Abstract
Vagus nerve stimulation (VNS) is an adjunctive treatment for adult patients with pharmacoresistant epilepsy. Little is known about VNS therapy for children with epilepsy. This article will: (1) Review the contemporary medical literature related to VNS therapy in children with epilepsy, (2) describe the experience of VNS treatment in 153 children less than 18 years of age, in the University of California, Los Angeles (UCLA) Pediatric Epilepsy Surgery Program, from 1998 to 2012, and (3) describe the surgical technique used for VNS implantation at UCLA. Review of the literature finds that despite different etiologies and epilepsy syndromes in children, VNS appears to show a similar profile of efficacy for seizure control compared to adults, and low morbidity and mortality. The UCLA experience is similar to that reported in the literature for children. VNS constitutes about 21% of our pediatric epilepsy surgery volume. We have implanted VNS in infants as young as six months of age and the most common etiology is the Lennox-Gastaut Syndrome. About 5% of the patients are seizure-free with VNS therapy and there is a low rate of surgically related complications. The UCLA surgical approach emphasizes minimal direct manipulation of the vagus nerve and adequate wire loops, to prevent a lead fracture. In summary, VNS is a viable palliative treatment for medically refractory epilepsy in children, with outcomes and complications equal to adult patients. Being a small child is not a contraindication for VNS therapy, if needed for refractory epilepsy.
Keywords: Intractable, pediatric, seizures, surgery
INTRODUCTION
Vagus nerve stimulation (VNS) using the Cyberonics system was approved by the US Food and Drug Administration (FDA), in 1997, as an adjunctive treatment for patients over the age of 12 years, with medically resistant focal epilepsy. This was based on a randomized controlled trial, which did not include young children. [1] Like all FDA approved anti-epilepsy drugs (AED), VNS was subsequently utilized in children without Class I data (randomized controlled trials). Hence, it is unclear if children who often have generalized epilepsies, such as Lennox-Gastaut syndrome, and developmental etiologies, such as, malformations of cortical development, [4] respond to VNS therapy differently than adults with focal epilepsy.
This article was designed to fulfill the following purposes. First, to review the use and efficacy of VNS therapy for epilepsy in children, based on contemporary medical literature. Next, to describe the experience of VNS therapy in the University of California, Los Angeles (UCLA) pediatric epilepsy surgery program, of 153 children, under age 18 years at time of implantation. Finally, to describe the surgical technique used for VNS implantation at UCLA. By design, we exclude a discussion on the role of VNS for non-epilepsy conditions, such as, movement disorder and depression. [10,27]
MATERIALS AND METHODS
A PubMed search using the keywords ((pediatric) OR (children)) AND (vagal nerve stimulation) AND (epilepsy)) was undertaken. Clinical articles from English-language journals were used, especially those published since 1999. For the UCLA cohort, an existing data base containing patients who had VNS implantation was used to identify individuals under the age of 18 years at the time of surgery. Abstracted, was information related to age at seizure onset, age at surgery, epilepsy duration, and etiology as previously described. [11]
Literature review
History of vagus nerve stimulation therapy
Vagus nerve stimulation for epilepsy was in use experimentally since the late 1980s, where more than 800 patients had been implanted between 1988 and 1997. [15] Early studies by Murphy and Hornig suggested that VNS treatment was well-tolerated by children and could result in a dramatic reduction in the seizure burden. [12,18] The landmark study for children was published in 1999, by the Pediatric VNS Study Group. [17] Sixty children (ages 3 to 18 years) with refractory epilepsy, were implanted under controlled or compassionate use protocols. At six months, 55 patients experienced a median reduction of 31% in the seizure burden, at 12 months 51 patients experienced a 34% median reduction, and at 18 months 46 patients experienced a 42% median reduction.
