Abstract
Background:
Spinal cord stimulation (SCS) has been used to treat neuropathic pain since 1967. Following that, technological progress, among other advances, helped SCS become an effective tool to reduce pain.
Methods:
This article is a non-systematic review of the mechanism of action, indications, results, programming parameters, complications, and cost-effectiveness of SCS.
Results:
In spite of the existence of several studies that try to prove the mechanism of action of SCS, it still remains unknown. The mechanism of action of SCS would be based on the antidromic activation of the dorsal column fibers, which activate the inhibitory interneurons within the dorsal horn. At present, the indications of SCS are being revised constantly, while new applications are being proposed and researched worldwide. Failed back surgery syndrome (FBSS) is the most common indication for SCS, whereas, the complex regional pain syndrome (CRPS) is the second one. Also, this technique is useful in patients with refractory angina and critical limb ischemia, in whom surgical or endovascular treatment cannot be performed. Further indications may be phantom limb pain, chronic intractable pain located in the head, face, neck, or upper extremities, spinal lumbar stenosis in patients who are not surgical candidates, and others.
Conclusion:
Spinal cord stimulation is a useful tool for neuromodulation, if an accurate patient selection is carried out prior, which should include a trial period. Undoubtedly, this proper selection and a better knowledge of its underlying mechanisms of action, will allow this cutting edge technique to be more acceptable among pain physicians.
Keywords: Failed back surgery syndrome, indications, neuromodulation, review, spinal cord stimulation
INTRODUCTION
Neuromodulation is defined as the application of either an electric current or pharmacological agents used to change the neuron membrane permeability to ions, leading to an increase or decrease in its threshold for action potentials. The International Neuromodulation Society established that: Neuromodulation is defined as, “the therapeutic alteration of activity in the central, peripheral or autonomic nervous systems, electrically or pharmacologically, by means of implanted devices”. The term neuromodulation is preferred over the term stimulation, as the former includes the excitation and inhibition techniques in a clearer fashion. Low frequency electrical stimulation has an excitatory effect, whereas, high frequency stimulation is applied to produce neuronal inhibition. At present, neuromodulation is used for several neurological conditions such as epilepsy, movement disorders, psychiatric disease, spasticity and pain. With regard to pain treatment, low frequency is applied to activate dorsal spinal tracts, periaqueductal gray matter, and motor cortex, while inhibitory stimulation is utilized for peripheral nerve, thalamic, and hypothalamic modulation. Spinal cord stimulation is a technique of neuromodulation, which consists of placing leads in the epidural space of the spinal cord, as a method to treat numerous types of disturbances. Due to its reversibility and safety, neuromodulative approaches are preferred over neuroablative procedures, which have been more commonly performed in the previous era. [33,42,72,73,83,108,129,161]
Electricity has been used in medicine since almost two thousand years ago. According to historical records, the first time electricity was used in medicine was in the time of the Roman Empire, where the torpedo fish was used to treat headaches and painful gout. In the eighteenth century, the use of electrical current for treating pain was widespread and indiscriminate. Both the poor results and frequent accidents led to the prohibition of the technique. [33,108,161] In spite of these antique reports, it was not until 1965 that Melzack and Wall published their gate theory, allowing SCS to emerge as a new technique for achieving pain relief. This theory proposed that nociceptive impulses, which were carried by Aδ and C fibers, might be blocked by simultaneous tactile stimuli or by the electrical stimulation of thick myelinated Aβ fibers. It represented the first scientific basis for the use of electrical stimulation for pain. [33,73,83,100,108,129,161] In 1967, Wall and Sweet started to use peripheral nerve stimulation to treat pain. Based on this theory, Shealy et al. implanted the first electrode in the spinal cord in the same year. [134] Undoubtedly, it was a milestone in neuromodulation. The number of implanted devices has increased enormously since then. However, SCS was not a successful treatment in the beginning, probably due to technical problems and poor patient selection.
Technological progress, a better knowledge of its mechanism of action, and careful patient selection, among other advances, helped SCS become an effective tool to reduce pain. In the early years, leads were placed in the subarachnoid space through a laminectomy, but later, electrodes were implanted in the epidural space to avoid some complications such as cerebrospinal fluid leakage and arachnoiditis. More recently, the percutaneuos technique was introduced. It allowed a trial stimulation with an external pulse generator, to assess if SCS was beneficial for the patient before the permanent implant, which was much more expensive. This trial was probably useful to improve the outcome of SCS, because it allowed for more accurate patient selection. Thus, this trial improved the cost-effectiveness, as 17 - 20% of the patients failed the trial stimulation and only the ones who exhibited a good response would undergo implantation of the permanent device.
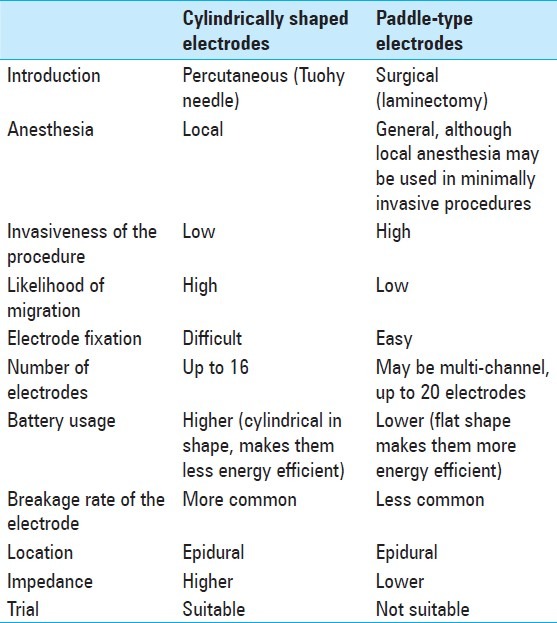
Nowadays, there are two types of electrodes available: Cylindrically shaped percutaneous electrodes and paddle-type surgical ones. The former are placed with a less invasive technique and are widely preferred now, whereas, the latter one requires a laminectomy, but it has less likelihood of migration. However, it is possible to introduce a paddle-type one by using a tubular retractor system under local anesthesia. [6,14,23,42,51,66,73,81,83,108,127,134,145,161] At present, the indications of SCS are being revised constantly, while new applications are being proposed and researched worldwide. Spinal cord stimulation is the most frequently applied neuromodulation method. [5,42] This article is a non-systematic review of its mechanism of action, indications, results, programming parameters, complications, and cost-effectiveness.
MECHANISM OF ACTION
Deafferentation pain may occur in patients with injuries in the central or peripheral nervous systems, as pain, either spontaneous or elicited by innocuous stimuli, may appear if there is a lesion anywhere in the sensory pathways. The principal mechanisms involved are: Sensitization of receptors, appearance of ectopic focus or pacemakers, and abnormal activity in the central processing units. For example, in case of a peripheral nerve injury, the neuroma formed in the proximal stump generates spontaneous action potentials that reach the posterior horn of the spinal cord. Also, the sensory ganglia cells increase their spontaneous electrical activity and undergo modifications in their nuclei, as a response to the peripheral injury. Ephaptic conduction may occur between the adjacent damaged fibers. This ectopic activity and the peripheral nerve injury itself generate functional modifications in the second order neurons in the dorsal horn of the spinal cord, known as central sensitization. Hyperactivity may occur in those neurons and its receptive field might increase, allowing pain to reach areas away from those innervated by the injured nerve structures. Furthermore, there is a decrease in the segmental inhibitory mechanisms as well as an increase in membrane excitability and synaptic efficacy of the second order neurons within the dorsal horn. [3,9,15,24,82,118,145,161] The objective of this example is to understand the basic mechanisms underlying deafferentation pain and its difference from nociceptive pain, in which the sensory pathways are intact.
In spite of the existence of several studies that try to prove the mechanism of action of SCS, it still remains unknown. Probably, the physiological bases of pain relief are different, depending on its cause. [51,81,83,102] Most experimental data regarding this topic are derived from animal models of neuropathic pain, in which pain must be evaluated in an indirect manner. As pain cannot be properly assessed and interpreted in animals, hypersensitivity after a nerve injury is the most common indirect behavioral sign of pain used in animal models. This is equivalent to allodynia in humans, but not to pain. Hence, if this data is translated to clinical practice, it may lead us to a wrong approach to knowledge, unless these findings have been confirmed in humans previously. [8,22,65,95,102,103,117,133,169]
The exact electrical target within the spinal cord is still unknown; however, there are several theoretical ones, such as, dorsal columns, dorsolateral funiculus, spinobulbar fibers, spinocerebellar tract, and dorsal root fibers. [31,45,51,117,129] Spinal cord stimulation arose as a direct consequence of the gate-control theory, which postulates that the electrical stimulation of the Aβ fibers within the dorsal columns inhibits the transmission of pain information to the brain via the Aδ and C fibers. The Aβ fibers carry non-nociceptive information, whereas, the Aδ and C fibers are small nociceptive projections. The gate-control theory does not explain the mechanism of action of SCS accurately, as it principally modulates neuropathic pain without having an adequate effect on nociceptive pain. Stimulation of Aβ fibers also elicits paresthesia in the corresponding dermatomes, which is thought to be needed to achieve pain relief. However, it may only be an epiphenomenon of neuromodulation, and thus, be actually unrelated to the mechanism of pain relief. [31,45,51,73,81,103,117,129] The longer the distance between a fiber and the electrode, the lower is the stimulation threshold of that fiber. Therefore, most dorsal fibers of the dorsal column have the lowest threshold for activation. [31] Probably, SCS selectively influences transmission of type A fibers, as it has been seen in rats. [103] The mechanism of action of SCS would be based in the antidromic activation of the dorsal column fibers, which activate inhibitory interneurons in or near the substantia gelatinosa and marginal layer of the dorsal horn. Dorsal columns contain Aβ fibers related to all dermatomes, from the caudal to the spinal segments, where the contact is located, and these fibers are arranged in a precise order: The more caudal the dermatome, the more medial the axon. [27,31,34,51,102,117,119,121,129,169] As referred to earlier, peripheral neuropathy appears to induce a hyperexcitability state in the dorsal horn neurons, and SCS may have an effect in normalizing the excitability of a wide-range neurons in response to non-nociceptive stimuli. [51,169] Dorsolateral funiculus contains descending fibers that modulate the activity of the nociceptive dorsal horn neurons in rats; thus, it may be another target of electrical neuromodulation. [64,96,122]
Diverse studies in humans and animals revealed that SCS produces several changes in neurotransmitter release. Spinal cord stimulation likely produces an elevation of substance P in the cerebrospinal fluid. There is an increase in the release of inhibitory neurotransmitters, such as, gamma-aminobutyric acid (GABA) and acetylcholine, whereas, there is a decrease in the liberation of excitatory ones like glutamate and aspartate. Probably, SCS may produce pain relief by restoring the normal GABA levels in the dorsal horn, and increasing the release of adenosine. In a study in animals, it was seen that the beneficial effects of SCS were abolished if an intrathecal GABA-B receptor antagonist was administered simultaneously. [20,51,85,87,101,117,121,129,143] As GABA is thought to be involved in this process, SCS was combined with the intrathecal administration of baclofen (a GABA-B receptor agonist) during stimulation, in some studies, whose results suggest that it may improve the effect of SCS. In patients who have an insufficient response to SCS, a trial with intrathecal baclofen may be an optimal alternative. [85,117]
The anti-ischemic and antianginal effects of SCS seem to be due to its attenuation of the sympathetic system, stabilization of the intracardiac neuronal activity, an increase in the release of adenosine, and the peripheral release of calcitonin, a gene-related peptide that leads to vasodilatation. Nitric oxide may be involved in this latter effect too. [51,86,117,121]
INDICATIONS AND RESULTS
Spinal cord stimulation is still an underutilized method, although several indications have been described [Table 1]. However, this statement must not be confused with a suggestion of overuse. Despite the existing clear indications for SCS, there are marginal indications, which may yet be best approached as part of a clinical trial instead of as part of the clinical routine; but on the other hand, there are suggestions that it may be effective for other conditions besides pain, such as, severe peripheral vascular disease, reducing diarrheal episodes, and decreasing insulin requirements. [18,54,68,98,150]
Table 1.
