Abstract
Aims
Cardiac dysfunction is a complication of sepsis and contributes to morbidity and mortality. Since raising plasma apolipoprotein (apo) A-I and high density lipoprotein (HDL) concentration reduces sepsis complications, we tested the hypothesis that the apoA-I mimetic peptide 4F confers similar protective effects in rats treated with lipopolysaccharide (LPS).
Methods and results
Male Sprague-Dawley (SD) rats were randomized to receive saline vehicle (n=13), LPS (10 mg/kg: n=16) or LPS plus 4F (10 mg/kg each: n=13) by intraperitoneal injection. Plasma cytokine and chemokine levels were significantly elevated 24 hrs after LPS administration. Echocardiographic studies revealed changes in cardiac dimensions that resulted in a reduction in left ventricular end-diastolic volume (LVEDV), stroke volume (SV) and cardiac output (CO) 24 hrs after LPS administration. 4F treatment reduced plasma levels of inflammatory mediators and increased LV filling, resulting in improved cardiac performance. Chromatographic separation of lipoproteins from plasma of vehicle, LPS and LPS+4F rats revealed similar profiles. Further analyses showed that LPS treatment reduced the agarose electrophoretic mobility of isolated HDL fractions. HDL-associated proteins were characterized by SDSPAGE and mass spectrometry. ApoA-I and apoA-IV were reduced while apoE content was increased in LPStreated rats. 4F treatment in vivo attenuated changes in HDL-associated apolipoproteins and increased the electrophoretic mobility of the particle.
Conclusions
The ability of 4F to reduce inflammation and improve cardiac performance in LPS-treated rats may be due to its capacity to neutralize endotoxin and prevent adverse changes in HDL composition and function.
Keywords: Lipopolysaccharide, Sepsis, ApoA-I mimetic peptide, HDL, Cardiac function, Inflammation
Introduction
Sepsis is a major cause of death in hospitalized patients. Approximately 50% of patients in intensive care units develop sepsis and the overall mortality rate of all affected patients is 29% [1]. Mortality is due, in large part, to the cytotoxic actions of LPS, a component of the outer membrane of Gram-negative bacteria. LPS is released from bacterial membranes and activates Toll-like receptors (TLR) on monocytes, neutrophils and other target cells [2–5]. TLRs transduce LPS action by activating NF-κB-dependent signaling [4–6]. By this mechanism, LPS stimulates the synthesis/release of inflammatory cytokines and chemokines that play an important role in the innate immune response [7,8]. Dysregulation of this response can lead to endothelial dysfunction, intravascular coagulation, multiple organ failure, including cardiovascular (CV) collapse, and death. CV failure, characterized by severe hypotension and cardiac dysfunction, is a principal cause of death in septic patients [1–2].
HDL and its major protein component apoA-I exert prominent anti-inflammatory and anti-oxidant effects [9–10]. Reduced plasma concentrations of HDL/apo A-I accompany the acute phase response to bacterial infection and overt sepsis, and increasing plasma HDL reduces complications associated with endotoxemia [11]. In this regard, a 2-fold increase in HDL was shown to enhance binding of intraperitoneally-administered LPS to HDL, reduce plasma cytokine levels and improve survival in apoA-I overexpressing mice [12]. Raising plasma HDL thus represents an important therapeutic goal in the treatment of sepsis and its complications. Therapeutic approaches to raise plasma HDL, however, have yielded variable results [13].
Apolipoprotein mimetic peptides, previously developed in our laboratories, represent an emerging area of HDL therapy [14–16]. The apoA-I mimetic peptide 4F, whose structure is based on the helical repeating domains of apoA-I, dramatically inhibits lesion formation and vessel wall thickness in dyslipidemic mouse models [14–17]. 4F improves HDL quality/function by increasing its antioxidant activity and by stimulating the formation of small pre β-HDL particles [16]. Additional CV protective effects of 4F include stimulation of the expression of the antioxidant enzymes heme oxygenase 1 (HO-1) and extracellular superoxide dismutase (EC-SOD) [18].
We previously reported that in vitro treatment of human umbilical vein endothelial cells with 4F reduces LPS-induced expression of cytokines, chemokines and adhesion molecules [19]. These responses were associated with the binding of 4F to LPS and neutralization of endotoxin activity. The current study extends our previous observations by examining in vivo effects of 4F in SD rats treated with LPS. We present data showing that LPS impairs LV filling and cardiac output (CO) in septic rats. LPS also induced changes in HDL-associated proteins that are characteristic of a dysfunctional lipoprotein particle. It is proposed that 4F, by neutralizing endotoxin, reduces circulating concentrations of pro-inflammatory mediators, prevents modifications to HDL-associated apolipoproteins and improves cardiac performance in LPS-treated rodents.
Methods
Materials
LPS (Escherichia coli, serotype 026:B6) was obtained from Sigma (St. Louis, MO). Antibodies to apoA-I were obtained from Brookwood Biomedical Inc (Birmingham, AL). Superose 6 columns were from Amersham Biosciences (NJ, USA). Total plasma cholesterol was measured using a commercially available kit (Wako, Inc).
ApoA-I mimetic peptide synthesis
4F, whose amino acid sequence is Ac-DWFKAFYDKVAEKFKEAFNH2, was synthesized using L-amino acids by the solid phase peptide synthesis method as previously described [15]. Peptide purity was ascertained by mass spectral analysis and analytical HPLC. Peptide concentration was determined using molar extinction coefficients of tryptophan and tyrosine [20].
Animals
Ten-week old, male SD rats were purchased from Charles Rivers Breeding Laboratories (Wilmington, MA) and allowed a 1 week recovery period prior to initiating experimental protocols. All rats received a standard laboratory chow (Teklad Diets, Inc.) and water ad libitum. Rats were maintained at constant humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (6 AM to 6 PM). All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and were consistent with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 96-01, revised 2002).
