Abstract
The neuro-hypophysial hormone oxytocin (OT) has been implicated in female reproductive and maternal behavior and in the formation of pair bonds in monogamous species. Here we measure variation in urinary OT concentrations in relation to reproductive biology and socio-sexual behavior in a promiscuously breeding species, the chacma baboon (Papio hamadryas ursinus). Subjects were members of a habituated group of baboons in the Okavango Delta, Botswana. We collected behavioral data and urine samples from n= 13 cycling females across their estrous cycles and during and outside of short-term, exclusive sexual consortships. Samples were analyzed via Enzyme Immunoassay (EIA) and we used linear mixed models (LMM) to explore the relationship between peripheral OT and a female’s estrous stage and consortship status, her previous reproductive experience and fertility. We also used a Pearson’s correlation to examine the relationship between OT concentrations of consorting females and their extent of behavioral coordination with their consort partners. The results of the LMM indicate that only estrous stage had a significant influence on OT levels. Females had higher OT levels during their periovulatory period than during other stages of their estrous cycle. There were no differences in OT levels between consorting and non-consorting periovulatory females. However, among consorting females, there was a significant positive relationship between urinary OT levels and the maintenance of close proximity between consort partners. Our results suggest that physiological and behavioral changes associated with the initiation and maintenance of short-term inter-sexual relationships in baboons correspond with changes in peripheral OT.
Keywords: Consortship, estrous cycle, oxytocin, chacma baboons, Papio hamadryas ursinus, socio-sexual behavior, non-invasive hormone sampling
Introduction
Oxytocin (OT) is an evolutionarily conserved neuro-hypophysial hormone that is released into the brain and blood stream in response to social stimuli in a range of mammalian species, including humans (Carter, 1992; Heinrichs and Domes, 2008; Lim and Young, 2006). OT is regulated by estrogen (E) and has an especially important role in mediating female reproductive and maternal behaviors (reviewed by Campbell, 2008). Vagino-cervical stimulation during mating or parturition and nipple stimulation during lactation are potent releasers of OT peripherally (Carmichael et al., 1987; Carter and Altemus, 1997) and centrally (Nyuyki et al., 2011; Ross et al., 2009). Centrally-released OT facilitates sexual receptivity and maternal behavior (Pedersen and Prange, 1979; Witt, 1995) and OT antagonists impair these behaviors in rodents (Benelli et al., 1994) and primates (Boccia et al., 2007).
OT has also been implicated in the formation and maintenance of selective social bonds between human mothers and infants (Feldman et al., 2011; Seltzer et al., 2010; Wismer-Fries et al., 2005) and between mates in monogamously breeding rodents (Williams et al., 1994; Witt et al., 1990) and primates (Smith et al., 2010; Snowdon et al., 2010). In the monogamously breeding prairie vole (Microtus ochrogaster), there is evidence that OT released centrally during mating interacts with the dopamine (DA) system to reinforce the rewarding nature of interactions with specific partners, leading to selective pair bonds (Ross et al., 2009). In comparison, relatively little is known about the role of OT in mediating sexual relationships in species with promiscuous mating systems. In addition, there have been few studies in any species to measure oxytocin during social interactions outside of a laboratory setting.
In this study we measure urinary OT levels in wild, free-ranging chacma baboons (Papio hamadryas ursinus) and explore the relationship between peripheral OT, changes in reproductive physiology and the initiation and maintenance of sexual relationships in a promiscuous species. Intact oxytocin can be reliably measured in urine (Polito et al., 2006; Seltzer and Ziegler 2007), and there is growing evidence for covariation between urinary OT levels and the social environment in humans (Feldman et al., 2011; Nagasawa et al., 2009; Seltzer et al., 2010; Wismer-Fries et al., 2005) and in other species (cotton-top tamarins, Saguinus oedipus: Seltzer and Ziegler, 2007; Snowdon et al., 2010; dogs, Canis familiarus: Mitsui et al., 2011). While oxytocin concentrations in urine do not always correspond with other systemic measurements of OT (e.g. Feldman et al., 2011), this is likely due to differences in the time course between changes in OT in plasma or saliva and excretion of OT in urine. In studies in different species where peripheral OT levels were monitored in plasma and urine over several hours following infusion of endogenous OT, increases in urinary OT concentrations were consistently found within 30– 60 minutes of increases in plasma OT (Amico et al., 1987; Mitsui et al., 2011), suggesting a reliable relationship between changes in systemic OT and changes in urinary OT concentrations. Importantly, urinary measures reflect accumulated levels of OT over several hours and may thus be most relevant for exploring biological responses to sustained social stimuli, while plasma measures may be more relevant for measuring biological responses to acute, transient events.
The relevance of peripheral measures of OT for examining the relationship between general OT system activation and social behavior remains controversial. Some experimental evidence supports coordinated release of OT centrally and peripherally (e.g. Wotjak et al., 1998), while other evidence suggests largely independent mechanisms of release (reviewed in Veening et al., 2010). However, recent neuroanatomical (Ross et al., 2009) and genetic (Feldman et al., 2012) evidence provides increasing support for a relationship between the central OT system and peripheral OT measurements. Moreover, even if central and peripheral OT release is largely independent, peripheral OT may still influence or respond to social behavior via activation of peripheral OT receptors, for example in the gonads, thymus and heart, which could then provide afferent feedback to the central nervous system (Cushing and Carter, 2000). Thus peripheral oxytocin has biological relevance for exploring mechanisms mediating social behavior, and may become an increasingly important physiological marker of variation in prosocial behavior and social attachment in free-ranging animals and in humans (Bartz and Hollander 2006).