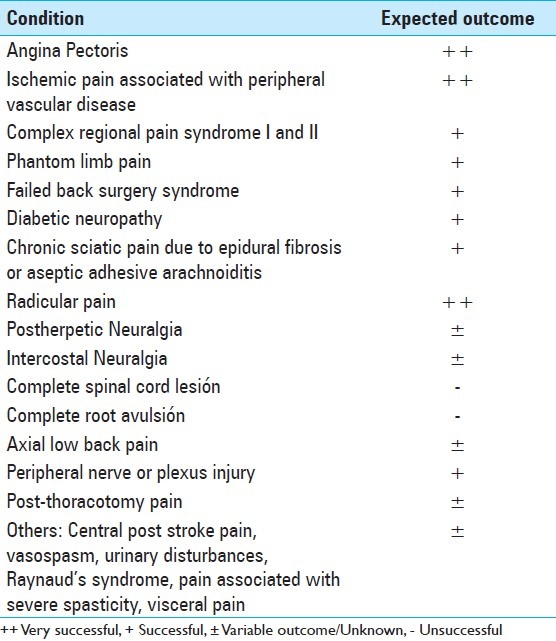
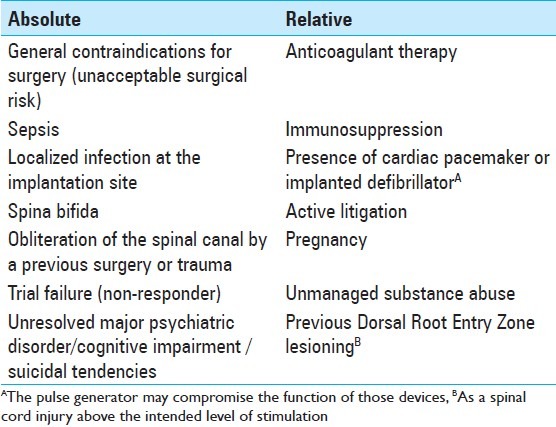
First, a correct pain analysis must be undertaken, because only the neuropathic component of pain is expected to be diminished. [18,73,88] Spinal cord stimulation should be used only if conservative treatments have failed previously. Patients with neuropathic pain have to undergo a magnetic resonance imaging (MRI) scan before considering SCS, to exclude any surgically curable cause of pain, such as, spinal canal or root compression, in which case, surgical decompression is indicated. There should not be any abnormality on the imaging studies or if any is present, it should not seem to be related to the patient's pain. A psychiatric evaluation should be done before the procedure is performed. [3,18,73,83] It is thought that the dorsal column fibers should retain at least some degree of functional integrity, to produce some inhibitory effect on the pain transmitting system, which is consistent with some findings of a lack of efficacy in patients with neuropathic pain, in whom the lesion is located proximal to the dorsal root ganglion, leading to extreme hypesthesia in the painful area. It would be due to orthodromic degeneration of the primary fibers emanating from the lesion up to the brainstem. Thus, those patients who present with complete central lesions may not be good candidates for SCS. It may be evaluated by somatosensory evoked potentials, as it is sometimes difficult to clinically determine the exact location of the lesion. In summary, in the absence of any contraindication, the proper candidates for SCS must present a well-defined, non-malignant, organic cause of pain, which cannot be treated by a curative surgical procedure, and they must have already failed a trial period of initial medical pain management, of at least six months. [3,44,81,88,137] Absolute and relative contraindications for SCS are listed in Table 2.
Table 2.
A trial stimulation with a temporary electrode, which usually lasts from three days to three weeks is almost always performed on each patient, to assess the efficacy of SCS, before a permanent pulse generator is implanted. The goal of the trial is to increase the cost-effectiveness of the method and to improve patient selection. The area of pain has to be covered by paresthesia during the trial. A pain relief of at least 50%, according to the visual analog scale (VAS), and patient satisfaction, are both considered as positive responses to the trial, which confirms the indication for definitive implantation of the pulse generator. There are approximately 20% of non-responder patients in whom permanent implantation is not indicated and the electrode is removed. Although an Outpatient trial is the gold standard, in some health centers the trial is conducted intraoperatively, through external stimulation, with the patient awake, under local anesthesia. [3,18,51,73,81,83,106,117,132,137,163,165]
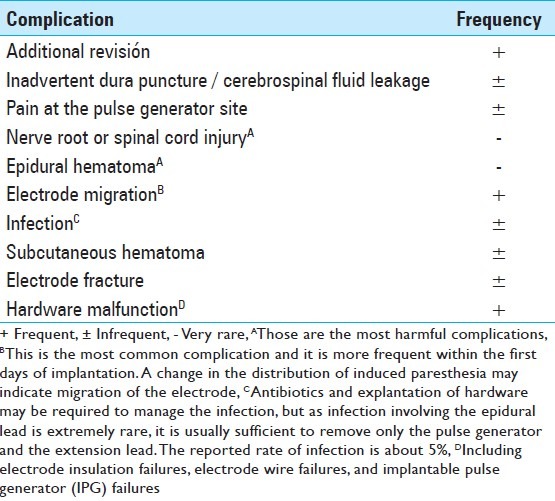
Several factors are suspected to be related to the SCS outcome [Table 3]. Williams et al. found that the variable most strongly associated with a negative result was experiencing <50% pain relief during the trial, which is coincident with the major criterion of permanent implantation. Furthermore, the presence of allodynia and/or hyperalgesia, two well-known hallmarks of neuropathic pain, is associated with a positive outcome, whereas, the history of substance abuse is correlated with a poor result. [165] Kumar and Wilson have published that the success rate is inversely proportional to the time interval, from the initial onset of symptoms to the time of implantation. They have concluded that patients had to be implanted within the first five years from the onset of their symptoms. This may be due to changes in the pain pathways over time, making it more resistant to treatment with SCS with the passage of time. [81] Complications of this technique, which are obviously related to the outcome, are shown in Table 4. Turner et al. have found that approximately 34% of the patients who received a stimulator had complications. [155]
Table 3.
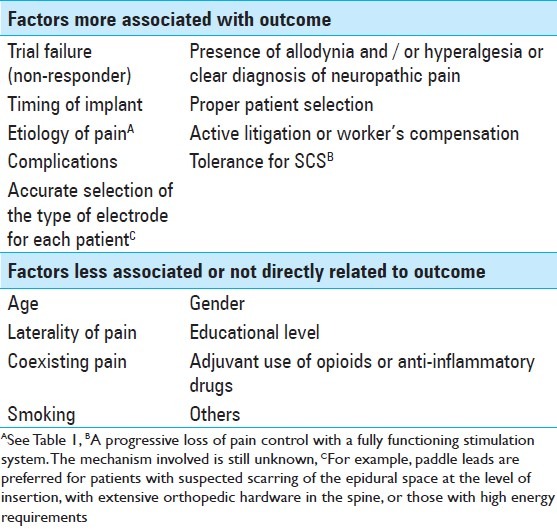
Table 4.
Failed back surgery syndrome
Failed back surgery syndrome (FBSS), also known as post laminectomy pain syndrome, is defined as a persistent or recurrent pain following surgery on the lumbosacral spine. It must be known that the surgery may have been successful in correcting the underlying spine pathology, but may have failed to achieve durable pain relief. Although the common symptoms of FBSS are diffuse, dull, and/or aching pain involving the back and/or legs, it may also include sharp, burning, pricking and/or stabbing pain in the extremities. Thus, in summary, it ranges from chronic axial low back pain to radiculopathy, but it does not necessarily mean that the surgery has fully failed. The incidence of FBSS varies widely among different studies, ranging from 10 to approximately 40% of patients who have undergone spine surgery. Forty to eighty percent of the patients obtain significant pain relief following open spine surgery for single-level fusions, whereas, only 15% reach this objective when a fusion of three levels is performed. FBSS is the most common indication for SCS. [3,25,51,72,84,91,92,97,98,108,125,157,162] Depending on each case, persistent pain following spinal surgery may be due to residual or recurrent disk herniation, persistent postoperative compression of the spinal nerves, like residual foraminal stenosis, altered joint mobility, pseudoarthrosis, joint hypermobility with instability, arachnoiditis or inflammation of the nerve roots in the thecal sac, depression, spinal muscular pain, epidural fibrosis, and discogenic pain, which may be from the disk above, below, or at the level of the fusion. It is essential to differentiate those patients in whom the pain is neuropathic in origin. [5,72,97,98,127,144,164] A clinical case of FBSS is described in Figure 1.
Figure 1.
