Abstract
Background
Enormous variation exists in HIV prevalence between countries in sub-Saharan Africa. The contribution of migration to the spread of HIV has long been recognized, but its effect at the population level has never been assessed. In this ecological analysis, we explore how much variation in HIV prevalence in urban sub-Saharan Africa is explained by in-migration.
Methods
We performed a linear regression to analyze the association between the proportion of recent in-migrants and HIV prevalence for men and women in urban areas, using 60 data points from 28 sub-Saharan African countries between 1987 and 2005.
Results
We found a strong association between recent in-migration and HIV prevalence for women (Pearson R2 = 57%, P < 0.001) and men (R2 = 24%, P = 0.016), taking the earliest data point for each country. For women, the association was also strong within east/southern Africa (R2 = 50%, P = 0.003). For both genders, the association was strongest between 1985 and 1994, slightly weaker between 1995 and 1999, and nonexistent as from 2000. The overall association for both men and women was not confounded by the developmental indicators GNI per capita, income inequalities, or adult literacy.
Conclusions
Migration explains much of the variation in HIV spread in urban areas of sub-Saharan Africa, especially before the year 2000, after which HIV prevalences started to level off in many countries. Our findings suggest that migration is an important factor in the spread of HIV, especially in rapidly increasing epidemics. This may be of relevance to the current HIV epidemics in China and India.
Enormous variation exists in HIV prevalence between countries in sub-Saharan Africa.1 Furthermore, HIV prevalence is typically much higher in east and southern Africa than in the west and central regions of the subcontinent. This variation remains poorly understood, which is unfortunate since a clear understanding may aid identification of effective interventions. Cross-country comparison suggests that development is associated with more rapid and extensive spread of HIV in Africa.2,3 Other studies suggest that biologic factors, notably male circumcision4-6 and HSV-2 infection7,8 may be more important at the population level than differences in individual behavior.9,10
The contribution of migration to the spread of HIV has long been recognized11-15 but its effect at the population level has never been assessed. There have been various attempts to identify factors that explain the variation in HIV prevalence at the population level,10,16 but these did not look at migration. We present measurements of the association between in-migration and HIV prevalence in urban areas for 28 countries in sub-Saharan Africa, based on data from Demographic and Health Surveys (DHS)17 and HIV sentinel surveillance of pregnant women.18 Separate analyses are presented for men and women, because in-migration behavior may be different for men and women.
MATERIALS AND METHODS
Data were analyzed for all publicly available DHS performed within sub-Saharan African before 2006 (i.e., between 1987 and 2005). The in-migration level was derived from each DHS by calculating the proportions of male and female residents aged 15 to 49 years in urban areas (cities and towns) who had moved into their current place of residence in the last 12 months.17 Thus, people moving within a town or city were not considered as recent migrants. HIV prevalence was derived from sentinel surveillance data by taking the median value reported for “major urban areas” (the capital city and other metropolitan areas) for the year(s) of the DHS survey(s), or by linear interpolation from adjacent years if no data were reported for the year of the DHS survey.18 In total, 12 of the 77 DHS were excluded because HIV data were lacking for the year of the DHS survey and could not be calculated by linear interpolation since a more recent or an older adjacent year was also lacking. Of the remaining 65 DHS, 5 were excluded because the question on in-migration was not asked in the DHS. The remaining 60 data points, covering 28 countries, were included in the analysis for women. Following the same procedures, for men 42 data points covering 24 countries could be analyzed (the DHS initially covered women only).
For men and women in urban areas, we related in-migration to HIV prevalence through linear regression, whereby Pearson R2 reflects the proportion explained variance. If more than one DHS was performed in a country, we only included the earliest measure point in our overall analyses. To explore whether any found association could be due to differences between east/ southern versus west/central Africa, we also analyzed the association within these regions, whereby countries were allocated to regions based on geographical proximity and existing UN regional groupings.19 We also analyzed the association between HIV prevalence and in-migration for each 5-year period (i.e.,1985–1989, 1990–1994, 1995–1999, 2000–2005). To avoid that some countries would have 2 data points within a 5-year period, some data points had to be allocated to an adjacent period (i.e., Ethiopia/Rwanda/Malawi ’00 were allocated to ’95-’99; Tanzania ’99 was allocated to ’00-’05).