Baboon social groups consist of several matrilines of philopatric females, along with a variable number of immigrant males. Males are organized in a linear dominance hierarchy characterized by high levels of reproductive skew (Bulger, 1993). Cycling females have sexual swellings that reach maximal tumescence coinciding with the periovulatory period (POP), typically considered to last between five to seven days prior to detumescence (Bulger, 1993; Weingrill et al., 2000). During this period, females form short-term, exclusive mating relationships, or consortships, with males that last on average 3–4 days in chacma baboons (Bulger, 1993). While in consortships, males and females typically maintain close proximity and engage in frequent mating and other affiliative behaviors such as grooming (Seyfarth, 1978; Weingrill et al., 2000). Males compete for access to estrous females and alpha males monopolize consorts during the periovulatory period (Gesquiere et al., 2007; Moscovice et al., 2010). However, due to cycle overlap among females, periovulatory females also form consortships with lower-ranking males, and females are not necessarily consorted on all of their periovulatory days (Moscovice et al., 2010; Weingrill et al., 2000).
We characterized fluctuations in peripheral OT in female baboons in relation to their stage of estrous and their involvement in consortships. Based on the excitatory effects of E on OT, we predicted that peripheral OT levels would track changes in E across the baboon estrous cycle, which steadily increases during the follicular stage, peaks prior to the Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) surge that induces ovulation and then falls rapidly to baseline during the luteal phase (Honore and Tardif, 2009). We predicted that peripheral OT levels would be higher during the periovulatory period compared with the follicular and luteal phases of the estrous cycle. This pattern would be consistent with studies in humans (Salonia et al., 2005; Shukovski et al., 1989; Stock et al., 1991) and rhesus macaques (Macaca mulatta, Falconer et al., 1980) that indicate mid-cycle peaks in plasma OT levels. There is also evidence that estrogen levels can vary with differences in reproductive experience or fertility outcomes in female primates. Human females who have previously experienced at least one full-term pregnancy exhibit long-term reductions in E, both during and outside of pregnancy, compared to primiparous females (Arslan et al., 2006; Bernstein et al., 1986). In yellow baboons, females tend to have higher fecal estrogen concentrations during conceptive compared to non-conceptive cycles (Gesquiere et al. 2007). Since differences in parity and fertility are associated with differences in E, it is also possible that these factors may be associated with differences in OT levels.
In addition to estrogen-mediated changes in OT, it is likely that salient socio-sexual stimuli may influence peripheral OT levels. Based on the relatively prolonged changes in social context that occur during consortships, including increased sexual behavior, and evidence that sexual arousal and mating are associated with increases in peripheral OT in female mammals (Carmicheal et al., 1987; Salonia et al., 2005), we predicted that periovulatory females in consortships would have higher urinary OT levels than periovulatory females who were not in consortships. Within consortships as well, variation in OT levels may relate to variation in the quality of relationships between consort partners, similar to evidence for co-variation between peripheral OT and affiliation among pair-bonded cotton top tamarins (Snowdon et al. 2010).
Methods
Study Site and Data Collection
Subjects were members of a habituated group of wild chacma baboons located in the Moremi Wildlife Reserve in the Okavango Delta, Botswana. All research was conducted using noninvasive methods approved by the Animal Care and Use Committee of the University of Pennsylvania (Protocol no. 19001). During the study period from June 2006– June 2007, group size ranged from 62–73 individuals, including 28 adult females over five years of age. Complete reproductive histories of all adult females were known and female reproductive cycles were monitored throughout the study period. Estrous cycles were identified post-hoc as conceptive or non-conceptive based on whether the cycle resulted in a pregnancy.
For cycling females, daily data were recorded on estrous state and consortship status six days per week, between 6–13:30 hours. Estrous state was inferred based on changes in size, color and firmness of sexual swellings. Visual assessments of changes in swelling size are correlated with changes in fecal estrogen concentrations in female baboons (Gesquiere et al., 2007) and are an effective means of distinguishing between different stages of the estrous cycle. For the purpose of this study, estrous state was classified into three phases: Pre-ovulatory (increasing tumescence, lasting 12.9 ± 0.97 days), periovulatory (maximally tumescent, lasting 5.3 ± 0.59 days) and post-ovulatory (detumescent, lasting 14.3 ± 1.24 days). Each of these phases is associated with distinct patterns of estrogen release: A gradual increase during the pre-ovulatory period, a large surge during the periovulatory period and rapid return to baseline levels during the post-ovulatory period (Honore and Tardiff, 2009). When females exhibited irregular cycles, indicated by prolonged periods of time in pre- or post-ovulatory states and/or a lack of a distinct period of maximal tumescence, data and samples from these cycles were excluded from analyses.
Consortships were recorded ad libitum and were readily identified by conspicuous mate-guarding by males, as well as frequent mating. On a subset of days when females were in consortships, we also conducted ten-minute focal follows of females during which we measured the duration of time females spent within close proximity (defined as within two meters) of their consort partners. These focals represent gross measures of the extent of behavioral coordination between consort partners on the day of observation.