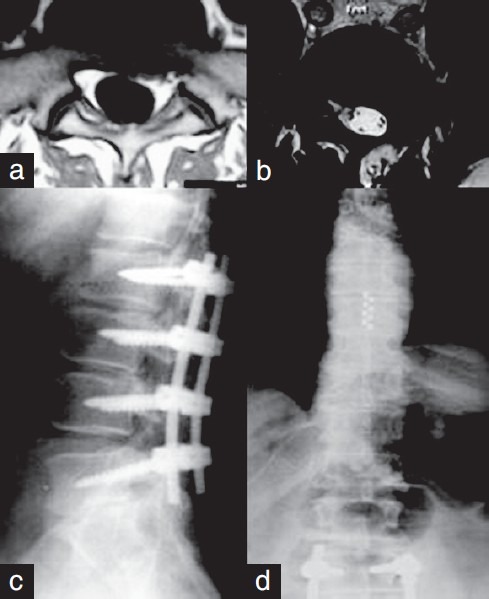
A case of failed back surgery syndrome is described. (a) A patient underwent lumbar microdiscectomy because of radicular pain. A left L5-S1 herniated disk was detected in the preoperative images. (b and c) The patient continued with radicular and lumbar pain after the surgery. Although magnetic resonance imaging showed arachnoiditis surrounding the root (b), he underwent posterior lumbar instrumentation (c). (d): The second surgery did not produce pain relief. Subsequently, the patient was referred to our Neurosurgical Department. A paddle-type lead with eight contacts was placed and Spinal cord stimulation led to pain relief
There are only few randomized and controlled trials to evaluate the effectiveness of SCS in treating FBSS, while most of the studies are retrospective case series. [74,75,77,78,90,98,112,149] Taylor et al. carried out a systematic review on SCS for FBSS, in which more than three thousand patients who had undergone implantation were included. Sixty-two and seventy percent of them had experienced >50% pain relief and had been satisfied with the treatment received. [149] Burchiel et al. reported that SCS successfully managed pain in 55% of the patients who were available for a one-year follow-up. [13] In 1991, North et al. presented a series of 50 patients with FBSS, who underwent spinal cord stimulator implantation. A successful outcome was defined, as in both, at least 50% sustained pain relief and patient satisfaction with the result, and it was recorded in 53% of the patients, two years postoperatively, and in 47% of them, five years postoperatively. [111]
North et al. conducted a prospective, randomized, and controlled trial to compare the outcome of SCS and repeated spine surgery in FBSS patients. They accepted patients with axial low back pain, but only if the intensity of this pain was equal to or less than that of their radicular pain, because they aimed to evaluate patients with the latter type of pain. It should be remembered that axial low back pain was more difficult to treat by SCS than radicular pain, as it was clearly explained in that article. A total of 50 patients were randomized to SCS or reoperation, but if the results of the randomized treatment were unsatisfactory, the patients could cross over to the alternative one; however, the crossover was an outcome measure, as were pain relief and patient satisfaction. Those patients randomized to reoperation could cross over to SCS after a six-month postoperative period. A therapeutic trial with a percutaneous electrode was carried out in patients who were to undergo SCS, before permanent implantation. Only 45 patients were available for follow-up. They concluded that SCS was more successful than reoperation (nine of 19 patients had a good outcome versus three of 26 patients, respectively; P < 0.01). One of the most important results of the study was the fact that the patients initially randomized to SCS were significantly less likely to cross over than those randomized to reoperation (five of 24 patients versus 14 of 26 patients, P = 0.02). Furthermore, those patients who were assigned to reoperation required increased opioid analgesics significantly more often than those randomized to SCS (P < 0.025), whereas, other measures of activities of daily living and work status did not differ significantly between the two groups. [112] Currently, the first multicenter, multinational, randomized, controlled trial to assess the effectiveness and cost-effectiveness of SCS with rechargeable pulse generator versus reoperation, through a 36-month follow-up in patients with failed back surgery syndrome, is being performed. [115]
The Prospective Randomized Controlled Multicenter Trial of the Effectiveness of Spinal Cord Stimulation (PROCESS) randomized 100 FBSS patients with predominant leg pain of neuropathic radicular origin, between spinal cord stimulation and conventional medical management (CMM) or CMM alone, and its results at six months showed that SCS achieved better pain relief, health-related quality of life, and functional capacity. Conventional medical management included oral medication (such as opioids, nonsteroidal anti-inflammatory drugs, antidepressants, antiepileptic drugs, and others), nerve blocks, epidural infiltration of steroids, physical and psychological rehabilitative therapy, and/or chiropractic care. In the intention-to-treat analysis at six months, 24 SCS patients (48%) and four CMM patients (9%) (P < 0.001) achieved the primary outcome, which was defined as the proportion of patients achieving 50% or more pain relief in the legs. It is essential to mention that five SCS patients crossed over to CMM, and 32 CMM patients crossed to SCS, between six and 12 months after the beginning of the treatment. [75,77,90] At 24 months, 46 of the 52 patients randomized to SCS and 41 of the 48 randomized to CMM were available for follow-up, and the primary outcome was achieved by 17 (37%) of the former versus one (2%) of the latter (P = 0.003). Moreover, this outcome was achieved by 34 (47%) of the 72 patients who received SCS as a final treatment versus one (7%) of the 15 for CMM (P = 0.02). [78]
In 1999, Krames proposed that SCS had to be a final treatment option, after all other therapies for FBSS had been exhausted. Thus, an algorithm of care, which ordered therapies by its invasiveness and costs, was recommended. Despite this, the algorithm was used for several years. If it is analyzed with the SAFE (Safety, Appropriateness, Fiscal Neutrality, and Effectiveness) principles, some changes should be done, although this issue is still controversial. They expressed that the risk of significant injury from the chronic use of opioids and nonsteroidal anti-inflammatory drugs or reoperation appeared to be greater than the risk of SCS. Fiscal Neutrality meant that the cost of a new therapy did not result in greater financial expenditure than the current therapy, with equivalent efficacy, during the same time period. [69–72,91,92] Several studies have analyzed the cost-effectiveness of SCS for FBSS. [7,12,35,43,72,74,91,92,147,148,166] Kumar et al. concluded that SCS was cost-effective in the long term, despite the initial high costs of the implantable devices. [74] On the other hand, pain medications contributed substantially to healthcare costs. SCS for FBSS was fiscally neutral on day one and at 3.5 years, when compared with reoperation and medical management, respectively. [72] Cost-effectiveness of SCS was highly sensitive to the device cost and its longevity. [136] Taylor et al. reported that rechargeable pulse generators should be considered when the battery life was likely to be short, despite their initial increased expense. [147] Therefore, in summary, Krames et al. demonstrated that SCS treatment for FBSS obeyed the four principles, and they proposed that it should be used before the institution of long-term opioid therapy, intrathecal therapies, or reoperation. [72] However, Frey et al. and some guidelines for management of low back pain established that more evidence was needed to determine at which point in the treatment continuum SCS should be considered. [35,91,92] New algorithms and paradigms for clinical management of these patients may be developed in the future, when higher quality evidence becomes available.
Complex regional pain syndrome
Complex regional pain syndrome (CRPS) may be divided into two types: type 1 (also known as reflex sympathetic dystrophy) and type 2 (or causalgia). Both types have the same signs and symptoms, but the former does not have a nerve injury, whereas, the latter has it. About 90% of the people with CRPS have type 1. The CRPS is generally preceded by trauma or surgery, and the affected area is usually greater than the region of the original injury, as it happens in other types of neuropathic pain. Nerve conduction studies may be used to confirm or exclude peripheral neuropathic disease, thus, differentiating between the two types. The symptoms, which are increased with exertion, are a combination of continuous and excruciating pain, which is disproportionate to the trigger event, hyperalgesia, allodynia, changes in skin color and asymmetry of skin temperature due to abnormal skin blood flow, edema, sweating abnormalities like hyper-/ hypohidrosis, decreased range of motion of the affected joints, muscle weakness, tremors, involuntary movements, bradykinesia, dystonia and trophic signs, such as, abnormal hair and nail growth. [3,11,40,49,98,135,141,142,159]
Spinal cord stimulation may be considered for patients with CRPS, in whom conservative medical and rehabilitation therapy or sympathetic blocks have not been successful. [159] This is the second most common indication for SCS, in USA, and it would be an effective therapy in the management of patients with CRPS types 1 and 2. [51,135] The clinical effects of SCS include decreased edema, allodynia, and symptoms of movement and vasomotor disorders. It produces an increased blood flow too, however, its pain-relieving effect is apparently not related to these changes in the blood flow. It is particularly effective in helping to restore function in the affected extremities. [76,98,121,141] It is essential to treat these patients early in the disease course, as there is evidence which suggests that it is associated with better outcomes. Stanton-Hicks has recommended that SCS be considered for patients with CRPS type 1 if no response to conventional treatment is noted within 12 - 16 weeks. [76,98,141,146] The presence of brush-evoked allodynia may predict a poor outcome in these patients. [158]
Kemler et al. carried out a randomized study on patients with CRPS, to compare SCS plus physical therapy with physical therapy alone. Thirty-six patients were assigned to SCS and physical therapy, and 18 were randomized to receive physical therapy alone; however, the trial stimulation was successful only in 24 patients; so the other 12 patients did not receive implanted stimulators. In an intention-to-treat analysis at six months, the group assigned to receive SCS and physical therapy had a mean reduction of 2.4 cm according to the VAS, while there was an increase of 0.2 cm in the group assigned to physical therapy alone (P < 0.001). [59] At two years, the intention-to-treat analysis showed improvements in the group randomized to SCS plus physical therapy with regard to pain intensity (- 2.1 vs. 0.0 cm; P < 0.001). Health-related quality of life improved only in the group receiving SCS. They concluded that SCS resulted in a long-term pain relief and health-related quality of life improvement in CRPS, but accurate patient selection is required. [60] Nevertheless, at five years, implanted patients had similar results to those who were treated with physical therapy for pain relief and all other measured variables, although 95% of the patients with an implant would repeat the treatment for the same result. [61,62]
Phantom limb pain
Up to 80% of the patients, who have undergone amputation, may be affected by phantom limb pain, although the real percentage is controversial. [10,28,30,109,130,163] In a study of 25 unilateral upper limb amputees, the prevalence of clinical symptoms of phantom sensation, phantom pain, and stump pain were 64, 32, and 24%, respectively. [28] Phantom limb pain is a painful sensation referred to the absent limb, but if it is localized particularly in the stump, it is called stump pain, which is common in the early post-amputation period, but in 5 - 10% of the cases it persists and may even get worse with time. Phantom limb sensation is any other sensation in the absent limb, except pain. These elements often coexist in each patient and may be hard to separate. [109] It is difficult to evaluate the success of SCS in each type of pain individually, as the available studies usually group these different categories together. [98] Spinal cord stimulation can relieve phantom pain, but the effect often decreases with time. [67,109]
