Abstract
The major challenge of performing root canal treatment in an open apex pulp-less tooth is to obtain a good apical seal. MTA has been successfully used to achieve a good apical seal, wherein the root canal obturation can be done immediately. MTA and White Portland Cement has been shown similarity in their physical, chemical and biological properties and has also shown similar outcome when used in animal studies and human trials. In our study, open apex of three non vital upper central incisors has been plugged using modified white Portland cement. 3 to 6 months follow up revealed absence of clinical symptoms and disappearance of peri-apical rarefactions. The positive clinical outcome may encourage the future use of white Portland cement as an apical plug material in case of non vital open apex tooth as much cheaper substitute of MTA.
Keywords: Apical plug, nonvital tooth, open apex, white portland cement
Introduction
Treatment of a pulpless tooth with an open apex is always a challenging task. Apical surgery was the only successful management until 1966, when Frank described the apexification procedure.[1] He has shown in repeated clinical trials that placement of Ca(OH)2 and CMCP paste as intracanal medication for a longer period brings about resumption of apical development or formation of a calcific bridge just coronal to apex, so that the root canal can be obliterated using conventional root canal filling material.[1,2] This procedure gives a relief from traumatic apical surgery and soon become the most popular way of management of nonvital teeth with open apex. The major drawback of the apexification procedure is high requirement of patient compliance, which needs repeated appointments for changing the intracanal dressing until the treatment completes. Moreover, immature tooth may more prone to fracture when kept in Ca(OH)2 treatment for long time.[3] Besides the said apexification procedure, several other procedures utilizing different other materials have been proposed to induce apical barrier formation. These are freeze-dried allogenic dentin powder, bone ceramic, tricalcium phosphate, osteogenic protein, collagen, calcium gel, etc. Mineral trioxide aggregate (MTA) apical plugging has also been used successfully for this purpose. The use of MTA has been advantageous over the other materials as less number of visits required to achieve the apical repair. The major disadvantage of MTA is its very high cost which refrain it from using for the benefit of the common people. In last one decade, portland cement (PC) has been under review to see whether it can be a much cheaper replacement of MTA as both of them have a similar composition and have similar physical, chemical, and biological properties. We present here three cases of repair of apical rarefaction at nonvital, open apex, upper central incisors using modified white portland cement (WPC).
Materials and Methods
All cases mentioned in this article were the patients visiting to the pedodontic department as general outpatient department (OPD) patients. Permission is sought from the Ethical Committee of the institution to undertake this kind of human trial. A consent form according to declaration of Helsinki (Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended in 59th WMA General Assembly, Seoul, Korea, October 2008) was signed by the parents of the patients to allow us to work with PC in their ward's teeth, after they have been explained in detail about the nonconventional material that we were using. This article demonstrated three cases of apical plugging, where we use rapid setting WPC from JK White Cement Works, Gotan, Rajasthan, India. The cement is modified by adding 20 wt.% bismuth oxide. The mixed cement is used to place an apical plug in a root canal with a wide-open apex with apical radiolucency. All three cases responded well in the absence of clinical symptoms and radiographic healing of apical lesions.
A custom-made plunger is made by inserting a long 24 gauge hypodermic needle into a long 20 gauge hypodermic needle. The sharp part of both needles in the inserted position is removed using a diamond disc. The cut opening of the inner (24) needle is blocked with zinc phosphate cement, so that it can push the material present in 20 needle bore [Figures 1 and 2].
Figure 1.
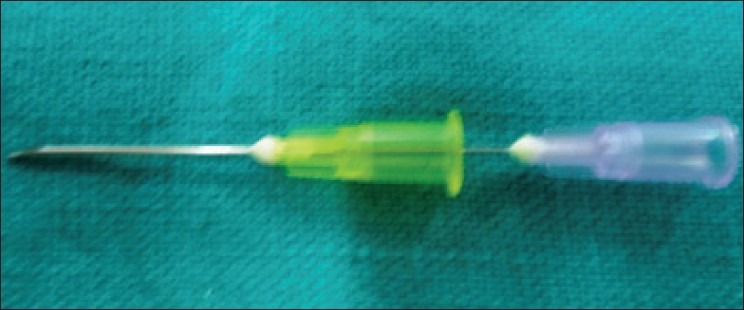
24 gauge hypodermic needle inserted into a long 20 gauge hypodermic needle
Figure 2.
