ABSTRACT
BACKGROUND
There are no life-tables quantifying the average life-spans of post-hospitalized heart failure populations across various strata of risk.
OBJECTIVE
To quantify the life-expectancies (i.e., average life-spans) of heart failure patients at the time of hospital discharge according to age, gender, predictive 30-day mortality heart failure risk index, and comorbidity burden.
DESIGN
Population-based retrospective cohort study.
SETTING
Ontario, Canada.
PATIENTS
7,865 heart failure patients discharged from Ontario hospitals between 1999 and 2000.
MEASUREMENTS
Data were obtained from the Enhanced Feedback for Effective Cardiac Treatment EFFECT provincial quality improvement initiative. All patients were linked to administrative data, and tracked longitudinally until March 31, 2010. Detailed clinical variables were obtained from medical chart abstraction, and death data were obtained from vital statistics. Average life-spans were calculated using Cox Proportion Hazards models in conjunction with the Declining Exponential Approximation of Life Expectancy (D.E.A.L.E) method to extrapolate life-expectancy, adjusting for age, gender, predicted 30-day mortality, left ventricular function and comorbidity, and was reported according to key prognostic risk-strata.
RESULTS
The average life-span of the cohort was 5.5 years (STD +/− 10.0) ranging from 19.5 years for low-risk women of less than 50 years old to 2.9 years for high-risk octogenarian males. Average life-spans were lower by 0.13 years among patients with impaired as compared with preserved left ventricular function, and by approximately one year among patients with three or more as compared with no concomitant comorbidities. In total, 17.4 % and 27 % of patients had died within 6 months and 1 year respectively, despite having predicted life-spans exceeding one-year.
LIMITATIONS
Data regarding changes in patient clinical status over time were unavailable.
CONCLUSIONS
The development of risk-adjusted life-tables for heart failure populations is feasible and mirrored those with advanced malignant diseases. Average life span varied widely across clinical risk strata, and may be less accurate among those at or near their end of life.
KEY WORDS: life-expectancy, heart failure, risk, survival, comorbidity
INTRODUCTION
Heart failure remains among the leading causes of death from cardiovascular disease in North America.1 Heart failure has been determined to be a significant determinants of survival among patients with other chronic conditions.2 Furthermore, the prognosis associated with heart failure is comparable with many advanced cancers.3–5 However, no study has generated life-tables or quantified the average life-spans of heart failure populations, particularly from the point of hospital discharge onward—a point in time associated with clinical vulnerability and fragility in which management decisions, regarding end-of-life decision-making may be important.6–9 Life-expectancy data may allow physicians to better advise their patients on prognosis,10–18 may help physicians make medical and surgical decisions (e.g., implantable defibrillators, cardiac re-synchronization therapy, transplantation),19 and may help in the medical decision-making process where treatment options exist, given that patient treatment preferences may vary according to their life-expectancy.20,21 Life-expectancy data may also have implications for policy, especially in the prioritization and allocation of scarce resources.22,23
Life-expectancy data are derived from the area under a survival curve of a population. Many studies are limited by their durations of follow-up, leaving many individuals alive at the end of a follow-up period. In such circumstances, area under the survival curve may underestimate life-expectancy. Consequently, most heart failure studies have relied on presenting median survival rather than mean survival or life-expectancy (i.e., herein termed average life-spans).24 However, life-expectancy data may allow for better estimations of life-years lost or gained as a result of heart failure therapies.11 Few studies have followed a population-based heart failure cohort long enough to allow for a sufficient number of deaths to permit estimation of average lifespan. Moreover, no study has ever determined the average life-span of heart failure populations within strata defined by different clinical risk profiles.
Accordingly, the objective of this long-term natural history study was to quantify the average life-span of heart failure patients from the point of hospital discharge, according to age, gender, clinical risk-severity, and comorbid disease burden.
