Abstract
Hair loss (alopecia) can result from a variety of metabolic, endocrine, immunologic, and environmental causes. This investigation was undertaken to determine the mechanisms underlying the sporadic development of alopecia in litters from C57BL/6 interleukin-10-deficient (Il10−/−) mice. All pups in affected litters demonstrated alopecia by postnatal days 17–19, with hair loss from their trunks but not from their head, base of tail, or feet. Histopathology revealed distorted hair follicles containing broken hair shafts and prominent dermal infiltrates containing increased numbers of activated mast cells. Hair re-growth began soon after weaning, suggesting that the alopecia was triggered by factors transmitted during lactation. Milk from Il10−/− dams induced macrophage secretion of pro-inflammatory cytokines in vitro regardless of whether or not their pups developed alopecia. Feeding dams a diet containing 3–6 ppm iron increased the percentage of litters with alopecia to 100% for pups with mast cells, with 0% alopecia in mast cell-deficient pups. When dams were fed diet containing 131 ppm iron, significantly lower hemoglobin and hematocrit values were observed in pups from litters with alopecia (71%; 5 of 7 litters) compared to litters without alopecia. Genetic or pharmacologic inhibition of c-kit that resulted in depletion of mast cells in pups prevented hair loss in at-risk litters. These studies demonstrate that maternal iron-restricted diets enhance the incidence of alopecia in IL-10-deficient mouse pups and suggest mast cells as potential effector cells. Further studies are indicated to further explore the mechanisms involved and to determine how mast cells may contribute to alopecia in humans.
Keywords: alopecia, iron-deficiency, mast cells
Introduction
Hair follicle morphogenesis and cycling involves multiple cell types and cell signaling pathways. Accordingly, the appearance of alopecia (abnormal hair loss) in a mutant mouse strain may provide new insight into the functional significance of a gene product in relation to hair follicle morphogenesis and cycling (1–3). Congenital hair defects are rare and typically caused by mutations in keratins or other structural proteins (4). Acquired alopecia can result from both inflammatory and non-inflammatory etiologies. Inflammatory damage to hair follicles can result from bacterial infections, dermatophytosis, external parasites, immune-mediated diseases, trauma, or exposure to toxins (e.g. mercury, thallium, or iodine). Non-inflammatory diseases that affect hair follicles include nutritional deficiencies, endocrine abnormalities, parturition, anemia, and therapeutic drug treatments (4). Understanding the underlying etiology of alopecia that occurs in mice is important, since it reflects their health status and may also provide critical insight into mechanisms of alopecia that occur in humans.
Normal hair cycling in mammals requires a complex differential interaction of the follicular epithelium with the mesenchymal dermal papilla during three defined stages: growth (anagen), regression (catagen), and rest (telogen). The molecular signals and factors that are involved in initiation of anagen and transition to catagen and telogen are still not fully characterized. Most rodents exhibit synchronous follicular cycling in waves that sweep posteriorly and dorsally (5) and decrease in frequency with age. Although this rhythm is inherent in the follicles themselves, environmental and systemic factors influence the hair growth cycle. Synchronization of the hair cycle combined with a growth insult may result in massive hair loss and alopecia, whereas a similar growth insult to non-synchronized hair follicles may just result in sporadic hair depletion that may not be clinically evident.
Hair follicle cycling in rodents is associated with substantial alterations in the number, location, and activation of immune cells, including macrophages, Langerhans cells, T cells, and perifollicular mast cells (5). Mast cells are found in the bulge region and close to the dermal papilla in normal murine skin, in intimate association with hair follicle stem cells and mesenchymal tissue involved in hair growth regulation (6). Previous studies have implicated mast cells as potential effector cells in the murine hair growth cycle (7–9). When anagen is induced experimentally or occurs spontaneously in the mouse hair cycle, mast cell numbers decline in early anagen and increase in mid-anagen. Towards the end of anagen, mast cell numbers decrease gradually. In models of anagen induction by depilation or cyclosporine injection, mast cells exhibit evidence of degranulation (7). In a non-depilated murine model of alopecia, foot shock stress prolonged telogen and delayed the induction of anagen and was associated with increased mast cell degranulation (8). Stimulation of mast cell degranulation using the mast cell stimulatory compound 48/80 can induce anagen in mouse telogen follicles and antagonists of mast cell products can inhibit the development of anagen (7). The neuropeptide substance P increases mast cell degranulation and accelerates hair follicle regression in a murine model of the autoimmune hair loss syndrome alopecia areata (10) and has been further implicated in stress-associated telogen effluvium and alopecia areata (11). Increased numbers of activated mast cells have also been documented in scarring alopecias in humans, including male pattern baldness (12, 13).
This study was initated by our observation of sporadic post-natal alopecia that appeared to result from mast cell activation in mouse pups deficient in the immunoregulatory cytokine IL-10. The purpose of this study was to further investigate the association between mast cell activation and alopecia in Il10−/− pups. The development of alopecia in humans has been associated with iron deficiency (14) and was also recently reported in mice with iron-resistant, iron deficiency anemia due to mutation of the transmembrane serine protease 6 (Tmprss6) gene that regulates dietary iron absorption (15). Therefore, we also investigated the effect of maternal iron-restricted diets on the incidence of alopecia in Il10−/− pups.