Transthoracic echocardiography
Echocardiographic studies were performed to determine effects of 4F administration on left ventricular (LV) function in LPS-treated rats. Eleven week old rats were anesthetized with 2.0% isoflurane, and baseline echocardiographic images were obtained using a 15 MHz transducer attached to an Agilent Sonos 5500 echocardiography instrument (Philips, Inc). Both two-dimensional and M-mode echocardiograms were used to measure the following cardiac dimensions, as previously described: interventricular septum width (IVS), posterior wall width (PW), LV end-diastolic dimension (LVEDd) and LV end-systolic dimension (LVESd) [21]. At least three cardiac cycles were averaged for each measurement. End-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV) and cardiac output (CO) were derived from LV dimensional measurements using the Teicholz method. Fractional shortening (FS) was derived from M-mode images.
After obtaining baseline measurements, rats were randomized to receive saline vehicle, LPS (10 mg/kg) or LPS (10 mg/kg) plus 4F (10 mg/kg) by IP injection. This dosage of 4F was previously shown to inhibit aortic VCAM-1 expression in LPS-treated rats and is consistent with a clinical study showing that 4F administration at 6 mg/kg significantly improves anti-inflammatory properties of HDL in humans [19,22]. After twenty-four hours, echo measurements were repeated. Next, a high-fidelity pressure-transducing catheter (Millar SPR-671 connected to Biopak MP100 data acquisition unit) was inserted into the carotid artery and advanced to the level of the ascending aorta. Aortic mean arterial pressure (MAP) was measured, followed by advancement of the catheter across the aortic valve into the LV. Pressure tracings were analyzed using data acquisition software (AcqKnowledge: Biopac Systems Inc.) to determine HR, LV end-diastolic pressure (LVEDP), and LV end-systolic pressure (LVESP). At least ten cycles were averaged per parameter. Each animal served as its own control, and data are expressed as both absolute values and percent changes over the 24 hr study period.
Multiplex cytokine array
Blood samples were collected by cardiac puncture from rats 24 hrs after receiving vehicle, LPS or LPS+4F. SearchLight protein array technology (ThermoFisher, Inc) was used to measure circulating concentrations of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and cytokine-induced neutrophil chemoattractant-2α (CINC-2α).
Column lipoprotein analyses
Plasma was collected from vehicle, LPS and LPS+4F-treated rats. Plasma lipoprotein profiles were determined using column chromatography as previously described [23]. Cholesterol profiles were decomposed into component peaks and analyzed for relative area using PeakFit (SPSS Science, Chicago, IL). The concentration of individual lipoproteins was then determined as a percentage of total cholesterol.
Electrophoretic mobility of plasma HDL
Plasma lipoproteins were separated on the basis of charge by agarose electrophoresis. Ten μl of plasma was loaded onto a 0.75% agarose gel, and proteins were transferred to a nitrocellulose membrane. The relative electrophoretic mobility of HDL was determined by probing with an anti-apoA-I antibody.
Isolation of HDL by sequential flotation ultracentrifugation
In additional experiments, HDL was isolated from rat plasma by a two-step sequential flotation ultracentrifugation procedure. Plasma density was initially adjusted to 1.06 g/mL with potassium bromide (KBr), and centrifuged (541,100 g) for 24 hours at 7°C. The upper layer, containing very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL), was removed. The density of the remaining, HDL-containing fraction was then adjusted to 1.25 g/mL, mixed and centrifuged at 541,100 g for another 24 hours. At the end of this step, HDL, localized in the upper (more buoyant) zone, was collected and dialyzed against 3 changes of 1XPBS + 100 μM diethylenetriamine pentaacetic acid (DTPA). On the following day, HDL was collected and protein concentration was determined using the Bradford protein assay (Bio-Rad). Proteins in isolated HDL fractions (5 μg) were then separated by gradient (4–20%) SDS-PAGE. Bands were visualized by Coomassie Blue staining. The relative concentration of each protein in a given sample was then determined and expressed as a percentage of the total protein in each lane. In order to identify specific HDL-associated proteins, bands were excised and submitted to the UAB Mass Spectrometry Center for identification using a MALDI-TOF mass spectrometer.
Statistical methods
All results are reported as means ± SEM. Statistical analysis was performed using SigmaStat 3.5 software (Systat Software, Inc). Differences between groups were assessed by analysis of variance (ANOVA) with post hoc testing (Student-Neuman-Keuls test). A P value <0.05 was considered statistically significant.
Results
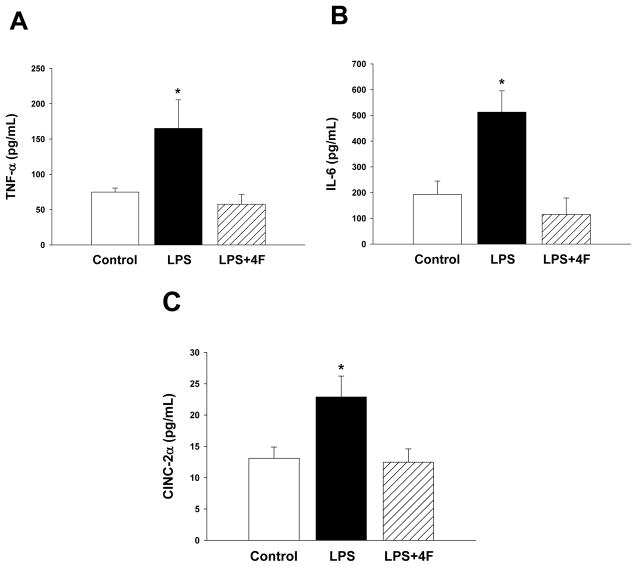
Results of these studies show that LPS administration increases the release of the proinflammatory mediators TNF-α, IL-6 and CINC- 2α in rats (Figure 1). Concurrent treatment of rats with LPS+4F significantly blunted the increase in plasma cytokines and chemokines (Figure 1). Since these inflammatory mediators are associated with the development of sepsis complications, we assessed cardiac function in vehicle, LPS and LPS+4F treated rats non-invasively by transthoracic echocardiography. Echocardiograms were recorded at baseline and 24 hrs after treatment. There were no significant changes in the absolute values for cardiac dimensions in vehicle controls at baseline or after 24 hrs. Accordingly, percent changes in these parameters were minimal (Table 1). In contrast to controls, dimensional changes in LPS-treated rats were observed during diastole. IVSd and PWd widths were significantly increased (20% and 30%, respectively) compared to vehicle controls at 24 hrs (Table 1). Changes in cardiac wall thickness in LPS-treated rats corresponded with a 13% reduction in LVEDd (Table 1), reflecting a decrease in ventricular chamber diameter at rest. These dimensional changes in LPS-treated rats were associated with a 32% decrease in EDV (Table 1; Figure 2). Vehicle treatment did not influence EDV in control rats. In contrast to diastole, changes in cardiac dimensions (IVSs, PWs, LVESd) and volume (ESV) during systole were similar in vehicle and LPS-treated rats over the 24 hr study period (Table 1). After measuring cardiac dimensions at the 24 hr time point, a highfidelity catheter was inserted in rats for measurement of systemic arterial and LV pressures. While aortic MAP was similar in both vehicle- and LPS-treated rats, advancement of the catheter into the LV revealed a significant reduction in LVEDP in LPS-treated rats compared to controls (Table 2). In contrast to LVEDP, LVESP was similar in both groups.
