Abstract
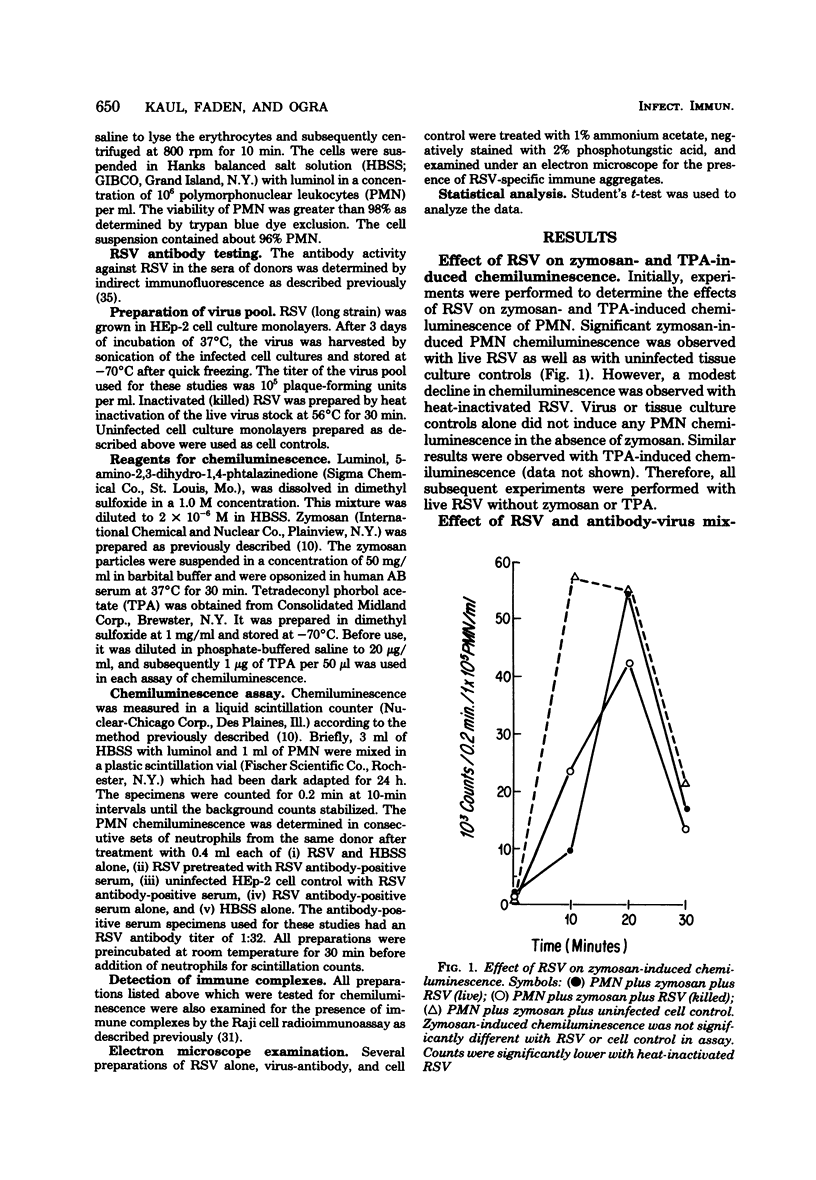
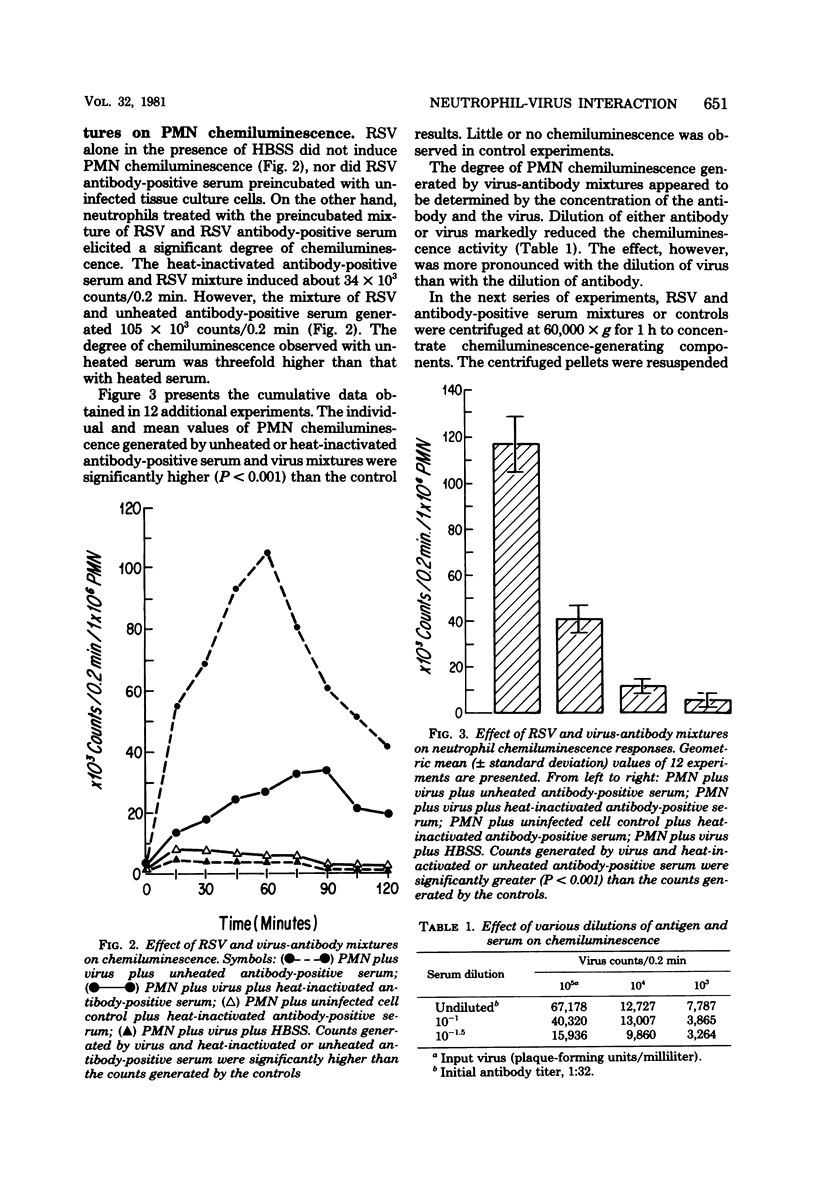
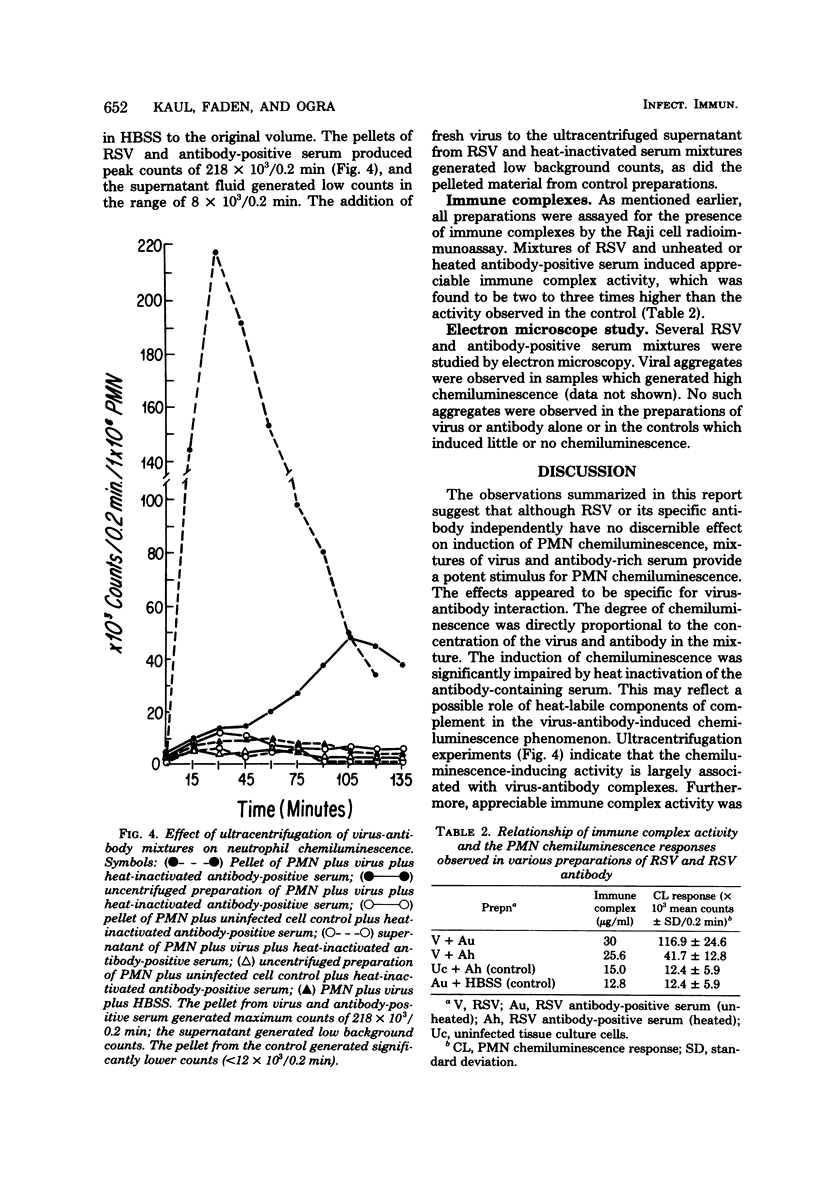
The effect of respiratory syncytial virus (RSV) or mixtures of RSV and its specific antibody on the oxidative metabolic activity of human polymorphonuclear leukocytes was studied by the technique of luminol-dependent chemiluminescence. Peripheral blood neutrophils obtained from normal healthy donors were used. RSV alone failed to induce any chemiluminescent response by the neutrophils. However, mixtures of RSV and RSV antibody-positive serum regularly elicited significant neutrophil chemiluminescence. Ultracentrifugation, electron microscopy, and Raji cell immune complex assays of virus-antibody mixtures suggested that the neutrophil chemiluminescent response was related to the presence of specific immune complexes of RSV antigen-antibody. Heat inactivation of the serum significantly reduced the polymorphonuclear leukocyte chemiluminescence, and the response also appeared to be dependent on the dose of the virus and the antibody in the reaction mixture. It is proposed that interaction between the neutrophil and RSV-specific immune complexes may contribute to the pathogenesis of RSV infection via the possible release of metabolic products from the activated neutrophils.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Brogan M. D., Sagone A. L., Jr The metabolic response of human phagocytic cells to killed mumps particles. J Reticuloendothel Soc. 1980 Jan;27(1):13–22. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRANE C. G., WEIGLE W. O., DIXON F. J. The role of polymorphonuclear leukocytes in the initiation and cessation of the Arthus vasculitis. J Exp Med. 1959 Sep 1;110:481–494. doi: 10.1084/jem.110.