Materials and Methods
Generation of Mouse Pups
IL-10-deficient mice (strain name = B6.129P2-Il10tm1Cgn/J; stock # 002251) (16) and mast cell-deficient KitW-sh/KitW-sh (“sash”) mice (strain name = B6.Cg-KitW-sh/HNihrJaeBsmJ; stock #005051) (17) were obtained from Jackson Laboratories (Bar Harbor, ME). These mice were cross-bred to generate Il10−/− mast cell-deficient (KitW-sh/KitW-sh) double mutant mice. The KitW-sh allele of the c-kit gene contains a promoter inversion that specifically prevents transcription of c-kit in melanocytes and mast cells, while allowing near normal transcription in other cell types (18). The strong dependence of mast cell and melanocyte development on c-kit activity results in mice that are deficient in mast cells and have white coats since they are also deficient in melanocytes. Genotype was determined by pigmentation (19) and by PCR genotyping for the targeted mutation in IL-10 as described (http://jaxmice.jax.org/pubcgi/protocols/protocols.sh?objtype=protocol&protocol_id=346; accessed 3/31/2005). Mice were housed in polycarbonate micro-isolator cages in individually ventilated racks with free access to food and water. Diets used were LabDiet 5001 (270 ppm iron) or PicoDiet 5058 (200 ppm iron) (Purina, Framingham, MA), except where noted. Each room was maintained at 22 ± 2°C on a light-dark cycle of 12h light and 12h dark. Sentinel mice housed on soiled bedding from the mice studied were negative for the presence of endo- and ectoparasites and serum samples were negative for a comprehensive panel of murine viral and bacterial pathogens by serology, PCR and microbiological assays (Research Animal Diagnostic Laboratory, Columbia, MO). All animal studies were approved by the Duke University Institutional Animal Care and Use Committee.
Breeding for studies designed to generate a single litter per female was performed in triads consisting of 2 females and 1 male, typically beginning when females were 6 – 8 wks of age. For studies designed to generate multiple litters per female, one male and one female were typically co-housed throughout the study. Thus, the next litter of pups was typically born soon after the previous litter was weaned. For both study designs, the number of pups born and the number surviving to weaning on P21 were determined for each successful pregnancy. The incidence of alopecia, defined as visually detectable loss of hair following initial pelage development, was determined by visual observation and was confirmed histologically.
Alopecia Treatments
To deplete skin mast cells, half of the pups from selected litters at risk were injected intraperitoneally (IP) with anti-c-kit monoclonal antibody ACK-2 in 0.1 ml saline. The following dosing schedules were tested: 1) ACK-2 every 3 days, beginning on day 5 of life and continuing through day 17 (5 doses); 2) ACK-2 on day 5 only (1 dose); 3) ACK-2 on days 5, 8, and 11 (3 doses); and 4) ACK-2 on days 5, 8, 11, and 14 (4 doses). Doses of ACK-2 were 500 µg (day 5), 200 µg (days 8 and 11), 300 µg (day 14), and 400 µg (day 17). The remaining littermates of ACK-2-injected pups received injections of saline only.
Sample Collection
Blood samples were obtained from the maxillary vein of live mice or from the inferior vena cava after euthanasia. Milk was collected on P19 essentially as described (20). Lactating females were separated from their litters for 1 – 3 hrs, then milk ejection was induced by i.p. injection with 0.5U oxytocin. Milk was expressed by manual massage and 40 – 100 µl was collected using a capillary tube. All animals were euthanized according to acceptable methods within the American Veterinary Medical Association Guidelines on Euthanasia (http://www.avma.org/issues/animal_welfare/euthanasia.pdf). Skin biopsies were obtained from a subset of pups to document histology. Additional tissues, including colon, were obtained from dams to document their inflammation status. Tissue samples were fixed in Carnoy’s solution (60% ethanol, 30% chloroform, 10% glacial acetic acid) for 2 – 4 hrs then placed into 100% ethanol for processing into paraffin blocks using standard techniques. Sections were stained with hematoxylin and eosin or with toluidine blue to document mast cell granules. Mast cells were counted at a magnification of x400 using a calibrated microscope stage, with at least 4 mm2 examined per mouse skin analyzed.
In vitro studies
Samples of milk obtained from dams with and without alopecia in their litters were incubated for 30 minutes at 37°C with DMEM media ±10 µg/ml lipoprotein lipase (LPL) to release fatty acids. The milk samples were diluted 1:1000 in serum-free DMEM, sterile-filtered, then added to RAW267.4 murine macrophage cells (American Type Culture Collection, Manassas, VA) that had been previously plated into 96 well plates, allowed to adhere, and then cultured for 18 hrs in serum-free DMEM, as previously described (20). Media for cytokine analysis was harvested after a 20 hr incubation at 37°C in a humidified atmosphere containing 5% CO2. The cytokines and chemokines present were quantitated using a Luminex bead-based fluorescent multiplex immunoassay (Bio-Plex, BioRad, Hercules, CA).
Iron studies
Complete blood counts were performed on EDTA-anticoagulated whole blood using a CELL-DYN 3700 Hematology Analyzer (Abbott Diagnostics, Abbott Park, IL). For studies of effects of dietary iron on alopecia development, custom-prepared iron-deficient (2–6 ppm Fe) and matched iron-adjusted (200 ppm Fe) control casein-based defined diets (TD.07605 and TD.08420, respectively; Harlan-Teklad, Madison, WI) were initially begun on day 10 and continued through lactation day 19. Initiation of a similarly iron-poor diet on gestational day 10–11 in pregnant rats was previously shown to result in development of severe anemia by lactation day 1 (21). However, due to severe runting and high mortality, the iron-deficient diet was instituted on day 16 of gestation for the remaining studies. Alternately, mice were placed on the custom prepared 5SH5 diet (identical to PicoDiet 5058, but with 131 ppm Fe; Purina) on day 0 of gestation and continued through day 19 of lactation. Diets containing 35 ppm iron have been found to provide sufficient iron to prevent the development of anemia in normal adult mice (22), thus the 5SH5 diet is not considered to be iron-deficient.
Statistical Analysis
Differences in development of alopecia between groups were tested for using Fisher’s exact test. Differences in litter sizes, mast cell numbers, or cytokine production were determined using Student’s t test, or analysis of variance (ANOVA) followed by the least significant differences method for multiple comparisons. P values of < 0.05 were considered to represent significant differences.