Figure 1. 4F administration reduces plasma levels of inflammatory mediators.
Plasma was collected from rats 24 hrs after treatment with vehicle, LPS or LPS+4F for measurement of pro-inflammatory molecules. Units are pg/ml. *denotes a significant difference (P<0.05) compared to vehicle and 4F treated rats. N=5 for each treatment group.
Table 1. Effects of LPS administration and 4F treatment on cardiac dimensional changes.
Data are the mean ± SEM and are presented as absolute values and as percent changes within each group over the 24 hr study period.
| Vehicle (n=13) | LPS (n=16) | LPS+4F (n=13) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| baseline | 24hr | % change | baseline | 24hr | % change | baseline | 24hr | % change | |
| BW (g) | 316 ± 10 | 315 ± 11 | −1 ± 1 | 303 ± 8 | 279 ± 9 | −8 ± 1 * | 302 ± 10 | 282 ± 12 | −7 ± 1 * |
| IVSd (mm) | 1.51 ± 0.06 | 1.51 ± 0.04 | 1 ± 4 | 1.50 ± 0.04 | 1.79 ± 0.06 | 20 ± 3 * | 1.54 ± 0.06 | 1.62 ± 0.06 | 7 ± 6 # |
| PWd (mm) | 1.44 ± 0.07 | 1.45 ± 0.07 | 1 ± 4 | 1.41 ± 0.05 | 1.81 ± 0.09 | 30 ± 8 * | 1.54 ± 0.05 | 1.64 ± 0.04 | 8 ± 4 # |
| LVEDd (mm) | 8.10 ± 0.16 | 8.07 ± 0.14 | 1 ± 1 | 7.95 ± 0.12 | 6.95 ± 0.15 | −13 ± 1 * | 7.86 ± 0.19 | 7.43 ± 0.24 | −5 ± 2 *,# |
| EDV (mL) | 0.56 ± 0.03 | 0.56 ± 0.03 | −1 ± 4 | 0.53 ± 0.02 | 0.36 ± 0.02 | −32 ± 3 * | 0.52 ± 0.04 | 0.45 ± 0.04 | −13 ± 7 # |
| IVSs (mm) | 2.48 ± 0.11 | 2.43 ± 0.08 | −1 ± 4 | 2.53 ± 0.06 | 2.66 ± 0.06 | 6 ± 2 | 2.55 ± 0.06 | 2.43 ± 0.07 | −4 ± 5 |
| PWs (mm) | 2.44 ± 0.06 | 2.55 ± 0.09 | 5 ± 3 | 2.36 ± 0.09 | 2.68 ± 0.10 | 16 ± 6 | 2.44 ± 0.08 | 2.61 ± 0.07 | 8 ± 5 |
| LVESd (mm) | 5.06 ± 0.17 | 4.95 ± 0.12 | −1 ± 3 | 4.97 ± 0.15 | 4.38 ± 0.09 | −11 ± 2 | 4.89 ± 0.20 | 4.65 ± 0.27 | −4 ± 6 |
| ESV (mL) | 0.14 ± 0.01 | 0.13 ± 0.01 | −6 ± 8 | 0.13 ± 0.01 | 0.09 ± 0.01 | −27 ± 6 | 0.13 ± 0.02 | 0.12 ± 0.02 | −11 ± 14 |
| FS | 0.38 ± 0.02 | 0.39 ± 0.01 | 5 ± 6 | 0.38 ± 0.01 | 0.37 ± 0.01 | −1 ± 4 | 0.38 ± 0.01 | 0.38 ± 0.02 | −1 ± 6 |
| EF (%) | 75 ± 2 | 77 ± 1 | 3 ± 3 | 75 ± 2 | 74 ± 1 | −1 ± 3 | 76 ± 1 | 75 ± 3 | −1 ± 4 |
(P < 0.05) denotes a significant difference compared to sham-operated rats.
(P < 0.05) denotes a significant difference compared to LPS-treated rats.
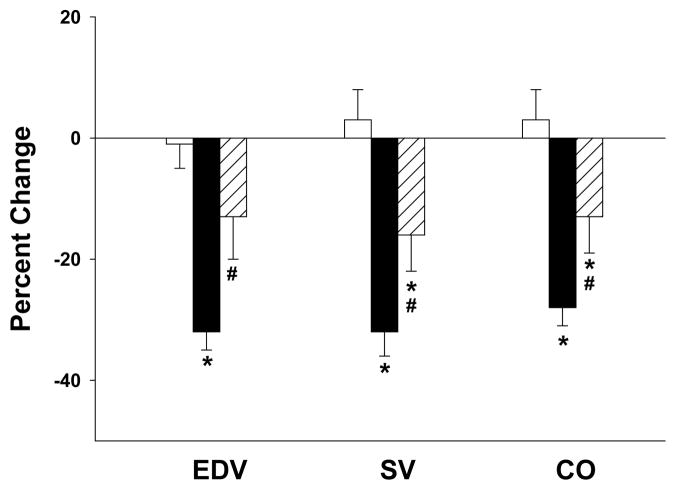
Figure 2. 4F administration attenuates LPS-induced changes in cardiac volumes.