Because migration is associated with socio-economic development, we adjusted our overall linear regression analyses for socio-economic factors, for both men and women. To this end, we included for each country Gross National Income (GNI) per capita,20,21 the Gini index (representing household inequalities),22,23 and male or female literacy rate22,24 as con-founders in the linear regression analyses. In case data for the correct year (i.e., corresponding to the DHS measurement) were lacking for any of these 3 indicators, we included data of the nearest available year; for 80% of the data points, the figures differed less than 5 years. We also looked at interaction between the significant factors in the multivariate models for men and women, but there was no interaction.
We did not control our analyses for other possible population level determinants such as male circumcision, HSV-2 infection, or religion, because our focus was on migration and there was no a priori justification for expecting these determinants to confound the relationship between migration and HIV prevalence. Moreover, we did not include population age composition as a confounder in our multivariate model, because there was no univariate association between female urban HIV prevalence and the proportion of women in urban areas aged 15 to 24 year old, as derived from the DHS datasets (R2 = 3%, P = 0.4).
RESULTS
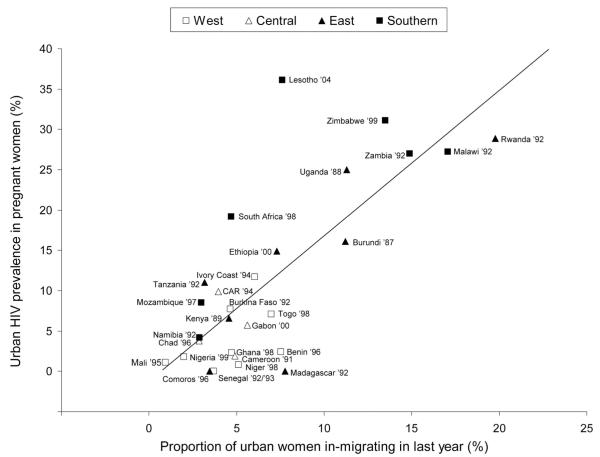
We found a strong association between recent in-migration and HIV prevalence for women (R2 = 57%, P < 0.001, n = 28) and men (R2 = 24%, P = 0.016, n = 24), taking the earliest data point for each country. Figure 1 shows the scatterplot and the fitted regression line of HIV prevalence versus proportion recent in-migrants for women, by country and by region. For women, the association remained strong when the analysis was restricted to countries in east/southern Africa (R2 = 50%, P = 0.003, n = 15); for west/central Africa, where the variation in HIV prevalence is small, there was no association (R2 = 13%, P = 0.2, n = 13). For men, there was no association in either region.
Figure 1.
Association between urban HIV prevalence in pregnant women (derived from sentinel surveillance data) and proportion of urban women who in-migrated in the last year (derived from Demographic and Health Surveys), for 28 sub-Saharan African countries (R2 = 57%, P < 0.001). The association remains strong when analysis is restricted to countries in east/southern Africa (R2 = 50%, P = 0.003).
We analyzed the 60 and 42 data points for women and men, respectively, by 5-year period. The association between in-migration and HIV prevalence was strongest in the late eighties (R2 = 88%, P = 0.061, n = 4 for women; no data available for men in this period) and early nineties (R2 = 75%, P < 0.001, n = 15 for women; and R2 = 74%, P = 0.06, n = 5 for men). The association slightly declined in the late nineties (R2 = 43%, P = 0.001, n = 22 for women; and R2 = 32%, P = 0.01, n = 19 for men). After the year 2000, there was no association between in-migration and HIV prevalence, for women (R2 = 1%, P = 0.7, n = 19) or men (R2 = 4%, P = 0.4, n = 18).