There are several case series on the treatment of phantom limb pain with SCS, which have reported heterogeneous results. It could be due to the fact that some of them were from the seventies and eighties, when the hardware was less technologically advanced and some leads were not located in the epidural space. [10,28,30,32,58,67,109,130,163] Viswanathan et al. treated four patients with phantom limb pain by SCS and all of them experienced excellent pain relief postoperatively. [163] Katayama et al. operated 19 patients with phantom limb pain. All of them underwent SCS, and if the SCS failed to reduce the pain, the patients were considered for deep brain stimulation and/or motor cortex stimulation. They found that satisfactory long-term pain control was achieved in six of the 19 patients, by SCS. They concluded that there was no evidence for an advantage of motor cortex stimulation over SCS and deep brain stimulation, in controlling phantom limb pain. [58] Fernandes Correa did not find any improvement in three patients who had been treated with SCS. [32]
Peripheral vascular disease
Although critical limb ischemia should be treated with open surgery or endovascular technique, some patients cannot undergo these procedures because they are contraindicated due to comorbidities. In other cases, these techniques may be not effective to achieve complete rest-pain relief. Patients with small vessel disease, where revascularization is not possible, can be also be candidates for SCS. The main indication is severe ischemic pain at rest, without tissue involvement, which corresponds to grade 3 according to the Fontaine classification; however, patients with necrosis or gangrene (grade 4) may also benefit from this treatment. Patients who have been already revascularized and present with transcutaneous oxygen pressure > 30 mmHg, but with persistent pain and / or ulcers that do not heal, in spite of the medical treatment, may also be considered for SCS. Spinal cord stimulation is thought to produce significant long-term pain relief in these patients. Furthermore, SCS may prevent the need for amputation in patients with critical limb ischemia, as also, its usage is associated with an increase in capillary blood flow and skin temperature, and enhanced healing of skin ulcers less than 3 cm. In spite of its effects on the peripheral circulation, patients must have adequate collateral blood flow in the affected areas, in order to be considered for SCS. [1,19,48,51,98,120] In all these cases, SCS may be indicated and a trial stimulation has to be performed prior to permanent implantation. Pain reduction and improved tissue perfusion during trial stimulation correlates with successful limb salvage, with SCS. The aching ischemic pain is expected to be reduced by this technique, but pain due to inadequate venous return is not relieved. [1,48,51,80,88,98,120,139] Spincemaille et al. suggests that those patients with greater than 50% pain relief and better than 15% increase in transcutaneous oxygen pressure during the trial stimulation must undergo permanent implantation. [139]
The European Peripheral Vascular Disease Outcome Study (SCS-EPOS), which is a prospective controlled multicenter study, has determined that SCS treatment of non-reconstructable critical leg ischemia provides a significantly better limb survival rate than conservative treatment. [1] Kumar et al. carried out a prospective study in which SCS was used in 46 patients, for pain associated with lower extremity ischemic vascular disease, which was considered to be non-reconstructable. The therapy was successful in 30 out of 39 cases, and the best results were seen in patients with severe claudication and rest pain, without trophic changes in the foot. [80] Horsch et al. studied 177 patients with ischemic pain caused by non-reconstructable severe peripheral arterial occlusive disease. After a mean follow-up of 35.6 months, 110 patients achieved more than 75% pain relief with limb salvage. [48] A prospective randomized controlled study in 51 patients who presented with chronic leg ischemia with rest pain and/or ischemic ulcerations, due to technically inoperable arterial occlusions, concluded that SCS might reduce the amputation levels in patients with severe inoperable leg ischemia, and it would be most effective in patients without arterial hypertension. [52] In 2004, a systematic review and meta-analysis comparing SCS in addition to any form of conservative treatment for inoperable chronic critical leg ischemia, found that the addition of SCS to standard conservative treatment improved limb salvage, ischemic pain, and the general clinical situation in these patients. [156]
Angina pectoris
Spinal cord stimulation is thought to improve the New York Heart Association functional class, reduce pain, decrease nitrate requirements, improve exercise capacity, prevent hospital admissions, and improve the quality of life in patients with refractory angina. Despite its effectiveness in preventing hospital admissions, SCS does not mask serious ischemic symptoms, which may lead to silent infarction. Mannheimer et al. have determined that these anti-anginal and anti-ischemic effects seem to be due to a decrease in myocardial oxygen consumption. Spinal cord stimulation can also improve blood flow, through the creation of collateral circulation, because of the enhanced physical activity of the patients after implantation. [26,39,51,93,98,107,151]
The ESBY study (Electrical Stimulation versus Coronary Artery Bypass Surgery in Severe Angina Pectoris) included 104 patients with severe angina and increased surgical risk, who were randomized to coronary artery bypass grafting or spinal cord stimulation. At five years, there was no significant difference in the survival rate or quality of life between the groups. Thus, they concluded that both treatments could be considered as effective options for patients with severe angina, increased surgical risks, and those estimated to have no prognostic benefits from coronary artery bypass grafting. [29] In summary, SCS could be an option for high-risk patients who could not undergo surgery because of comorbidities, increased risk of surgical complications, and other contraindications. In order to be candidates for SCS, patients should present with severe and stable angina pectoris, reduced quality of life, stenosis of the coronary arteries on coronary angiography, and pain refractory to optimal medical treatment. Patients who had unstable angina, valve defects, or cardiac pacemaker systems were not candidates for SCS implantation. It should be borne in mind that these latter systems would interfere with the SCS hardware and vice versa, thus, these patients should not undergo implantation. Small vessel disease cannot be treated surgically, however, it may be more appropriately treated with SCS, as this has been shown to improve microcirculation. [29,51,73,94,98]
Other indications
These and many other uses of SCS are described in the literature, while new applications are being proposed and researched worldwide, positioning it as a cutting edge technique within the healthcare environment [Table 1]. Spinal lumbar stenosis in patients who are not surgical candidates may be treated successfully with SCS. [2,98] Simultaneous use of SCS and peripheral nerve field stimulation appear to increase the efficacy of both methods for low back pain due to failed back surgery syndrome and / or spinal stenosis. [104]
Cervical SCS has been hypothesized to be useful in the treatment of cerebral vasospasm after subarachnoid hemorrhage, as several experiments in animals and research in humans have demonstrated an increase in cerebral blood flow (CBF). Spinal cord stimulation works in different ways, such as, preventing vasoconstriction of the cerebral arteries by functional sympathectomy, acting at the lower cervical levels, and increasing CBF through the central pathways at the upper cervical levels. Slavin et al. carried out a prospective study in 12 aneurysmal subarachnoid hemorrhage patients, who underwent implantation of an eight-contact electrode, with the aim of establishing the safety of this intervention. There were no complications related to the electrode insertion and patients were stimulated for 14 consecutive days or until discharge. [36,138] This is still under research.
Cervical SCS is also used for several pain conditions, such as, occipital neuralgia, Raynaud's syndrome, and chronic intractable pain located in head, face, jaw, neck, shoulder, or upper extremities. [98,105,154,167] Tomycz et al. have implanted paddle leads in the cervicomedullary junction in patients with intractable head or facial pain, and they conclude that patients suffering from trigeminal deafferentation pain, trigeminal neuropathic pain, and post-herpetic neuralgia may respond favorably to SCS, whereas, patients with occipital neuralgia are rarely relieved by this technique. [154] The results of SCS for post-herpetic neuralgia are controversial. [41,50,98] Herke et al. have reported that 23 out of 28 patients with post-herpetic neuralgia and four out of four patients with acute herpes zoster have experienced pain relief after SCS. [41] Iseki et al. believe that limited-duration SCS for subacute post-herpetic neuralgia is a useful treatment, which may be able to prevent the pain from progressing to chronic post-herpetic neuralgia. [50]
Post-thoracotomy pain syndrome is defined as pain that occurs or persists in the area of the thoracotomy incision for at least two months following the initial procedure, and SCS may be effective in treating this type of pain, as the neuropathic component can be predominant in these patients. [38,89]
Spinal cord stimulation could increase exercise tolerance and produce pain relief in patients with painful diabetic neuropathy. The technique should be considered in these patients when they do not respond to conventional treatment, and its effect could last for a long time. [21,54,98,152] Furthermore, Kapural et al. reported a case of diabetic neuropathy in which SCS had produced not only significant pain relief, but also better blood glucose control. [54]
Some articles suggest that SCS may be useful in relieving chronic visceral abdominal pain, although the criteria for patient selection are still not clear. Spinal cord stimulation has been used to treat abdominal pain in several conditions, such as, mesenteric ischemia, chronic pancreatitis, diffuse abdominal adhesions, and chronic pelvic pain after endometriosis. [53,55,56,57,63,153] Kapural et al. has found that SCS seems to produce long-term pain relief and a decrease in opioid use in these patients. [53] Yakovlev and Resch have reported a case in which SCS has produced excellent relief of urinary incontinence symptoms in a patient with both urinary incontinence and low back pain related to FBSS. In this patient, SCS has been effective in controlling the urinary voiding dysfunction symptoms, but the use of SCS to treat these urinary voiding problems needs further investigation. [170]
HARDWARE AND PROGRAMMING PARAMETERS
The hardware consists of electrodes, extension wires, a pulse generator, and a programmer. The extension wires connect the leads to the pulse generator device. As mentioned earlier, two types of electrodes are currently commercially available: Cylindrically shaped percutaneous electrodes and paddle-type surgical ones. The features of each type and their differences can be seen in Table 5. Also, the leads vary in type according to other features, such as, the number of contacts (up to 16 or 20, depending on the type of electrode), electrode shape, configuration, spacing, and length. The number of electrodes used in each case depends on the disease to be treated, as well as the surgeon's preference. Many physicians prefer to implant a generous number of electrodes, as the pain pattern may change or the lead can migrate; thus, if this occurs, it can be solved electronically. [23,83,108,126,127]
Table 5.