EDV, SV and CO in control (n=13), LPS (n=16) and LPS+4F (n=13) rats were calculated using dimensional measurements at baseline and 24 hrs post-treatment. Data are expressed as percent changes over the 24 study period and are means ± SEM. *(P < 0.05) denotes a significant difference compared to vehicle treated controls. # (P < 0.05) denotes a significant difference compared to LPS rats.
Table 2. Pressure measurements in control, LPS and LPS+4F treated rats.
Data were obtained 24 hrs post treatment and are means ± SEM.
| Vehicle (n=12) | LPS (n=13) | LPS+4F (n=12) | |
|---|---|---|---|
| MAP (mmHg) | 94 ± 2 | 97 ± 6 | 92 ± 5 |
| Systolic BP (mmHg) | 114 ± 2 | 116 ± 5 | 110 ± 5 |
| Diastolic BP (mmHg) | 75 ± 2 | 76 ± 5 | 73 ± 4 |
| HR (beats/min) | 340 ± 5 | 382 ± 13 * | 378 ± 9 * |
| LVEDP (mmHg) | 6 ± 1 | 2 ± 1 * | 4 ± 1 *,# |
| LVESP (mmHg) | 70 ± 3 | 64 ± 3 | 64 ± 3 |
| Heart wet-dry weight ratio | 3.38 ± 0.05 | 3.77 ± 0.14 * | 3.48 ± 0.04# |
(P < 0.05) denotes a significant difference compared to vehicle treated rats.
(P < 0.05) denotes a significant difference compared to LPS-treated rats.
Parameters of cardiac performance were calculated from dimensional measurements. Vehicle treatment in control rats did not induce significant changes in SV or CO (Figure 2) over the 24 study period. In contrast, SV and CO were reduced 32 ± 4% and 28 ± 3%, respectively, in LPS-treated rats over this time period (Figure 2). HR was significantly elevated in LPS-treated rats compared to sham operated controls (Table 2). FS and EF%, indices of LV contractile function, were similar in both groups at baseline and were not altered by vehicle or LPS treatment (Table 1).
4F administration blunted changes in LV diastolic, but not systolic, dimensions in LPS-treated rats (Table 1). LV wall thickness (i.e., IVSd and PWd) was reduced and LVEDd was increased in LPS+4F rats (Table 1). These changes resulted in a significant increase in EDV, SV and CO (Table 1, Figure 2). Despite improvement in these cardiac functional parameters, SV and CO were significantly different from vehicle treated controls. 4F treatment did not alter FS or EF%, reinforcing our observation that systolic function is not degraded in LPS-treated rats over the 24 hr study period (Table 1). MAP and HR were also similar in LPS rats in the presence and absence of 4F treatment. 4F, however, significantly attenuated the reduction in LVEDP observed in LPS-treated rats (Table 2). The ability of 4F to blunt changes in diastolic dimensions (IVSd, PWd, LVEDd) and improve cardiac performance (SV, CO) imply a role for the peptide in preventing the impaired LV filling associated with LPS administration. Our data further suggest that LPS induces edema in a manner that is prevented by 4F treatment. In support of this, 4F administration was associated with a significant reduction in the heart wet-dry weight ratio in LPS-treated rats (Table 2).
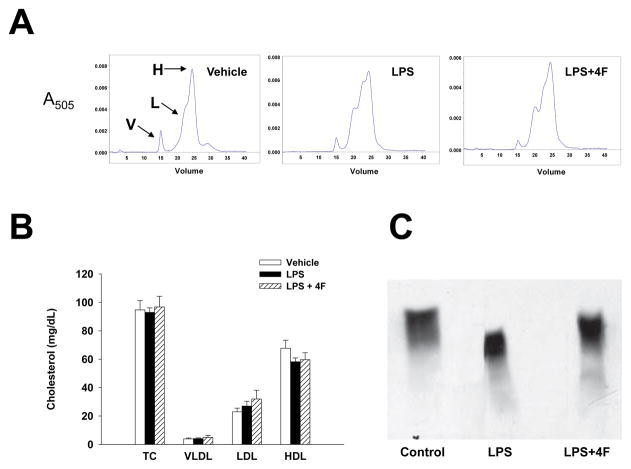
Effects of LPS and 4F administration on plasma lipoprotein profiles were monitored in rats. Plasma profiles for VLDL, LDL and HDL in samples from vehicle, LPS and LPS+4F treated rats were obtained by gel filtration using a Superose 6 column. Figure 3A depicts representative elution profiles for lipoproteins isolated from plasma of each group. As indicated by the figure, HDL (peak H) represents the major lipoprotein component of normal rat plasma, with smaller quantities of VLDL (peak V) and LDL (peak L) present. Total cholesterol was similar in all groups at the 24 hr time point (Figure 3B). There was a tendency for a reduction in HDL and an increase in LDL cholesterol in LPS and LPS+4F treated rats but these values were not significantly different from vehicle controls. Electrophoresis experiments showed that LPS treatment altered the physical characteristics of HDL and its major protein component apoA-I. Plasma samples from vehicle, LPS and LPS+4F treated rats were separated by charge on an agarose gel. Proteins were then transferred to a nitrocellulose membrane and probed with an anti-apoA-I antibody to verify the presence of HDL (Figure 3C). ApoA-I immunoreactive bands were associated with mature α-HDL particles. LPS treatment shifted the position of apoA-I on agarose gels, indicating that the charge and the electrophoretic mobility of α-HDL particles was reduced in these samples compared to HDL from control rats (Figure 3C). The mobility of α-HDL particles was restored in plasma samples from LPS+4F rats (Figure 3C).