METHODS
Data Source
The Enhanced Feedback for Effective Cardiac Treatment (EFFECT) project was a cluster randomized trial conducted in Ontario, Canada to evaluate the effectiveness of public report cards in improving quality and outcomes of cardiac care. The EFFECT heart failure project was designed to be representative of the heart failure population of Ontario, and consisted of consecutive patients hospitalized with a most responsible diagnosis of heart failure across 86 Ontario acute care institutions from April 1, 1999 to March 31, 2000.25 Any patients who had a previous heart failure hospitalization within 3 years were excluded. Each patient was tracked longitudinally forward until March 31, 2010 using administrative data linkage. Mortality outcomes were determined by linking the EFFECT dataset to the Ontario Registered Persons Database (a database derived from vital statistics data) using unique encrypted patient identifiers to protect patient confidentiality. The Registered Persons Database is maintained by the Ministry of Health and Long Term Care and contains mortality information including date of death for each patient who has a valid OHIP health card in Ontario. The accuracy of the mortality data has been previously validated by linking the myocardial infarction data directly to provincial vital statistics data at Cancer Care Ontario with agreement rates of 99 %.26 In our study, no patients were lost to follow-up, with evidence of active health care claims during the last year of follow-up (or in the last year of life, in the case of death). This study was approved by Sunnybrook Health Sciences Centre’s Research Ethics Board.
Study Sample
Patients with heart failure were initially identified from the Canadian Institute for Health Information discharge abstract database using International Classification of Disease (ICD) 9th code 428 for heart failure. Trained nurse abstractors further validated the diagnosis of heart failure with hospital chart records using Framingham criteria.27 Only those EFFECT patients who met a clinical diagnosis of heart failure and survived until hospital discharge were included in this study.
Clinical Risk Parameters
The probability of 30-day mortality was determined using the EFFECT-Heart Failure mortality prediction risk score. This score consists of age, admission characteristics (respiratory rate, systolic blood pressure, cerebrovascular disease, dementia, COPD, hepatic cirrhosis, cancer), and laboratory values (urea nitrogen, sodium concentration, hemoglobin). The EFFECT-heart failure 30-day mortality risk score has been previously validated.28,29 In addition to the 30-day mortality risk score, other indices of risk included age, gender, left ventricular function, and the number of comorbid illnesses, as determined from medical chart abstraction.
Statistical Analyses
The average life span for patients in each subgroup was derived from the area under the survival curve. Life-expectancy data were reported as means and standard deviations. Average life-spans (i.e., life-expectancies) were calculated for the following five subgroups: Age (<50 years, 51–60 years, 61–70 years, 71–80 years, and >80 years), gender (male vs. female), predicted 30-day mortality risk (stratified about the median for the population), left ventricular function (impaired as defined as < = 40 %, preserved as defined as > 40 %), and the number of concomitant comorbid illnesses (0, 1 or 2, 3 or more).
The Declining Exponential Approximation of Life Expectancy (D.E.A.L.E) method was used to extrapolate life-expectancy.30,31 D.E.A.L.E is based on the assumption that survival follows a simple declining exponential function over time when extrapolating the survival curve from the last observed duration of follow-up to the time point at which all subjects were predicted to have died (survival function = 0). The mortality rates were determined using the first and last available time points on the survival curve.
Cox Proportional Hazards, in conjunction with the D.E.A.L.E methodology above, was used to generated risk-adjusted survival curves, adjusting for age, gender, predicted 30-day mortality risk, left ventricular function (impaired vs. preserved) and comorbidity (number of comorbid illnesses), such that average life-spans were derived for each covariate pattern of each patient represented in our cohort. Time to death was regressed on baseline patient characteristics, and a predicted survival curve was generated for each subject.
