Abstract
Platelet-rich fibrin has long been used as a wound healing therapy in skin wounds and recently evidence has suggested its usage in oral cavity for different treatment procedures. This article proposes an overview of use of platelet-rich fibrin in management of complicated oral wounds. Excessive hemorrhage of the donor area, necrosis of epithelium, and morbidity associated with donor site have been described as the possible complications after harvesting subepithelial connective tissue graft, but little has been mentioned about their management. The article includes a case report of a 45-year-old male patient who showed a delayed wound healing after subepithelial connective tissue graft harvestation, which was treated with platelet-rich fibrin.
Keywords: Delayed, palatal, platelet-rich fibrin, subepithelial connective tissue graft, wound healing
Introduction
In a healing wound, the increased metabolic demands made by the inflammation and cellular activity require the surgeon to critically appraise and selectively use the growing array of biologic approaches that seek to assist healing by favorably modulating the wound microenvironment. One of such biologic approaches to wound healing is platelet-rich-fibrin (PRF).[1]
Following an injury (e.g., by surgical trauma), the most important initial reactions leading to immediate blood coagulation are mainly mediated by platelets and blood vessel wall changes.[2] At the site of the injury, platelets release an arsenal of potent inflammatory and mitogenic substances that are involved in all aspects of the wound-healing process [Figure 1]. Based on this, platelet in the form of activated PRF has been extensively used for the topical therapy of various clinical conditions, including both soft and hard tissue injuries, e.g., diabetic foot ulcer.[1,3] The relative risk for a “hard-to-heal” wound to heal after treatment with platelet releasate (1.14), compared with standard care (1.59)[3] as estimated in general medicine, inspires us to evaluate the effect of PRF in complicated oral wounds.
Figure 1.
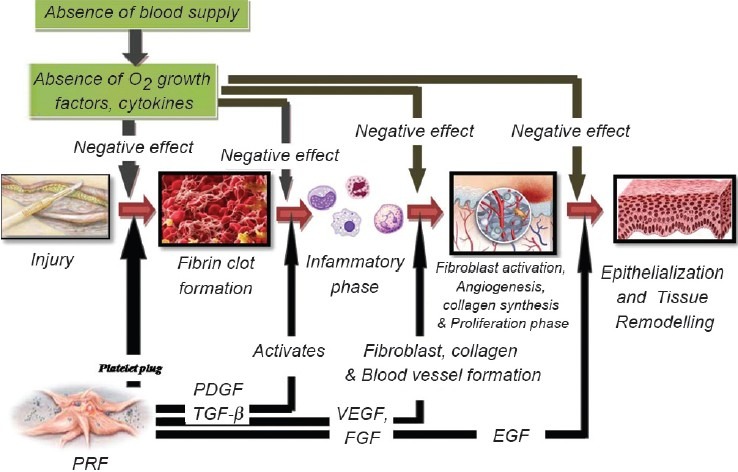
Phases of wound healing with application of PRF at each step
Platelet-rich fibrin (PRF) is an immune and platelet concentrate collecting on a single fibrin membrane all the constituents of a blood sample favorable to healing and immunity.[4] It has a rich source of growth factors and its application has been reported as an effective way to induce tissue response and regeneration. There are at least 60 different biologically active substances present in platelets which are involved in tissue-repair mechanisms such as chemotaxis, cell proliferation and differentiation, angiogenesis, intracellular matrix deposition, immune modulation, antimicrobial activity, and remodeling.
Studies have shown that platelet-rich fibrin supports the three main keys of wound healing mechanism that is “angiogenesis,” “immunity,” and “epithelial proliferation” and thus has implicated its use to protect open wounds and accelerate healing. Its utilization seems to be of high interest mainly in the case of nonhealing wounds.[4]
The present case report displays a case of uncontrolled hemorrhage subsequent to subepithelial connective tissue harvesting followed by delayed wound healing and its management with platelet-rich fibrin and Kollagen membrane.
Case Report
A 45-year-old systemically healthy male patient was treated in Department Of Periodontics, Bapuji Dental College and Hospital, Davangere for root exposure in relation to lower front teeth (31) using the subepithelial connective tissue grafting procedure.