Sample Collection and Analysis
We collected 48 urine samples from n= 13 cycling females. Subjects were nulliparous (n= 2), primiparous (n= 8) or multiparous (n= 3), but none had dependent offspring during the period of sample collection. Samples were collected between 6– 13:30 hours, opportunistically and during continuous focal observations. We collected samples either from the ground or directly in a plastic bag when a female urinated from a tree. Between 0.5– 3 ml of urine were pipetted into an epindorf tube and stored at −20° C within three hours of collection. Samples were transported to the USA on dry ice and stored at −80° C at the Wisconsin National Primate Assay Lab prior to analyses. Samples were analyzed by Enzyme Immunoassay (EIA) using the Assay Designs EIA kit (Assay Designs, Inc. Ann Arbor, MI, USA). This kit has been validated in a range of species and across different biological media including urine (Feldman et al., 2011; Gray et al., 2007; Seltzer et al., 2010; Seltzer and Ziegler, 2007; Snowdon et al., 2010; Wismer-Fries et al., 2005), plasma and saliva (Carter et al., 2007; Feldman et al., 2011) and CSF (Toni Ziegler, unpublished data). The specificity of the antibody used in this assay has been repeatedly validated via high-performance liquid chromatography (HPLC) and results across different species and biological media indicate that the assay antibody binds only to intact OT and does not show cross-reactivity with other peptide hormones (Carter et al., 2007; Seltzer and Ziegler, 2007; Wismer-Fries et al., 2005). This assay should be considered a reliable but conservative measure of OT, since the assay antibody responds only to the intact OT molecule and not to any OT metabolites (Seltzer and Ziegler 2007).
Samples were first purified by solid phase extraction (SPE). Urine was diluted to 1 ml with purified water and 10 µ l of phosphoric acid and added to SPE columns (Phenomenex Strata X) conditioned with 1 ml methanol and 1 ml purified water. Samples were washed with 10% acetonitrile, 1% TFA (trifluoroacetic acid) in water and eluted with 1 ml 80:20 acetonitrile:water. Samples were dried and resuspended in 200 µl assay buffer and added to the microtiter plates in duplicate according to the assay kit directions. The assay accuracy was 104.37 ± 5.11% (mean ± SEM), n= 14. Serially diluted pooled baboon urine was parallel to oxytocin standards (t= 2.02, df= 22, p> 0.05). Mean intra-and inter-assay coefficients of variation were 2.41 (n= 8) and 12.02 (n= 8) respectively. Initially, nine samples had OT values that exceeded the upper sensitivity limit of the assay. These samples were re-run at reduced concentrations until they could be reliably measured on the standard curve. Following Ziegler and colleagues (1995), we controlled for differences in fluid variability in urine samples by determining Creatinine (Cr) levels for each sample via microtiterplate-assay. Creatinine was then divided into oxytocin concentrations and oxytocin samples are presented as pg/mg Cr.
Statistical Analysis
We used R version 2.15.1 (R Core Team, 2012) for statistical analyses. Due to small sample sizes and violations of normality, we used the Wilcoxon Exact test to compare within-subject variation in OT of n= 6 females who were sampled during their periovulatory period (including during and outside of consortships) and during their following post-ovulatory period when they were detumescing. We also used linear mixed models (LMM) to assess the effects of estrous state and consortship status on urinary OT concentrations of all females who were sampled in at least two different estrous states (n= 13). The response variable was log transformed OT concentrations. The transformation reduced the influence of outliers and resulted in normally-distributed residuals. The predictor variable combined estrous state and consortship status into one categorical factor with four levels: pre-ovulatory, periovulatory but not in a consortship, periovulatory and in a consortship, and post-ovulatory. We also included parity (nulliparous, primiparous or multiparous) and fertility outcomes (conceptive or non-conceptive cycle) as categorical fixed effect control variables. We included female identity as a random factor and used the ‘lmer’ function (Bates et al., 2012) to fit the full model, examining all main effects. Due to small sample sizes we did not examine interactions. To test the significance of the full model, we fit a simpler model including only the main effects of parity and fertility and used a likelihood ratio test to compare the full model to the reduced model (R function ‘anova’). We used ‘language R’ to determine p-values for all individual effects in the final model. In this function p-values are based on Markov Chain Monte Carlo (MCMC) sampling with 10,000 simulations (Baayen, 2011). We first fit the models with all of the data included and then re-fit the models after excluding all OT samples that were identified as outliers, based on values greater than two standard deviations from the mean (n= 2 samples). We did this to examine the robustness of the models with respect to outliers. Results of the models with and without outliers included were identical with respect to general patterns and significance outcomes, so we only present the results of the models with all of the data included.
We used matched endocrine samples and behavioral data from focal observations of consorting females to examine the relationship between urinary OT and the extent of behavioral coordination between consort partners. This analysis included n= 7 females, representing n= 11 different consort days where urine samples were collected from a subject within 24 hours of completion of a focal follow on that individual. This time frame was chosen based on evidence that following injection of radioactively labeled OT into plasma in cotton top tamarins, labeled OT can be detected in urine for similarly extended periods (Seltzer and Ziegler, 2007). We used a Pearson correlation to measure the dependence between log transformed OT and log transformed proportion of time in close proximity to consort partners. We used log transformed data to reduce the impact of outliers. We performed a permutation test (Manly, 1997) with 1000 iterations to compute the two-sided p-value to account for repeated sampling of two subjects. All results are presented as mean (±SEM).