Figure 3. Chromatographic and electrophoretic separation of plasma lipoproteins.
(Panel A) Plasma samples from control, LPS and LPS+4F rats were collected and separated by column chromatography. A representative chromatographic profile of plasma VLDL (V), LDL (L) and HDL (H) cholesterol are shown for each group. (Panel B) Peak areas for each lipoprotein were derived from chromatographic profiles of control (n=5), LPS (n=5) and LPS+4F (n=5) rats. The plasma concentration for each lipoprotein was then calculated as a percentage of total plasma cholesterol. Data are means ± SEM. (Panel C) Plasma samples were separated on an agarose gel by charge. Bands were then transferred to nitrocellulose membranes for apoA-I immunoblotting. The electrophoretic mobility of HDL was reduced in samples from LPS rats compared to vehicle control and LPS+4F treatment.
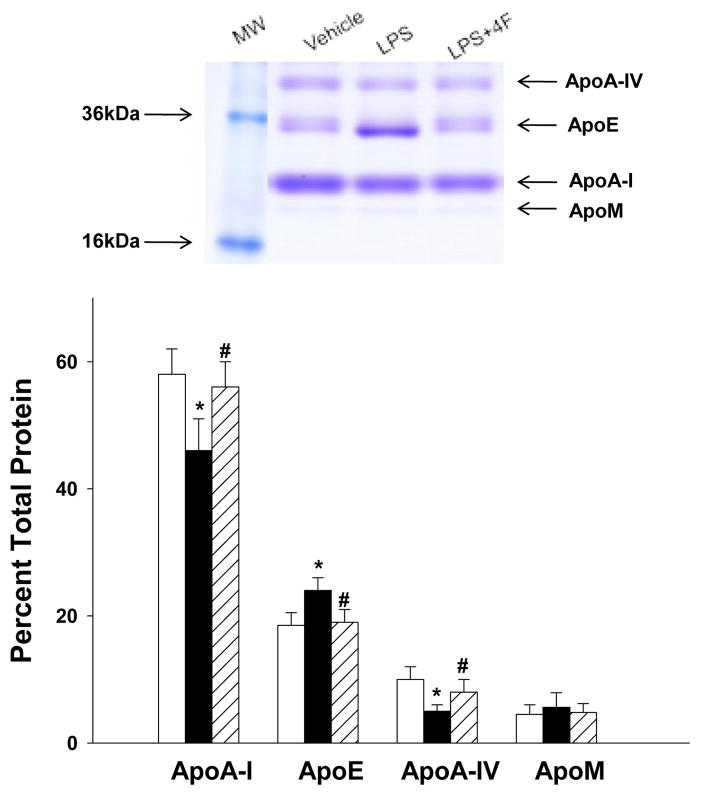
To determine whether changes in HDL electrophoretic mobility were related to alterations in the protein composition of HDL, we isolated HDL from plasma of vehicle, LPS and LPS+4F treated rats by ultracentrifugation. Proteins in each isolated HDL fraction were then separated by gradient (4–20%) SDS-PAGE. Staining with Coomassie blue revealed four principal protein bands on these gels (Figure 4). The bands were excised and their identity determined by mass spectrometry. Results of MALDITOF mass spectrometry experiments confirmed the presence of apoA-I, apoE, apoA-IV and apoM in HDL fractions isolated from each treatment group (Figure 4). The characterization of the bands was consistent with the known molecular weights of these apolipoproteins. The distribution of each apolipoprotein in a given sample was determined by calculating band density as a percentage of total band density for each lane. Results showed that LPS treatment was associated with a significant reduction in HDL-associated apoA-I and apoA-IV (21% and 50% reduction, respectively) and an increase in apoE (30% increase) compared to vehicle treated rats. In contrast, 4F treatment in LPS rats resulted in apoA-I, apoA-IV and apoE levels that were similar to vehicle controls (Figure 4).
Figure 4. HDL-associated apolipoproteins are altered by LPS treatment.
HDL from control, LPS and LPS+4F rats was isolated by ultracentrifugation. A representative SDS-PAGE gel showing Coomassie blue staining for separated proteins is depicted in the top panel. The identity of bands was determined by MALDI-TOF mass spectrometry. The density of each band was measured to determine changes in the relative concentration of each apolipoprotein in the HDL fraction. Data are means ± SEM. * (P < 0.05) denotes a significant difference compared to vehicle controls. # (P < 0.05) denotes a significant difference compared to LPS rats. N=5 for each treatment group.
Discussion
Results of the current study show that 4F treatment reduces circulating levels of proinflammatory mediators, attenuates changes in HDL-associated apolipoproteins and improves cardiac performance in LPS-treated rats. The activation of inflammatory cascades is an early response to sepsis. At later stages, the risk for development of multiple organ dysfunction syndrome (MODS) is increased [2,6,24,25]. Cardiac failure and cardiovascular collapse are major causes of death in patients with severe sepsis [1–2]. Clinical and experimental studies suggest that decreased contractile efficiency and ventricular dilation are important components of sepsis-induced cardiac failure [26–30]. Effects of LPS administration per se on cardiac function have been studied using in vivo and ex vivo animal models [24,27,28,31]. Echocardiographic assessment of LPS-treated rats revealed a decrease in EF and ESV 24 hrs post-treatment [24]. In the isolated perfused rat heart, LV developed pressure was significantly reduced in animals that were pretreated with LPS. Degradation of cardiac function in this study was associated with increased TNF-α synthesis and activation of the P38 MAPK signaling pathway [31]. LPS-induced alterations in contractile protein expression may also influence cardiac function by reducing α-actin expression in rat cardiomyocytes [32].