We included GNI per capita, the Gini index, and female literacy rate as possible confounders in the overall regression models for men and women. In the multivariate model for women, recent in-migration remained very strongly associated with HIV prevalence (P < 0.001); none of the confounders was significantly associated with HIV prevalence, although R2 increased from 57% to 67%. In univariate analyses, female literacy explained 20% of the variance in HIV prevalence (P = 0.02) whereas GNI per capita and the Gini index were not significant. In the multivariate model for men, in-migration remained very strongly associated with HIV prevalence (P = 0.006), whereas male literacy (P = 0.010) and the Gini index (P = 0.04) were also significant and R2 increased from 24% to 53%. In univariate analyses, male literacy explained 20% of the variance in HIV prevalence (P = 0.03), whereas GNI per capita or the Gini index were not significant. Of the variables that we considered, recent in-migration had the strongest association with HIV prevalence for women as well as men, and potential socio-economic confounders could not account for this effect.
DISCUSSION
In this exploratory ecological analysis of data from 28 countries in sub-Saharan Africa, we found an unexpectedly strong association (R2 = 57%, P < 0.001) between the proportion of recent female in-migrants and HIV prevalence for urban areas. Differences in urban HIV prevalence within the east/southern region were also highly associated with the proportion of female recent in-migrants. The association between in-migration and HIV prevalence was strongest in the late eighties and early nineties, when the HIV epidemic rapidly increased in many countries. The association disappeared after the year 2000, probably because the HIV epidemic leveled off in many countries due to sexual behavior change and selective AIDS-induced mortality. The difference between women and men in the overall analyses (R2 = 57% vs. R2 = 24%), proved to be largely because most data points for men were more recent than for women; when stratifying data by 5-year period the R2 for men and women was comparable.
Our study is the first that demonstrates a strong association between in-migration and the spread of HIV at the population level. The observed association could not be explained by confounding with socio-economic development. Economic development often results in higher mobility due to labor migration and improvement in transport infrastructure.3,11,14 However, large-scale migration also occurs independently of development (e.g., in times of war and famine) and some aspects of development may constrain the spread of HIV (e.g., greater access to secondary education could facilitate the spread of HIV awareness25,26).
In our study, we only took confounding by socioeconomic development into account, because this is a priori associated with migration. Confounding by other factors, such as male circumcision, HSV-2 infection, or religion, were not taken into account, because this was beyond the scope of our study. Nevertheless, it would be interesting to see if the association between migration and HIV prevalence is confounded by the mentioned factors.
A well-known limitation of ecological analyses is the inability to indicate the direction of causality. The association could also result if larger HIV epidemics induce or intensify migration. Although few studies indicate such a reverse-causal mechanism,27,28 possible results from large HIV epidemics— such as job loss, widowhood, and returning home to be cared for or to die—would mean that most people move from urban to rural areas rather than vice versa.29 On the other hand, a strong causal link between migration into cities and the spread of HIV at the population level is highly plausible. Migration enhances casual and commercial contacts, because of spousal separation and the weaker social control in towns.30-33 Armed conflicts can increase the risk of HIV infection due to rape, disruption of sexual norms, collapse of health systems, and lack of access to condoms.1 Moreover, migration increases the size of sexual networks by linking networks from different locations.34
Our study has more limitations. Sentinel surveillance data are biased towards high-prevalent sites and they reflect HIV prevalence in (pregnant) women rather than men.35 National data on male HIV prevalence have been lacking till recently, when DHS+ started to collect HIV prevalence data in national representative samples.36 Because these data have been collected for only a dozen of countries in sub-Saharan Africa, and all but one after the year 2000, we could not use them for our analyses. Furthermore, we linked migration in “urban areas” as defined in DHS to HIV prevalence in “major urban areas” as defined in sentinel surveillance, which may not completely overlap. Another limitation is that the figures for the socio-economic confounders could often not be found for the correct year (i.e., the year of the DHS), nor adjacent years. Lastly, since one would expect a delay between migration and HIV infection if assuming a causal link through high-risk sexual behavior, we alternatively could have included people who moved longer than a year ago. We performed these analyses, including people who in-migrated in the last 5 years, and found similar results as for in-migration in the past year.
