perforin dependent mechanism 122.
Figure 3.
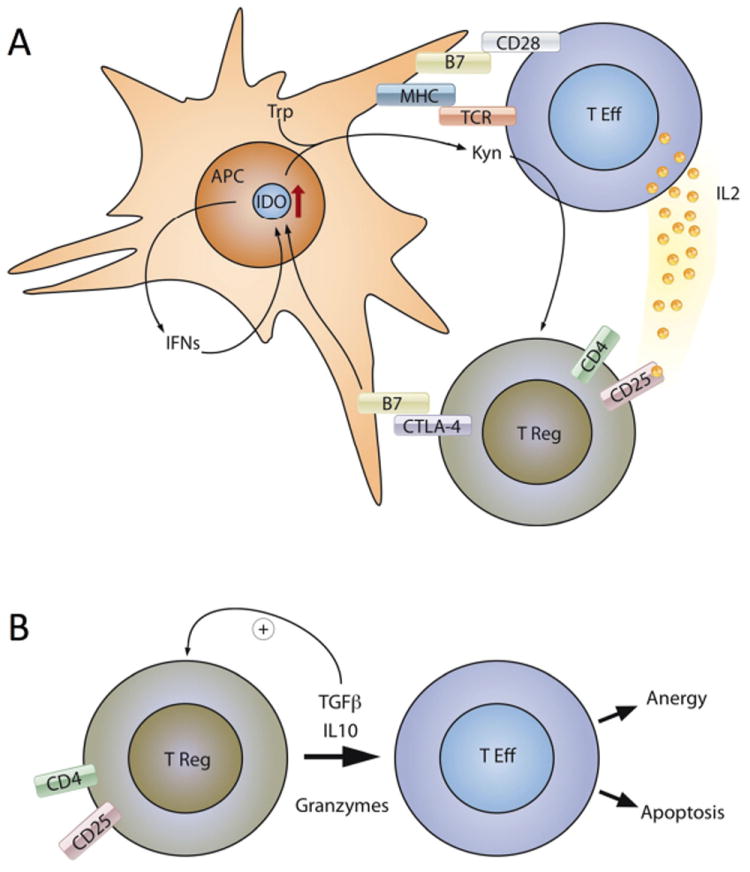
Main mechanisms of T regulatory cell function. Treg suppress effector T cell function through cell contact (Figure 3A) and inhibitory cytokine and granzyme secretion (Figure 3B). T Eff; effector T cells, IDO; indoleamine 2,3-dioxygenase, Trp; Tryptophan, Kyn; kynurenines, IFNs; interferons
Transplant tolerance and T regulatory cells
CD4+CD25+ T cells play a role in regulating the allo response and tolerance induction. Depletion of CD25+ T cells results in faster rejection of allogeneic skin grafts and increases graft versus host disease (GVHD) related mortality in murine models 90,123. The presence of CD4+CD25+ T cells in in vitro mixed lymphocte reaction cultures can induce tolerance to alloantigen 124. Infusion of CD4+CD25+ regulatory T cells after stem cell transplantation decreased GVHD related mortality in recipients of allogeneic HSCT 123,125. Treg induce tolerance to minor histocompatibility antigen-mismatched skin grafts 126,127 and are involved in tolerance and prevention of graft rejection after organ transplant 128-130. Treg may be a long-awaited T cell population for adoptive therapy to induce tolerance in clinical settings specifically after transplantation (reviewed in 131). Hippen and coworkers from University of Minnesota reported large scale expansion of human natural regulatory cells from peripheral blood with well-preserved suppressive function 132. The same group also showed that the infusion of ex vivo expanded Treg into adults transplanted with umbilical cord blood is safe and might be able to prevent GVHD in clinical settings 133. Finally Di Lanni and colleagues evaluated the effects of pre-transplant adoptive transfer of Treg lymphocytes in 28 patients with haploidentical HSCT. They found that Treg infusion prevented GVHD, promoted immune reconstitution and improved immunity to opportunistic infections 134. These data suggest that adoptive therapy with donor derived Treg can prevent GVHD after allogeneic HSCT.