Seizure control
Most studies of VNS in children have shown significant reductions in seizure burden, and efficacy appears to improve if the the system is used for longer periods. It must be remembered, however, that most of the studies reported to date have been observational, non-randomized, open-label trials. Benifla et al., in their report on the Hospital for Sick Children, described their experience with VNS implantation, and found that after a mean follow-up of 31 months, 38% of the patients had a reduction in seizure frequency of more than 90%. They also noted that 38% of children were non-responders. [6] Murphy et al. found that 45% of the children with VNS experienced greater than 50% reduction in seizure frequency at six months, with 18% of the children being seizure-free. [19] Rossignol and colleagues followed a cohort of 28 adolescents and children implanted with VNS, for nonsurgical refractory epilepsy, and reported that 68% experienced greater than 50% reduction in seizure frequency at two years, with 14% being seizure-free. [26] Rychlicki et al. reported that in 34 children who received VNS, a 39% reduction in seizure burden was seen at three months, 49% reduction at one year, 61% reduction at two years, and 71% at three years. [29] In their cohort, they found that patients with partial epilepsy had better outcomes than those with the Lenox-Gastaut syndrome.
A larger study by Colicchio et al. examined VNS efficacy in 135 patients with refractory epilepsy, of which 81 were children. [8] The children's cohort consisted of patients with Lenox-Gastaut syndrome, multifocal epilepsy, and partial epilepsy. All experienced a statistically significant reduction in seizure frequency, with an increase in response, over time. Interestingly, adolescents and children had the best clinical responses, as did patients with multifocal epilepsy, compared to those with Lenox-Gastaut. In fact, those implanted at younger ages tended to have the best response in seizure reduction at follow-up. Kabir et al. described that 55% of the adult and pediatric patients had a satisfactory seizure frequency reduction following VNS, although they failed to find a correlation between the outcome and age at implantation. [14] Thus, there was an unconfirmed suggestion in the literature that implanting children at younger ages may be of greater benefit than waiting. However, further studies with larger cohorts are needed for validation.
Studies also suggest that VNS therapy may be more beneficial for certain seizure types or syndromes in children. Zamponi and colleagues examined the efficacy of VNS in patients with Lennox-Gastaut Syndrome or severe epilepsy with multiple independent spike foci that experienced drop attacks and pharmacoresistant seizures. [34] They found a 41% reduction in seizure frequency at six months, a 50% reduction at one year, and 54% reduction at three years. The efficacy appeared to be less for drop attacks, where, about 20% of then patients had a reduction of 50% or more and 17% experienced a reduction in intensity and duration, but not in frequency.
Vagus nerve stimulation has been explored as a potential treatment option for less common epileptic disorders. In a study of ten patients with tuberous sclerosis and refractory epilepsy, treated with VNS, nine patients experienced at least 50% reduction in seizure burden, with 50% having a 90% or greater reduction in seizure burden. [22] Another study looked at VNS efficacy in the Dravet syndrome (severe myoclonic epilepsy in infancy (SMEI)) and found a 12% reduction in seizure frequency at three months, 6% reduction at six months, and 31% reduction at one year. [33] Almost two-thirds of the Dravet patients experienced at least a 33% reduction in seizure frequency at one year, and half of patients experienced a reduction in seizure burden of greater than 50%. Many of the parents of Dravet patients reported an increase in alertness and communication skills after VNS therapy for one year. One case of VNS in a child with ring chromosome 20 suggested a significant reduction in seizure burden. [7] One report of VNS for refractory seizures in children with hypothalamic hamartoma suggested beneficial effects on seizure frequency as well as autistic behaviors. [20]
Quality of life and development