Results
Pups born to a subset of Il10−/− dams develop alopecia
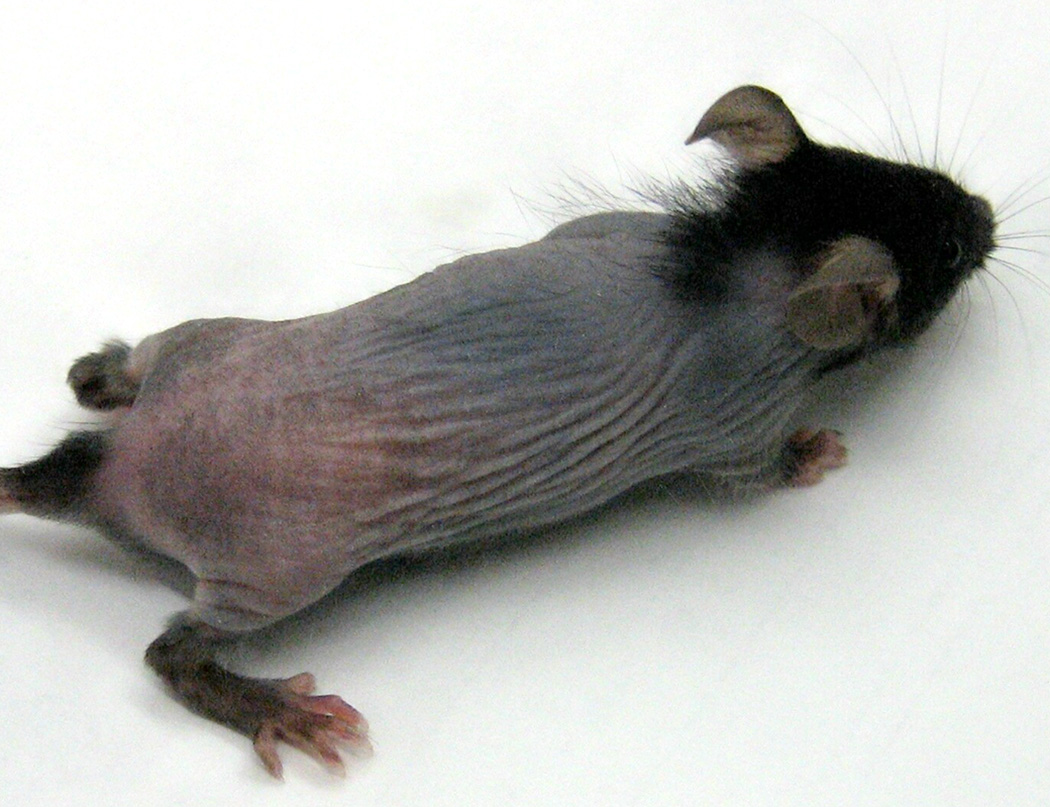
In the course of maintaining breeding colonies of Il10−/− mice on the C57BL/6 background, we observed several litters with alopecia. Initial hair growth in these pups occurred normally on post-natal (P) days 5 – 7, but progressive hair loss became apparent on P15. Dorsal skin of affected pups typically exhibited near-complete alopecia by P18 - 19, with marked thinning of the ventral pelage and sparing of the head, base of the tail, and feet (Figure 1). The incidence of alopecia in first litters from Il10−/− females who were 6 – 8 weeks of age at initial breeding was 18% (6 of 34 litters). Litter size did not differ in litters with (8 ± 2 pups) and without alopecia (6 ± 1 pups; p = 0.18).
Figure 1. Alopecia in IL-10−/− mice.
Mouse is shown on day 27, representative of appearance on days 17 – 35.
The epidemiology of the alopecia suggested an environmental rather than a genetic cause. Alopecia was sporadically seen in progeny of mice obtained directly from the supplier as well as from colony-bred mice. When alopecia occurred, all pups in the litter were affected. In most cases, subsequent litters from those dams also developed alopecia. However, increased age of the dam was not directly associated with hair loss, since no alopecia was seen in first litters born to 9 females that were 18 – 20 weeks old at the time of first breeding. Although Il10−/− mice can develop inflammatory bowel disease, colon inflammation in dams was not linked to alopecia in their offspring. In a set of 4 dams whose colon tissues were examined after they had one or more litters with alopecia, two had no evidence of colon inflammation, another had minimal colitis, and the fourth had severe colitis with rectal prolapse at the time of weaning.
Pups with alopecia were active and grew normally after weaning on P21. Hair re-growth began on P36–39 and proceeded rapidly. By P42, pups that had experienced neonatal alopecia were visually indistinguishable from pups that did not exhibit this phenotype.