Results
We analyzed 3.69 (± 0.33) urine samples from each female subject (insert Table 1). Samples were collected from n= 34 different estrous cycles, and were distributed across the pre-ovulatory period (n= 17), the periovulatory period during consortships (n= 12) and outside of consortships (n= 6) and during the post-ovulatory period (n= 13). Of the samples, 14.5% (n= 7) were from nulliparous females, 66.7% (n= 32) were from primiparous females and 18.7% (n=9) were from multiparous females. The majority of samples (66.7%, n=32) were collected during non-conceptive cycles, while the remaining n=16 samples were collected during conceptive cycles.
Table 1.
Attributes of cycling female baboons, listed in rank order, from whom urine samples were collected for analysis.
| Female ID | Parity | No. of urine samples analyzed |
Proportion of samples from consortships |
Proportion of samples from conceptive cycles |
Mean (+SEM) oxytocin (pg/mg creatinine) |
|---|---|---|---|---|---|
| SL | Multiparous | 4 | 0.50 | 0.25 | 812.62 (425.39) |
| PM | Multiparous | 2 | 0.50 | 0.50 | 100.89 (2.02) |
| NX | Nulliparous | 3 | 0.33 | 0.33 | 64.24 (13.50) |
| BE | Primiparous | 2 | 0.50 | 0.50 | 384.92 (138.07) |
| VD | Primiparous | 6 | 0.33 | 0.50 | 168.66 (42.09) |
| RU | Primiparous | 4 | 0.00 | 0.00 | 271.36 (187.26) |
| CO | Primiparous | 3 | 0.33 | 0.67 | 803.74 (773.16) |
| CD | Primiparous | 5 | 0.00 | 0.00 | 181.06 (138.41) |
| LS | Nulliparous | 4 | 0.00 | 0.75 | 278.40 (240.65) |
| DO | Primiparous | 3 | 0.00 | 0.00 | 24.4 (4.65) |
| WS | Primiparous | 4 | 1.00 | 0.75 | 489.62 (148.57) |
| LN | Primiparous | 5 | 0.00 | 0.00 | 74.06 (19.07) |
| JK | Multiparous | 3 | 0.00 | 0.00 | 243.62 (162.41) |
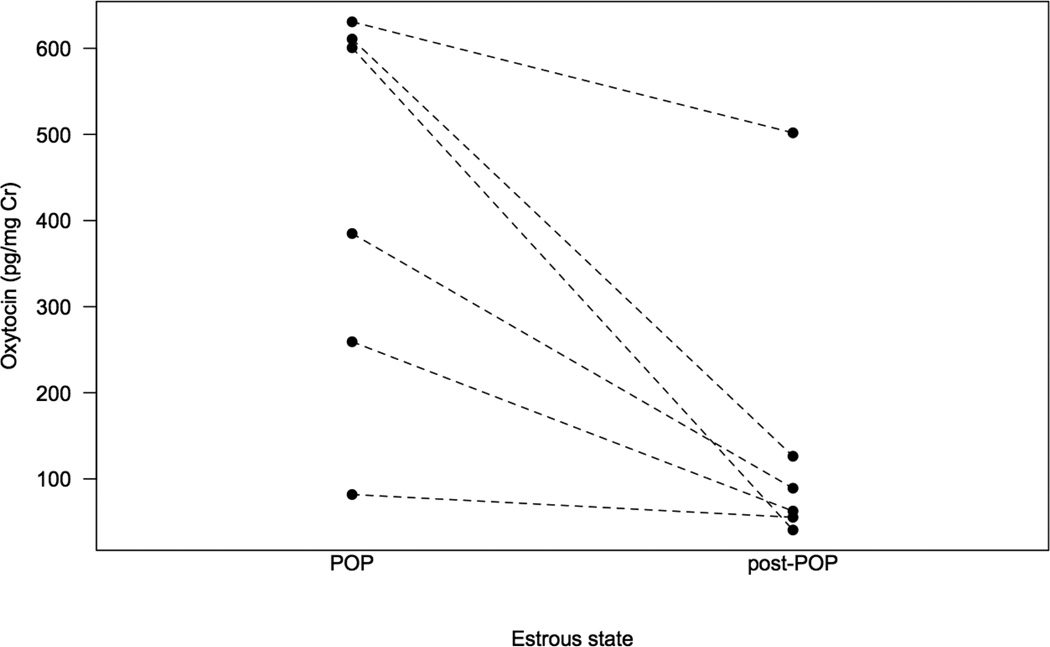
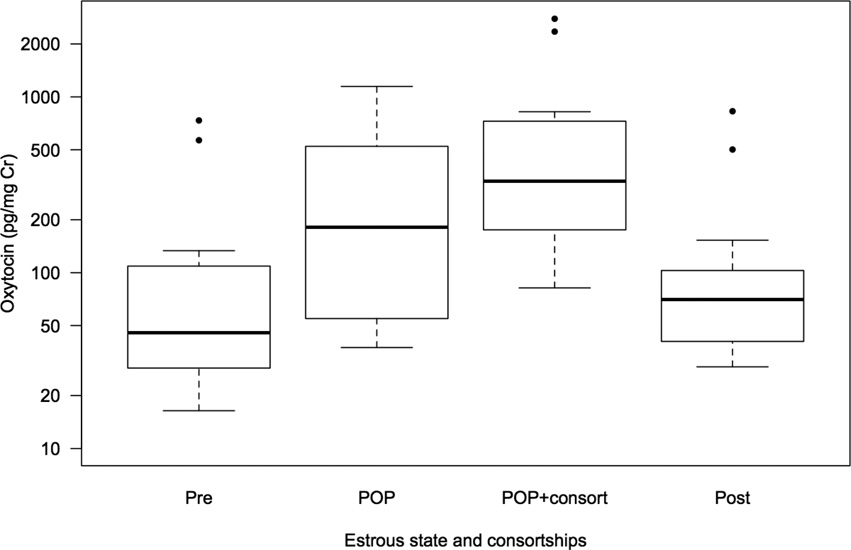
Females had higher average urinary OT levels during their periovulatory period (whether in consortships or not) than in the following post-ovulatory period (428.03 ± 92.10 pg/mg Cr vs. 146.01 ± 72.22 pg/mg Cr, Wilcoxon Exact Test: n= 6, T+= 21, 0 ties, p= 0.031, insert Fig. 1). The LMM allowed for further differentiation of the effects of multiple factors on peripheral OT levels, while controlling for repeated sampling of female subjects. The full model including the main effects of estrous state and consortships, parity and fertility explained a significantly greater amount of variance in OT levels than a reduced model that included only the main effects of parity and fertility (Full model: AIC= 74.14, df= 9, n= 48, reduced model: AIC= 