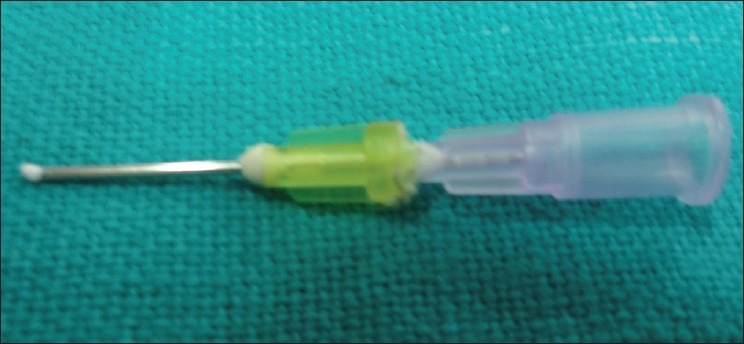
WPC is pushed through a custom-made plunger
Access cavity is prepared for all cases followed by measurement of working length was done. The working length of the needle plunger is set to 3 mm short of the apex using a rubber stopper. Intracanal instrumentation was done by circumferential filing followed by irrigation with 2.5% sodium hypochlorite. The canals dried with absorbent paper points.
The modified cement is mixed with sterile water for injection to a creamy consistency. The mixed cement is loaded into the open needle by repeatedly pressing it into the mix. The plunger filled with cement is then placed into the canal till the measured working length. The cement is then slowly pushed into the canal apex area by pressing the plunger. The material is slowly pushed toward the apex by using 90/100 sized Gutta-Percha (GP ) point up to the desired measurement of 3–4 mm. A repeated radiograph is taken to determine the correct placement of the cement. In two cases, some amount of the cement mix is painted on the canal wall while removing the needle plunger from the canal. A cotton pellet moistened with sterile water was placed in the pulp chamber, and the access cavity was filled with zinc oxide-eugenol cement. Patients were recalled after 24 h, and the canals were obturated with GP cones using a lateral and vertical condensation technique.
Results
Case I
An 8-year-old boy reported to the pedodontic department with severe pain and tenderness in relation to 21. The boy had a history of trauma with fractured enamel and dentin 6 months back. It was repaired with a poorly done composite restoration elsewhere. On examination, there were mild labial swelling, and the offending tooth was terribly tender. Radiographic (RVG) examination shows that 21 had an open apex along with radiographic periapical pathosis [Figure 3]. The access opened under the left-sided infraorbital block. There was purulent discharge from canal. The access kept open for 2 days for drainage, and amoxicillin + clavulanic acid was also prescribed for 5 days. After 5 days, the WPC apical plug procedure was performed following the technique as described above [Figure 4]. The tooth remained symptom-free and a review radiograph after 6 months showed an almost total radiographic apical healing [Figure 5].
Figure 3.
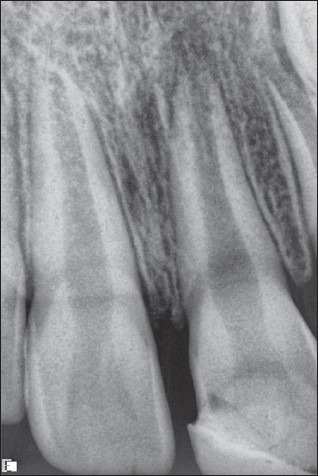
Case I – RVG picture of 21 with radiographic periapical rarefaction
Figure 4.
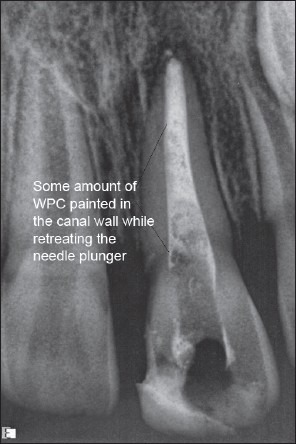
Case I – WPC apical plug placed. Some amount of the cement mix is painted on the canal wall while removing the needle plunger
Figure 5.