Skin histology in neonatal Il10−/− mice with alopecia
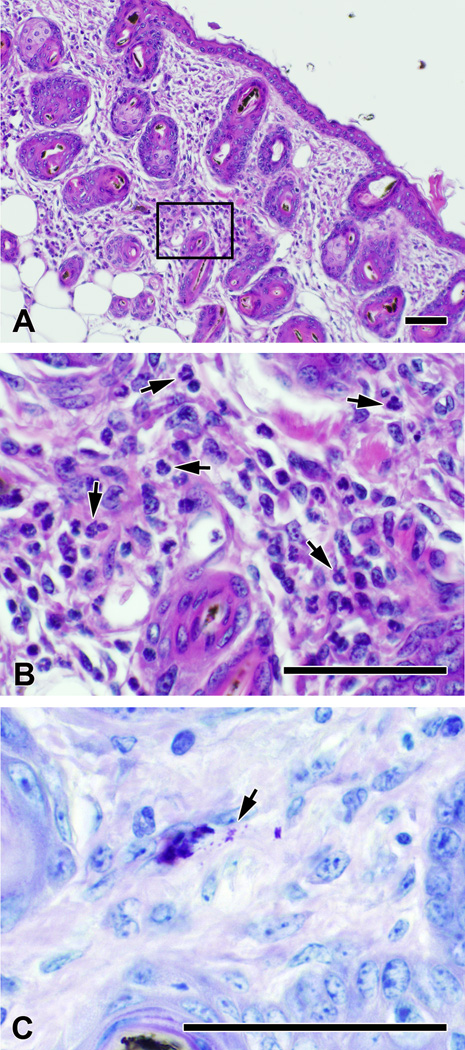
Hair follicle morphogenesis in littermates of mice that eventually developed alopecia was consistent with what has been previously reported for wild type C57BL/6 mice (1), with many follicles containing fully developed hairs on day 5. No abnormalities were seen on P9, however occasional follicles with irregular profiles that contained bent or broken hairs could be seen as early as P12. Kinked and broken hairs were present within enlarged and distorted follicles beginning on P14 (not shown), with a mild dermal inflammatory infiltrate containing of neutrophils and mononuclear cells. When alopecia was near total on P19, the inflammatory infiltrate was abundant and the dermis was full of dilated follicles containing bent and broken hairs (Figure 2A, B). Many papillae were still deep in the subcutaneous tissue, but others were beginning to rise into the upper subcutaneous region and the dermis, consistent with a transition to catagen (Figure 3A). Hair follicles in Il10−/− mice that did not develop alopecia cycled with timing similar to that published for wild type C57BL/6 mice (23), with anagen phase from days 5 – 16, catagen on days 16 – 19 (Figure 3B), telogen phase from days 19 – 27, and return to anagen phase by day 28. Toluidine blue staining revealed large numbers of dermal mast cells in the skin of pups with alopecia, many of which were degranulated or actively degranulating (Table 1, Figure 2C, 3I). Numbers of both activated and total mast cells present in the skin of pups with alopecia were increased relative to pups that did not develop alopecia (Table 1).
Figure 2. Skin histology of Il10−/− pups with alopecia.
On day 19, the skin of mice with alopecia contains dilated follicles with bent and broken hairs (A), as well as a prominent inflammatory infiltrate that includes neutrophils (arrows, B) and mononuclear cells. Numerous degranulating mast cells are also present throughout the dermis (arrow points to relased granules). Panels A and B were stained with hematoxylin and eosin. Panel C was stained with toluidine blue. Bar in A – C represents 50 µm.
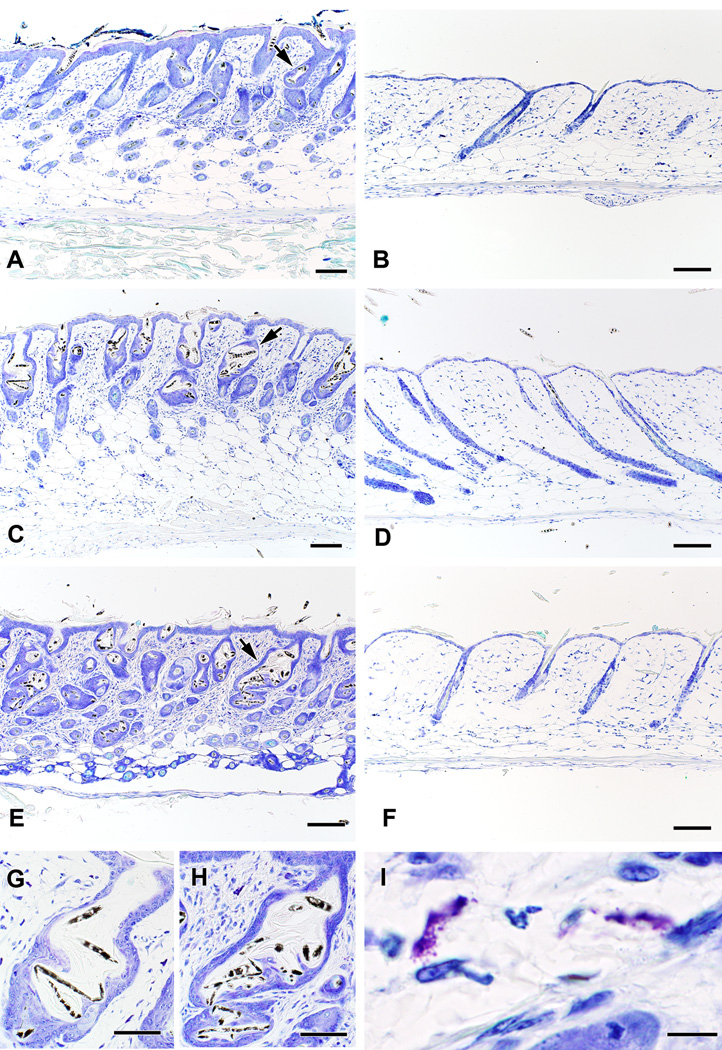
Figure 3. Skin histology from Il10−/− pups with and without alopecia.
Dilated follicles containing bent and broken hairs are evident in the skin of mice with alopecia (A), as well as a prominent inflammatory infiltrate that includes numerous degranulating mast cells (I). Il10−/− mice that do not develop alopecia have normal follicles in catagen or telogen on day 19 (B). Mast cell depletion using anti-c-kit mAb prevents the development of alopecia in at-risk litters. (D). Skin from a saline-treated littermate with alopecia is shown in C for comparison. Feeding dams an iron-deficient diet enhances the incidence of alopecia in litters of Il10−/− pups (E), but does not cause alopecia in mast cell-deficient KitW-sh/W-sh x Il10−/− pups (F). The abnormal hair follicles seen when alopecia is triggered by an iron-deficient diet (H) are indistinguishable from those seen in sporadic alopecia (G). All skin shown was obtained on P19 and was stained with toluidine blue. The scale bar represents 100 µm in panels A – H and 20 µm in panel I.
Table 1.