88.63, df= 6, n= 48; Likelihood ratio test: χ2= 20.492, df= 3, p< 0.001). Considering the full model, the categorical predictor ‘estrous state and consortships’ explained a significant amount of the variance in OT values (pmcmc< 0.001, insert Table 2 and Fig. 2). Periovulatory females in consortships had significantly higher urinary OT concentrations than pre- (pmcmc= 0.002) and post- (pmcmc= 0.004) ovulatory females. Non-consorting periovulatory females also had higher OT concentrations than pre- (pmcmc= 0.015) but not post- (pmcmc= 0.133) ovulatory females. There were no significant differences in OT values between periovulatory females in consortships and periovulatory females who were not in consortships (pmcmc= 0.305). There was no relationship between a female’s OT levels and her reproductive history (pmcmc= 0.103) or fertility outcomes (pmcmc= 0.541, see Table 2).
Figure 1.
Urinary OT levels of females during their periovulatory period (POP) and during the following period (post-POP), when they were detumescent (Wilcoxon, p= 0.031).
Table 2.
Results of the LMM examining main effects of the categorical predictor variables ‘Estrous State and Consortships’, ‘Parity’ and ‘Fertility’ on log transformed urinary OT concentrations (dependent variable). P-values are presented for the overall effect of each factor. Factors that contribute significantly to explaining variance in urinary OT levels appear in bold.
| Variables | Estimate | SE | pmcmc | |
|---|---|---|---|---|
| Intercept | 1.527 | 0.217 | ||
| Estrous State and Consortships | < 0.001 | |||
| POP without consortship | 0.602 | 0.215 | ||
| POP+consortship | 0.857 | 0.179 | ||
| Post-POP | 0.225 | 0.163 | ||
| Parity | 0.103 | |||
| Primiparous | 0.207 | 0.194 | ||
| Multiparous | 0.545 | 0.235 | ||
| Fertility | 0.589 | |||
| Conceptive cycle | −0.102 | 0.539 | ||
Figure 2.
Urinary OT concentrations of female baboons in relation to estrous state and involvement in consortships. OT values are presented on a logarithmic scale. Periovulatory females in consortships (POP+cons) had significantly higher OT levels compared with pre-ovulatory (Pre) or post-ovulatory (Post) periods (pmcmc< 0.01). Non-consorting periovulatory females (POP) also had higher OT levels compared with their pre-ovulatory period (pmcmc= 0.015). All other comparisons were non-significant (pmcmc > 0.133). Box plots indicate median values and second and third quartiles. Error bars represent minimum and maximum values. Circles represent outliers within each level of the factor.