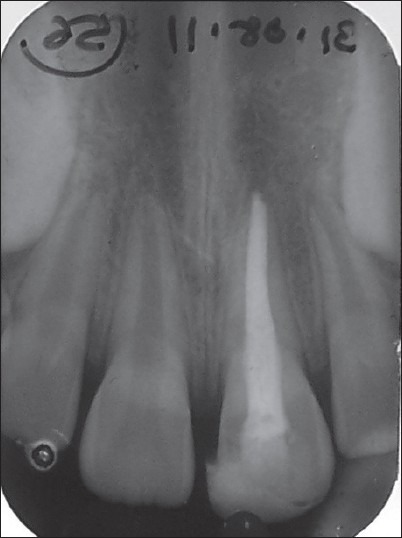
Case I – 6 months postoperative. An IOPA radiograph showing total healing of periapical rarefaction
Case II
A 9-year-old girl came to the pedodontic department with a complaint of discharging sinus in relation to her 11 tooth. Intra oral periapical (IOPA) X-ray reveals that 11 was having open apex and periapical radiolucency [Figure 6]. The offending tooth was treated with a WPC plug followed by gutta-percha obturation as described above. In 3 months postoperative review, the patient was free of complaints and an IOPA radiograph showed a definite radiographic healing in relation to 11 whereas the root of 21 was growing normally [Figure 7].
Figure 6.
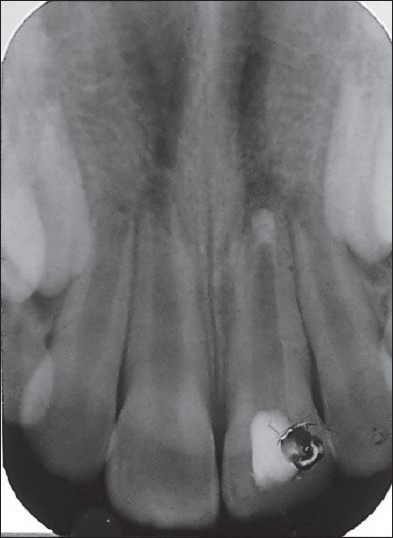
Case II – WPC plug 11 with an open apex and periapical radiolucency
Figure 7.
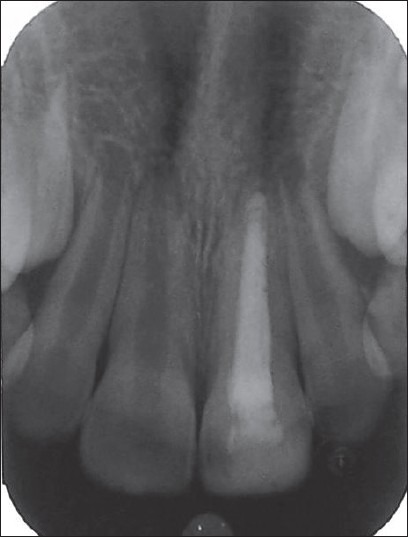
Case II – 3 months postoperative. Radiographic healing of periapical rarefaction of 11. The apex of 21 has grown normally
Case III
A 10-year-old boy presented himself with fractured 11. The patient had a trauma couple of years back. The broken 11 was then repaired elsewhere with composite restoration. The patient gave history that he had a second trauma 4 months back when he lost his restoration on 11. On examination, both 11 and 21 found nonvital. An IOPA radiograph showed 11 with an open apex and 21 had almost completed apex, but both teeth having periapical radiolucency [Figure 8]. An apical plug using WPC was planned to treat 11 and treatment of 12 was planned for a root canal treatment. The WPC apical plug was placed to 11, and the patient was asked to attain next day for obturation of the root canal of 11 [Figure 9]. Unfortunately, the patient did not turn up the next day. The patient came back to the clinic after 5 months with a swollen face. Radiographic examination revealed a total apical repair of 11 which had a WPC plug, whereas the lesion at the apex of 12 has enlarged to an extensive one [Figure 10] that lead to the present complaint.
Figure 8.