A major goal of the current study was to determine effects of 4F administration on LV function in LPS-treated rats. Our results show that LPS adversely affects cardiac performance in a manner that is partially reversed by 4F treatment. Measurements of cardiac dimensions revealed changes in LPS treated rats during diastole, but not systole. These were associated with a significant reduction in EDV and LVEDP, suggesting a reduction in LV filling. Accordingly, SV and CO were reduced by LPS treatment. In contrast, parameters of LV systolic function (LVESP, FS and EF) were not altered in LPS treated rats compared to controls. While MAP was similar in control and LPS rats, HR was significantly elevated in the latter group. These results suggest that compensatory mechanisms in LPS-treated rats are still intact at this time point. Further, histological assessment of LV sections of LPS rats did not show ultrastructural changes in the myocardium compared to control rats (not shown). Collectively, these data suggest that early stage cardiac dysfunction in LPS-treated rats is due to impaired LV filling rather than to a defect in myocardial contractility per se. These findings are in agreement with an echocardiographic study showing no changes in cardiac contractility during the early stage of endotoxic shock in dogs [33].
A decrease in CO in septic rats has been linked to a reduction in blood volume [34]. Two mechanisms may explain impaired LV filling in these animals. First, there may be a significant pooling of blood in capacitance vessels resulting in a decrease in venous return and CO. Second, blood volume reduction may occur secondary to fluid loss from edema in LPS rats. The role of cytokines in increasing vascular permeability and fluid loss from the vascular compartment is well known [1,2,6]. Our results suggest a role for edema in LPS-treated rats since the heart wet-dry weight ratios were significantly elevated compared to vehicle treated rats. Administration of 4F to LPS-treated rats attenuated these changes and was associated with an improvement in EDV, LVEDP, SV and CO.
Alterations in lipoprotein metabolism and function accompany sepsis, and are associated with increased risk/severity of sepsis complications and mortality [6,11,12,35]. Activation of the acute phase response increases the incorporation of serum amyloid A (SAA), secretory phospholipase A2 (sPLA2) and ceruloplasmin in the HDL particle and causes apo A-I, paraoxonase (PON) and platelet activating factor-acetylhydrolase (PAF-AH) to be displaced [35]. Under these conditions, HDL is converted to an acute phase lipoprotein that displays pro-oxidant and pro-inflammatory properties [11,35–37]. While plasma lipoprotein profiles were similar in all treatment groups in the current study, we noted that the electrophoretic mobility of HDL was reduced by LPS treatment. Since this response may reflect an alteration in the protein composition of the lipoprotein particle, we assessed the apolipoprotein content of HDL. LPS treatment was associated with a reduction in HDL-associated apoA-I and apoA-IV and an increase in apoE. The important role of apoA-I as a mediator of reverse cholesterol transport has been well established, and the apolipoprotein also possesses prominent anti-inflammatory and anti-oxidant properties [38]. ApoA-IV function plays a role in activation of the HDL-associated enzyme lecithincholesterol acyltransferase (LCAT). Increased expression of apoA-IV in a murine model of endotoxemia has also been shown to reduce circulating levels of interleukin-4, interferon-γ and TNF-α [39]. It follows that the reduction in HDL-associated apoA-IV noted in the current study may serve to stimulate the release of pro-inflammatory cytokines. The observed reduction in apoA-I and apoA-IV content of HDL may be related to the binding of acute phase proteins to the particle, since incorporation of sPLA2 and SAA is reported to increase the catabolism of HDL and its apolipoproteins [40–41]. Other data suggest that cytokines contribute to changes in HDL composition by inhibiting the hepatic synthesis of apolipoproteins [42].
A significant increase in the apoE content of HDL was noted in LPS-treated rats. ApoE is an exchangeable apolipoprotein that acts as a ligand for hepatic receptors that mediate cholesterol clearance. While some data suggest that an increase in circulating apoE serves a protective role in the context of inflammation, other reports implicate the apolipoprotein as a pathogenic mediator of sepsis [43,44]. Infusion of apoE induces natural killer T cell proliferation, enhances cytokine release and increases mortality in septic rats [45]. Results of the Leiden 85-plus Study showed that an elevation inplasma apoE levels in humans was associated with a significant increase in cardiovascular mortality [46]. Sepsis-induced changes in HDL-associated apolipoproteins likely play a key role in converting HDL from an anti-inflammatory to a proinflammatory state [47–49].