Authors have often reported an improvement in the qualify of life measures associated with VNS therapy in children. For example, Mikati et al. examined quality of life changes following VNS for refractory epilepsy and showed an improvement in the total quality of life and social indices that correlated with the reduction in seizure burden. [16] Others have reported similar beneficial effects on the quality of life assessments following VNS therapy in children. [25,35] A more focused review of very young children, less than three years of age, using VNS therapy for catastrophic epilepsy and cognitive impairment suggested increases in quality of life, parental satisfaction, and even achievement of developmental milestones. [35] In another series of VNS in young children, no changes in cognitive functioning were noted, but there were positive effects on alertness, playfulness, global interaction, and nighttime sleep. [14] That said, improvements in adaptive behavior have not been noted in all studies involving VNS therapy in children, and whether this is a direct effect of the stimulation or associated with the reduced seizure burden is not clear. [23]
Adverse effects and complications
Side effects with VNS therapy are usually transient and mild. They include local discomfort at the battery implantation site, throat pain, coughing, and voice changes. [6,26,29,31] Hoarseness appears to be the most common complaint in older children, and a small number of cases may experience mild sleep apnea. [28] Endoscopic evaluation of the larynx of patients who have undergone VNS has shown some vocal cord dysfunction that may be a transient paresis, secondary to vagal manipulation during surgery or stimulation-induced contraction of the layngeal musculature. [32] Sleep apnea, however, may be secondary to respiratory pattern changes from VNS activation, including reductions in effort and tidal volume. [21] A single case report described obstructive sleep apnea resulting from recurrent vocal cord adduction during VNS. [3] VNS has not been shown to cause aspiration, which was an initial possible concern during the pivotal VNS trial. [30]
Concerns were voiced about VNS affecting systemic inflammation and the cardiac autonomic tone. Barone et al., performed Holter monitoring and checked the serum cytokine levels in patients on VNS therapy. [5] They found no significant effect on either process. In a study looking at autonomic regulation and heart rate variability, Jansen et al. noted that VNS restored autonomic modulation and vagal tone that was dysfunctional in patients with refractory epilepsy. [13] That study suggested that changes in autonomic function could be related to morbidity and mortality in refractory epilepsy, and that VNS could have cardioprotective effects in addition to its antiepileptic efficacy.
Surgically related infections with VNS hardware are reported at a frequency of approximately 3-5%. [2,19,31] Patel and Edwards have reported that in all cases of VNS pocket infections, removal of the device is necessary to achieve a cure. [24] Some authors, however, suggest that IV antibiotics without hardware removal may be an option for the management of these cases. [2]
UNIVERSITY OF CALIFORNIA, LOS ANGELES EXPERIENCE WITH VAGUS NERVE STIMULATION IN CHILDREN
The UCLA experience with VNS for children with epilepsy, mirror reports from other centers. From 1998 to July 2012, we implanted VNS systems in 153 children under the age of 18 years. This represents 49% of the 317 VNS implantations at UCLA for all ages. For children, 96 (63%) were less than 12 years of age, and 18 (12%) less than five years of age at surgery. The youngest case was implanted at six months of age, after being in the Pediatric Intensive Care Unit for uncontrolled status epilepticus for many weeks. The device reduced the seizure frequency, but did not terminate the status epilepticus.
Vagus nerve stimulation implantation accounted for 21% of all pediatric surgical cases (resections, corpus callosotomy, and VNS). The introduction of VNS therapy, in 1998, reduced the number of corpus callosotomy operations, as seen when comparing 1986 - 1997 with 1998 - 2009. [5] The epilepsy syndromes and etiologies varied considerably within this pediatric cohort. Lennox-Gastaut Syndrome with multiple seizure types and drop attacks was the most frequent, with 88 cases (57%). Other syndromes included, atypical absence seizures (n = 6), history of prior viral or bacterial central nervous system (CNS) infections (n = 4), Rett's syndrome (n = 3), Doose syndrome (n = 2), Noonam's syndrome (n = 1), tuberous sclerosis complex (n = 2), and rarer conditions, such as, bilateral nodular heterotopia, ring chromosome-14, and Down's syndrome. Nine cases were implanted after failed surgery, which involved resections or multiple subpial transections. Four patients eventually underwent corpus callosotomy after failure of a trial of VNS.