Three pulse generator systems are currently available: Radiofrequency system, external pulse generator, and the a fully implantable one. The former is a telemetric system in which a receiver is implanted under the skin and the transmitter is placed externally on a belt, without using a battery. Energy is telemetrically transmitted from the outside. Fully implantable pulse generators have replaced radiofrequency receivers and external pulse generators, which have been commonly seen in the past. Nowadays, external pulse generators are used to stimulate the electrodes through a disposable lead during the trial, which lasts from three days to three weeks. Throughout the trial period, the external pulse generator can be activated or deactivated by the patient, allowing him/her to become familiar with the basic control of the amplitude and the sensation of paresthesia, which must correspond with the area of pain. Implantable ones are more comfortable for the patient, but they require additional procedures to replace the battery. Depending on its parameter configurations and its usage, most patients can expect a battery life of 2.5 to 4.5 years. Rechargeable and implantable pulse generators are commercially available, and a battery life of up to 10 years is expected. Fully implantable, multi-channel, and multi-programmable pulse generators connected to dual-lead, multi-contact electrodes are the most versatile tools for SCS today. [49,51,83,108,126,127,135]
The programmer is used to adjust the stimulation parameters of the pulse generator, such as, the amplitude, frequency, pulse width, and polarity. [83,108,161] Biphasic discontinuous or alternating electric current, also known as Lilly pulses, is used in neuromodulation. The amplitude refers to the amount of charge generated, which is provided in volts (V) or amperes (A), whereas, the frequency is the pulse rate and is measured in Hertz (Hz). The pulse width is the duration of the current delivered and it has been seen that the area of paresthesia extends caudally with increasing pulse width. The smallest dorsal column fibers can be activated only when the pulse width is sufficiently large. Impedance is the resistance of the leads to the current flow and is given in Ohms. The physician must keep in mind that the higher the energy utilized, the shorter the battery life. It is thought that electric current over 800 μA can create a neuronal lesion. Lower frequency and amplitude are used to elicit neuronal excitation (5 - 15 μA, 10 - 40 Hz), while higher values of these variables produce neuronal inhibition (40 - 80 μA, > 60 Hz). The stimulus amplitude must be less than the threshold for motor responses and uncomfortable sensations, which is generally 1.4 times the amplitude related to the initial paresthesia. [31,46,49,83,108,110,117,123,128,160,161,172] A cathode is a negatively charged electrode, which may make a neuron nearby more electrically positive or depolarized, leading it to trigger an action potential or facilitating it. A neuron can be hyperpolarized, with the subsequent raising of its threshold for action potential, by a positively charged contact or anode. Stimulation between two contacts is known as bipolar stimulation, in which one contact is used as a cathode and the other as an anode. The electrons run from the former to the latter, going through the stimulated tissue, which is determined by the distance between the two contacts. During monopolar stimulation, the electrons go from the contact (cathode) to all directions, as the anode, which is usually the implanted pulse generator, is placed very far from it. Thus, the stimulated area is less predictable in monopolar stimulation. Although the programming parameters depend on each patient, they are usually within the following ranges: Amplitude: 3 - 10 mA, frequency: 10 - 40 Hz, pulse width: 60 - 450 msec. Spinal cord stimulation is used either in an intermittent or in a continuous stimulation mode; the last is probably more effective for patients who have shorter pain-free intervals following the cessation of stimulation. [16,46,83,117,161,168,171]
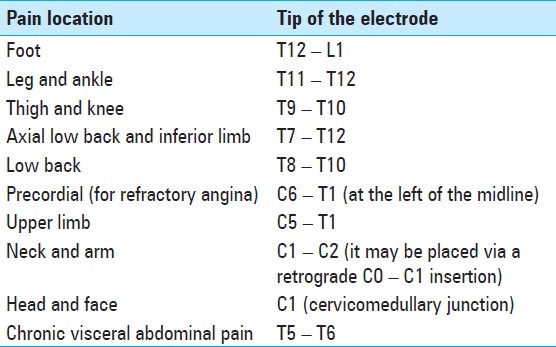
Spinal cord stimulation must produce an electrical field that stimulates the spinal cord structures, without stimulating the nearby nerve root. Typically, the lead is several levels above the desired area to be covered by paresthesia [Table 6]. If the lead is close to the midline, the electrical field will reach the spinal cord before reaching the nerve root, whose stimulation produces paresthesia only in its corresponding dermatoma. As the dorsal root sensory fibers have a low threshold, it is essential that the lead be located close to the physiological spinal cord midline, to avoid recruitment of the root. Moreover, the cerebrospinal fluid layer at the thoracic spinal cord is thicker than at other levels, leading to an increase in the amplitude needed to reach the spinal cord targets, which increase the possibility of stimulation of the dorsal root nerves. Thus, it is difficult to relieve axial low back pain, as the fibers that innervate it are located lateral to the sacral segment΄s axons, within the dorsal column. It is difficult to evoke paresthesia along the physiological midline. Spinal cord stimulation has been carried out with single electrodes for decades. However, it has been noted that a single percutaneous electrode cannot cover pain in the midline and in the bilateral dermatomes accurately. Dual multi-contact electrode systems have been developed, with the idea that two electrodes can be positioned close to the midline on each side, which will extend the electrical field, and therefore, allow for generation of paresthesia in every necessary dermatome. Separate programming of each electrode and contact has led to an almost unlimited number of combinations of the contacts on each electrode and between the pairs. In spite of these advances and some clear advantages, there is no adequate evidence in the literature which establishes that the results of a dual electrode SCS are definitely better than the outcome of a single electrode SCS. Transverse tripolar configuration with one central cathode (+, -, +) may be used to achieve a more accurate delimitation of the stimulated area, as it can recruit the deeper dorsal column fibers, but without producing dorsal root activation. Tripolar stimulation seems to be useful in reducing axial back pain, which is more difficult to treat than the radicular one. [47,51,98,116,117,126] A five-column paddle electrode has recently been developed and approved by the Food and Drug Administration. This lead is thought to have more precise field control, allowing for modulation of a greater lateral area of the spinal cord, which is useful for treating low back pain.
Table 6.
The desired location of the tip of the electrode in most cases is shown. Nevertheless, this is just a schematic view and the definitive location of the lead depends on each patient[37,51,53,105,131,154,165]
CONCLUSION
Spinal cord stimulation is a useful tool for neuromodulation, if accurate patient selection is carried out previously, which must include a trial period. Undoubtedly, this proper selection and a better knowledge of its underlying mechanisms of action, will allow this cutting edge technique to be accepted more among pain physicians.
Footnotes
Disclaimer: The authors of this paper have received no outside funding, and have nothing to disclose.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/5/275/103019
Contributor Information
Claudio Yampolsky, Email: claudio.yampolsky@hospitalitaliano.org.ar.
Santiago Hem, Email: Santiago.hem@hospitalitaliano.org.ar.
Damián Bendersky, Email: damian.bendersky@hospitalitaliano.org.ar.
REFERENCES
- 1.Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. European Peripheral Vascular Disease Outcome Study SCS-EPOS. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: Results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS) Eur J Vasc Endovasc Surg. 2003;26:280–6. doi: 10.1053/ejvs.2002.1876. [DOI] [PubMed] [Google Scholar]
- 2.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: Conservative or surgical management.: A prospective 10-year study? Spine (Phila Pa 1976) 1976;2000(25):1424–35. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson L, Sundaraj SR, Brooker C, O’Callaghan J, Teddy P, Salmon J, et al. Recommendations for patient selection in spinal cord stimulation. J Clin Neurosci. 2011;18:1295–302. doi: 10.1016/j.jocn.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Barolat G. Experience with 509 plate electrodes implanted epidurally from C1 to L1. Stereotact Funct Neurosurg. 1993;61:60–79. doi: 10.1159/000100624. [DOI] [PubMed] [Google Scholar]
- 5.Barolat G, Sharan AD. Future trends in spinal cord stimulation. Neurol Res. 2000;22:279–84. doi: 10.1080/01616412.2000.11740671. [DOI] [PubMed] [Google Scholar]
- 6.Beems T, van Dongen RT. Minimally invasive placement of epidural plate electrodes under local anaesthesia in spinal cord stimulation. Acta Neurochir Suppl. 2007;97(Pt 1):105–9. doi: 10.1007/978-3-211-33079-1_14. [DOI] [PubMed] [Google Scholar]
- 7.Bell GK, Kidd D, North RB. Cost-effectiveness analysis of spinal cord stimulation in treatment of failed back surgery syndrome. J Pain Symptom Manage. 1997;13:286–95. doi: 10.1016/s0885-3924(96)00323-5. [DOI] [PubMed] [Google Scholar]
- 8.Bennett GJ, Xie YK. Peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 9.Berson BS, Berntson GG, Zipf W, Torello MW, Kirk WT. Vasopressin-induced antinociception: An investigation into its physiological and hormonal basis. Endocrinology. 1983;113:337–43. doi: 10.1210/endo-113-1-337. [DOI] [PubMed] [Google Scholar]
- 10.Bosmans JC, Geertzen JH, Post WJ, van der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 31/2-year prospective study. Clin Rehabil. 2010;24:444–53. doi: 10.1177/0269215509360645. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria.International Association for the Study of Pain. Pain. 1999;81:147–54. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 12.Budd K. Spinal cord stimulation: Cost-benefit study. Neuromodulation. 2002;5:75–8. doi: 10.1046/j.1525-1403.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- 13.Burchiel KJ, Anderson VC, Brown FD, Fessler RG, Friedman WA, Pelofsky S, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine (Phila Pa. 1976;1996(21):2786–94. doi: 10.1097/00007632-199612010-00015. [DOI] [PubMed] [Google Scholar]
- 14.Burchiel KJ, Anderson VC, Wilson BJ, Denison DB, Olson KA, Shatin D. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995;36:1101–10. doi: 10.1227/00006123-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Burchiel KJ, Russell LC. Spontaneous activity of ventral root axons following peripheral nerve injury. J Neurosurg. 1985;62:408–13. doi: 10.3171/jns.1985.62.3.0408. [DOI] [PubMed] [Google Scholar]