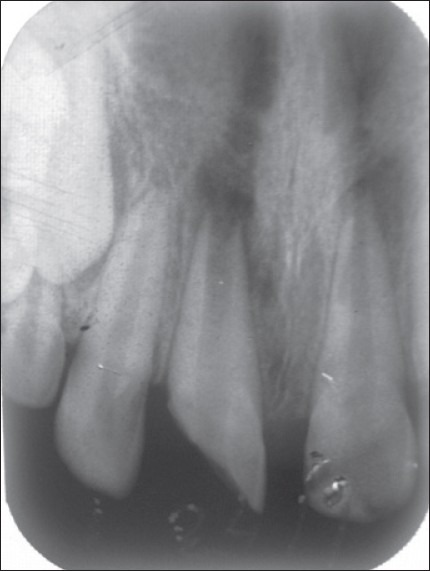
Case III – 11 (open apex) and 21 (closed apex). Both having periapical radiolucency
Figure 9.
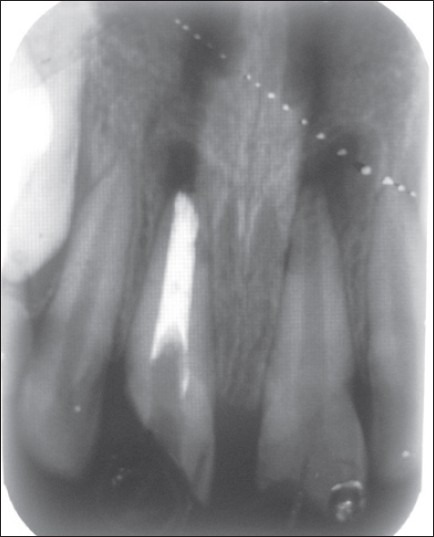
Case III – 11 with WPC apical plug (some amount of the cement mix is painted on the canal wall)
Figure 10.
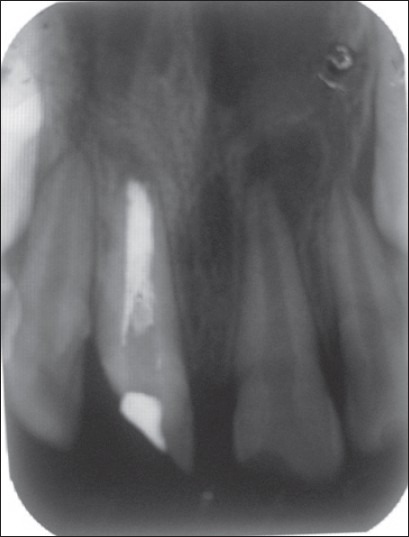
Case III – 5 months postoperative. Apical repair of 11 which had a WPC plug, whereas the lesion at the apex of 12 has enlarged to an extensive one
Discussion
Since 1993, the mineral trioxide aggregate has been successfully used for all kinds of dental hard tissue repair. The use of MTA had simplified the procedure of repairing an open apex, where MTA can be used as an apical plug over an open apex and have the same result of using Ca(OH)2 apexification in a single or maximum in couple of sittings. The MTA apical plug is introduced by Shabahang and Torabinejad[4] and later recommended by several studies.[5–8] The success of MTA apical plug has been attributed mainly to its good sealing ability,[9] biocompatibility,[10] and ability to promote pulpal and periradicular tissue regeneration.[11] However, the major disadvantage of MTA is highly expansive and is difficult for common people to enjoy its benefit, especially in developing countries.