The connective tissue graft was harvested using Bruno's technique. Immediately after harvesting SCTG, the palatal donor site showed profuse bleeding. Digital pressure was applied for 15 minutes to control the bleeding and to find its origin. Slight reflection of the epithelial flap at the palate revealed pulsating bleeding with the artery involved. Bleeding was controlled using heat coagulation and later palatal wound was sutured using interrupted sutures and periodontal dressing was given. The patient was kept under observation for another half an hour to check bleeding. Postoperative instructions including oral hygiene maintenance, antibiotics, and analgesics were given and the patient was recalled after 10 days for further evaluation and suture removal.
Ten days postoperatively, periodontal dressing was removed and the surgical site was irrigated using a 5% iodine solution. Debridement of the wound showed an exposed bone with yellowish slough formation at the donor site with surrounding erythema and edema [Figure 2]. Overlying epithelium showed necrosis and the patient complained of moderate pain in relation to the same. After evaluation of the site, it was decided to use PRF to initiate healing along with Kollagen to stabilize the PRF. A written informed consent was taken from the patient for the same.
Figure 2.
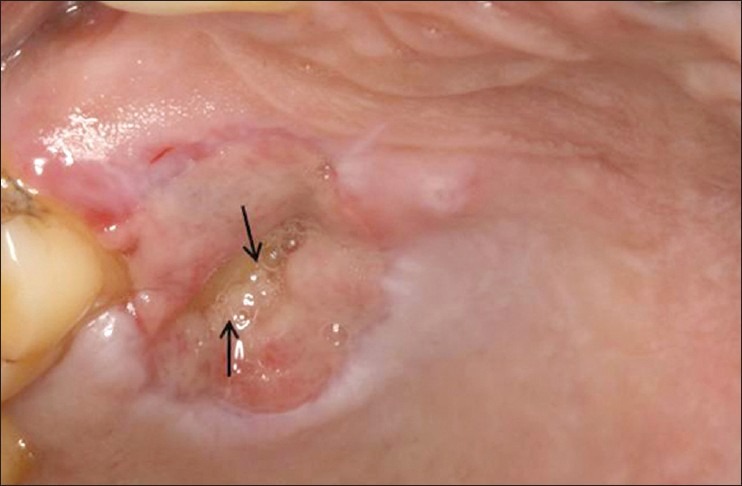
Delayed wound healing with exposed bone, epithelial necrosis, and slough
Then 5 ml of venous blood was collected from anticubital fossa of the patient and was immediately transferred to a sterile test tube. The blood was centrifuged at 3000 rpm for 10 minutes, following which the platelet-rich fibrin was obtained. A Kollagen membrane containing bovine collagen was rinsed in sterile saline water and then was spread uniformly on a clean glass slab. The platelet-rich fibrin obtained from patient blood was placed over the kollagen membrane and the latter was folded in two folds over PRF [Figure 3] and was further cut to the size of the palatal wound.
Figure 3.
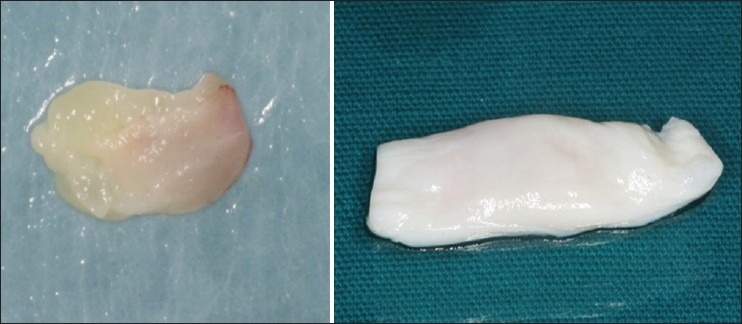
Preparation of PRF with Kollagen
The palate was anesthesized and bleeding was induced at the site of infection from the surrounding tissues. A layer of platelet-rich fibrin was covered on the wound surface and a Kollagen membrane was further placed over PRF as a scaffold [Figure 4]. Seven days after placement of PRF + kollagen, the palatal stent was removed and the site was evaluated. The clinical examination of wound site showed complete resorption of the kollagen and PRF with reduced erythema, edema, and absence of slough [Figure 5]. Margins of the wound were seen to be merging with the periphery of the wound and the bone was covered with thin layer of epithelium. After 14 days complete epithelization was observed [Figure 5]. The wound margins could not be differentiated from the rest of the palate. No signs of erythema or edema were seen at the wound site. After 21 days the healing site could not be differentiated from the surrounding tissue [Figure 6]. The patient did not present any discomfort. At 1-month palpation of soft tissue in the wound region showed resistance to probe thus depicting formation of new connective tissue.