Mast Cells in Skin of IL-10−/− Mouse Pups1
| Total mast cells/mm2 | Activated mast cells/mm2 | |
|---|---|---|
| Alopecia (n = 12) | 37 ± 42 | 13 ± 33 |
| No alopecia (n = 13) | 23 ± 2 | 5 ± 1 |
| Alopecia (dam on Fe-deficient diet) | 50 ± 24 | 39 ± 24 |
| (n = 6) |
All pups were assessed on day 19 of life. Mast cells were identified using toluidine blue-stained sections as described in the Methods and are presented as mean ± SEM/mm2.
p = 0.009 vs. no alopecia
p = 0.02 vs. no alopecia
p ≤ 0.007 vs. pups with alopecia on standard diet and p ≤ 2 × 10−5 vs. no alopecia
Genetic mast cell deficiency prevents development of alopecia in Il10−/− pups
To test the hypothesis that mast cells contributed to the development of alopecia in Il10−/− pups, we created IL-10-deficient mice that additionally lacked mast cells (Il10−/− x KitW-sh/KitW-sh). Alopecia was observed in 7 of 11 litters (64%) born to 3 mast cell-sufficient Il10−/− females that were allowed to breed repeatedly to Il10−/− males in stable pairs. In contrast, no alopecia (0%) was observed in a total of 17 litters born to 4 Il10−/− x KitW-sh/KitW-sh mast cell-deficient females that were similarly allowed to breed repeatedly to Il10−/− x KitW-sh/KitW-sh males (p= 0.0003; Fisher’s exact test).
Pharmacologic depletion of mast cells protects against alopecia
Systemic administration of anti-c-kit monoclonal antibody ACK-2 was used as previously described (24) to deplete dermal mast cells of C57BL/6 Il10−/− pups. Mast cells were abundant (21/mm2) in the skin of control mice and decreased in a dose-dependent fashion to 9, 5, and 2 /mm2 on P19 in the skin of pups who received 1, 3, and 4 doses of ACK-2, respectively. Mast cells were almost totally absent (0.2/mm2) on P19 in the skin of mice that received a total of 5 doses of ACK-2. Mast cell deficiency in these mice persisted (0.6/mm2) at least through P35, the latest time point tested. None of the ACK-2-treated pups had demonstrable hair loss (Figure 3D; Figure 4B) when marked alopecia was observed in their saline-treated littermates (Figure 3C; Figure 3A). Interestingly, through its documented inhibitory effects on melanocytes (25), ACK-2 treatment also altered the color of hair shafts, yielding pink skin with black-tipped white dorsal and white ventral hair colors (Figure 4B). Of note, mast cells in the pups seemed to be required for the development of alopecia; a mast cell-deficient Il10−/− x KitW-sh/KitW-sh pup fostered from P2 – P19 by an Il10−/− dam who transmitted alopecia to her saline-treated Il10−/− offspring did not develop alopecia (Figure 4C).
Figure 4. Treatment with c-kit antibody or genetic mutation of c-kit protects against Il10−/− pups against alopecia.
A–C. Pups from an Il10−/− dam were randomized to receive i.p. injections with anti-c-kit mAb ACK2 or saline every 3 days, beginning on day 5. In addition, one mast cell-deficient pup from a KitW-sh/W-sh x Il10−/− dam was fostered to this litter on day 2 of life. Photographs were taken on day 17. Pharmacologic (ACK-2, panel B) or genetic lack of mast cells (panel C) prevented the spontaneous development of alopecia in pups nursed by this dam. D – F. Kit+/W-sh x Il10−/− mice were mated to generate black pups with normal numbers of mast cells (D), black and white-sashed pups with intermediate numbers of mast cells (E), and white pups lacking mast cells within the same litter (F). In this experiment, dams were placed on an iron-deficient (3 – 6 ppm) diet on day 16 of gestation. Similar results were obtained when a diet containing 131 ppm iron was initiated on day 0 of gestation. As shown, pups with mast cells (D, E) developed alopecia, while pups lacking mast cells (F) showed no evidence of hair loss. Photographs were obtained on day 21.
Macrophage stimulation by murine milk does not correlate with alopecia
We next tested the hypothesis that milk produced by Il10−/− dams whose pups developed alopecia had direct inflammatory effects. Addition of a 1:1000 dilution of milk from dams whose pups developed alopecia induced RAW267.4 macrophages to secrete all 9 of the cytokines and chemokines tested (IL-12, IFN-γ, KC, MCP-1, TNF, IL-1β, IL-4, IL-6, and IL-10). The amounts of these cytokines and chemokines produced by RAW267.4 cells in the absence of milk exposure were at or below the limit of detection. Addition of lipoprotein lipase (LPL) to the milk samples to release free fatty acids further increased macrophage secretion of TNF, IL-1β, and IL-6 cytokine proteins (p = 0.035, 0.003, and 0.024, respectively; Figure 5); addition of LPL alone had no effect on cytokine secretion. Exposure to murine milk clearly has pro-inflammatory effects on RAW267.4 macrophages, however no differences were observed in cytokines stimulated by milk samples obtained from dams whose litters developed alopecia and those that did not (Figure 5). Thus, although Il10−/− dams appear to produce “inflammatory milk”, this is not sufficient to induce alopecia in their pups.
Figure 5. Milk from IL-10−/− dams induces production of pro-inflammatory cytokines by RAW267.4 macrophages.
Milk samples from dams whose litters did or did not develop alopecia were cultured with RAW267.4 macrophages and cytokine production was determined as described in Methods. On each graph, the mean ± SEM for each cytokine or chemokine is shown for milk samples from dams whose litters developed alopecia (n = 3) and from dams whose litters did not develop alopecia (n = 5). Note differences in scale between the 2 graphs. * indicates p < 0.05 compared with absence of lipoprotein lipase (LPL).