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock R. M., Kapikian A. Z., Mills J., Kim H. W., Parrott R. H. Influence of immunological factors in respiratory syncytial virus disease. Arch Environ Health. 1970 Sep;21(3):347–355. doi: 10.1080/00039896.1970.10667249. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., BARON S. The role of antibody in recovery from infection with vaccinia virus. J Immunol. 1961 Oct;87:379–382. [PubMed] [Google Scholar]
- Faden H. S., Keller N., Ogra P. L. Effect of human and murine neutrophils on encephalomyocarditis, vesicular stomatitis, and reo type 3 virus infections in tissue culture. J Reticuloendothel Soc. 1978 Dec;24(6):629–636. [PubMed] [Google Scholar]
- Faden H., Sutyla P., Ogra P. L. Effect of viruses on luminol-dependent chemiluminescence of human neutrophils. Infect Immun. 1979 Jun;24(3):673–678. doi: 10.1128/iai.24.3.673-678.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Gardner P. S., McQuillin J., Court S. D. Speculation on pathogenesis in death from respiratory syncytial virus infection. Br Med J. 1970 Feb 7;1(5692):327–330. doi: 10.1136/bmj.1.5692.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Malmsten C. L., Kindahl H., Kaplan H. B., Rådmark O., Samuelsson B., Weissmann G. Thromboxane generation by human peripheral blood polymorphonuclear leukocytes. J Exp Med. 1978 Sep 1;148(3):787–792. doi: 10.1084/jem.148.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower R. G., Sausker W. F., Kohler P. F., Thorne G. E., McIntosh R. M. Small vessel vasculitis caused by hepatitis B virus immune complexes. Small vessel vasculitis and HBsAG. J Allergy Clin Immunol. 1978 Oct;62(4):222–228. doi: 10.1016/0091-6749(78)90211-7. [DOI] [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Mechanisms of recovery from viral infections: destruction of infected cells by neutrophils and complement. J Immunol. 1980 Jan;124(1):312–319. [PubMed] [Google Scholar]