Figure 4.
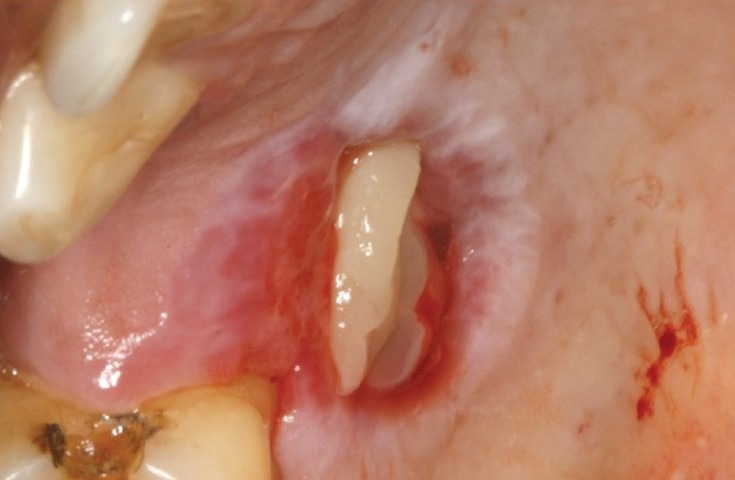
Placement of PRF followed by kollagen membrane
Figure 5.
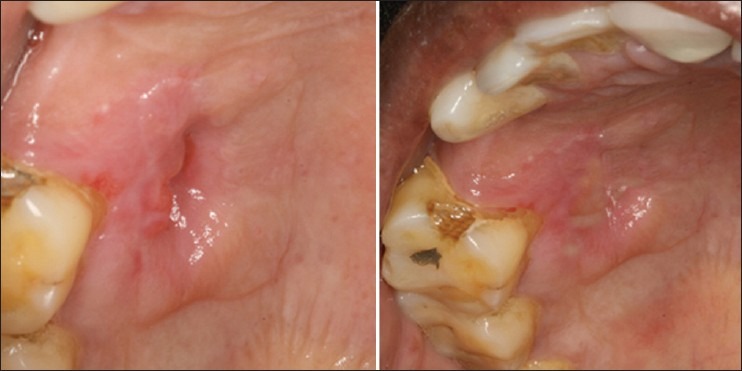
7-day and 14-day healing following placement of PRF + Kollagen membrane
Figure 6.