Clinical epilepsy variables were similar compared with other pediatric series involving vagus nerve stimulator implantation. Age at seizure onset was generally very young, before the age of three years [Figure 1a], age at surgery was between the ages of five and 17 years [Figure 1b], and epilepsy duration averaged about eight years [Figure 1c]. We typically take an average from 10 to 15 new VNS implantations per year [Figure 1d]. Patients were taking a mean (± SD) of 2.8 ± 1.0 different anti-epilepsy drugs before VNS implantation. Acute complications included three infections requiring removal of the device and leads, one case of clinically evident temporary vocal cord paralysis, and two cases of fractured leads, often months to years after surgery. These two cases had complete replacement of the leads without complication.
Figure 1.
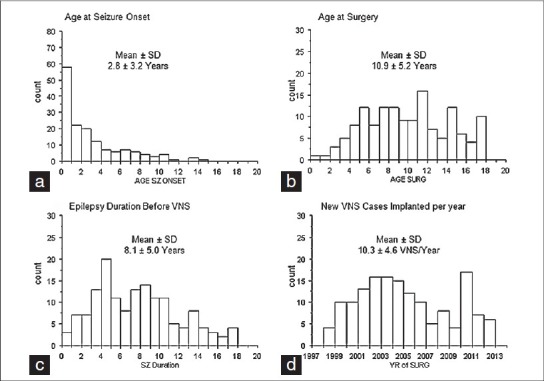
Bar graphs showing the age at seizure onset (a), age at surgery (b), epilepsy duration, and (c) number of new vagus nerve stimulation implantations per year (d) for children (less than age 18 years) at UCLA. Mean (± SD) are shown above each graph
Complete seizure freedom with VNS therapy occurred in about 5% of the cases in our series. In many of these cases it can be directly attributed to the stimulator therapy, as seizures have returned, because the battery became depleted and seizure control was re-established once the generator was replaced. Of the 118 patients with VNS systems in place for five years or greater, 34 (29%) had returned for replacing the generator, due to depletion of the battery. Another ten patients had asked for the VNS system to be removed due to being ineffective or because they did not like the therapy. These result outcomes should be taken cautiously, as we presume that many children and families transferred VNS care to other facilities after implantation at UCLA. In our pediatric cohort, we do not know of any cases of sudden unexpected death related to epilepsy (SUDEP).
UNIVERSITY OF CALIFORNIA, LOS ANGELES SURGICAL TECHNIQUE FOR VAGUS NERVE STIMULATION
This technique was developed by the senior author (GWM), and has worked well in the pediatric population The procedure is performed under general endotracheal anesthesia as a same day operation. Usually, peripheral intravenous access is all that is necessary and no Foley catheter is inserted. Appropriate intravenous antibiotics are given before the start of the procedure. The left side of the neck and chest region is prepped and draped. Several cubic centimeters of 0.25% Marcaine with epinephrine is infused in the two-planned incisions [Figure 2b], with each described below.
Figure 2.
