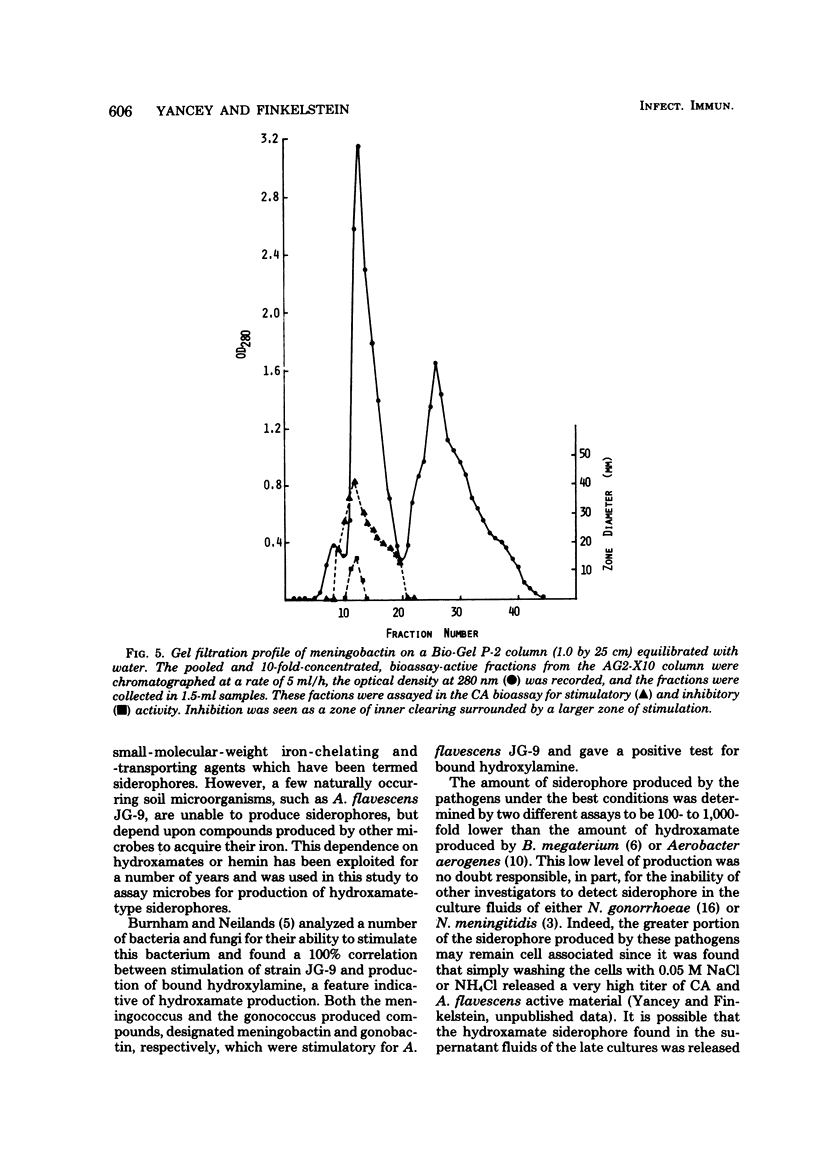
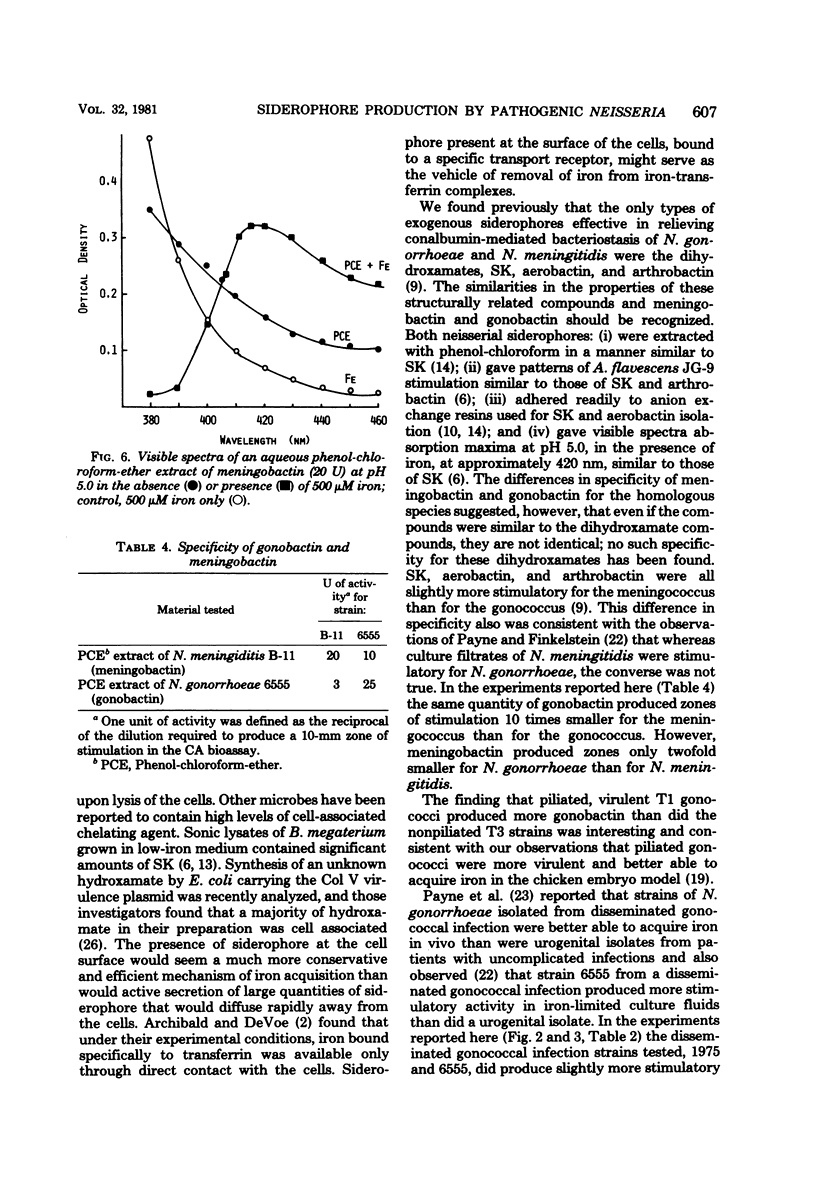
Abstract
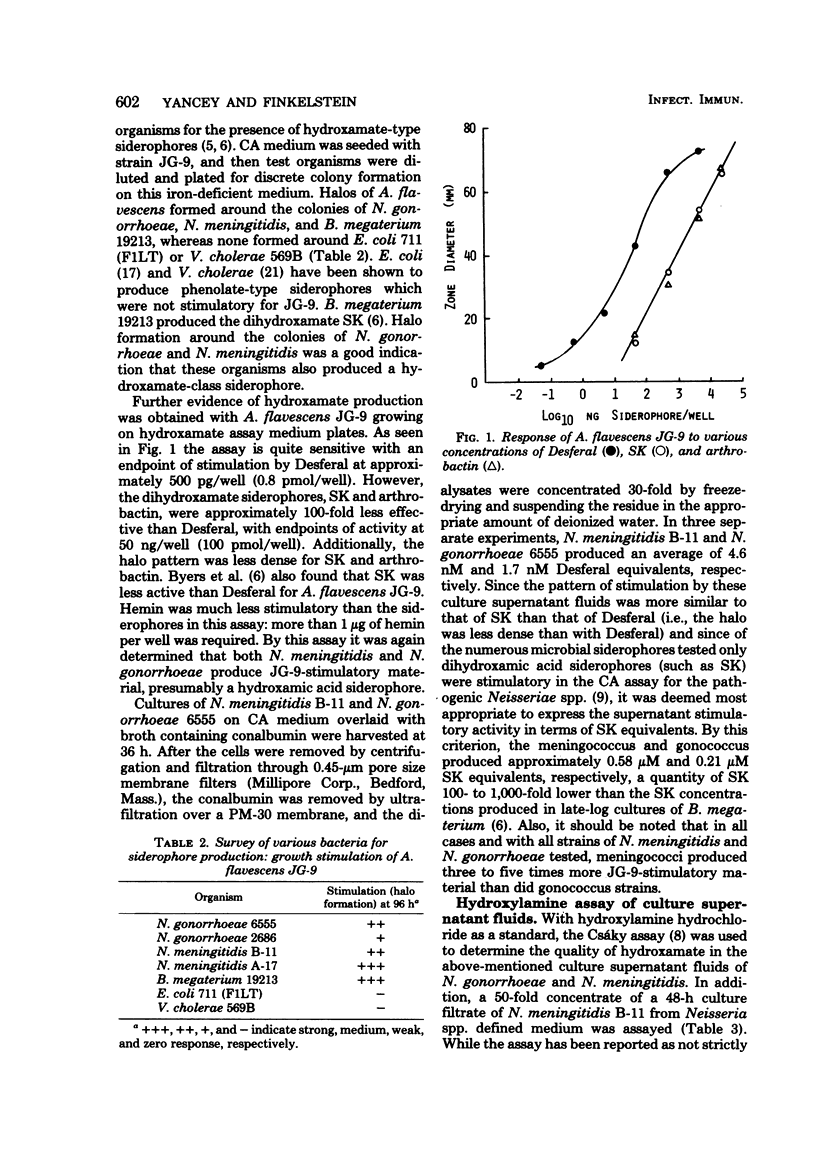
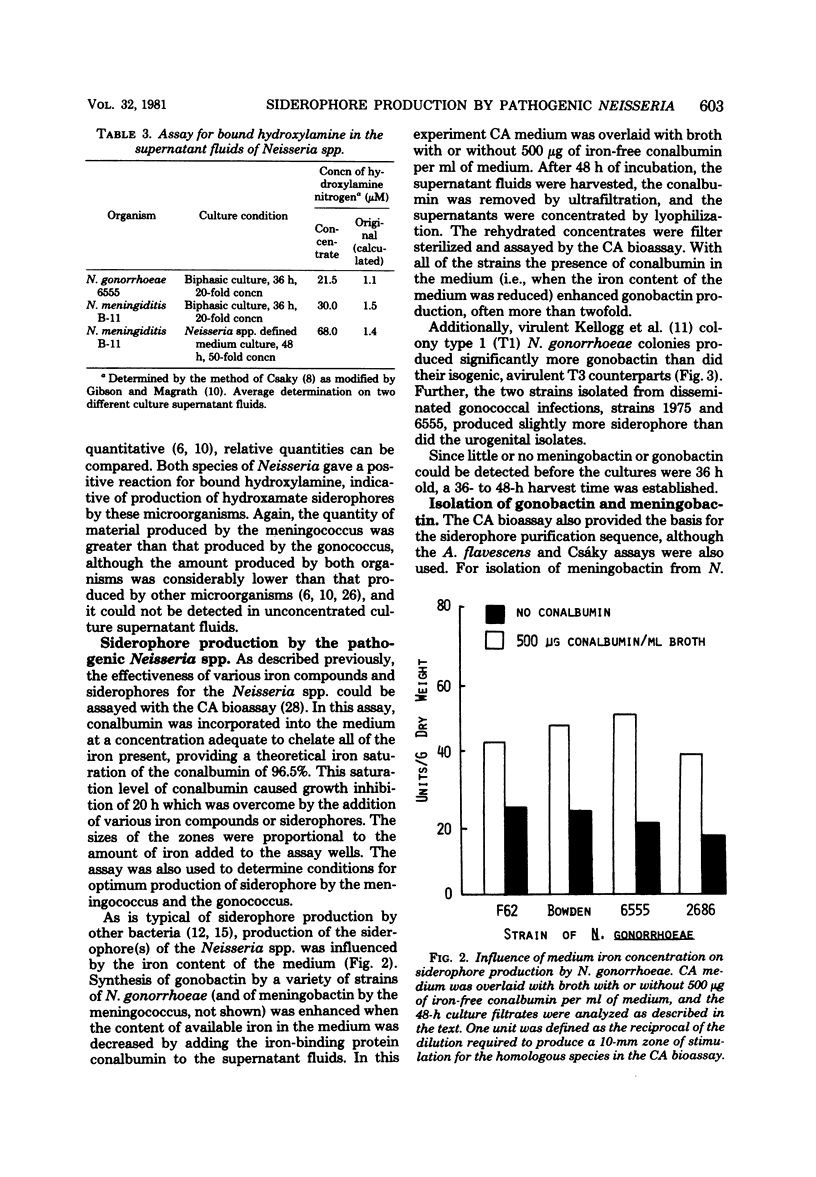
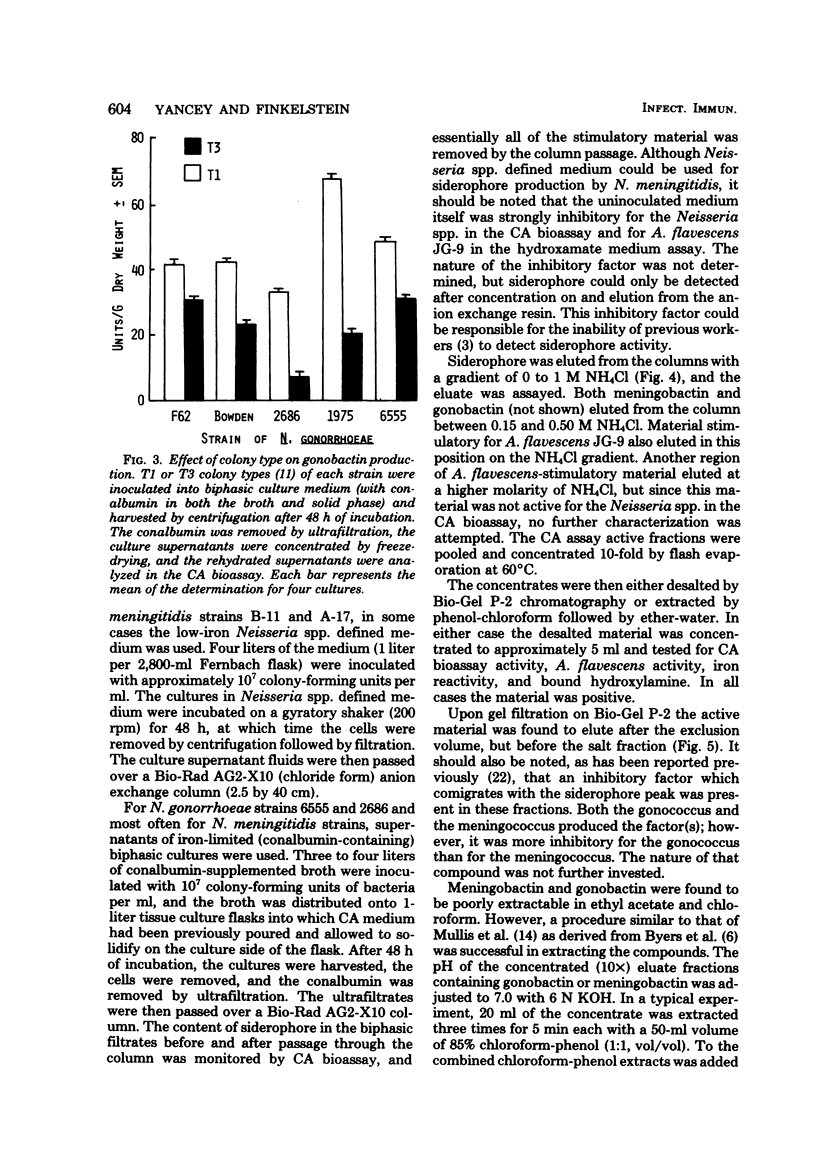
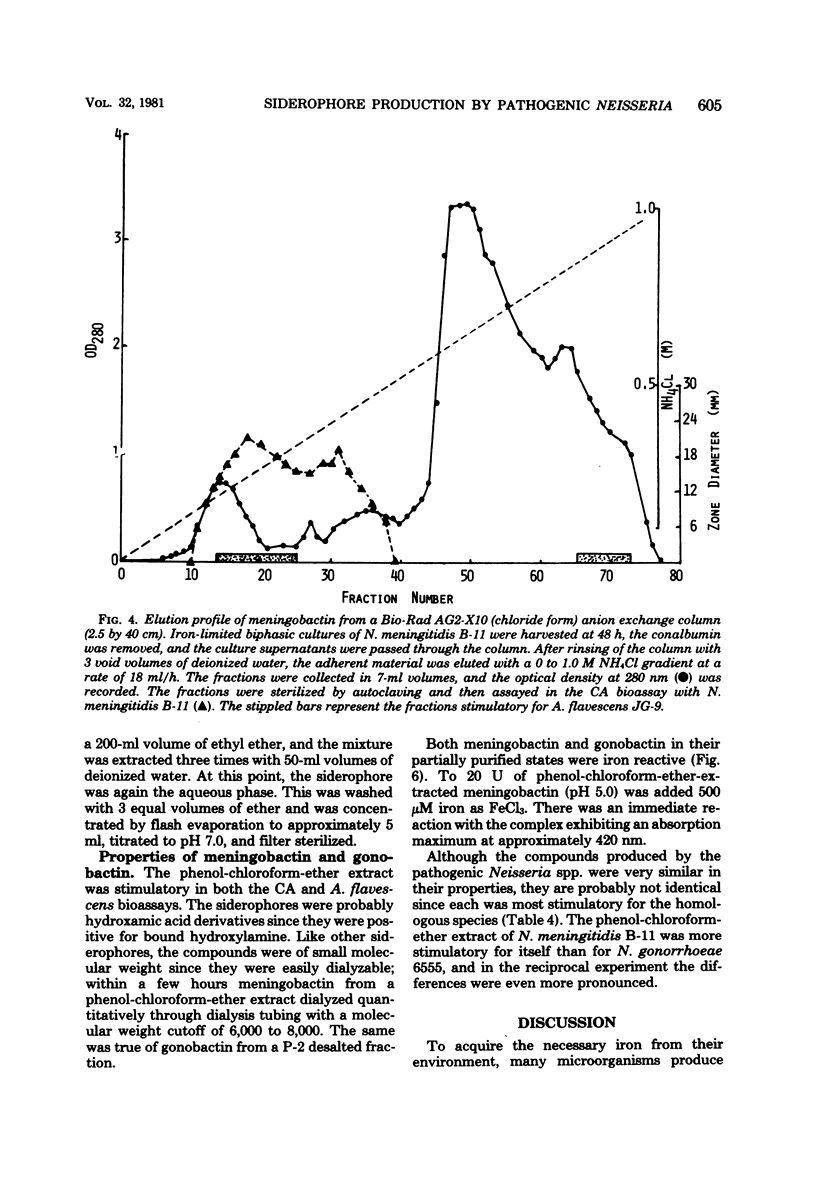
Previous studies have established the importance of iron acquisition to the growth and virulence of Neisseria meningitidis and Neisseria gonorrhoeae. Although preliminary evidence that the Neisseria spp. produce siderophores has been presented, the exact mechanism of iron acquisition has remained obscure. Siderophore production by N. gonorrhoeae and N. meningitidis was induced in two different low-iron media. The iron-reactive siderophores, “gonobactin” and “meningobactin,” were partially purified by ion exchange chromatography followed by extraction