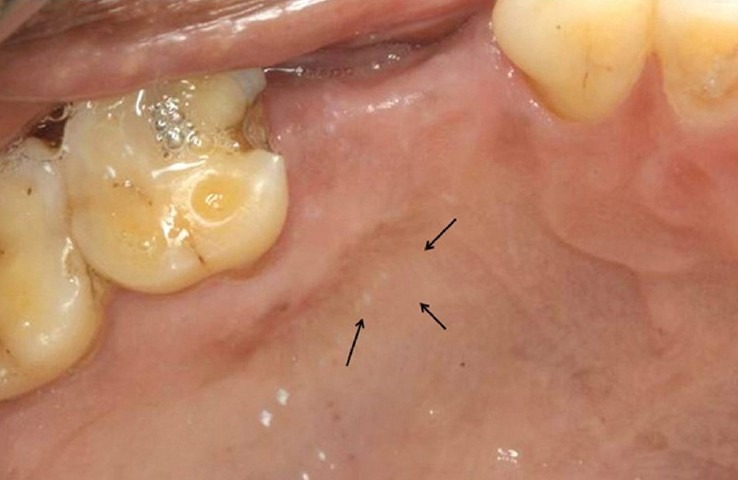
21 days postoperative
Discussion
Use of PRF in oral cavity has been implicated in different procedures such as extraction socket preservation, intrabony defects, sinus augmentation, and sinus lift procedures for implant placement, bone augmentation, root coverage procedures, and healing in donor site with successful results.[4]
During harvesting connective tissue graft, the operative risk that is most dreaded by clinicians is the occurrence of hemorrhage involving collateral branches. An accidental injury causing necrosis of palate mucosa may also occur if the main artery is not protected. It has been seen that it is nearly impossible to individually localize the palatine vessels for ligation.[5]
Although subepithelial connective tissue graft (SCTG) procedure show a better wound healing over free gingival grafts, primary closure is not always achieved at donor site in SCTG procedures. Del Pizzo[6] in his study compared palatal healing after harvesting a standardized connective tissue graft with single incision, trap door, and free gingival graft technique. The postoperative bleeding was encountered in all the three techniques ranging from 8% to 32% and the differences were not statistically significant. The hemorrhagic complications following a palatal harvest of the connective tissue graft are infrequent, but are always difficult to manage. In the case of abundant bleeding, persistent firm pressure has been advised. Some authors also recommend use of haemostatic agents such as oxidized regenerated cellulose, gelatin sponge or tranexamic acid.[5]
The present case report shows a case of uncontrolled hemorrhage and denuded bone following subepithelial connective tissue graft which was successfully managed. Hemostasis was achieved using firm persistent pressure and heat coagulation. The presence of denuded bone has been related to increased incidence of infection which can further complicate the wound.
The prevention of local hemorrhage and the resulting blood clot contributes to stopping the bleeding and then functions as a provisional matrix for the wound healing. Blood vessels play an important role in sustaining cell metabolism by providing oxygen and nutrients. The integrity of the granulation tissue depends on the presence of growth factors that are released by activated platelets in the wounded areas.[1]
Kahnberg and Thilander[7] conducted a study on palatal healing of rats and observed that epithelization progressed from wound borders and reduction of wound surface proceeded by contraction of the wound margins and by epithelial cell migration, with complete healing in 3 weeks. In the present case report due to the control of hemorrhage by heat coagulation, there is a probability that there was an absence of blood supply to the area for a long time, which means the absence of various growth factors and cells derived from blood and their interaction. Blood supports the wound with the oxygen, nutrients, and inflammatory cells, all of which are necessary for healing. Oxygen is further critical to wound healing, stimulating collagen production, and migration of epithelial cells.[8] Thus the absence of blood supply deprives the tissue of the access to substances necessary for its metabolic sustenance which, in turn, may have resulted in necrosis of the overlying epithelium, delayed wound healing, and infection causing bone exposure.
PRF releases high quantities of three main growth factors transforming growth factor b-1 (TGFbeta-1), platelet-derived growth factor AB (PDGF-AB), vascular endothelial growth factor (VEGF), and an important coagulation matricellular glycoprotein (thrombospondin-1, TSP-1) during 7 days. It is believed to contain platelets in a concentration seven times that of blood. Apart from these PRF also secrete EGF, FGF, and three important proinflammatory cytokines- IL-1β, IL-6, and TNF-α. Due to its mechanical function, a rapid angiogenesis promoting ability and an easier remodeling of fibrin in a more resistant connective tissue, PRF membranes are viable material for all types of superficial cutaneous and mucosal healing.[9]
A recent report in an ex vivo model of gingival repair showed that sustained PDGF delivery promoted wound closure.[10] Soileau and Brannon[11] reported that in conventional autogenous grafting procedures at least 9 weeks are necessary for remodeling of the palatal wound to appear complete histologically. A recent study by Alec Yen et al.[12] evaluated the effect of Platelet concentrate on palatal donor site healing after harvesting of SCTG and noticed that platelet concentrate could accelerate soft tissue healing and regeneration of palatal tissue thickness at 6-week intervals at clinical and histologic levels.