Other suppressive cells
Natural Killer (NK)-T Cells
These are T cells that express NK cell associated markers (CD16 and CD56 in humans, NK1.1, DX5 in mouse) together with T cell markers and TCR αβ on their surface. NK-T cells secrete high levels of IL-4 and IFN-γ. In mice, NK1 T cells express a restricted TCR repertoire with an invariant TCR alpha chain and recognize the products of the conserved family of MHC class I-like CD1 genes 135. The presence of allogeneic donor NK1.1+ TCRα+ T cells in the BM can protect mice against lethal GVHD in recipients of allogeneic HSCT 136. Total lymphoid irradiation (TLI) can induce tolerance after organ and HSC transplantation by selectively increasing NK-T cells in the host 137. Host conditioning with TLI and ATG prevents GVHD by an NK-T cell dependent mechanism 138. Recent reports suggest that NK-T cells promote immune tolerance through an interaction with Treg by increasing expression of negative costimulatory molecules (like PD-1) on Treg, which is mostly dependent on enhanced IL-4 secretion by NK-T cells 139. Taken together, NK-T cells play a specific role in immune tolerance and strategies to enrich NK-T cells can induce transplant tolerance. Cytokine stimulation with IFN-γ, IL-2 and anti-CD3 expands human CD3+ CD56+ NK-T cells, and these cells show MHC-independent cytotoxicity (cytokine induced killer cells-CIK). The safety and feasibility of CIK cell infusion have been shown after autologous HSCT 140. Adoptive immunotherapy with CIK cells induces anti-tumor responses with minimal GVHD in patients with relapsed hematological malignancies after allogeneic HSCT 141
Veto cells
Veto activity was defined in 1980 by Miller as the capacity to suppress cytotoxic T lymphocyte (CTL) precursors directed against antigens expressed on veto cells, but not against third party antigens 142,143. Interestingly, the most potent veto cell activity was found in CD8+ CTL lines or clones 144,145. The specificity of the veto effect mediated by CTL clones is antigen specific and MHC restricted. Veto activity occurs by apoptosis through the Fas/FasL pathway 145,146. Other cells, including varying BM subsets, have been reported to have veto activity and augment immune tolerization 147-149. Murine and human hematopoietic stem cells also have veto cell activity 150,151, and tolerance can be induced by using high doses of stem cells especially in MHC-mismatched donor/host combinations (reviewed in 152. Megadose human CD34+ stem cells have been successfully used in haploidentical transplantation, which results in high-level engraftment of MHC disparate stem cells 153
Inducing tolerance; clinically applicable strategies
Multiple strategies have been explored to optimize immune tolerance after transplantation. T cell depletion is one of the well-known strategies to enhance immune tolerance and decrease graft versus host disease. Increased susceptibility to infections and increased relapse have been observed after T cell depletion in recipients of allogeneic HSCT.
Pre or post-transplant chemotherapy can be used to induce immune tolerance to transplanted organs or to the host. Cyclophosphamide (CTX) is a well-known chemotherapeutic agent; preclinical and clinical studies demonstrated that pre or post-transplant CTX promotes immune tolerance in recipients of allogeneic HSCT 154-156. Recent studies have demonstrated that in particular post-transplant cyclophosphamide can improve engraftment and reduce GVHD 157. This topic is discussed in detail in another section in this issue.
Generation of mixed chimerism is another strategy that has been explored in clinical settings. As previously discussed, intrathymic clonal deletion is one of the main mechanisms leading to the development of transplant tolerance. Combining thymic irradiation with T cell depleting antibodies results in a multi-lineage mixed chimerism and skin graft tolerance without GVHD in recipients of MHC-mismatched BMT in murine models 158,159. Addition of CTX to conditioning protocols with TI provides successful engraftment and long-term chimerism in fully-MHC mismatched murine BMT recipients. Durable chimerism after transplantation prevents chronic rejection and induces graft survival as shown in experimental animal models 160. This strategy was successfully translated to the clinic to induce organ tolerance in recipients of both BM and kidney transplantation 161-163. A similar strategy combining kidney transplantation and Hematopoietic stem cell transplantation has been reported with durable chimerism and donor specific tolerance 164. Interestingly, immunosuppression was discontinued one year after transplant in patients who achieved durable chimerism after HSC+ facilitating cell (FC) infusion. BM derived FC were initially identified as CD8+ cells that did not express TCR on their surface and enhanced engraftment of allogeneic HSC 148. Subsequently, FCs were shown to be predominantly composed of a plasmacytoid precursor dendritic cell subpopulation that produces IFN-alpha and TNF-alpha, and can be activated by toll-like receptor (TLR)-9 ligand stimulation, and expanded by Flt-3 ligand 165.