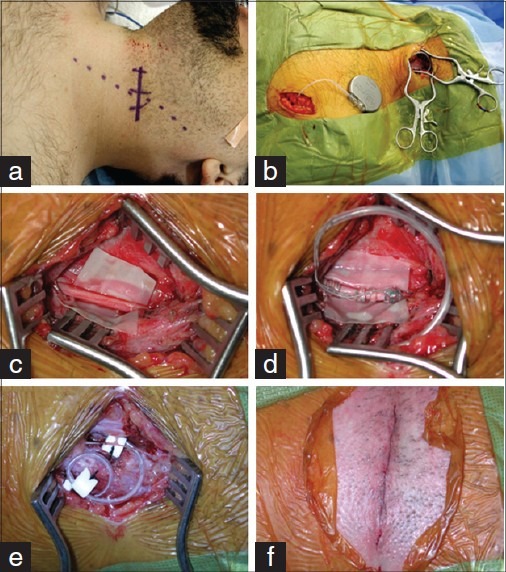
Operative approach for vagus nerve stimulation implantation used at UCLA. (a) Example of marked neck skin incision about halfway between the clavicle and mastoid process, based on the palpated medial edge of the sternocleidomastoid muscle. (b) View of the two incisions (left neck and chest), implanted leads, tunneled leads, and generator, prior to closure. (c) Higher magnified view of the left vagus nerve with a cut piece of glove under it, to help with placement of the leads. (d) View of the vagus nerve after placement of the leads. (e) Lead loops in the neck dissection. We typically place the fi rst along the medial edge of the sternocleidomastoid muscle and the second over the body of the muscle. (f) Neck incision after subcuticular closure
We start with the chest dissection. The skin incision is made parallel to the pectoralis major on the left side [Figure 2d]. The dissection, best accomplished with curved scissors, should be directly above the fascia of the pectoralis muscle. Once tested, to be sure that the pocket is large enough for the generator and any extra lead wire, especially in younger children, a wet sponge is placed for hemostasis, while work continues on the neck dissection.
The neck incision is transverse, approximately halfway between the clavicle and the mastoid process, based on the medial edge of the sternocleidomastoid muscle [Figure 2a]. The skin is sharply incised with a #15 or #10 blade (depending on the size of the child), and hemostasis is obtained with bipolar coagulation. Sharp dissection is carried down to the platysma. The platysma and the fascia of the sternocleidomastoid muscle are incised parallel to the skin incision, and a pocket is created between the muscle and the fascia that will be the location of the stress wire loops at the end of the case [Figure 2e]. Care is taken to expose and preserve the external jugular vein and any superficial cutaneous nerves in the field. Self-retaining retractors are used (either Wheatlander's or Henderson's), with blunt tips.
The medial edge of the sternocleidomastoid muscle and the body of the muscle is dissected and brought laterally to expose the carotid sheath. Releasing a long portion of the muscle helps convert what has been a transverse approach into a longitudinal dissection, parallel to the nerve and carotid artery. The carotid sheath is entered parallel to the major vessels. The medial edge of the internal jugular vein is identified and brought laterally, sometimes sacrificing the facial vein or other large veins crossing the field. It is not uncommon to find several, often large, lymph nodes within the carotid sheath in patients with a long-standing history of taking AEDs. These should be dissected carefully as each has a small draining vein.
The internal jugular vein is retracted laterally and the carotid artery medially. Between these two, the vagus nerve must be identified making sure that it is not confused with the cardiac branch of the vagus nerve, which can cross the field more superficially. After the vagus nerve is dissected a piece of glove cut into a square or rectangle is placed underneath it [Figure 2c]. The retractors are temporally removed with the piece of glove marking the vagus nerve.
The tunneling tool is passed between the neck and the chest incision. After visual inspection of the leads (to ensure they are intact and the covering is not stripped), they are carefully passed from the neck to the chest. The proximal leads are then attached around the vagus nerve. We prefer to do this using fine DeBakey forceps with the left (non-dominant) hand holding the spiral electrode at its base and the right (dominant) hand manipulating the electrode around the nerve. The nerve itself is not grasped, and this is performed under loupe magnification. It is important to make sure the leads are in adequate contact and are placed in the correct orientation ([Figure 2d]; positive proximal, negative in the middle, and anchor distal). We prefer to have the hubs of the electrodes pointed toward the surgeon in case the leads need to be eventually removed or replaced. That way the lead system in encountered before the nerve. The distal lead exiting out of the chest incision is attached to the generator with a self-locking system.
At this point the VNS system is ready for the initial programming and testing. The programming paddle and computer are wrapped in a sterile plastic sheet, and the programming is performed through it. The device is first interrogated to confirm the model and serial number and these are recorded for the operative report. The patient's initials and the implant date are programmed into the device. A Systems Diagnostic program is initiated and the impedance is recorded, for the record. The device is then programmed for 30 seconds at 1 milliamp. At this point we assess that the patient is not paralyzed and we watch for bradycardia or abnormal diaphragmatic movement. It is useful to bathe the proximal lead in situ with sterile saline during the programming.