In the manufacturer's material safety data sheet (MSDS), it has been found that MTA_Angelus is composed of 80% portland cement and 20% bismuth oxide, but the mostly used Pro-Root MTA is containing 75% portland cement, 20% bismuth oxide, and 5% dehydrated calcium sulfate. The basic major content of these two materials are tricalcium silicate, dicalcium silicate, tricalcium aluminate, and tricalcium oxide. Besides these, silica, alumina, ferric oxide, magnesium oxide, etc. are also present. The basic difference between these two materials is that the PC does not contain bismuth oxide. In 1999, Wucherpfennig and Green showed that the MTA and PC were almost identical in their macroscopic and microscopic structure when evaluated through X-ray diffraction.[12] Then, Estrela et al. used ordinary PC as a reference material to MTA and showed that these two materials are chemically identical except the presence of bismuth oxide and both have the same antimicrobial property.[13] Later, in more than one decade, innumerable in vitro, in vivo, and animal studies had shown that PC had a similar physical, chemical, and biological properties with MTA with respect to its similar pH,[14,15] compressive strength and setting time,[16] sealing ability and prevention of microleakage,[17,18] and biocompatibility.[19,20] The arsenic release (0.002–0.007 ppm) from both materials are similar and which is much lower than the set limit (0.01 ppm in drinking water) by Environmental Protection Agency (EPA)/FDA.[21,22] Both materials have shown successful pulpotomies and a hard tissue formation procedure in dogs and pigs.[23–26]
De-Deus G and colleagues tried the first clinical trial of apical plugging in an immature 35 apex using PC. One year follow-up of that case revealed adequate clinical function, absence of clinical symptoms, and no signs of periapical rarefaction.[27] Four anterior teeth with an open apex were treated with a single step apexification plug with white PC. In addition, 3–24 months follow-up demonstrated successful apical repair.[28] Pulpotomy was carried out in two mandibular first molars and one mandibular second molar using PC, which were further followed up at 3, 6, and 12 months. Clinical and radiographic examinations of the pulpotomized teeth and their periradicular area revealed that the treatments were successful in maintaining the teeth asymptomatic and preserving pulpal vitality.[29] In another study, pulpotomy was carried out in 15 primary mandibular molars using PC and 14 primary mandibular molars using MTA. The result showed no statistically significant difference regarding dentine bridge formation between both groups throughout the follow-up period. The authors concluded that PC may serve as an effective and less expensive MTA substitute in primary molar pulpotomies.[30]
Conclusion
Review of several articles mentioned above showed that PC presents a nearly equal outcome with MTA in almost all in vivo, in vitro, and animal trials with respect to its chemistry, physical properties like compressive strength, setting time, dimensional changes, radioopacity, microleakage with sealing ability, etc. PC is equally biocompatible like MTA and calcium hydroxide, and its antimicrobial property is also at par with MTA.
The successful clinical outcome in these cases mentioned above is encouraging to use WPC in non-vital open apex conditions. The few clinical studies carried before had also shown successful clinical outcomes while using WPC instead of MTA. PC is almost 1000 times less expensive than MTA in the Indian market. A cheaper substitute of MTA will certainly benefit millions of people in the world who cannot bear the cost of MTA. The existing literature and success in few clinical studies give a firm base and strong indication that PC could be recommended in the future for clinical use by replacing MTA. However, a lot more, well-designed long-term studies are required before PC can be taken as an equally effective and a less expensive substitute for MTA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87–93. doi: 10.14219/jada.archive.1966.0017. [DOI] [PubMed] [Google Scholar]
- 2.Steiner JC, Dow PR, Cathey GM. Inducing root end closure of non-vital permanent teeth. J Dent Child. 1968;35:47–54. [PubMed] [Google Scholar]
- 3.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 4.Shabahang S, Torabinejad M. Treatment of teeth with open apices using mineral trioxide aggregate. Pract Periodont Aesthet Dent. 2000;12:315–20. [PubMed] [Google Scholar]