Mast cell-dependent alopecia can be triggered by an iron-deficient maternal diet
Il10−/− mice with enterocolitis have been previously reported to have iron deficiency anemia, but the incidence of anemia in mice of breeding age without colitis was unclear (16). Complete blood counts (CBCs) obtained from adult helicobacter-free Il10−/− mice including breeders whose litters did not develop alopecia (13 – 28 wk; n = 9) showed normal hematocrit (Hct, 45 ± 3%), hemoglobin (Hgb, 14.3 ± 1.0 mg/dL), and mean cellular volume of erythrocytes (MCV, 44.1 ± 0.7 fL). Thus, anemia is not a typical characteristic of Il10−/− mice without colitis. However, a CBC obtained on day 19 of lactation from an Il10−/− dam whose litter demonstrated sporadic alopecia showed severe anemia, with Hgb = 6.4 mg/dL and Hct = 22%. A second Il10−/− dam whose litter developed alopecia also showed dyserythropoiesis, with anisocytosis and microcytosis (MCV = 40.8 fL; normal = 43.6 – 55.9 fL), although her Hgb and Hct were within the normal range.
Based on this data, we determined whether an iron-restricted maternal diet influenced the development of alopecia in litters from Il10−/− dams with and without mast cell deficiency. Pups born to dams fed an iron-deficient diet (3–6 ppm iron) exhibited growth-retardation (weight = 4.0 ± 0.4 g on day 19; normal weights = 7.8 ± 0.1) and increased mortality with maternal cannibalism. Alopecia was seen on day 19 in 100% (8 of 8) surviving Il10−/− pups from 3 different litters. These pups had hypochromic, microcytic anemia consistent with iron deficiency by blood smear (not shown). Skin histology in alopecic pups from dams on the iron-deficient diet was identical to that seen in pups with sporadic alopecia (Figure 3E), with elevated numbers of total and activated mast cells in the skin (Table 1). KitW-sh/W-sh x Il10−/− pups deficient in both mast cells and IL-10 (n = 9 surviving from 3 litters) also developed hypochromic, microcytic anemia when their dams were fed the iron-deficient diet, but had 0% alopecia (Figure 3F). No consistent differences in pup mortality or the occurrence of alopecia were observed when the iron-deficient diet was instituted on gestational day 10 vs. day 16.
To remove maternal variables and to avoid the difficulties associated with cross-fostering, Kit+/W-sh x Il10−/− males and females were mated to generate Il10−/− black pups with normal numbers of mast cells (genotype Kit+/+ x Il10−/−), black and white-sashed pups with fewer mast cells (genotype Kit+/W-sh x Il10−/−), and white pups lacking mast cells (genotype KitW-sh/W-sh x Il10−/−) within the same litter. Dams were placed on either the 3–6 ppm iron-deficient diet on day 16 of gestation or a diet containing 131 ppm iron on day 0 of gestation (n = 3 – 7 dams per group). Black pups with normal numbers of mast cells developed alopecia and white pups lacking mast cells did not (Figure 4D, F). Sashed pups developed near total alopecia of dorsal hair from black regions, with partial retention of hair in white regions (Figure 4E), a pattern that appears to correlate with regional differences in mast cell distribution (data not shown). Alopecia was observed in 71% (5 of 7) of litters from dams fed the 131 ppm diet and 100% (3 of 3) of litters from dams fed the iron-deficient (3–6 ppm) diet, compared with the previously observed 18% (6 of 34) of litters from dams fed a diet containing 200 ppm iron. Thus, a maternal diet containing decreased iron increases the incidence of alopecia in C57BL/6 Il10−/− pups, in a mast cell-dependent fashion.
To further explore the role of iron deficiency in alopecia in this model, we obtained CBCs as an indicator of iron status in Il10−/− pups from 7 dams fed the diet with 131 ppm iron. Data were analyzed as a function of mast cell status and the presence or absence of alopecia in their litter. Hgb and Hct did not differ between black, sashed, and white Il10−/− pups based on their Kit genotype (Table 2). However, Il10−/− pups from litters with alopecia had significantly decreased Hgb and Hct compared to Il10−/− pups from litters without alopecia (Table 2). The Hgb, Hct, and MCV of the dams did not differ according to the presence or absence of alopecia in their litter (Table 2).
Table 2.
Alopecia Correlates with Pup Iron Status
| Hgb (g/dL) | Hct (%) | MCV (fL) | ||
|---|---|---|---|---|
| Alopecia in Litter | ||||
| Black pups (n=12) | 7.6 ± 0.2* | 26.5 ± 0.8* | 39.4 ± 0.3 | |
| Sashed pups (n=9) | 7.4 ± 0.3* | 25.9 ± 1.0* | 41.1 ± 0.4 | |
| White pups (n=4) | 6.9 ± 0.4 | 23.1 ± 0.8 | 41.1 ± 0.4 | |
| No Alopecia in Litter | ||||
| Black pups (n=7) | 9.1 ± 0.3 | 30.7 ± 1.2 | 42.1 ± 0.3 | |
| Sashed pups (n=3) | 9.1 ± 0.4 | 31.0 ± 1.3 | 40.6 ± 0.7 | |
| Dams | ||||
| Alopecia in litter (n=5) | 15.0 ± 0.3 | 47.8 ± 0.8 | 45.0 ± 0.7 | |
| No alopecia in litter (n=2) | 15.6 ± 0.2 | 49.1 ± 0.7 | 45.9 ± 1.2 | |
Indicates p < 0.05 compared to corresponding genotype of pups without alopecia
Discussion
This study demonstrates that alopecia in litters from Il10−/− dams is associated with increased mast cell infiltration and degranulation in the skin of the pups and that hair loss can be prevented by either pharmacologic or genetic inhibition of c-kit that leads to depletion of pup mast cells. The epidemiologic pattern of incidence suggested an environmental rather than a genetic trigger for hair loss. Although Il10−/− mice generally exhibit exaggerated inflammatory responses, pre-existing inflammation (e.g. in the colon of dams) was not required for transmission of alopecia to pups. Furthermore, “inflammatory milk” was not the cause of alopecia, since milk from Il10−/− dams induced macrophage secretion of pro-inflammatory cytokines in vitro regardless of whether or not their pups developed alopecia. Our studies ultimately showed that a maternal iron-restricted diet enhanced the incidence of alopecia in Il10−/− pups in a mast cell-dependent fashion.