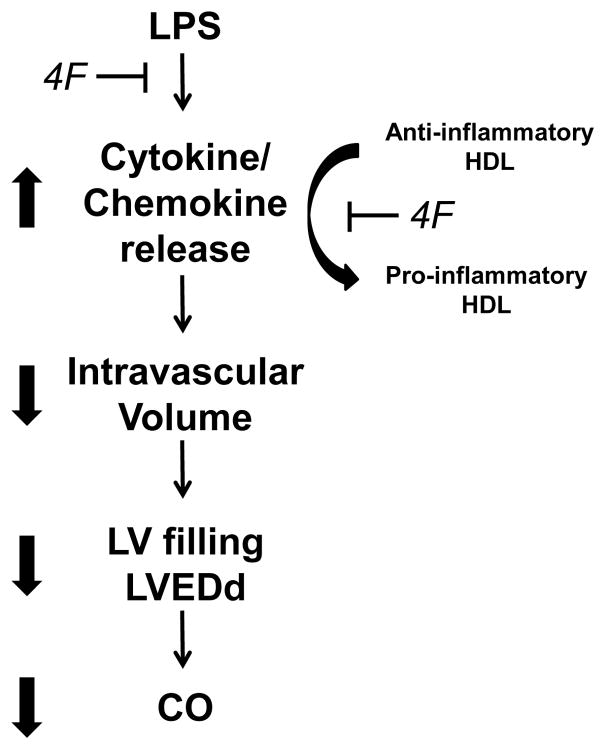
Conclusions
Raising circulating levels of functional HDL reduces complications associated with sepsis. Administration of reconstituted HDL to humans treated with low dose LPS reduces TNF-α, IL-6 and IL-8 release and decreases expression of monocytic mCD14 [50]. Similarly, overexpression of apoA-I in mice results in an increase in circulating HDL levels that confer protection against the administration of exogenous LPS compared to wildtype controls [12]. The binding of endotoxin to plasma HDL is associated with reduced LPS toxicity in vivo and in vitro [44,51]. It has been suggested that HDL neutralizes LPS via insertion and masking of the lipid A domain of LPS in the phospholipid leaflet on the surface of the HDL particle [12]. This modification is associated with diminished activation of LPS receptors on target cell membranes. We previously reported that 4F neutralizes LPS, under in vitro conditions, by a mechanism involving direct binding of LPS with 4F or a 4F-phospholipid complex [19]. Similarly, 4F administration in LPS-treated rats results in the rapid co-localization of 4F and LPS in a plasma fraction composed of HDL particles and neutralization of plasma endotoxin activity [52]. The protective mechanism of 4F action in LPS rats is summarized in Figure 5 and is likely due to the ability of the peptide to interact with plasma lipoproteins to form HDL-like particles that effectively bind and neutralize LPS [52]. By scavenging LPS, 4F may prevent the release of pro-inflammatory mediators from circulating monocytes/macrophages. In the current study, 4F also attenuated endotoxin-induced changes in HDL composition, thus potentially negating secondary effects of altered apolipoproteins (reduced apoA-I and apoA-IV; increased apoE) on cytokine release. 4F may also reduce inflammatory injury by reducing the activation state of macrophages. In this regard, we recently reported that 4F favors the differentiation of monocyte-derived macrophages to an anti-inflammatory M2 phenotype [53]. In the context of LPS-induced inflammation, 4F likely prevents the activation of pro-inflammatory cascades and improves cardiac performance via one or more of these mechanisms. It is proposed that this apoA-I mimetic peptide may be effective in reducing complications associated with sepsis in humans.
Figure 5. Proposed mechanism of 4F action in LPS-treated rats.
An increase in circulating levels of pro-inflammatory mediators is an early response to LPS administration. Cytokines induce injury at the cellular level and promote the loss of fluid to the extravascular compartment. Accordingly, edema reduces circulating blood volume and thus LV filling and cardiac output. LPS may also facilitate the hepatic release of acute phase proteins that bind to and modulate the protein composition of the HDL particle. This interaction is associated with the conversion of HDL from an anti-inflammatory to a pro-inflammatory particle. 4F inhibits this inflammatory cascade via binding and neutralization of LPS. In this manner, the peptide prevents LPS from interacting with target cells and averts the formation of dysfunctional HDL particles.
Acknowledgments
These studies were supported by National Institutes of Health [DK070040 to CRW, GM082952 to CRW/GD, HL34343 to GMA, HL085282 to HG] and a grant from the American Heart Association to GD.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Disclosure
GMA is a Principal in Bruin Pharma, Inc.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Tobias PS, Tapping RI, Gegner JA. Endotoxin interactions with lipopolysaccharide responsive cells. Clin Infectious Diseases. 1999;28:476–481. doi: 10.1086/515163. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Rivest S. Is survival possible without arachidonate metabolites in the brain during systemic infection? News Physiol Sci. 2003;18:137–142. doi: 10.1152/nips.01415.2002. [DOI] [PubMed] [Google Scholar]
- 4.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cellular Signalling. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 5.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 6.Opal SM, Gluck T, Thomas Endotoxin as a drug target. Crit Care Med. 2003;31:S57–S64. doi: 10.1097/00003246-200301001-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kalogeris TJ, Kevil CG, Laroux FS, Coe LL, Phifer TJ, et al. Differential monocyte adhesion and adhesion molecule expression in venous and arterial endothelial cells. Am J Physiol. 1999;276:L9–L19. doi: 10.1152/ajplung.1999.276.1.L9. [DOI] [PubMed] [Google Scholar]
- 8.Danenberg HD, Welt FG, Walker M, Seifert P, Toegel GS, et al. Systemic inflammation induced by lipopolysaccharide increases neointimal formation after balloon and stent injury in rabbits. Circulation. 2002;105:2917–2922. doi: 10.1161/01.cir.0000018168.15904.bb. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Nissen SE. New targets of high-density lipoprotein therapy. Curr Opin Lipidol. 2007;18:421–426. doi: 10.1097/MOL.0b013e32821f603b. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen HJ, Heezius ECJM, Dallinga GM, van Strijp JAG, Verhoef J, et al. Lipoprotein metabolism in patients with severe sepsis. Crit Care Medicine. 2003;31:1359–1366. doi: 10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]
- 12.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah PK, Kaul S, Nilsson J, Cerecek B. Exploiting the vascular protective effects of high density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, Part II. Circulation. 2001;104:2498–2502. doi: 10.1161/hc4501.098468. [DOI] [PubMed] [Google Scholar]
- 14.Garber DW, Datta G, Chaddha M, Palgunachari MN, Hama SY, et al. A new synthetic class A amphipathic peptide analogue protects mice from diet induced atherosclerosis. J Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 15.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- 16.Navab M, Anantharamaiah GM, Reddy ST, van Lenten BJ, Datta G, et al. Human apolipoprotein A-I and A-I mimetic peptide: potential for atherosclerosis reversal. Curr Opin Lipidol. 2004;15:645–649. doi: 10.1097/00041433-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res. 2005;97:1190–1197. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, et al. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 19.Gupta H, Dai L, Datta G, Garber DW, Grenett H, et al. Inhibition of lipopolysaccharide induced inflammatory responses by an apolipoprotein A-I mimetic peptide. Circulation Res. 2005;97:236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 20.Lund-Katz, Anantharamaiah GM, Venkatachalapathi YV, Segrest JP, Phillips MC. Nuclear magnetic resonance investigation of interactions of an amphipathic α-helix forming peptide of an apolipoprotein class. J Biol Chem. 1990;265:12217–12223. [PubMed] [Google Scholar]