- Milgrom M., Albini B., Noble B., O'Connell D., Brentjens J., Andres G. A. Antibodies in guinea-pigs immunized with kidney and lung basement membranes. Clin Exp Immunol. 1979 Nov;38(2):249–258. [PMC free article] [PubMed] [Google Scholar]
- Nowoslawski A., Krawczyński K., Brzosko W. J., Madaliński K. Tissue localization of Australia antigen immune complexes in acute and chronic hepatitis and liver cirrhosis. Am J Pathol. 1972 Jul;68(1):31–56. [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Ozawa T., Stewart J. A. Immune-complex glomerulonephritis associated with cytomegalovirus infection. Am J Clin Pathol. 1979 Jul;72(1):103–107. doi: 10.1093/ajcp/72.1.103. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A., Henson P. M. Neutrophils are mediators of antiviral immunity. Experientia. 1978 Mar 15;34(3):346–348. doi: 10.1007/BF01923026. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A., Mukkur T. K. The role of neutrophils in antiviral defense--in vitro studies on the mechanism of antiviral inhibition. J Immunol. 1977 Jun;118(6):1957–1961. [PubMed] [Google Scholar]
- Sieber O. F., Wilska M. L., Riggin R. Elevated nitroblue tetrazolium dye reduction test response in acute viral respiratory disease. Pediatrics. 1976 Jul;58(1):122–124. [PubMed] [Google Scholar]
- Stagno S., Volanakis J. E., Reynolds D. W., Stroud R., Alford C. A. Immune complexes in congenital and natal cytomegalovirus infections of man. J Clin Invest. 1977 Oct;60(4):838–845. doi: 10.1172/JCI108838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. A., Ferrington R. A., Jordan G. W., Merigan T. C. Cellular events in zoster vesicles: relation to clinical course and immune parameters. J Infect Dis. 1975 May;131(5):509–515. doi: 10.1093/infdis/131.5.509. [DOI] [PubMed] [Google Scholar]