Although our analyses are exploratory, the results are striking. They suggest that in-migration has been an important factor in the spread of HIV in sub-Saharan Africa, especially in the rapidly increasing epidemics of eastern and southern Africa. This finding may be of relevance to other rapidly increasing HIV epidemics such as currently in China and India. Because of its potential importance, further study of the mechanisms underlying the association between in-migration and HIV prevalence is recommended.
Acknowledgments
Supported by the European Commission (contract B7.6211/99/010). The sponsor of the study had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit for publication.
REFERENCES
- 1.UNAIDS . 2006 Report on the Global AIDS Epidemic. Geneva, Switzerland: 2006. [Google Scholar]
- 2.Gregson S, Waddell H, Chandiwana SK. School education and HIV control in sub-Saharan Africa: From discord to harmony? J Int Dev. 2001;13:467–485. [Google Scholar]
- 3.Whiteside A. How the transport sector drives HIV/AIDS and how HIV/AIDS drives transport. AIDS Anal Afr. 1998;8:5–6. 15. [PubMed] [Google Scholar]
- 4.Auvert B, Buve A, Lagarde E, et al. Male circumcision and HIV infection in four cities in sub-Saharan Africa. AIDS. 2001;15(suppl 4):S31–S40. doi: 10.1097/00002030-200108004-00004. [DOI] [PubMed] [Google Scholar]
- 5.Moses S, Bradley JE, Nagelkerke JC, et al. Geographical patterns of male circumcision practices in Africa: association with HIV seroprevalence. Int J Epidemiol. 1990;19:693–697. doi: 10.1093/ije/19.3.693. [DOI] [PubMed] [Google Scholar]
- 6.Orroth KK, Freeman EE, Bakker R, et al. Understanding differences between contrasting HIV epidemics in East and West Africa: results from a simulation model of the Four Cities Study. Sex Transm Inf. 2007;83(suppl 1):i5–i16. doi: 10.1136/sti.2006.023531. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other STI in driving HIV prevalence in Africa. PLoS ONE. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss HA, Buvé A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(suppl 4):S97–S108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 9.Buvé A, Carael M, Hayes RJ, et al. The multicentre study on factors determining the differential spread of HIV in four African cities: summary and conclusions. AIDS. 2001;15(suppl 4):S127–S131. doi: 10.1097/00002030-200108004-00014. [DOI] [PubMed] [Google Scholar]
- 10.Drain PK, Smith JS, Hughes JP, et al. Correlates of National HIV seroprevalence: An ecologic analysis of 122 developing countries. J Acquir Immune Syndr. 2004;35:407–420. doi: 10.1097/00126334-200404010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Hunt CW. Migrant labor and STD: AIDS in Africa. J Health Soc Behav. 1989;30:353–373. [PubMed] [Google Scholar]
- 12.Lurie M, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South-Africa: a study of migrant men and non-migrant men and their partners. Sex Transm Dis. 2003;30:149–156. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Nunn AJ, Wagner HU, Kamali A, et al. Migration and HIV-1 sero-prevalence in a rural Ugandan population. AIDS. 1995;9:503–506. [PubMed] [Google Scholar]
- 14.Pison G, Le Guenno B, Lagarde E, et al. Seasonal migration: a risk factor for HIV infection in rural Senegal. J Acquir Immun Defic Syndr. 1993;6:196–200. [PubMed] [Google Scholar]
- 15.Quinn TC. Population migration and the spread of types 1 and 2 human immunodeficiency viruses. Proc Natl Acad Sci USA. 1994;91:2407–2414. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Over M. The effect of societal variables on urban rates of HIV infection in developing countries: an exploratory analysis. In: Ainsworth M, Fransen L, Over M, editors. Confronting AIDS: Evidence From the Developing World; Selected Background Papers for the World Bank Policy Research Report, Confronting AIDS: Public Priorities in a Global Epidemic. Office for the official publications of the European Communities; Luxemburg, Germany: 1998. pp. 39–51. [Google Scholar]
- 17.Measure DHS. Demographic and Health Surveys. Available at: http://www.measuredhs.com.