Collagen dressing is another material which has been used extensively in the field of medicine and dentistry due to its ability to achieve hemostasis, chemotactic to fibroblasts and platelets, and inducing mesenchymal cell proliferation and differentiation. A clinical and histologic study comparing the use of collagen sponge to coe-pak dressing in healing of palatal wounds found dampening of inflammation during healing with less pain and burning sensation in sites treated with collagen sponge. Also there was a comparative acceleration in wound healing with greater firmness in the tissues 2 weeks postoperatively with collagen sponge application.[13]
In the present case report Kollagen membrane was used along with platelet-rich fibrin with former acting as a scaffold for PRF. PRF may have supplied all the necessary growth factors important for collagen synthesis, cell recruitment, epithelial closure, and blood vessel growth, whereas Kollagen acting as stent for PRF, also helped in strengthening of newly formed matrix. These two acting together accelerated wound healing. The 2-week results showed complete epithelization without any signs of infection or inflammation and complete resorption of Kollagen and PRF. Although histologic analysis was not performed in the present case report, the clinical evaluation showed excellent healing and newly formed tissue blend well with color and texture to the surrounding tissue.
Conclusion
The present case report substantiates the use of PRF in a case of delayed and complicated healing following sub epithelial connective tissue graft harvestation which was successfully managed. PRF has proved to be effective in reducing donor site morbidity of free grafts. Thus further studies to evaluate its use are warranted.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156–65. [PubMed] [Google Scholar]
- 2.Everts PA, Knape JT, Weibrich G, Schonberger JP, Hoffmann J, Overdevest EP, et al. Platelet rich plasma and platelet gel, A review. J Extra Corpor Technol. 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropatic neuropatic foot ulcers. Diabetes Care. 2001;24:483–8. doi: 10.2337/diacare.24.3.483. [DOI] [PubMed] [Google Scholar]
- 4.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Dridi SM, Chousterman M, Danan M, et al. hemorrhagic risk when harvesting palatal connective tissue grafts: A reality? Perio. 2008;5:231–40. [Google Scholar]
- 6.Del Pizzo M, Modica F, Bethaz N, Priotto P, Romagnoli R. The connective tissue graft: A comparative clinical evaluation of wound healing at the palatal donor site. A preliminary study. J Clin Periodontol. 2002;29:848–54. doi: 10.1034/j.1600-051x.2002.290910.x. [DOI] [PubMed] [Google Scholar]
- 7.Kahnberg KE, Thilander H. Healing of experimental excision wounds in the rat palate.(1) Histological study of the interphase in wound healing after sharp dissection. Int J Oral Surg. 1982;11:44–51. doi: 10.1016/s0300-9785(82)80048-3. [DOI] [PubMed] [Google Scholar]
- 8.Anderson L, Kahnberg K, Pogrel MA. Oral and maxillofacial surgery. United Kingdom: Wiley-Blackwell; 2010. [Google Scholar]
- 9.Del Corso M, Toffler M, Ehrenfest DM, et al. Use of an Autologous Leukocyte and Platelet-Rich Fibrin (L-PRF) Membrane in Post-Avulsion Sites: An Overview of Choukroun's PRF. J Implant Adv Clin Dent. 2010;1:27–35. [Google Scholar]
- 10.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–56. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soileau KM, Brannon RB. A histologic evaluation of various stages of palatal healing following subepithelial connective tissue grafting procedures: A comparison of eight cases. J Periodontol. 2006;77:1267–73. doi: 10.1902/jop.2006.050129. [DOI] [PubMed] [Google Scholar]
- 12.Yen CA, Griffin TJ, Cheung WS, Chen J. Effects of Platelet Concentrate on Palatal Wound Healing After Connective Tissue Graft Harvesting. J Periodontol. 2007;78:601–10. doi: 10.1902/jop.2007.060275. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugam M, Kumar TS, Arun KV, Arun R, Karthik SJ. Clinical and histological evaluation of two dressing materials in the healing of palatal wounds. J Indian Soc Periodontol. 2010;14:241–4. doi: 10.4103/0972-124X.76929. [DOI] [PMC free article] [PubMed] [Google Scholar]