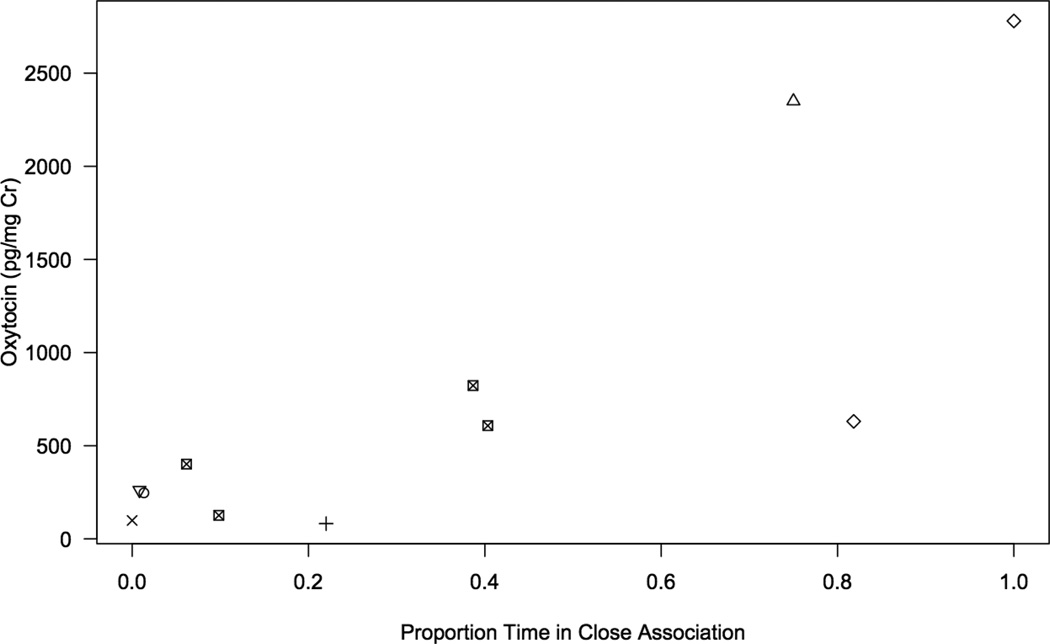
In eleven instances we were able to collect a urine sample from a consorting female within twenty-four hours of conducting a ten-minute focal observation of that female. Urine samples were collected 14.12 (± 3.58) hours following the focal observation. Samples were collected while subjects were in consortships with the alpha male (n= 6 observations), the beta male (n= 4) or a low ranking male (n= 1). Females spent 34 (± 11) % of focal observation time in close proximity to their consort partners. There was a significant positive correlation between a female’s urinary OT levels and the proportion of observation time spent in close proximity to her consort partner (Pearson correlation, r= 0.823, 2-sided p-value= 0.044, insert Fig. 3).
Figure 3.
The relationship between urinary OT levels of consorting females and the proportion of observation time spent within close proximity to their consort partners (Pearson, p= 0.044). Distinct symbols represent the n=7 females in the sample. Replicated symbols indicate the n=2 females who were sampled more than once. A permutation test was performed to account for repeated sampling of two subjects.
Discussion
This is the first study to examine fluctuations in peripheral OT in relation to female reproductive physiology and socio-sexual behavior in wild primates. Even with a relatively small sample size, our results confirm a pattern of increased peripheral OT concentrations during the periovulatory period in cycling female baboons that is in agreement with patterns found in humans (e.g. Salonia et al., 2005) and in rhesus macaques (Falconer et al., 1980), and is likely triggered by the surge in E coinciding with ovulation. There is evidence that peripheral OT contributes importantly to female reproductive function by regulating the timing of the LH surge (Einspanier et al., 1995; Evans et al., 2003), and facilitating the development and function of the corpus luteum during pregnancy (Khan et al., 1993).
Peripheral OT also plays a role in facilitating female sexual receptivity in rodents (Caldwell et al., 1986; Pedersen and Boccia, 2002) and humans (Salonia et al., 2005), and has been implicated in the maintenance of pair bonds in monogamously breeding species (Snowdon et al., 2010). Contrary to our predictions, there were no differences in urinary OT concentrations between consorting and non-consorting periovulatory females. This is surprising, given the high frequency of mating associated with consortships, and evidence that vagino-cervical stimulation associated with mating coincides with increased peripheral OT levels in humans (Carmichael et al., 1987) and rabbits (Todd and Lightman, 1986). The relatively small number of samples from periovulatory females who were not in consortships, or the lack of controlled sampling at set time periods following copulations, may have influenced the results.
It is also possible that variation in peripheral OT in female baboons may be more closely related to the quality of relationships with consort partners than to mere involvement in a consortship. This hypothesis is supported by the significant positive relationship between OT concentrations of consorting females and the maintenance of close proximity between consort partners. It is likely that brief focal observations captured more prolonged differences in the extent of affiliation and behavioral coordination among consort partners. Similar evidence for covariation between peripheral OT levels in females and the extent of non-sexual affiliative behavior with mates has been found in monogamously breeding cotton-top tamarins (Snowdon et al., 2010) and in humans (Grewen et al., 2005).
Subordinate male chacma baboons closely track the status of consortships and may take even momentary changes in proximity between consort partners as opportunities to attempt to mate sneakily with the female (Crockford et al., 2007). Thus any behavioral or endocrine mechanisms that promote affiliation and synchronized activity during consortships may have important reproductive consequences for male and female baboons. Related to this, males may have different behavioral strategies for maintaining consortships, which could correspond with differences in oxytocin levels among consorting females.
More intensive endocrine sampling of periovulatory females across multiple consortships and within shorter time frames following affiliative and sexual behaviors will help to determine how within-subject variation in OT relates to the identity of consort partners and to socio-sexual interactions during consortships. Our results also indicate extensive variation in urinary OT concentrations among periovulatory females who were not in consortships (from 37.50– 1146.49 pg/mg Cr, see Fig. 2), suggesting that factors other than the estrous cycle and interactions with consort partners may influence peripheral OT levels in female baboons. This area also deserves further investigation.