Depletion of mast cells by administration of ACK-2 antibody or by KitW-sh/ KitW-sh mutation prevented hair loss in susceptible pups. Both of these interventions interfere with the c-kit pathway that is required for mast cell survival and activation. Taken together with the enhanced mast cell numbers and activation seen in pups with alopecia, the data strongly suggest mast cells as effector cells for the alopecia phenotype but do not rule out potential contributions from other c-kit-positive cell types. The usual method of proving mast cell-dependence, e.g. restoring sensitivity of KitW-sh/ KitW-sh x Il10−/− pups from iron-deficient dams to alopecia following adoptive transfer of bone marrow-derived mast cells, is not practical in this case since such reconstitution requires a minimum of 4 weeks and the process of alopecia is already histologically evident as early as postnatal day 12. c-kit is normally expressed at high levels on melanocytes and mast cells, but has been documented at low levels on a variety of cell types including hair matrix keratinocytes (26). Since IL-10 has been shown to decrease c-kit expression (27), it is possible that c-kit expression is increased on a variety of cell types in IL-10-deficient mice. The role of IL-10 deficiency in mast cell activation is also not currently understood. Thus, further studies will be required to establish mast cells as the sole effector cells for this phenotype and to determine the specific contributions of IL-10 deficiency to the enhanced mast cell proliferation and degranulation observed in the Il10−/− mice reported here.
Histologic samples from alopecic mice demonstrated enlarged and dilated follicles with bent or broken hair shafts and prominent dermal infiltrates consisting of neutrophils, mononuclear inflammatory cells, and activated mast cells. The follicular changes may indicate that alopecia is a result of abnormal morphogenesis (e.g hair matrix fragility that leads to hair breakage), that may or may not be related to hair cycle abnormalities. We hypothesize that the pattern of alopecia observed, with loss of hair primarily from the back and retention on the head and limbs, reflects the differential sensitivity of hair follicles to inflammatory mediators at different stages of the hair cycle, since follicular cycle lengths are known to vary according to body location (5). Additional studies will be required to elucidate the mechanisms by which inflammation may affect follicular morphogenesis and cycling.
Alopecia was observed in a litter of double heterozygotes (IL-10+/- Kit+/W-sh) generated from a cross between an Il10−/− female and a KitW-sh/KitW-sh male, suggesting that IL-10-deficiency in dams but not pups was required for the development of alopecia in pups. A dependence on maternal genotype, combined with return of hair after weaning, led us to initially hypothesize that the alopecia might result from inflammatory mediators that were transmitted in milk. Such a scenario was recently suggested by Wan et al, who elegantly documented the presence of oxidized fatty acids that stimulated cytokine mRNA production by the RAW267.4 murine macrophage cell line in milk from dams with Tie-2/cre-driven deletion of PPARγ (20). Pups from these Tie-2/cre-Pparγ−/− dams uniformly exhibited a hair loss phenotype and histology similar to what we report here, however mast cells were not investigated in that study. Our studies showed that milk from Il10−/− dams induced macrophage secretion of pro-inflammatory cytokines in vitro similar to what was reported by Wan et al, however this was unrelated to whether their pups developed alopecia. Thus, “inflammatory milk” by itself was not the cause of alopecia. This suggests that additional environmental mechanisms must trigger the onset of alopecia in affected litters.
The observations reported here clearly indicate that maternal iron restriction can trigger a mast cell-mediated alopecia in their pups. However most cases of spontaneous alopecia in Il10−/− pups occurred before our identification of iron as a potential trigger; thus CBC data was not available for most dams and litters. The gross appearance and histology seen in sporadic alopecia and alopecia triggered by dietary iron deficiency were similar and abnormal erythropoiesis was documented in 2 Il10−/− dams whose litters exhibited sporadic alopecia. Furthermore, the epidemiologic pattern of sporadic alopecia that was exacerbated by repeated breeding during the post-partum estrus and relieved by long intervals on an iron-supplemented diet between litters is consistent with a hypothesized relationship to maternal iron deficiency. However, the mechanisms that may have led to iron deficiency on some Il10−/− dams despite consumption of an iron-sufficient diet (200 ppm iron) remain undocumented at present.
Our CBC data indicate that Il10−/− mice without enterocolitis generally have robust erythropoiesis without anemia. IL-10 regulates the growth, differentiation, and activation of many hematopoietic cells. IL-10 also upregulates expression of transferrin receptor and ferritin, favoring receptor-mediated uptake of iron and its storage within macrophages (28). Thus, lack of IL-10 might be predicted to decrease iron reserves, placing Il10−/− mice at risk for development of iron deficiency anemia if iron intake is insufficient, demand for iron is high (e.g. pregnancy, lactation, or colitis-associated blood loss), or if iron absorption is decreased due to chronic inflammation. An iron-deficient diet clearly increases the incidence of alopecia (from 18% to 100%) in litters of Il10−/− dams. However, fully determining the mechanisms that trigger mast cell-mediated alopecia in offspring of Il10−/− dams on iron-sufficient (131 or 200 ppm iron) diets and how iron and mast cells regulate hair growth will require additional study. Such studies should also address the best route of iron supplementation, since oral iron is poorly absorbed when levels of the iron regulatory hormone hepcidin are elevated (e.g. in the setting of chronic inflammation) (28).