- 18.UNAIDS Epidemiological fact sheets on HIV/AIDS and Sexually Transmitted Infections. 2008 update (and 2006 update; 2004 update; 2002 update; 2000 update). Available at: http://www.who.int/GlobalAtlas/predefinedReports/EFS2008/index.asp.
- 19.United Nations Statistics Division Standard Country and Area Codes Classification (M49) Available at: http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm.
- 20.The Worldbank World Development Indicators. Available at: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,contentMDK:20398986~isCURL:Y~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html.
- 21.UC Atlas of Global Inequality Atlas of Global Inequality Database. Available at: http://ucatlas.ucsc.edu/gnp/gnp.html.
- 22.United Nations Development Programme Human Development Reports. Available at: http://hdr.undp.org/en/reports/global/hdr2007-2008/
- 23.United Nations University World Institute for Development Economics Research World Income Inequality Database Available at: http://www.wider.unu.edu/research/Database/en_GB/wiid/
- 24.United Nations Statistics Division Common Database. Available at: http://unstats.un.org/unsd/cdb/cdb_series_xrxx.asp?series_code= 25600.
- 25.Glynn JR, Carael M, Buve A, et al. Does increased general schooling protect against HIV infection? A study in four African cities. Trop Med Int Health. 2004;9:4–14. doi: 10.1046/j.1365-3156.2003.01168.x. [DOI] [PubMed] [Google Scholar]
- 26.Michelo C, Sandoy I, Fylkesnes K. Marked HIV prevalence declines in higher educated young people: evidence from population-based surveys (1995-2003) in Zambia. AIDS. 2006;20:1031–1038. doi: 10.1097/01.aids.0000222076.91114.95. [DOI] [PubMed] [Google Scholar]
- 27.Knodel J, VanLandingham M. Return migration in the context of parental assistance in the AIDS epidemic: the Thai experience. Soc Sci Med. 2003;57:327–342. doi: 10.1016/s0277-9536(02)00361-1. [DOI] [PubMed] [Google Scholar]
- 28.London AS, Wilmoth JM, Fleishman JA. Moving for care: Findings from the US HIV cost and services utilization Study. AIDS Care. 2004;16:858–875. doi: 10.1080/09540120412331290149. [DOI] [PubMed] [Google Scholar]
- 29.Clark SJ, Collinson MA, Kahn K, et al. Returning home to die: Circular labour migration and mortality in South Africa. Scand J Public Health Suppl. 2007;69:35–44. doi: 10.1080/14034950701355619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chirwa WC. Migrant labour, sexual networking and multi-partnered sex in Malawi. Health Transit Rev. 1997;7(suppl 3):5–15. [Google Scholar]
- 31.Kishamawe C, Vissers DC, Urassa M, et al. Mobility and HIV in Tanzanian couples: Both mobile persons and their partners show increased risk. AIDS. 2006;20:601–608. doi: 10.1097/01.aids.0000210615.83330.b2. [DOI] [PubMed] [Google Scholar]
- 32.Coffee MP, Garnett GP, Mlilo M, et al. Patterns of movement and risk of HIV infection in rural Zimbabwe. J Infect Dis. 2005;191(suppl 1):S159–S167. doi: 10.1086/425270. [DOI] [PubMed] [Google Scholar]
- 33.Bassett MT, Mhloyi M. Women and AIDS in Zimbabwe: the making of an epidemic. Int J Health Serv. 1991;21:143–156. doi: 10.2190/N0NJ-FKXB-CT25-PA09. [DOI] [PubMed] [Google Scholar]
- 34.Ghani AC, Swinton J, Garnett GP. The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis. 1997;24:45–56. doi: 10.1097/00007435-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Zaba B, Boerma T, White R. Monitoring the AIDS epidemic using HIV prevalence data among young women attending antenatal clinics: prospects and problems. AIDS. 2000;14:1633–1645. doi: 10.1097/00002030-200007280-00020. [DOI] [PubMed] [Google Scholar]
- 36.Mishra V, Vaessen M, Boerma JT, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ. 2006;84:537–545. doi: 10.2471/blt.05.029520. [DOI] [PMC free article] [PubMed] [Google Scholar]