The rubber pledget under the proximal lead and nerve is removed. Two Teflon pledgets are used in the neck to secure the wire loops, which prevents unintended wire breakage or migration [Figure 2e]. The first pledget typically holds the loop between the carotid sheath and the medial edge of the muscle, and the second loop is between the muscle and fascia. The wound is then copiously irrigated. The platysma is closed with inverted interrupted 3-0 Vicryls. The subcutaneous layer is closed with inverted interrupted 3-0 Vicryls, and the skin is closed with a 4-0 Monocryl subcuticular stitch [Figure 2f]. Additional Marcaine with epinephrine, Mastisol, and Steri-Strips are used, as well as a local dressing.
The generator is placed into the chest pocket and secured to the fascia of the muscle, using an O silk suture. A second and final systems diagnostic check is performed with the programming paddle on the skin over the generator, to ensure adequate impedance and to check that the system has been turned off. The wound is then copiously irrigated and closed in layers, similar to the neck incision.
The patient is typically extubated in the Operating Room and transferred to the post-anesthesia care unit (PACU). It is our policy to have the patients fully awake, having taken their normal AEDs without vomiting, before release. Patients are given oral antibiotics for 24 hours and a few pain medications. Children and parents are instructed to keep the local dressing on for five days, and can get it wet thereafter. For children with very frequent seizures, the device may be first programed in the PACU once, fully away and able to protect their airway. Children typically return to school within a few days post surgery and are seen at a one-month visit to check the wound sites.
CONCLUSIONS
Vagus nerve stimulation is a viable palliative surgical strategy in children with refractory epilepsy. The treatment is efficacious for many children and is generally well-tolerated. Seizure control and complications are very similar to what is observed in adult VNS therapy. Future studies still need to revolve if some seizure types and epilepsy syndromes respond better than others in children, and if early VNS therapy improves the chance for seizure control and improved quality of life measures. [9] It will also be important to begin to understand how VNS therapy compares and contrasts with other neurostimulation devices in the control of epilepsy, in medically refractory children, so that optimal and cost-effective management strategies can be developed.
Footnotes
Disclaimer: This study was partially supported by the NIH grant R01 NS38992 to GWM.
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/5/269/103017
Contributor Information
Jason S. Hauptman, Email: jhauptman@mednet.ucla.edu.
Gary W. Mathern, Email: gmathern@ucla.edu.
REFERENCES
- 1.A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995;45:224–30. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 2.Air EL, Ghomri YM, Tyagi R, Grande AW, Crone K, Mangano FT. Management of vagal nerve stimulator infections: Do they need to be removed? J Neurosurg Pediatr. 2009;3:73–8. doi: 10.3171/2008.10.PEDS08294. [DOI] [PubMed] [Google Scholar]
- 3.Aron M, Vlachos-Mayer H, Dorion D. Vocal cord adduction causing obstructive sleep apnea from vagal nerve stimulation: Case report. J Pediatr. 2012;160:868–70. doi: 10.1016/j.jpeds.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: Update 2012. Brain. 2012;135(Pt 5):1348–69. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone L, Colicchio G, Policicchio D, Di Clemente F, Di Monaco A, Meglio M, et al. Effect of vagal nerve stimulation on systemic inflammation and cardiac autonomic function in patients with refractory epilepsy. Neuroimmunomodulation. 2007;14:331–6. doi: 10.1159/000127360. [DOI] [PubMed] [Google Scholar]