Selective removal of allo-reactive T cells is another approach to decrease GVH reaction and induce tolerance after stem cell transplantation. The T cell proliferation that occurs in haplotype-mismatched mixed lymphocyte reactions provides a platform for elimination of allo-reactive T cells. An immunotoxin coupled to a T cell activation marker (CD25) successfully depletes allo-reactive T cells without having any harmful effects on stem cells 166. The same group reported successful ex vivo depletion of host-reactive donor T cells from peripheral blood stem cell (PBSC) transplant allografts using an anti-CD25 immunotoxin, while the remaining T cell population had an intact third-party response in most cases 167. However, possible depletion of CD4+CD25+ regulatory cells by this method raised a concern regarding the induction of long-term immune tolerance and efficacy in the mitigation of GVHD risk. A non-FCR binding anti-CD3 antibody has been used to induce apoptosis in selectively antigen–activated and cycling T cell populations 168. This antibody might have a role in the therapy of GVHD 169,170 but it has not yet been reported as a tolerance-inducing agent in clinical trials. Campath is an antibody against CD52 that was evaluated in recipients of renal allografts and found to be ineffective for inducing tolerance despite successful T cell depletion 171,172, suggesting that the degree of T cell depletion may not always be correlated with better tolerance to alloantigens.
Many challenges to optimize transplant tolerance remain 173. Many successful preclinical studies in mice and rats have not resulted in positive results when translated to humans 174. Large animal models including nonhuman primate models are necessary to confirm the studies done in rodents. A number of examples teach us to be careful in designing early stage clinical trials. One such example is a first in human clinical trial with a CD28 superagonist, which resulted in life-threatening cytokine storm following administration of this agent to normal healthy volunteers 175. In this clinical trial, the superagonist antibody was used at a small fraction of the dose that was used safely in nonhuman primates. The difference in CD28 superagonist binding affinity in humans and non-human primates might explain the disparity in severe side effects. Anti-CD154 antibody administration has also induced unexpected side effects including thrombosis, which precludes conducting further clinical trials of this agent 176,177. The lack of standardized clinical tests for the evaluation of immune tolerance is another problem in developing clinical trials.
Conclusion
Induction of transplant tolerance improves outcomes after HSCT and solid organ transplantation. Adoptive immunotherapy with immune suppressor cells including regulatory T cells, NK-T cells, veto cells and facilitating cells, achieving mixed chimerism with thymic irradiation, costimulatory molecule blockade with/without inhibitory signal activation and elimination of alloreactive T cells with varying methods including pre or post-transplant cyclophosphamide administration appear to be effective methods to induce transplant tolerance. Clinically applicable transplant tolerance induction methods will broaden options to treat GVHD, facilitate anti-tumor effects, and shorten the period of post-transplant immune deficiency, thus leading to the development of new treatment strategies.
Acknowledgments
The authors thank EnricoVelardi from Memorial Sloan-Kettering Cancer Institute (NY, NY) for drawing Figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Onder Alpdogan, Email: onder.alpdogan@jefferson.edu.
Marcel RM van den Brink, Email: vandenbm@mskcc.org.