OT concentrations were not related to differences in reproductive experience among female baboons and did not differ between conceptive and non-conceptive cycles. Based on the relatively small reported changes in E with reproductive experience and fertility in other species (e.g. Arslan et al., 2006, Gesquiere et al., 2007), it is possible that any changes in estrogen with reproductive experience or fertility in baboons are also relatively small and are not associated with changes in OT. It is also possible that the smaller sample sizes of nulliparous females and conceptive cycles may make it difficult to detect differences in OT.
Due to limited sample volumes we were not able to directly measure estrogen concentrations. As a result we are not able to rule out the possibility that differences in peripheral OT among periovulatory females may reflect fine-scale differences in E, either within or across cycles. Differences in E could in turn influence the motivation of both males and females to initiate and maintain consortships. At several study sites, alpha males preferentially consort with females during conceptive cycles (Bulger, 1993; Gesquiere et al., 2007; Weingrill et al., 2003) and within cycles on days closer to ovulation (Gesquiere et al., 2007), suggesting that alpha males may detect and use information about fine-scale differences in ovulatory state when making consort decisions. In yellow baboons (Papio cynocephalus), fecal estrogen levels exhibit minor fluctuations within the periovulatory period and between conceptive and non-conceptive cycles (Gesquiere et al. 2007), but it is unlikely that such minor fluctuations in E could explain the extensive variation in OT levels among periovulatory females in this study. Moreover, we found no evidence for differences in females’ OT levels between conceptive and non-conceptive cycles. While alpha males appear to invest selectively in consortships that represent an increased probability of paternity, males of other ranks consort more broadly across the periovulatory period, and are as likely to consort during fertile as during non-fertile cycles (Bulger, 1993; Gesquiere et al., 2007). These rank-based differences in male consort strategies increase the chances that females will be consorted across their periovulatory periods, regardless of fine-scale differences in estrogen levels. The positive relationship between periovulatory females’ OT levels and their extent of close association with consort partners provides further evidence for a relationship between peripheral OT and socio-sexual environment in chacma baboons.
Lactating female baboons form selective 006Eon-sexual bonds, or ‘friendships’, with males, which in chacma baboons appear to function as protection against the threat of infanticide (Palombit, 2000). Females form friendships preferentially with males with whom they consorted the most during their last conceptive cycle (Moscovice et al., 2010). Consortships thus represent initial investment in more long-term inter-sexual bonds that exhibit some behavioral parallels with pair bonds in monogamous species. Our results suggest that the maintenance of sexual consortships in baboons is associated at the physiological level with increases in peripheral OT in females, similar to patterns observed during the formation of pair bonds in monogamous species (Cushing and Carter, 2000).
Peripheral OT has well-known functions in female reproductive physiology and there is increasing evidence that peripheral OT also fluctuates with changes in social context in humans (Feldman et al., 2011; Grewen et al., 2005; Seltzer et al., 2010; Wismer-Fries et al., 2005) and in non-human primates (Seltzer et al., 2007; Snowdon et al., 2010). In addition to the established relationship between OT and affiliative pair-bonds in monogamous species (Snowdon et al., 2010; Young and Wang, 2004), this preliminary study suggests that peripheral OT is related to the maintenance of short-term, exclusive consortships in a promiscuous species.
Highlights.
We non-invasively measured peripheral oxytocin levels in wild female chacma baboons
Periovulatory females had higher oxytocin levels than at other estrous stages
Females who spent more time near consort partners had higher oxytocin levels
Oxytocin may be involved in maintaining short-term sexual relationships in baboons
Acknowledgements
We are grateful to the Office of the President of the Republic of Botswana and the Botswana Department of Wildlife and National Parks for permission to conduct research in the Moremi Game Reserve. We thank Marlies Heesen, Alec Mokopi, Chantelle Shaw and Werner Smith for their assistance with field data collection. Dorothy Cheney, Robert Seyfarth and two anonymous reviewers provided helpful comments on initial manuscripts. William Stein, Roger Mundry and Leo Polansky provided important statistical support. Research was supported by the National Institutes of Health grant No. MH62249.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amico JA, Ulbrecht JS, Robinson AG. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J Clin Endocrinol Metab. 1987;64:340–345. doi: 10.1210/jcem-64-2-340. [DOI] [PubMed] [Google Scholar]
- Arslan AA, Zeleniuch-Jacquotte A, Lukanova A, Afanasyeva Y, Katz J, Levitz M, Del Priore G, Toniolo P. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidem Biomar. 2006;15:2123–2130. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- Baayen RH. languageR: Data sets and functions with "Analyzing Linguistic Data: A practical introduction to statistics". R package version 1.4. 2011 http://CRAN.R-project.org/package=languageR. [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation : Forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0. 2012 http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, Arletti R. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27:245–250. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Depue RH, Ross RK, Judd HL, Pike MC, Henderson BE. Higher maternal levels of free estradiol in first compared to second pregnancy: early gestational differences. J Natl Cancer I. 1986;76:1035–1039. [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger JB. Dominance rank and access to estrous females in male savanna baboons. Behaviour. 1993;127:67–103. [Google Scholar]