- 5.Giuliani V, Baccetti T, Pace R, Pagavino G. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18:217–21. doi: 10.1034/j.1600-9657.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Shimizu A, Ebisu S. MTA for obturation of mandibular central incisors with open apices: Case report. J Endod. 2004;30:120–2. doi: 10.1097/00004770-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Pace R, Giuliani V, Pini Prato L, Baccetti T, Pagavino G. Apical plug technique using mineral trioxide aggregate: Results from a case series. Int Endod J. 2007;40:478–84. doi: 10.1111/j.1365-2591.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- 8.Annamalai S, Mungara J. Efficacy of mineral trioxide aggregate as an apical plug in non-vital young permanent teeth: Preliminary results. J Clin Pediatr Dent. 2010;35:149–55. doi: 10.17796/jcpd.35.2.9061h7g718834017. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 10.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Felippe WT, Felippe MCS, Rocha MJC. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39:2–9. doi: 10.1111/j.1365-2591.2005.01037.x. [DOI] [PubMed] [Google Scholar]
- 12.Wucherpfennig AL, Green DB. Mineral trioxide vs. Portland cement: two compatible filling materials. J Endod. 1999;25:308. [Google Scholar]
- 13.Estrela C, Bammann LL, Estrela CR, Silva RS, Pecora JD. Antimicrobial and chemical study of MTA, Portland cement, Ca(OH)2 paste, Sealapex and Dycal. Braz Dent J. 2000;11:3–9. [PubMed] [Google Scholar]
- 14.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40:462–70. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 15.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod. 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Islam I, Chng HK, Yap AU. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006;39:220–5. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 17.Storm B, Eichmiller FC, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J Endod. 2008;34:80–2. doi: 10.1016/j.joen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.De-Deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T. MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage model. Int Endod J. 2006;39:293–8. doi: 10.1111/j.1365-2591.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 19.De-Deus G, Ximenes R, Gurgel-Filho ED, Plotkowski MC, Coutinho-Filho T. Cytotoxicity of mineral trioxide aggregate and Portland cement on human ECV 304 endothelial cells. Int Endod J. 2005;38:604–9. doi: 10.1111/j.1365-2591.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro DA, Sugui MM, Matsumoto MA, Duarte MA, Marques ME, Salvadori DM. Genotoxicity and cytotoxicity of MTA and regular and white Portland cement on chinese hamster ovary cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:258–61. doi: 10.1016/j.tripleo.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 21.Duarte MA, De Oliveira Demarchi AC, Yamashita JC, Kuga MC, De Campos Fraga S. Arsenic release provided by MTA and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:648–50. doi: 10.1016/j.tripleo.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spångberg LS, et al. Presence of arsenic in different types of MTA and white and gray Portland cement. Oral surgery Oral Med Oral Pathol Oral Radiol Endod. 2008;106:909–13. doi: 10.1016/j.tripleo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Holland R, de Souza V, Murata SS, Nery MJ, Bernabé PF, Otoboni Filho JA, et al. Healing prozess of dog dental pulp after pulpotomy and pulp covering with MTA or Portland cement. Braz Dent J. 2001;12:109–13. [PubMed] [Google Scholar]
- 24.Menezes R, Bramante CM, Letra A, Carvalho VG, Garcia RB. Histologic evaluation of pulpotomies in dog using two types of MTA and regular and white Portland cements as wound dressings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:376–9. doi: 10.1016/S107921040400215X. [DOI] [PubMed] [Google Scholar]
- 25.Shayegan A, Petein M, Abbeele AV. Beta-tricalzium phosphate, white MTA, white Portland cement, ferric sulfate and formocresol used as pulpotomy agents in primary pig teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:536–42. doi: 10.1016/j.tripleo.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Shayegan A, Petein M, Abbeele AV. The use of beta-tricalcium phosphate, white MTA, white Portland cement and calcium hydroxide for direct pulp capping of primary pig teeth. Dent Traumatol. 2009;25:413–9. doi: 10.1111/j.1600-9657.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 27.De-Deus G, Coutinho-Filho T. The use of white Portland cement as an apical plug in a tooth with a necrotic pulp and wide-open apex: A case report. Int Endod J. 2007;40:653–60. doi: 10.1111/j.1365-2591.2007.01269.x. [DOI] [PubMed] [Google Scholar]
- 28.Hegde RS, Sharathchandra R, Rao R. Single step apexification using white portland cement - Case series. Endodontology. 2010;22:107–11. [Google Scholar]
- 29.Conti TR, Sakai VT, Fornetti AP, Moretti AB, Oliveira TM, Lourenço Neto N, et al. Pulpotomies with Portland cement in human primary molars. J Appl Oral Sci. 2009;17:66–9. doi: 10.1590/S1678-77572009000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai VT, Moretti AB, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al. Pulpotomy of human primary molars with MTA and Portland cement: A randomised controlled trial. Br Dent J. 2009;207:E5. doi: 10.1038/sj.bdj.2009.665. [DOI] [PubMed] [Google Scholar]