- 6.Benifla M, Rutka JT, Logan W, Donner EJ. Vagal nerve stimulation for refractory epilepsy in children: Indications and experience at The Hospital for Sick Children. Childs Nerv Syst. 2006;22:1018–26. doi: 10.1007/s00381-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 7.Chawla J, Sucholeiki R, Jones C, Silver K. Intractable epilepsy with ring chromosome 20 syndrome treated with vagal nerve stimulation: Case report and review of the literature. J Child Neurol. 2002;17:778–80. doi: 10.1177/08830738020170101805. [DOI] [PubMed] [Google Scholar]
- 8.Colicchio G, Policicchio D, Barbati G, Cesaroni E, Fuggetta F, Meglio M, et al. Vagal nerve stimulation for drug-resistant epilepsies in different age, aetiology and duration. Childs Nerv Syst. 2010;26:811–9. doi: 10.1007/s00381-009-1069-2. [DOI] [PubMed] [Google Scholar]
- 9.Crumrine PK. Vagal nerve stimulation in children. Semin Pediatr Neurol. 2000;7:216–23. doi: 10.1053/spen.2000.9218. [DOI] [PubMed] [Google Scholar]
- 10.Handforth A, Ondo WG, Tatter S, Mathern GW, Simpson RK, Jr, Walker F, et al. Vagus nerve stimulation for essential tremor: A pilot efficacy and safety trial. Neurology. 2003;61:1401–5. doi: 10.1212/01.wnl.0000094355.51119.d2. [DOI] [PubMed] [Google Scholar]
- 11.Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, et al. Improved outcomes in pediatric epilepsy surgery: The UCLA experience, 1986-2008. Neurology. 2010;74:1768–75. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornig GW, Murphy JV, Schallert G, Tilton C. Left vagus nerve stimulation in children with refractory epilepsy: An update. South Med J. 1997;90:484–8. doi: 10.1097/00007611-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jansen K, Vandeput S, Milosevic M, Ceulemans B, Van Huffel S, Brown L, et al. Autonomic effects of refractory epilepsy on heart rate variability in children: Influence of intermittent vagus nerve stimulation. Dev Med Child Neurol. 2011;53:1143–9. doi: 10.1111/j.1469-8749.2011.04103.x. [DOI] [PubMed] [Google Scholar]
- 14.Kabir SM, Rajaraman C, Rittey C, Zaki HS, Kemeny AA, McMullan J. Vagus nerve stimulation in children with intractable epilepsy: Indications, complications and outcome. Childs Nerv Syst. 2009;25:1097–100. doi: 10.1007/s00381-009-0849-z. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan RS. Vagus nerve stimulation for intractable epilepsy: A review. J Clin Neurophysiol. 1997;14:358–68. doi: 10.1097/00004691-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Mikati MA, Ataya NF, El-Ferezli JC, Baghdadi TS, Turkmani AH, Comair YG, et al. Quality of life after vagal nerve stimulator insertion. Epileptic Disord. 2009;11:67–74. doi: 10.1684/epd.2009.0244. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy.The Pediatric VNS Study Group. J Pediatr. 1999;134:563–6. doi: 10.1016/s0022-3476(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JV, Hornig G, Schallert G. Left vagal nerve stimulation in children with refractory epilepsy.Preliminary observations. Arch Neurol. 1995;52:886–9. doi: 10.1001/archneur.1995.00540330064016. [DOI] [PubMed] [Google Scholar]
- 19.Murphy JV, Torkelson R, Dowler I, Simon S, Hudson S. Vagal nerve stimulation in refractory epilepsy: The first 100 patients receiving vagal nerve stimulation at a pediatric epilepsy center. Arch Pediatr Adolesc Med. 2003;157:560–4. doi: 10.1001/archpedi.157.6.560. [DOI] [PubMed] [Google Scholar]