Iron deficiency anemia has been documented in 12% of females from ages 12–49 in the United States, rising to 19–22% in African- and Mexican-American females and to 29% in low income pregnant women of all races (29). Anemia of chronic disease, the next most common form of anemia, occurs in patients with acute or chronic immune activation (28). Most of the few studies that have been performed to investigate the relationship between iron deficiency, anemia, and alopecia in humans have dealt exclusively with females and with non-scarring forms of alopecia. Results are mixed (reviewed in 14), with some studies suggesting a relationship between iron deficiency and alopecia areata, chronic telogen effluvium, and androgenic alopecia and others failing to find such a connection. Overall, the available studies are weakened by their small size, imprecision in classifying the cause for alopecia, and lack of rigorous statistical analysis. Thus, insufficient data currently exist to recommend universal screening for iron deficiency in human patients with alopecia (14).
In summary, we have identified a model of c-kit-dependent alopecia in Il10−/− mouse pups that is associated with mast cell activation and can be enhanced by iron deficiency. Further studies are indicated to fully explore the mechanisms involved and to assess how these mechanisms may contribute to pathogenesis and treatment of alopecia in humans.
Acknowledgements
The authors would like to thank Chau Trinh for expert technical assistance with mouse husbandry, Rebecca Whittlesey and Dr. Karin A. Finberg for assistance with the assays of iron status, and Drs. Herman F. Staats, George Cianciolo, Ron E. Banks, and Nancy C. Andrews for helpful discussions over the course of this project. This work was funded by the National Institutes of Health R21-DK075522.
References
- 1.Paus R, Muller-Rover S, van der Veen C, Maurer M, et al. A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 2.Sundberg JP, editor. Handbook of Mouse Mutations with Skin and Hair Abnormalities: Animal Models and Biomedical Tools. Boca Raton: CRC Press; 1994. p. 544. [Google Scholar]
- 3.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 5.Stenn KS, Paus R. Controls of Hair Follicle Cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 6.Kumamoto T, Shalhevet D, Matsue H, Mummert ME, Ward BR, Jester JV, Takashima A. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood. 2003;102:1654–1660. doi: 10.1182/blood-2003-02-0449. [DOI] [PubMed] [Google Scholar]
- 7.Paus R, Maurer M, Slominski A, Czarnetzki BM. Mast cell involvement in murine hair growth. Dev Biol. 1994;163:230–240. doi: 10.1006/dbio.1994.1139. [DOI] [PubMed] [Google Scholar]
- 8.Katayama M, Aoki E, Suzuki H, Kawana S. Foot shock stress prolongs the telogen stage of the spontaneous hair cycle in a non-depilated mouse model. Exp Dermatol. 2007;16:553–560. doi: 10.1111/j.1600-0625.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 9.Maurer M, Fischer E, Handjiski B, et al. Activated skin mast cells are involved in murine hair follicle regression (catagen) Lab Invest. 1997;77:319–332. [PubMed] [Google Scholar]
- 10.Siebenhaar F, Sharov AA, Peters EMJ, Sharova TY, Syska W, Mardaryev AN, Freyschmidt- Paul P, Sundbery JP, Maurer M, Botchkarev VA. Substance P as an immunomodulatory peptide in a mouse model for autoimmune hair loss. J Invest Dermatol. 2007;127:1489–1497. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- 11.Peters EMJ, Liotiri S, Bodo E, Hagen E, Biro T, Arcj PC, Paus R. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Amer J Pathol. 2007;171:1872–1886. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Solky B, Elenitsas R, Cotsarelis G. Scarring alopecia associated with mastocytosis. J Cutan Pathol. 2003;30:561–565. doi: 10.1034/j.1600-0560.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 13.Won CH, Kwon OS, Kim YK, Kang YJ, Kim BJ, Choi CW, Eun HC, Cho KH. Dermal fibrosis in male pattern hair loss: a suggestive implication of mast cells. Arch Dermatol Res. 2008;300:147–152. doi: 10.1007/s00403-007-0826-x. [DOI] [PubMed] [Google Scholar]
- 14.Trost LB, Bergfield WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol. 2006;54:824–844. doi: 10.1016/j.jaad.2005.11.1104. [DOI] [PubMed] [Google Scholar]
- 15.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 17.Wolters PJ, Mallen-St. Clair J, Lewis CC, et al. Tissue selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berrozpe G, Timokhina I, Yukl S, Tajima Y, Ono M, Zelenetz AD, Besmer P. The Wsh W57, and Ph Kit expression mutations define tissue-specific control elements located between -23 and -154 kb upstream of Kit. Blood. 1999;94:2658–2666. [PubMed] [Google Scholar]
- 19.Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982;39:315–322. doi: 10.1017/s001667230002098x. [DOI] [PubMed] [Google Scholar]
- 20.Wan Y, Saghatelian A, Chong L-W, Zhang C-L, Cravatt BF, Evans RM. Maternal PPARγ protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21:1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research (ILAR) Nutrient Requirements of Laboratory Animals. Fourth Revised Edition. 1995. (accessed on 12/23/2008 at http://booksnapedu/openbookphp?record_id=4758&page80) [Google Scholar]
- 23.Muller-Rover S, Handjiski B, van der Veen C, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 24.Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okura M, Maeda H, Nishikawa S, Mizoguchi M. Effects of Monoclonal Anti-c-Kit Antibody (ACK2) on Melanocytes in Newborn Mice. J Invest Dermatol. 1995:322–328. doi: 10.1111/1523-1747.ep12319939. [DOI] [PubMed] [Google Scholar]
- 26.Peters EMJ, Maurer M, Botchkarev VA, Jensen KD, Welker P, Scott GA, Paus R. Kit is expressed by epithelial cells in vivo. J Invest Dermatol. 2003;121:976–984. doi: 10.1046/j.1523-1747.2003.12478.x. [DOI] [PubMed] [Google Scholar]
- 27.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 28.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 29.Clark SF. Iron deficiency anemia. Nutr Clin Pract. 2008;23:128–141. doi: 10.1177/0884533608314536. [DOI] [PubMed] [Google Scholar]