References
- 1.Sykes M. Immune tolerance: mechanisms and application in clinical transplantation. Journal of internal medicine. 2007;262:288–310. doi: 10.1111/j.1365-2796.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 2.Griesemer AD, Sorenson EC, Hardy MA. The role of the thymus in tolerance. Transplantation. 2010;90:465–74. doi: 10.1097/TP.0b013e3181e7e54f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller DL. Mechanisms maintaining peripheral tolerance. Nature immunology. 2010;11:21–7. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–30. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 5.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 7.Allman D, Sambandam A, Kim S, et al. Thymopoiesis independent of common lymphoid progenitors. Nature immunology. 2003;4:168–74. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 8.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–89. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–45. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 11.Sambandam A, Maillard I, Zediak VP, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nature immunology. 2005;6:663–70. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 12.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nature immunology. 2005;6:671–9. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 13.Huang EY, Gallegos AM, Richards SM, Lehar SM, Bevan MJ. Surface expression of Notch1 on thymocytes: correlation with the double-negative to double-positive transition. J Immunol. 2003;171:2296–304. doi: 10.4049/jimmunol.171.5.2296. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004;200:469–79. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciofani M, Schmitt TM, Ciofani A, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172:5230–9. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 16.Petrie HT, Livak F, Schatz DG, Strasser A, Crispe IN, Shortman K. Multiple rearrangements in T cell receptor alpha chain genes maximize the production of useful thymocytes. J Exp Med. 1993;178:615–22. doi: 10.1084/jem.178.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Huang CY, Kanagawa O. Rapid deletion of rearranged T cell antigen receptor (TCR) Valpha-Jalpha segment by secondary rearrangement in the thymus: role of continuous rearrangement of TCR alpha chain gene and positive selection in the T cell repertoire formation. Proc Natl Acad Sci U S A. 1998;95:11834–9. doi: 10.1073/pnas.95.20.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CY, Sleckman BP, Kanagawa O. Revision of T cell receptor {alpha} chain genes is required for normal T lymphocyte development. Proc Natl Acad Sci U S A. 2005;102:14356–61. doi: 10.1073/pnas.0505564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yannoutsos N, Wilson P, Yu W, et al. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J Exp Med. 2001;194:471–80. doi: 10.1084/jem.194.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 21.Weerkamp F, Baert MR, Brugman MH, et al. Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood. 2006;107:3131–7. doi: 10.1182/blood-2005-08-3412. [DOI] [PubMed] [Google Scholar]
- 22.Six EM, Bonhomme D, Monteiro M, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085–93. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- 24.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 25.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–3. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 26.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459–67. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 27.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nature reviews Immunology. 2009;9:833–44. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 28.Alam SM, Travers PJ, Wung JL, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–20. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 29.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990;248:1342–8. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–71. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J Exp Med. 1998;187:1427–38. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999;163:1817–26. [PubMed] [Google Scholar]
- 33.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–13. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+ thymocytes. J Immunol. 1993;151:1868–80. [PubMed] [Google Scholar]
- 35.Buhlmann JE, Elkin SK, Sharpe AH. A role for the B7-1/B7-2:CD28/CTLA-4 pathway during negative selection. J Immunol. 2003;170:5421–8. doi: 10.4049/jimmunol.170.11.5421. [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J Exp Med. 1998;187:1427–38. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 38.Gardner JM, Fletcher AL, Anderson MS, Turley SJ. AIRE in the thymus and beyond. Current opinion in immunology. 2009;21:582–9. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferber I, Schonrich G, Schenkel J, Mellor AL, Hammerling GJ, Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674–6. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 40.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–7. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 41.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166:1439–42. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 42.Heath WR, Kurts C, Miller JF, Carbone FR. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–53. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davey GM, Kurts C, Miller JF, et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–55. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–30. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Hutcheson J, Scatizzi JC, Siddiqui AM, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–17. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–6. [PubMed] [Google Scholar]
- 47.Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144–51. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 48.Boonen GJ, van Dijk AM, Verdonck LF, van Lier RA, Rijksen G, Medema RH. CD28 induces cell cycle progression by IL-2-independent down-regulation of p27kip1 expression in human peripheral T lymphocytes. Eur J Immunol. 1999;29:789–98. doi: 10.1002/(SICI)1521-4141(199903)29:03<789::AID-IMMU789>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 50.Rowell EA, Walsh MC, Wells AD. Opposing roles for the cyclin-dependent kinase inhibitor p27kip1 in the control of CD4+ T cell proliferation and effector function. J Immunol. 2005;174:3359–68. doi: 10.4049/jimmunol.174.6.3359. [DOI] [PubMed] [Google Scholar]