- 16.Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–70. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J Neurosurg. 2004;100(3 Suppl Spine):S254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 18.Compton AK, Shah B, Hayek SM. Spinal cord stimulation: A review. Curr Pain Headache Rep. 2012;16:35–42. doi: 10.1007/s11916-011-0238-7. [DOI] [PubMed] [Google Scholar]
- 19.Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities.Electric stimulation of spinal cord and posterior roots. N Y State J Med. 1976;76:366–8. [PubMed] [Google Scholar]
- 20.Cui JG, O’Connor WT, Ungerstedt U, Meyerson BA, Linderoth B. Spinal Cord Stimulation attenuates augmented dorsal horn release of excitatory amino acids inmononeuropathy via a GABAergic mechanism. Pain. 1997;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 21.Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabet Med. 2005;22:393–8. doi: 10.1111/j.1464-5491.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 22.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 23.Deer T, Bowman R, Schocket SM, Kim C, Ranson M, Amirdelfan K, et al. The prospective evaluation of safety and success of a new method of introducing percutaneous paddle leads and complex arrays with an epidural access system. Neuromodulation. 2012;15:21–9. doi: 10.1111/j.1525-1403.2011.00419.x. [DOI] [PubMed] [Google Scholar]
- 24.Devor M, Wall PD. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981;199:277–91. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery - the case for restraint. N Engl J Med. 2004;350:722–6. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 26.Diedrichs H, Zobel C, Theissen P, Weber M, Koulousakis A, Schicha H, et al. Symptomatic relief precedes improvement of myocardial blood flow in patients under spinal cord stimulation. Curr Control Trials Cardiovasc Med. 2005;6:7. doi: 10.1186/1468-6708-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubuisson D. Effect of dorsal-column stimulation on gelatinosa and marginal neurons of cat spinal cord. J Neurosurg. 1989;70:257–65. doi: 10.3171/jns.1989.70.2.0257. [DOI] [PubMed] [Google Scholar]
- 28.Ebrahimzadeh MH, Fattahi AS, Nejad AB. Long-term follow-up of Iranian veteran upper extremity amputees from the Iran-Iraq war (1980-1988) J Trauma. 2006;61:886–8. doi: 10.1097/01.ta.0000236014.78230.77. [DOI] [PubMed] [Google Scholar]
- 29.Ekre O, Eliasson T, Norrsell H, Währborg P, Mannheimer C. Electrical Stimulation versus Coronary Artery Bypass Surgery in Severe Angina Pectoris. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002;23:1938–45. doi: 10.1053/euhj.2002.3286. [DOI] [PubMed] [Google Scholar]
- 30.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Feirabend HK, Choufoer S, Ploeger S, Holsheimer J, van Gool JD. Morphometry of human superficial dorsal and dorsolateral column fibres: Significance to cord stimulation. Brain. 2002;125:1137–49. doi: 10.1093/brain/awf111. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes Correa C. Spinal cord stimulation (SCS) in painful neuropathy. In: Cukiert A, editor. Neuromodulation. Sao Paulo: Alaúde Editorial; 2010. pp. 67–105. [Google Scholar]
- 33.Fodstad H, Hariz M. Electricity in the treatment of nervous system disease. Acta Neurochir Suppl. 2007;97(Pt 1):11–9. doi: 10.1007/978-3-211-33079-1_2. [DOI] [PubMed] [Google Scholar]
- 34.Foreman RD, Beall JE, Coulter JD, Willis WD. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39:534–46. doi: 10.1152/jn.1976.39.3.534. [DOI] [PubMed] [Google Scholar]
- 35.Frey ME, Manchikanti L, Benyamin RM, Schultz DM, Smith HS, Cohen SP. Spinal cord stimulation for patients with failed back surgery syndrome: A systematic review. Pain Physician. 2009;12:379–97. [PubMed] [Google Scholar]
- 36.Goellner E, Slavin KV. Cervical spinal cord stimulation may prevent cerebral vasospasm by modulating sympathetic activity of the superior cervical ganglion at lower cervical spinal level. Med Hypotheses. 2009;73:410–3. doi: 10.1016/j.mehy.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 37.Golovac S. Spinal cord stimulation: Uses and applications. Neuroimaging Clin N Am. 2010;20:243–54. doi: 10.1016/j.nic.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Graybill J, Conermann T, Kabazie AJ, Chandy S. Spinal cord stimulation for treatment of pain in a patient with post thoracotomy pain syndrome. Pain Physician. 2011;14:441–5. [PubMed] [Google Scholar]
- 39.Greco S, Auriti A, Fiume D, Gazzeri G, Gentilucci G, Antonini L, et al. Spinal cord stimulation for the treatment of refractory angina pectoris: A two-year follow-up. Pacing Clin Electrophysiol. 1999;22(1 Pt 1):26–32. doi: 10.1111/j.1540-8159.1999.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 40.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–31. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 41.Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002;94:694–700. doi: 10.1097/00000539-200203000-00040. [DOI] [PubMed] [Google Scholar]
- 42.Hatzis A, Stranjalis G, Megapanos C, Sdrolias PG, Panourias IG, Sakas DE. The current range of neuromodulatory devices and related technologies. Acta Neurochir Suppl. 2007;97(Pt 1):21–9. doi: 10.1007/978-3-211-33079-1_3. [DOI] [PubMed] [Google Scholar]
- 43.Hollingworth W, Turner JA, Welton NJ, Comstock BA, Deyo RA. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: An observational study in a workers’ compensation population. Spine(Phila Pa 1976) 1976;2011(36):2076–83. doi: 10.1097/BRS.0b013e31822a867c. [DOI] [PubMed] [Google Scholar]
- 44.Holsheimer J. Effectiveness of spinal cord stimulation in the management of chronic pain: Analysis of technical drawbacks and solutions. Neurosurgery. 1997;40:990–6. doi: 10.1097/0006123-199705000-00023. [DOI] [PubMed] [Google Scholar]
- 45.Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531–40. doi: 10.1038/sj.sc.3100717. [DOI] [PubMed] [Google Scholar]
- 46.Holsheimer J, Buitenweg JR, Das J, de Sutter P, Manola L, Nuttin B. The effect of pulse width and contact configuration on paresthesia coverage in spinal cord stimulation. Neurosurgery. 2011;68:1452–61. doi: 10.1227/NEU.0b013e31820b4f47. [DOI] [PubMed] [Google Scholar]
- 47.Holsheimer J, Wesselink WA. Optimum electrode geometry for spinal cord stimulation: The narrow bipole and tripole. Med Biol Eng Comput. 1997;35:493–7. doi: 10.1007/BF02525529. [DOI] [PubMed] [Google Scholar]
- 48.Horsch S, Claeys L. Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Ann Vasc Surg. 1994;8:468–74. doi: 10.1007/BF02133067. [DOI] [PubMed] [Google Scholar]
- 49.Hyatt KA. Overview of complex regional pain syndrome and recent management using spinal cord stimulation. AANA J. 2010;78:208–12. [PubMed] [Google Scholar]
- 50.Iseki M, Morita Y, Nakamura Y, Ifuku M, Komatsu S. Efficacy of limited-duration spinal cord stimulation for subacute postherpetic neuralgia. Ann Acad Med Singapore. 2009;38:1004–6. [PubMed] [Google Scholar]
- 51.Jeon Y, Huh BK. Spinal cord stimulation for chronic pain. Ann Acad Med Singapore. 2009;38:998–1003. [PubMed] [Google Scholar]
- 52.Jivegård LE, Augustinsson LE, Holm J, Risberg B, Ortenwall P. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: A prospective randomised controlled study. Eur J Vasc Endovasc Surg. 1995;9:421–5. doi: 10.1016/s1078-5884(05)80010-3. [DOI] [PubMed] [Google Scholar]
- 53.Kapural L, Deer T, Yakovlev A, Bensitel T, Hayek S, Pyles S, et al. Technical aspects of spinal cord stimulation for managing chronic visceral abdominal pain: The results from the national survey. Pain Med. 2010;11:685–91. doi: 10.1111/j.1526-4637.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- 54.Kapural L, Hayek SM, Stanton-Hicks M, Mekhail N. Decreased insulin requirements with spinal cord stimulation in a patient with diabetes. Anesth Analg. 2004;98:745–6. doi: 10.1213/01.ane.0000102674.41527.1e. [DOI] [PubMed] [Google Scholar]
- 55.Kapural L, Nagem H, Tlucek H, Sessler DI. Spinal cord stimulation for chronic visceral abdominal pain. Pain Med. 2010;11:347–55. doi: 10.1111/j.1526-4637.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 56.Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7:440–3. doi: 10.1111/j.1526-4637.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 57.Kapural L, Rakic M. Spinal cord stimulation for chronic visceral pain secondary to chronic non-alcoholic pancreatitis. J Clin Gastroenterol. 2008;42:750–1. doi: 10.1097/01.mcg.0000225647.77437.45. [DOI] [PubMed] [Google Scholar]
- 58.Katayama Y, Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C. Motor cortex stimulation for phantom limb pain: Comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:159–62. doi: 10.1159/000064593. [DOI] [PubMed] [Google Scholar]
- 59.Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnée CA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618–24. doi: 10.1056/NEJM200008313430904. [DOI] [PubMed] [Google Scholar]
- 60.Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: Two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13–8. doi: 10.1002/ana.10996. [DOI] [PubMed] [Google Scholar]
- 61.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Spinal cord stimulation for chronic reflex sympathetic dystrophy--five-year follow-up. N Engl J Med. 2006;354:2394–6. doi: 10.1056/NEJMc055504. [DOI] [PubMed] [Google Scholar]
- 62.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: Five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292–8. doi: 10.3171/JNS/2008/108/2/0292. [DOI] [PubMed] [Google Scholar]
- 63.Khan YN, Raza SS, Khan EA. Application of spinal cord stimulation for the treatment of abdominal visceral pain syndromes: Case reports. Neuromodulation. 2005;8:14–27. doi: 10.1111/j.1094-7159.2005.05216.x. [DOI] [PubMed] [Google Scholar]
- 64.Khasabov SG, Ghilardi JR, Mantyh PW, Simone DA. Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus. J Neurophysiol. 2005;93:998–1006. doi: 10.1152/jn.01160.2003. [DOI] [PubMed] [Google Scholar]
- 65.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 66.Kirkpatrick AF. Percutaneous electrodes versus electrodes placed by laminotomy for spinal cord stimulation. Neurosurgery. 1991;28:932–3. doi: 10.1097/00006123-199106000-00033. [DOI] [PubMed] [Google Scholar]
- 67.Krainick JU, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation. Long-term follow-up study over 5 years. J Neurosurg. 1980;52:346–50. doi: 10.3171/jns.1980.52.3.0346. [DOI] [PubMed] [Google Scholar]
- 68.Krames E, Mousad DG. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: A case report. Neuromodulation. 2004;7:82–8. doi: 10.1111/j.1094-7159.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 69.Krames E, Poree L, Deer T, Levy R. Implementing the SAFE principles for the development of pain medicine therapeutic algorithms that include neuromodulation techniques. Neuromodulation. 2009;12:104–13. doi: 10.1111/j.1525-1403.2009.00197.x. [DOI] [PubMed] [Google Scholar]