- Svensson J., Hamberg M., Samuelsson B. Prostaglandin endoperoxides IX. Characterization of rabbit aorta contracting substance (RCS) from guinea pig lung and human platelets. Acta Physiol Scand. 1975 Jun;94(2):222–228. doi: 10.1111/j.1748-1716.1975.tb05881.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Wilson C. B., Dixon F. J. The Raji cell radioimmune assay for detecting immune complexes in human sera. J Clin Invest. 1976 Jan;57(1):169–182. doi: 10.1172/JCI108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Cohen S., Flanagan T. D. Leukotactic factors elaborated by virus-infected tissues. J Exp Med. 1972 May 1;135(5):1095–1103. doi: 10.1084/jem.135.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. Antibody dependent cytotoxicity mediated by neutrophils: a possible mechanism of antiviral defense. J Reticuloendothel Soc. 1976 May;19(5):323–332. [PubMed] [Google Scholar]
- Welliver R. C., Kaul A., Ogra P. L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979 Mar;94(3):370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Kaul T. N., Ogra P. L. The appearance of cell-bound IgE in respiratory-tract epithelium after respiratory-syncytial-virus infection. N Engl J Med. 1980 Nov 20;303(21):1198–1202. doi: 10.1056/NEJM198011203032103. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Kaul T. N., Putnam T. I., Sun M., Riddlesberger K., Ogra P. L. The antibody response to primary and secondary infection with respiratory syncytial virus: kinetics of class-specific responses. J Pediatr. 1980 May;96(5):808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]