- 51.Boise LH, Minn AJ, Noel PJ, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 52.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunological reviews. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells AD, Liu QH, Hondowicz B, Zhang J, Turka LA, Freedman BD. Regulation of T cell activation and tolerance by phospholipase C gamma-1-dependent integrin avidity modulation. J Immunol. 2003;170:4127–33. doi: 10.4049/jimmunol.170.8.4127. [DOI] [PubMed] [Google Scholar]
- 54.Rowell EA, Wang L, Hancock WW, Wells AD. The cyclin-dependent kinase inhibitor p27kip1 is required for transplantation tolerance induced by costimulatory blockade. J Immunol. 2006;177:5169–76. doi: 10.4049/jimmunol.177.8.5169. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 56.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 57.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 58.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 61.Pearson TC, Alexander DZ, Hendrix R, et al. CTLA4-Ig plus bone marrow induces long-term allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation. 1996;61:997–1004. doi: 10.1097/00007890-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 62.Blattman JN, Greenberg PD. PD-1 blockade: rescue from a near-death experience. Nature immunology. 2006;7:227–8. doi: 10.1038/ni0306-227. [DOI] [PubMed] [Google Scholar]
- 63.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–70. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 64.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends in immunology. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 67.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nature reviews Immunology. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 68.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 69.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–33. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–55. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–8. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynoso ED, Elpek KG, Francisco L, et al. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182:2102–12. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–36. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 76.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 77.Habicht A, Kewalaramani R, Vu MD, et al. Striking dichotomy of PD-L1 and PD-L2 pathways in regulating alloreactive CD4(+) and CD8(+) T cells in vivo. Am J Transplant. 2007;7:2683–92. doi: 10.1111/j.1600-6143.2007.01999.x. [DOI] [PubMed] [Google Scholar]
- 78.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175:1091–101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L) Critical reviews in immunology. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 80.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci U S A. 1995;92:4342–6. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunological reviews. 2011;244:115–33. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clarkson MR, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Transplantation. 2005;80:555–63. doi: 10.1097/01.tp.0000168432.60022.99. [DOI] [PubMed] [Google Scholar]
- 84.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 85.Larsen CP, Alexander DZ, Hollenbaugh D, et al. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 86.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–93. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 88.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 89.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature immunology. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 90.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 91.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunological reviews. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 92.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 94.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 95.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 96.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 97.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 98.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–8. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 101.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 102.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 104.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175:7728–37. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 106.Tsai YG, Yang KD, Niu DM, Chien JW, Lin CY. TLR2 agonists enhance CD8+Foxp3+ regulatory T cells and suppress Th2 immune responses during allergen immunotherapy. J Immunol. 2010;184:7229–37. doi: 10.4049/jimmunol.1000083. [DOI] [PubMed] [Google Scholar]
- 107.Robb RJ, Lineburg KE, Kuns RD, et al. Identification and expansion of highly suppressive CD8+FoxP3+ regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 108.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nature reviews Immunology. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 109.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 110.Sadlack B, Lohler J, Schorle H, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–9. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 111.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 113.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nature reviews Immunology. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 114.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nature immunology. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 115.Piccirillo CA, Letterio JJ, Thornton AM, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–46. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 119.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 120.Birebent B, Lorho R, Lechartier H, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–96. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 124.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558–65. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 127.Cobbold SP, Castejon R, Adams E, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–10. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 128.Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–89. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 129.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–6. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 130.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Seminars in immunology. 2004;16:119–26. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 131.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol. 2011;23:462–8. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Science translational medicine. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 135.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annual review of immunology. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 136.Zeng D, Lewis D, Dejbakhsh-Jones S, et al. Bone marrow NK1.1(-) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189:1073–81. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Higuchi M, Zeng D, Shizuru J, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–70. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 138.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9:355–63. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 139.Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581–9. doi: 10.1182/blood-2011-08-371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–7. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 141.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1679–87. doi: 10.1016/j.bbmt.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller RG. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980;287:544–6. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- 143.Fink PJ, Rammensee HG, Bevan MJ. Cloned cytolytic T cells can suppress primary cytotoxic responses directed against them. J Immunol. 1984;133:1775–81. [PubMed] [Google Scholar]
- 144.Zhang L, Shannon J, Sheldon J, Teh HS, Mak TW, Miller RG. Role of infused CD8+ cells in the induction of peripheral tolerance. J Immunol. 1994;152:2222–8. [PubMed] [Google Scholar]
- 145.Reich-Zeliger S, Zhao Y, Krauthgamer R, Bachar-Lustig E, Reisner Y. Anti-third party CD8+ CTLs as potent veto cells: coexpression of CD8 and FasL is a prerequisite. Immunity. 2000;13:507–15. doi: 10.1016/s1074-7613(00)00050-9. [DOI] [PubMed] [Google Scholar]
- 146.Sambhara SR, Miller RG. Programmed cell death of T cells signaled by the T cell receptor and the alpha 3 domain of class I MHC. Science. 1991;252:1424–7. doi: 10.1126/science.1828618. [DOI] [PubMed] [Google Scholar]
- 147.Sugiura K, Inaba M, Ogata H, et al. Wheat germ agglutinin-positive cells in a stem cell-enriched fraction of mouse bone marrow have potent natural suppressor activity. Proc Natl Acad Sci U S A. 1988;85:4824–6. doi: 10.1073/pnas.85.13.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–46. [PubMed] [Google Scholar]
- 149.Hiruma K, Nakamura H, Henkart PA, Gress RE. Clonal deletion of postthymic T cells: veto cells kill precursor cytotoxic T lymphocytes. J Exp Med. 1992;175:863–8. doi: 10.1084/jem.175.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bachar-Lustig E, Li HW, Marcus H, Reisner Y. Tolerance induction by megadose stem cell transplants: synergism between SCA-1+ Lin- cells and nonalloreactive T cells. Transplantation proceedings. 1998;30:4007–8. doi: 10.1016/s0041-1345(98)01320-7. [DOI] [PubMed] [Google Scholar]
- 151.Gur H, Krauthgamer R, Berrebi A, et al. Tolerance induction by megadose hematopoietic progenitor cells: expansion of veto cells by short-term culture of purified human CD34(+) cells. Blood. 2002;99:4174–81. doi: 10.1182/blood.v99.11.4174. [DOI] [PubMed] [Google Scholar]
- 152.Reisner Y, Gur H, Reich-Zeliger S, Martelli MF, Bachar-Lustig E. Hematopoietic stem cell transplantation across major genetic barriers: tolerance induction by megadose CD34 cells and other veto cells. Annals of the New York Academy of Sciences. 2005;1044:70–83. doi: 10.1196/annals.1349.010. [DOI] [PubMed] [Google Scholar]
- 153.Reisner Y, Martelli MF. From ‘megadose’ haploidentical hematopoietic stem cell transplants in acute leukemia to tolerance induction in organ transplantation. Blood cells, molecules & diseases. 2008;40:1–7. doi: 10.1016/j.bcmd.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 154.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–8. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 155.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 156.Grosso D, Carabasi M, Filicko-O’Hara J, et al. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood. 2011;118:4732–9. doi: 10.1182/blood-2011-07-365338. [DOI] [PubMed] [Google Scholar]
- 157.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sykes M, Szot GL, Swenson KA, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nat Med. 1997;3:783–7. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 159.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–98. [PubMed] [Google Scholar]
- 160.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–81. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Buhler LH, Spitzer TR, Sykes M, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–9. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 162.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–8. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 163.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Science translational medicine. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fugier-Vivier IJ, Rezzoug F, Huang Y, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–83. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mavroudis DA, Jiang YZ, Hensel N, et al. Specific depletion of alloreactivity against haplotype mismatched related individuals by a recombinant immunotoxin: a new approach to graft-versus-host disease prophylaxis in haploidentical bone marrow transplantation. Bone Marrow Transplant. 1996;17:793–9. [PubMed] [Google Scholar]
- 167.Solomon SR, Mielke S, Savani BN, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–9. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. Anti-CD3 epsilon F(ab’)2 prevents graft-versus-host disease by selectively depleting donor T cells activated by recipient alloantigens. J Immunol. 2001;166:5835–9. doi: 10.4049/jimmunol.166.9.5835. [DOI] [PubMed] [Google Scholar]
- 169.Carpenter PA, Lowder J, Johnston L, et al. A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:465–71. doi: 10.1016/j.bbmt.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 170.Carpenter PA, Appelbaum FR, Corey L, et al. A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease. Blood. 2002;99:2712–9. doi: 10.1182/blood.v99.8.2712. [DOI] [PubMed] [Google Scholar]
- 171.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 172.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–9. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 173.Salama AD, Womer KL, Sayegh MH. Clinical transplantation tolerance: many rivers to cross. J Immunol. 2007;178:5419–23. doi: 10.4049/jimmunol.178.9.5419. [DOI] [PubMed] [Google Scholar]
- 174.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 175.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 176.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 177.Koyama I, Kawai T, Andrews D, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–2. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]