- 70.Krames E, Poree LR, Deer T, Levy R. Rethinking algorithms of pain care: The use of the S.A.F.E. principles. Pain Med. 2009;10:1–5. doi: 10.1111/j.1526-4637.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 71.Krames ES. Interventional pain management.Appropriate when less invasive therapies fail to provide adequate analgesia. Med Clin North Am. 1999;83:787–808. doi: 10.1016/s0025-7125(05)70134-6. [DOI] [PubMed] [Google Scholar]
- 72.Krames ES, Monis S, Poree L, Deer T, Levy R. Using the SAFE principles when evaluating electrical stimulation therapies for the pain of failed back surgery syndrome. Neuromodulation. 2011;14:299–311. doi: 10.1111/j.1525-1403.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 73.Kuchta J, Koulousakis A, Sturm V. Neurosurgical pain therapy with epidural spinal cord stimulation (SCS) Acta Neurochir Suppl. 2007;97(Pt 1):65–70. doi: 10.1007/978-3-211-33079-1_8. [DOI] [PubMed] [Google Scholar]
- 74.Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: Cost-effectiveness analysis. Neurosurgery. 2002;51:106–15. doi: 10.1097/00006123-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Kumar K, North R, Taylor R, Sculpher M, Van den Abeele C, Gehring M, et al. Spinal cord stimulation vs.conventional medical management: A prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS Study) Neuromodulation. 2005;8:213–8. doi: 10.1111/j.1525-1403.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- 76.Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: Fact or fiction. Neurosurgery. 2011;69:566–78. doi: 10.1227/NEU.0b013e3182181e60. [DOI] [PubMed] [Google Scholar]
- 77.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 78.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63:762–70. doi: 10.1227/01.NEU.0000325731.46702.D9. [DOI] [PubMed] [Google Scholar]
- 79.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998;50:110–20. doi: 10.1016/s0090-3019(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 80.Kumar K, Toth C, Nath RK, Verma AK, Burgess JJ. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation: A prospective study. J Neurosurg. 1997;86:662–9. doi: 10.3171/jns.1997.86.4.0662. [DOI] [PubMed] [Google Scholar]
- 81.Kumar K, Wilson JR. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. Acta Neurochir Suppl. 2007;97(Pt 1):91–9. doi: 10.1007/978-3-211-33079-1_12. [DOI] [PubMed] [Google Scholar]
- 82.Kumar SP, Saha S. Mechanism-based classification of pain for physical therapy management in palliative care: A clinical commentary. Indian J Palliat Care. 2011;17:80–6. doi: 10.4103/0973-1075.78458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lanner G, Spendel MC. Spinal cord stimulation for the treatment of chronic non-malignant pain. Acta Neurochir Suppl. 2007;97(Pt 1):79–84. doi: 10.1007/978-3-211-33079-1_10. [DOI] [PubMed] [Google Scholar]
- 84.Leveque JC, Villavicencio AT, Bulsara KR, Rubin L, Gorecki JP. Spinal cord stimulation for failed back surgery syndrome. Neuromodulation. 2001;4:1–9. doi: 10.1046/j.1525-1403.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 85.Lind G, Schechtmann G, Winter J, Linderoth B. Drug-enhanced spinal stimulation for pain: A new strategy. Acta Neurochir Suppl. 2007;97(Pt 1):57–63. doi: 10.1007/978-3-211-33079-1_7. [DOI] [PubMed] [Google Scholar]
- 86.Linderoth B, Foreman RD. Physiology of spinal cord stimulation: Review and update. Neuromodulation. 1999;2:150–64. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 87.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289–97. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 88.Linderoth B, Meyerson BA. Spinal cord Stimulation. Techniques, indications and outcome. In: Lozano A, Gildenberg P, Tasker R, editors. Textbook of stereotactic and functional neurosurgery. Berlin: Springer-Verlag; 2009. pp. 2305–30. [Google Scholar]
- 89.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29:800–5. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial) Eur J Pain. 2008;12:1047–58. doi: 10.1016/j.ejpain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 91.Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. ASIPP-IPM.Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699–802. [PubMed] [Google Scholar]
- 92.Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, et al. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: Part 2. Therapeutic interventions. Pain Physician. 2010;13:E215–64. [PubMed] [Google Scholar]
- 93.Mannheimer C, Eliasson T, Andersson B, Bergh CH, Augustinsson LE, Emanuelsson H, et al. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. BMJ. 1993;307:477–80. doi: 10.1136/bmj.307.6902.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mannheimer C, Eliasson T, Augustinsson LE, Blomstrand C, Emanuelsson H, Larsson S, et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris: The ESBY study. Circulation. 1998;97:1157–63. doi: 10.1161/01.cir.97.12.1157. [DOI] [PubMed] [Google Scholar]
- 95.Mao J. Translational pain research: Bridging the gap between basic and clinical research. Pain. 2002;97:183–7. doi: 10.1016/S0304-3959(02)00109-4. [DOI] [PubMed] [Google Scholar]
- 96.Marvizón JC, Chen W, Murphy N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: An immunofluorescence colocalization study. J Comp Neurol. 2009;517:51–68. doi: 10.1002/cne.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mekhail N, Wentzel DL, Freeman R, Quadri H. Counting the costs: Case management implications of spinal cord stimulation treatment for failed back surgery syndrome. Prof Case Manag. 2011;16:27–36. doi: 10.1097/NCM.0b013e3181e9263c. [DOI] [PubMed] [Google Scholar]
- 98.Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T. Clinical applications of neurostimulation: Forty years later. Pain Pract. 2010;10:103–12. doi: 10.1111/j.1533-2500.2009.00341.x. [DOI] [PubMed] [Google Scholar]
- 99.Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011;11:148–53. doi: 10.1111/j.1533-2500.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 100.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 101.Meyerson BA, Brodin E, Linderoth B. Possible neurohumoral mechanisms in CNS stimulation for pain suppression. Appl Neurophysiol. 1985;48:175–80. doi: 10.1159/000101124. [DOI] [PubMed] [Google Scholar]
- 102.Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31:6–12. doi: 10.1016/j.jpainsymman.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 103.Meyerson BA, Ren B, Herregodts P, Linderoth B. Spinal cord stimulation in animal models of mononeuropathy: Effects on the withdrawal response and the flexor reflex. Pain. 1995;61:229–43. doi: 10.1016/0304-3959(94)00171-A. [DOI] [PubMed] [Google Scholar]
- 104.Mironer YE, Hutcheson JK, Satterthwaite JR, LaTourette PC. Prospective, twopart study of the interaction between spinal cord stimulation and peripheral nerve field stimulation in patients with low back pain: Development of a new spinal-peripheral neurostimulation method. Neuromodulation. 2011;14:151–4. doi: 10.1111/j.1525-1403.2010.00316.x. [DOI] [PubMed] [Google Scholar]
- 105.Moens M, De Smedt A, Brouns R, Spapen H, Droogmans S, Duerinck J, et al. Retrograde C0-C1 insertion of cervical plate electrode for chronic intractable neck and arm pain. World Neurosurg. 2011;76:352–4. doi: 10.1016/j.wneu.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 106.Moriyama K, Murakawa K, Uno T, Oseto K, Kawanishi M, Saito Y, et al. A prospective, open-label, multicenter study to assess the efficacy of spinal cord stimulation and identify patients who would benefit. Neuromodulation. 2012;15:7–11. doi: 10.1111/j.1525-1403.2011.00411.x. [DOI] [PubMed] [Google Scholar]
- 107.Murray S, Carson KG, Ewings PD, Collins PD, James MA. Spinal cord stimulation significantly decreases the need for acute hospital admission for chest pain in patients with refractory angina pectoris. Heart. 1999;82:89–92. doi: 10.1136/hrt.82.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicholson CL, Korfias S, Jenkins A. Spinal cord stimulation for failed back surgery syndrome and other disorders. Acta Neurochir Suppl. 2007;97(Pt 1):71–7. doi: 10.1007/978-3-211-33079-1_9. [DOI] [PubMed] [Google Scholar]
- 109.Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anaesth. 2001;87:107–16. doi: 10.1093/bja/87.1.107. [DOI] [PubMed] [Google Scholar]
- 110.North R, Shipley J, Prager J, Barolat G, Barulich M, Bedder M, et al. American Academy of Pain Medicine.Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(Suppl 4):S200–75. doi: 10.1111/j.1526-4637.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 111.North RB, Ewend MG, Lawton MT, Kidd DH, Piantadosi S. Failed back surgery syndrome: 5-year follow-up after spinal cord stimulator implantation. Neurosurgery. 1991;28:692–9. [PubMed] [Google Scholar]
- 112.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery. 2005;56:98–106. doi: 10.1227/01.neu.0000144839.65524.e0. [DOI] [PubMed] [Google Scholar]
- 113.North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design: Prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I: technical outcomes. Neurosurgery. 2002;51:381–9. [PubMed] [Google Scholar]
- 114.North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design: A prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: Part II-clinical outcomes. Neurosurgery. 2005;57:990–6. doi: 10.1227/01.neu.0000180030.00167.b9. [DOI] [PubMed] [Google Scholar]
- 115.North RB, Kumar K, Wallace MS, Henderson JM, Shipley J, Hernandez J, et al. Spinal cord stimulation versus re-operation in patients with failed back surgery syndrome: An international multicenter randomized controlled trial (EVIDENCE study) Neuromodulation. 2011;14:330–5. doi: 10.1111/j.1525-1403.2011.00371.x. [DOI] [PubMed] [Google Scholar]
- 116.Oakley JC, Espinosa F, Bothe H, McKean J, Allen P, Burchiel K, et al. Transverse Tripolar Spinal Cord Stimulation: Results of an International Multicenter Study. Neuromodulation. 2006;9:192–203. doi: 10.1111/j.1525-1403.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 117.Oakley JC, Prager JP. Spinal cord stimulation: Mechanisms of action. Spine (Phila Pa 1976) 1976;2002(27):2574–83. doi: 10.1097/00007632-200211150-00034. [DOI] [PubMed] [Google Scholar]
- 118.Ovelmen-Levitt J. Abnormal physiology of the dorsal horn as related to the deafferentation syndrome. Appl Neurophysiol. 1988;51:104–16. doi: 10.1159/000099953. [DOI] [PubMed] [Google Scholar]
- 119.Parker JL, Karantonis DM, Single PS, Obradovic M, Cousins MJ. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153:593–601. doi: 10.1016/j.pain.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 120.Pedrini L, Magnoni F. Spinal cord stimulation for lower limb ischemic pain treatment. Interact Cardiovasc Thorac Surg. 2007;6:495–500. doi: 10.1510/icvts.2006.150185. [DOI] [PubMed] [Google Scholar]
- 121.Prager JP. What does the mechanism of spinal cord stimulation tell us about complex regional pain syndrome? Pain Med. 2010;11:1278–83. doi: 10.1111/j.1526-4637.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 122.Prasad A, Sahin M. Characterization of neural activity recorded from the descending tracts of the rat spinal cord. Front Neurosci. 2010;4:21. doi: 10.3389/fnins.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pudenz RH, Bullara LA, Jacques S, Hambrecht FT. Electrical stimulation of the brain. III. The neural damage model. Surg Neurol. 1975;4:389–400. [PubMed] [Google Scholar]