- Caldwell JD, Prange AJ, Jr, Pedersen CA. Oxytocin facilitates the sexual receptivity of estrogen-treated female rats. Neuropeptides. 1986;7:175–189. doi: 10.1016/0143-4179(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann NY Acad Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann NY Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Seyfarth RM, Cheney DL. Baboons eavesdrop to deduce mating opportunities. Anim Behav. 2007;73:885–890. [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Einspanier A, Ivell R, Hodges JK. Oxytocin: a follicular luteinisation factor in the marmoset monkey. Adv Exp Med Biol. 1995;395:517–522. [PubMed] [Google Scholar]
- Evans JJ, Reid RA, Wakeman SA, Croft LB, Benny PS. Evidence that oxytocin is a physiological component of LH regulation in non-pregnant women. Hum Reprod. 2003;18:1428–1431. doi: 10.1093/humrep/deg291. [DOI] [PubMed] [Google Scholar]
- Falconer J, Mitchell MD, Mountford LA, Robinson JS. Plasma oxytocin concentrations during the menstrual cycle in the rhesus monkey, Macaca mulatta. J Reprod Fertil. 1980;59:69–72. doi: 10.1530/jrf.0.0590069. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 Genes. Biol Psych. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Gray PB, Parkin JC, Samms-Vaughan ME. Hormonal correlates of human paternal interactions: A hospital-based investigation in urban Jamaica. Horm Behav. 2007;52:499–507. doi: 10.1016/j.yhbeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Honore EK, Tardif SD. Reproductive Biology of Baboons. In: VandeBerg JL, Williams-Blangero S, Tardiff SD, editors. The Baboon in Biomedical Research. New York: Springer; 2009. pp. 89–110. [Google Scholar]
- Khan DF, Kanu EJ, Dawood MY. Baboon corpus luteum: presence of oxytocin receptors. Biol Reprod. 1993;49:262–266. doi: 10.1095/biolreprod49.2.262. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. New York: Chapman & Hall; 1997. [Google Scholar]
- Mitsui S, Yamamoto M, Nagasawa M, Kazutaka M, Kikusui T, Ohtani N, Ohta M. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm Behav. 2011;60:239–245. doi: 10.1016/j.yhbeh.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Moscovice LR, Di Fiore A, Crockford C, Kitchen DM, Wittig R, Seyfarth RM, Cheney DL. Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Anim Behav. 2010;79:1007–1015. [Google Scholar]
- Nagasawa M, Kikusui T, Onaka T, Ohta M. Dog's gaze at its owner increases owner’s urinary oxytocin during social interaction. Horm Behav. 2009;55:434–441. doi: 10.1016/j.yhbeh.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Nyuyki KD, Waldherr M, Baeumi S, Neumann ID. Yes, I am ready now: differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. PLoS One. 2011;6:e23599. doi: 10.1371/journal.pone.0023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombit RA. Infanticide and the evolution of male-female bonds in animals. In: van Schaik CP, Janson CH, editors. Infanticide by Males and its Implications. Cambridge: Cambridge University Press; 2000. pp. 239–268. [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin maintains as well as initiates female sexual behaviour: effects of a highly selective oxytocin antagonist. Horm Behav. 2002;41:170–177. doi: 10.1006/hbeh.2001.1736. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc of the Natl Acad of Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito AB, III, Goldstein DL, Sanchez L, Cool DR, Morris M. Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides. 2006;27:2877–2884. doi: 10.1016/j.peptides.2006.05.007. [DOI] [PubMed] [Google Scholar]
- R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2012. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-project.org/. [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE. Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm Behav. 2007;51:436–442. doi: 10.1016/j.yhbeh.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM. Social relationships among adult male and female baboons. I. Behaviour during sexual consortship. Behaviour. 1978;64:204–226. [Google Scholar]
- Shukovski L, Healy DL, Findlay JK. Circulating immunoreactive oxytocin during the human menstrual cycle comes from the pituitary and is estradiol dependent. J Clin Endocrinol Metab. 1989;68:455–460. doi: 10.1210/jcem-68-2-455. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock S, Bremme K, Uvnas-Moberg K. Plasma level of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum Reprod. 1991;6:1056–1062. doi: 10.1093/oxfordjournals.humrep.a137484. [DOI] [PubMed] [Google Scholar]
- Todd K, Lightman SL. Oxytocin release during coitus in male and female rabbits: effect of opiate receptor blockade with naloxone. Psychoneuroendocrinology. 1986;11:367–371. doi: 10.1016/0306-4530(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: Behavioral effects; a review. Physiol Behav. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Lycett JE, Barrett L, Hill RA, Henzi SP. Male consortship behaviour in chacma baboons: the role of demographic factors and female conceptive probabilities. Behaviour. 2003;140:405–427. [Google Scholar]
- Weingrill T, Lycett JE, Henzi SP. Consortship and mating success in chacma baboons (Papio cynocephalus ursinus) Ethology. 2000;106:1033–1044. [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrin. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Wismer-Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM. Oxytocin and rodent sociosexual responses: From behavior to gene expression. Neurosci Biobehav Rev. 1995;19:315–324. doi: 10.1016/0149-7634(95)00006-z. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster) Pharmacol Biochem Behav. 1990;37:63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female Cotton-Top tamarins, S. oedipus. Horm. Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]