- 20.Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol. 2000;23:167–8. doi: 10.1016/s0887-8994(00)00170-3. [DOI] [PubMed] [Google Scholar]
- 21.Nagarajan L, Walsh P, Gregory P, Stick S, Maul J, Ghosh S. Respiratory pattern changes in sleep in children on vagal nerve stimulation for refractory epilepsy. Can J Neurol Sci. 2003;30:224–7. doi: 10.1017/s0317167100002638. [DOI] [PubMed] [Google Scholar]
- 22.Parain D, Penniello MJ, Berquen P, Delangre T, Billard C, Murphy JV. Vagal nerve stimulation in tuberous sclerosis complex patients. Pediatr Neurol. 2001;25:213–6. doi: 10.1016/s0887-8994(01)00312-5. [DOI] [PubMed] [Google Scholar]
- 23.Parker AP, Polkey CE, Binnie CD, Madigan C, Ferrie CD, Robinson RO. Vagal nerve stimulation in epileptic encephalopathies. Pediatrics. 1999;103(4 Pt 1):778–82. doi: 10.1542/peds.103.4.778. [DOI] [PubMed] [Google Scholar]
- 24.Patel NC, Edwards MS. Vagal nerve stimulator pocket infections. Pediatr Infect Dis J. 2004;23:681–3. doi: 10.1097/01.inf.0000131632.25375.c7. [DOI] [PubMed] [Google Scholar]
- 25.Patwardhan RV, Stong B, Bebin EM, Mathisen J, Grabb PA. Efficacy of vagal nerve stimulation in children with medically refractory epilepsy. Neurosurgery. 2000;47:1353. [PubMed] [Google Scholar]
- 26.Rossignol E, Lortie A, Thomas T, Bouthiller A, Scavarda D, Mercier C, et al. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure. 2009;18:34–7. doi: 10.1016/j.seizure.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: A multicenter study. Biol Psychiatry. 2000;47:276–86. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 28.Rychlicki F, Zamponi N, Cesaroni E, Corpaci L, Trignani R, Ducati A, et al. Complications of vagal nerve stimulation for epilepsy in children. Neurosurg Rev. 2006;29:103–7. doi: 10.1007/s10143-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 29.Rychlicki F, Zamponi N, Trignani R, Ricciuti RA, Iacoangeli M, Scerrati M. Vagus nerve stimulation: Clinical experience in drug-resistant pediatric epileptic patients. Seizure. 2006;15:483–90. doi: 10.1016/j.seizure.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Schallert G, Foster J, Lindquist N, Murphy JV. Chronic stimulation of the left vagal nerve in children: Effect on swallowing. Epilepsia. 1998;39:1113–4. doi: 10.1111/j.1528-1157.1998.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 31.Smyth MD, Tubbs RS, Bebin EM, Grabb PA, Blount JP. Complications of chronic vagus nerve stimulation for epilepsy in children. J Neurosurg. 2003;99:500–3. doi: 10.3171/jns.2003.99.3.0500. [DOI] [PubMed] [Google Scholar]
- 32.Zalvan C, Sulica L, Wolf S, Cohen J, Gonzalez-Yanes O, Blitzer A. Laryngopharyngeal dysfunction from the implant vagal nerve stimulator. Laryngoscope. 2003;113:221–5. doi: 10.1097/00005537-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Zamponi N, Passamonti C, Cappanera S, Petrelli C. Clinical course of young patients with Dravet syndrome after vagal nerve stimulation. Eur J Paediatr Neurol. 2011;15:8–14. doi: 10.1016/j.ejpn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Zamponi N, Passamonti C, Cesaroni E, Trignani R, Rychlicki F. Effectiveness of vagal nerve stimulation (VNS) in patients with drop-attacks and different epileptic syndromes. Seizure. 2011;20:468–74. doi: 10.1016/j.seizure.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Zamponi N, Rychlicki F, Corpaci L, Cesaroni E, Trignani R. Vagus nerve stimulation (VNS) is effective in treating catastrophic 1 epilepsy in very young children. Neurosurg Rev. 2008;31:291–7. doi: 10.1007/s10143-008-0134-8. [DOI] [PubMed] [Google Scholar]