- 124.Quigley DG, Arnold J, Eldridge PR, Cameron H, McIvor K, Miles JB, et al. Long-term outcome of spinal cord stimulation and hardware complications. Stereotact Funct Neurosurg. 2003;81:50–6. doi: 10.1159/000075104. [DOI] [PubMed] [Google Scholar]
- 125.Quigley MR, Bost J, Maroon JC, Elrifai A, Panahandeh M. Outcome after microdiscectomy: Results of a prospective single institutional study. Surg Neurol. 1998;49:263–7. doi: 10.1016/s0090-3019(97)00448-5. [DOI] [PubMed] [Google Scholar]
- 126.Rainov NG, Demmel W, Heidecke V. Dual electrode spinal cord stimulation in chronic leg and back pain. Acta Neurochir Suppl. 2007;97(Pt 1):85–9. doi: 10.1007/978-3-211-33079-1_11. [DOI] [PubMed] [Google Scholar]
- 127.Rainov NG, Heidecke V. Hardware failures in spinal cord stimulation (SCS) for chronic benign pain of spinal origin. Acta Neurochir Suppl. 2007;97(Pt 1):101–4. doi: 10.1007/978-3-211-33079-1_13. [DOI] [PubMed] [Google Scholar]
- 128.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 129.Raslan AM, McCartney S, Burchiel KJ. Management of chronic severe pain: Spinal neuromodulatory and neuroablative approaches. Acta Neurochir Suppl. 2007;97(Pt 1):33–41. doi: 10.1007/978-3-211-33079-1_4. [DOI] [PubMed] [Google Scholar]
- 130.Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain. 2006;22:353–8. doi: 10.1097/01.ajp.0000177793.01415.bd. [DOI] [PubMed] [Google Scholar]
- 131.Schechtmann G, Lind G, Meyerson B, Linderoth B. Estimulación espinal crónica (EEC). Mecanismos de acción y experiencia clínica en el dolor neuropático. In: Basso A, Carrizo G, Mezzadri JJ, Goland J, Socolovsky M, editors. Neurocirugia. Aspectos clínicos y quirúrgicos. Rosario: Corpus Libros médicosy científicos; 2010. pp. 755–65. [Google Scholar]
- 132.Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation. 2011;14:312–8. doi: 10.1111/j.1525-1403.2011.00372.x. [DOI] [PubMed] [Google Scholar]
- 133.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 134.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967;46:489–91. [PubMed] [Google Scholar]
- 135.Shrivastav M, Musley S. Spinal cord stimulation for complex regional pain syndrome. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2033–6. doi: 10.1109/IEMBS.2009.5334418. [DOI] [PubMed] [Google Scholar]
- 136.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: Systematic review and economic evaluation. Health Technol Assess. 2009;13:iii(ix-x):1–154. doi: 10.3310/hta13170. [DOI] [PubMed] [Google Scholar]
- 137.Sindou MP, Mertens P, Bendavid U, García-Larrea L, Mauguière F. Predictivevalue of somatosensory evoked potentials for long-lasting pain relief after spinal cord stimulation: Practical use for patient selection. Neurosurgery. 2008;52:1374–83. doi: 10.1227/01.neu.0000064570.17828.88. [DOI] [PubMed] [Google Scholar]
- 138.Slavin KV, Vannemreddy PS, Goellner E, Alaraj AM, Aydin S, Eboli P, et al. Use of cervical spinal cord stimulation in treatment and prevention of arterial vasospasm after aneurysmal subarachnoid hemorrhage. Neuroradiol J Digit. 2011;1:139–43. doi: 10.1177/197140091102400119. [DOI] [PubMed] [Google Scholar]
- 139.Spincemaille GH, de Vet HC, Ubbink DT, Jacobs MJ. The results of spinal cord stimulation in critical limb ischaemia: A review. Eur J Vasc Endovasc Surg. 2001;21:99–105. doi: 10.1053/ejvs.2000.1291. [DOI] [PubMed] [Google Scholar]
- 140.Spincemaille GH, Klomp HM, Steyerberg EW, van Urk H, Habbema JD. ESES study group. Technical data and complications of spinal cord stimulation: Data from a randomized trial on critical limb ischemia. Stereotact Funct Neurosurg. 2000;74:63–72. doi: 10.1159/000056465. [DOI] [PubMed] [Google Scholar]
- 141.Stanton-Hicks M. Complex regional pain syndrome: Manifestations and the role of neurostimulation in its management. J Pain Symptom Manage. 2006;31(4 Suppl):S20–4. doi: 10.1016/j.jpainsymman.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: Changing concepts and taxonomy. Pain. 1995;63:127–33. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 143.Stiller CO, Cui J-G, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of GABA in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–75. doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- 144.Straus BN. Chronic pain of spinal origin: The costs of intervention. Spine (Phila Pa 1976) 1976;27:2614–9. doi: 10.1097/00007632-200211150-00041. [DOI] [PubMed] [Google Scholar]
- 145.Sweet WH, Wepsic JG. Treatment of chronic pain by stimulation of fibers of primary afferent neuron. Trans Am Neurol Assoc. 1968;93:103–5. [PubMed] [Google Scholar]
- 146.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain / failed back surgery syndrome: Results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Suppl):S13–9. doi: 10.1016/j.jpainsymman.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 147.Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26:463–9. doi: 10.1097/AJP.0b013e3181daccec. [DOI] [PubMed] [Google Scholar]
- 148.Taylor RS, Taylor RJ, Van Buyten JP, Buchser E, North R, Bayliss S. The cost effectiveness of spinal cord stimulation in the treatment of pain: A systematic review of the literature. J Pain Symptom Manage. 2004;27:370–8. doi: 10.1016/j.jpainsymman.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 149.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: A systematic review and analysis of prognostic factors. Spine (Phila Pa1976) 1976;2005(30):152–60. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 150.Tedesco A, D’Addato M. Spinal cord stimulation for patients with critical limb ischemia: Immediate and long-term clinical outcome from the prospective Italian register. Neuromodulation. 2004;7:97–102. doi: 10.1111/j.1094-7159.2004.04013.x. [DOI] [PubMed] [Google Scholar]
- 151.TenVaarwerk IA, Jessurun GA, DeJongste MJ, Andersen C, Mannheimer C, Eliasson T, et al. Clinical outcome of patients treated with spinal cord stimulation for therapeutically refractory angina pectoris. The Working Group on Neurocardiology. Heart. 1999;82:82–8. doi: 10.1136/hrt.82.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348:1698–701. doi: 10.1016/S0140-6736(96)02467-1. [DOI] [PubMed] [Google Scholar]
- 153.Tiede JM, Ghazi SM, Lamer TJ, Obray JB. The use of spinal cord stimulation in refractory abdominal visceral pain: Case reports and literature review. Pain Pract. 2006;6:97–202. doi: 10.1111/j.1533-2500.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 154.Tomycz ND, Deibert CP, Moossy JJ. Cervicomedullary junction spinal cord stimulation for head and facial pain. Headache. 2011;51:418–25. doi: 10.1111/j.1526-4610.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 155.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: A systematic review of effectiveness and complications. Pain. 2004;108:137–47. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 156.Ubbink DT, Vermeulen H, Spincemaille GH, Gersbach PA, Berg P, Amann W. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91:948–55. doi: 10.1002/bjs.4629. [DOI] [PubMed] [Google Scholar]
- 157.van Buyten JP. Neurostimulation for chronic neuropathic back pain in failed back surgery syndrome. J Pain Symptom Manage. 2006;31(4 Suppl):S25–9. doi: 10.1016/j.jpainsymman.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 158.van Eijs F, Smits H, Geurts JW, Kessels AG, Kemler MA, van Kleef M, et al. Brush-evoked allodynia predicts outcome of spinal cord stimulation in complex regional pain syndrome type 1. Eur J Pain. 2010;14:164–9. doi: 10.1016/j.ejpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 159.van Eijs F, Stanton-Hicks M, Van Zundert J, Faber CG, Lubenow TR, Mekhail N, et al. Evidence-based interventional pain medicine according to clinical diagnoses. 16 Complex regional pain syndrome. Pain Pract. 2011;11:70–87. doi: 10.1111/j.1533-2500.2010.00388.x. [DOI] [PubMed] [Google Scholar]
- 160.Velasco F, Carrillo-Ruiz JD, Brito F, Velasco M, Velasco AL, Marquez I, et al. Double-blind, randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1071–81. doi: 10.1111/j.1528-1167.2005.70504.x. [DOI] [PubMed] [Google Scholar]
- 161.Velasco F, Velasco AL, Núñez JM, Castro G, Carrillo-Ruiz JD, Soto J, et al. Neuromodulation: History, principles and current trends. In: Cukiert A, editor. Neuromodulation. Sao Paulo: Alaúde Editorial; 2010. pp. 22–36. [Google Scholar]
- 162.Villavicencio AT, Leveque JC, Rubin L, Bulsara K, Gorecki JP. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 2000;46:399–405. doi: 10.1097/00006123-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 163.Viswanathan A, Phan PC, Burton AW. Use of spinal cord stimulation in the treatment of phantom limb pain: Case series and review of the literature. Pain Pract. 2010;10:479–84. doi: 10.1111/j.1533-2500.2010.00374.x. [DOI] [PubMed] [Google Scholar]
- 164.Waguespack A, Schofferman J, Slosar P, Reynolds J. Etiology of long-term failures of lumbar spine surgery. Pain Med. 2002;3:18–22. doi: 10.1046/j.1526-4637.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 165.Williams KA, Gonzalez-Fernandez M, Hamzehzadeh S, Wilkinson I, Erdek MA, Plunkett A, et al. A multi-center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain Med. 2011;12:1142–53. doi: 10.1111/j.1526-4637.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 166.Willis KD. A simple approach to outcomes assessment of the therapeutic and cost-benefit success rates for spinal cord stimulation therapy. Anesthesiol Clin North America. 2003;21:817–23. doi: 10.1016/s0889-8537(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 167.Wolter T, Kieselbach K. Spinal cord stimulation for Raynaud's syndrome: Long-term alleviation of bilateral pain with a single cervical lead. Neuromodulation. 2011;14:229–33. doi: 10.1111/j.1525-1403.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- 168.Wolter T, Winkelmüller M. Continuous versus intermittent spinal cord stimulation: An analysis of factors influencing clinical efficacy. Neuromodulation. 2012;15:13–9. doi: 10.1111/j.1525-1403.2011.00410.x. [DOI] [PubMed] [Google Scholar]
- 169.Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79:223–33. doi: 10.1016/s0304-3959(98)00169-9. [DOI] [PubMed] [Google Scholar]
- 170.Yakovlev AE, Resch BE. Treatment of urinary voiding dysfunction syndromes with spinal cord stimulation. Clin Med Res. 2010;8:22–4. doi: 10.3121/cmr.2010.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yearwood TL, Hershey B, Bradley K, Lee D. Pulse width programming in spinal cord stimulation: A clinical study. Pain Physician. 2010;13:321–35. [PubMed] [Google Scholar]
- 172.Yuen TG, Agnew WF, Bullara LA, Jacques S, McCreery DB. Histological evaluation of neural damage from electrical stimulation: Considerations for the selection of parameters for clinical application. Neurosurgery. 1981;9:292–9. [PubMed] [Google Scholar]