- 21.Stein AB, Tiwari S, Thomas P, Hunt G, Levent C, et al. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res Cardiol. 2007;102:28–41. doi: 10.1007/s00395-006-0627-y. [DOI] [PubMed] [Google Scholar]
- 22.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res. 2000;41:1020–1026. [PubMed] [Google Scholar]
- 24.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circulation Research. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 25.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenic concepts from a clinical perspective. Med Sci Monit. 2005;11:76–85. [PubMed] [Google Scholar]
- 26.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Critical Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson M, Kliewer A, Maass D, Becker L, White DJ, et al. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatric Research. 2000;47:669–676. doi: 10.1203/00006450-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Zhong J, Adams HR, Rubin LJ. Cytosolic Ca2+ concentration and contractionrelaxation properties of ventricular myocytes from Escherichia coli endotoxemic guinea pigs: effect of fluid resuscitation. Shock. 1997;7:383–388. doi: 10.1097/00024382-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, et al. Vascular cell adhesion molecule-1 expression is obligatory for endotoxin-induced myocardial neutrophil accumulation and contractile dysfunction. Surgery. 2001;130:319–325. doi: 10.1067/msy.2001.116410. [DOI] [PubMed] [Google Scholar]
- 30.McDonald TE, Grinman MN, Carthy CM, Walley KR. Endotoxin infusion in rats induces apoptotic and survival pathways in hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2053–H2061. doi: 10.1152/ajpheart.2000.279.5.H2053. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Sankula R, Tsai BM, Meldrum K, Turrentine M, et al. P38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock. 2004;21:170–174. doi: 10.1097/01.shk.0000110623.20647.aa. [DOI] [PubMed] [Google Scholar]
- 32.Patten M, Kramer E, Bunemann J, Wenck C, Thoenes M, et al. Endotoxin and cytokines alter contractile protein expression in cardiac myocytes in vivo. Pflugers Arch. 2001;442:920–927. doi: 10.1007/s004240100612. [DOI] [PubMed] [Google Scholar]
- 33.Pinsky MR, Rico P. Cardiac contractility is not depressed in early canine endotoxic shock. Am J Respir Crit Care Med. 2000;161:1087–1093. doi: 10.1164/ajrccm.161.4.9904033. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Ba ZF, Tait SM, Zhou M, Chaudry IH. Alterations in circulating blood volume during polymicrobial sepsis. Circ Shock. 1993;40:92–98. [PubMed] [Google Scholar]
- 35.Wu A, Hinds CJ, Thiemermann C. High density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock. 2004;21:210–221. doi: 10.1097/01.shk.0000111661.09279.82. [DOI] [PubMed] [Google Scholar]
- 36.Shah PK, Kaul S, Nilsson J, Cerecek B. Exploiting the vascular protective effects of high density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, Part I. Circulation. 2001;104:2376–2383. doi: 10.1161/hc4401.098467. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson J, Dahlgren B, Ares M, Westman J, Hultgardh Nilsson A, et al. Lipoprotein-like phospholipid particles inhibit the smooth muscle cell cytotoxicity of lysophosphatidylcholine and platelet-activating factor. Arterioscler Thromb Vasc Biol. 1998;18:13–19. doi: 10.1161/01.atv.18.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol. 2005;46:1792–1798. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 39.Recalde D, Ostos MA, Badell E, Garcia-Otin AL, Pidoux J, et al. Human apolipoprotein A-IV reduces secretion of pro-inflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24:756–761. doi: 10.1161/01.ATV.0000119353.03690.22. [DOI] [PubMed] [Google Scholar]
- 40.Tietge UJF, Maugeais C, Lund-Katz S, Grass D, deBeer FC, et al. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler Thromb Vasc Biol. 2002;22:1213–1218. doi: 10.1161/01.atv.0000023228.90866.29. [DOI] [PubMed] [Google Scholar]
- 41.Cabana VG, Siegel JN, Sabesin SM. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989;30:39–49. [PubMed] [Google Scholar]
- 42.Ettinger WH, Varma VK, Sorci-Thomas M, Parks JS, Sigmon RC, et al. Cytokines decrease apolipoprotein accumulation in medium from Hep G2 cells. Arterioscler Thromb. 1994;14:8–13. doi: 10.1161/01.atv.14.1.8. [DOI] [PubMed] [Google Scholar]
- 43.Van Oosten M, Rensen PCN, Van Amersfoort ES, Van Eck M, Van Dam AM, et al. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J Biol Chem. 2001;276:8820–8824. doi: 10.1074/jbc.M009915200. [DOI] [PubMed] [Google Scholar]
- 44.Berbee JF, Havekes LM, Rensen PC. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res. 2005;11:97–103. doi: 10.1179/096805105X35215. [DOI] [PubMed] [Google Scholar]
- 45.Kattan OM, Kasravi FB, Elford EL, Schell MT, Harris HW. Apolipoprotein E-mediated immune regulation in sepsis. J Immunol. 2008;181:1399–1408. doi: 10.4049/jimmunol.181.2.1399. [DOI] [PubMed] [Google Scholar]
- 46.Mooijaart SP, Berbee JFP, van Heemst D, Havekes LM, de Craen AJM, et al. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3:e176. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Lenten BJ, Hama SY, deBeer FC, Stafforini DM, McIntyre TM, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response: loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 49.Kontush A, Chantepie, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 50.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, et al. Anti-inflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, et al. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai L, Datta G, Zhang Z, Patel R, Honavar J, et al. The apolipoprotein A-I mimetic peptide 4F prevents defects in vascular function in endotoxemic rats. J Lipid Res. 2010;51:2695–2705. doi: 10.1194/jlr.M008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smythies L, White CR, Maheshwari A, Palgunachari M, Anantharamaiah GM, et al. The apolipoprotein A-I mimetic, 4F, alters the function of human monocyte-derived macrophages. Amer J Physiol. 2010;298:C1538–C1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]