with phenol-chloroform-ether or by gel filtration. The compounds were of low molecular weight, their synthesis was repressed by iron in the medium, and they appeared to be hydroxamic acids since they were stimulatory for Arthrobacter flavescens JG-9 (a hydroxamate auxotroph) and gave a positive Csáky reaction for bound hydroxylamine. In the iron form, the compounds had an absorption maximum of approximately 420 nm. Although meningobactin stimulated growth of the gonococcus in low-iron media and vice versa, the homologous activity was more marked, indicating that the compounds, though similar, were probably not identical. As determined by A. flavescens assay the meningococcus produced three to five times more siderophore than did the gonococcus; however, the amount of siderophore present in the culture fluids of even the meningococcus was 100- to 1,000-fold lower than the concentration of hydroxamate siderophores reported to be produced by Bacillus megaterium or Aerobacter aerogenes. Virulent, colony type 1 N. gonorrhoeae produced significantly more gonobactin than did the avirulent colony type 3 gonococci.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., NEILANDS J. B. Studies on the metabolic function of the ferrichrome compounds. J Biol Chem. 1961 Feb;236:554–559. [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Yancey R. J. Effect of siderophores on virulence of Neisseria gonorrhoeae. Infect Immun. 1981 May;32(2):609–613. doi: 10.1128/iai.32.2.609-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford C. E., Walker J. R., Reeves J. B., Nabbut N. H., Byers B. R., Jones R. J. Inoculum-dependent division lag of Bacillus cultures and its relation to an endogenous factor(s) ("schizokinen"). J Bacteriol. 1966 Mar;91(3):1070–1079. doi: 10.1128/jb.91.3.1070-1079.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Pollack J. R., Neilands J. B. Structure of schizokinen, an iron-transport compound from Bacillus megaterium. Biochemistry. 1971 Dec 21;10(26):4894–4898. doi: 10.1021/bi00802a010. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Parker C., Romig W. R. Self-transfer and genetic recombination mediated by P, the sex factor of Vibrio cholerae. J Bacteriol. 1972 Nov;112(2):707–714. doi: 10.1128/jb.112.2.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Imferon agar: improved medium for isolation of pathogenic Neisseria. J Clin Microbiol. 1977 Sep;6(3):293–297. doi: 10.1128/jcm.6.3.293-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Siderophore production by Vibrio cholerae. Infect Immun. 1978 Apr;20(1):310–311. doi: 10.1128/iai.20.1.310-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Holmes K. K., Finkelstein R. A. Role of iron in disseminated gonococcal infections. Infect Immun. 1978 May;20(2):573–574. doi: 10.1128/iai.20.2.573-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack J. R., Neilands J. B. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 1970 Mar 12;38(5):989–992. doi: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- Stuart S. J., Greenwood K. T., Luke R. K. Hydroxamate-mediated transport of iron controlled by ColV plasmids. J Bacteriol. 1980 Jul;143(1):35–42. doi: 10.1128/jb.143.1.35-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