We undertook sensitivity analyses in which we compared our primary method of analyses, with two other methodologies. The first method did not incorporate risk-adjustment techniques but rather estimated average life-spans by using estimated Kaplan–Meier survival curves in conjunction with the D.E.A.L.E extrapolation. The second method did incorporate risk-adjustment techniques and estimated average life-spans by using the multi-step left-truncated, right-censored survival analysis methodology as developed by Mark and colleagues at Duke university.32,33 This method incorporates empirical patient-level data to extrapolate survival beyond an observed, but limited, follow-up period that allows for the estimation of an entire survival distribution for a specific patient population,33,34 and estimates the hazards of death as a function of age (as opposed to time). These estimated survival distributions are made up of two components: (1) survival based on the observed survival period, and (2) the lifetime survival projection beyond the observed study follow-up period. This “age-based” adjusted survival prediction model avoids the use of parametric assumptions or simulation techniques,34 has been evaluated against traditional survival analytic techniques, and has been applied to clinical trial data, observational data, and cost-effectiveness analyses.32,33 The steps are summarized as follows: First, among patients experiencing acute life threatening events, such as acute myocardial infarction and heart failure hospitalizations, the initial months following hospitalizations are associated with significant changes in the hazard rates over time.24,35 Given that age-based model depends on the assumption that the hazard rate (as a function of age) remains stable over time, the survival distribution in the early stage of the follow-up was based on a traditional time-based Cox proportional hazards model in which time-to-death was adjusted for age, gender and all of the above covariates.33 For the time-to-death Cox proportional hazards phase of the analysis, patients were censored at 4 years following hospitalizations—4 years being a conservative time interval in which mortality hazards will have already stabilized following hospitalizations for our study populations.24,35 Second, among those individuals surviving 4 years and beyond, Cox Proportional Hazards was then used to model five year survival as a function of age (rather than time) in order to generate age-specific predicted survival curves that were adjusted for the specific covariates described above. Age-specific survival curves (conditional upon patients surviving the first four years) were then converted to time-specific survival curves (i.e., the probability of surviving for x number of additional years) for each patient based on his/her covariate pattern. Third, the survival probabilities derived from steps 1 and 2 above were then combined to determine an overall survival curve for each patient. The area under this survival curve was then calculated to predict the life expectancy of each patient, since the area under a survival curve is the expected lifetime or survival time.
We conducted the statistical analyses using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). All statistical tests were two-tailed and P values of < 0.05 were considered statistically significant.
Role of the Funding Source
This project was supported by CIHR operating grant number MOP 79514. The funding source had no role in the design, analyses, or interpretation of the study.
RESULTS
The EFFECT cohort included 7,865 hospitalized heart failure patients. The mean age was 75.37 years (SD: 11.47); 50.2 % were female; the mean baseline 30-day mortality risk, as derived using the EFFECT-Heart Failure mortality index, was 4.0 % (SD: 3.18) (Table 1); The median survival for the cohort was 1.97 years (IQR: 0.75-3.38), with 7.4 % and 27 % of patients having average life-spans of less than 6 months and 12 months respectively.
Table 1.
Baseline Characteristics of Heart Failure Patients in Ontario, Canada
| Heart failure, N = 7,865 | |
|---|---|
| Characteristics | N (%) |
| Demographics | |
| Age (y), mean ± SD | 75.37 ± 11.47 |
| 65-74 | 1,928 (24.5 %) |
| 75-84 | 2,968 (37.7 %) |
| 85+ | 1,700 (21.6 %) |
| Female | 3,949 (50.2 %) |
| Admission characteristics | |
| SBP, mean ± SD | 149.81 ± 33.16 |
| DBP, mean ± SD | 81.81 ± 19.78 |
| Heart rate, mean ± SD | 94.37 ± 25.25 |
| Respiration rate, breaths/min, mean ± SD | 25.89 ± 7.22 |
| Serum creatinine, umol/L, mean ± SD | 124.41 ± 76.32 |
| Blood urea nitrogen, mg/dL, mean ± SD | 27.78 ± 17.47 |
| Sodium, mmol/L, mean ± SD | 138.38 ± 4.70 |
| Sodium < 136 mmol/L | 1,613 (20.5 %) |
| Hemoglobin, g/L, mean ± SD | 124.26 ± 20.57 |
| Hemoglobin < 100 g/L | 947 (12.0 %) |
| Cardiac risk factors and comorbidities | |
| Hypertension | 3,916 (49.8 %) |
| Diabetes | 2,726 (34.7 %) |
| COPD/asthma | 1,746 (22.2 %) |
| Prior myocardial infarction | 2,887 (36.7 %) |
| Prior stroke or TIA | 1,259 (16.0 %) |
| Dementia | 537 (6.8 %) |
| Hepatic cirrhosis | 57 (0.7 %) |
| Cancer | 886 (11.3 %) |
| Hospital characteristics | |
| Cardiac surgery suite | 675 (8.6 %) |
| Cardiac catheterization lab | 670 (8.5 %) |
| No invasive facilities | 6,520 (82.9 %) |
| Teaching hospitals | 1,191 (15.1 %) |
| Baseline risk | |
| Predicted 30-day mortality, mean ± SD | 4.01 ± 3.18 |
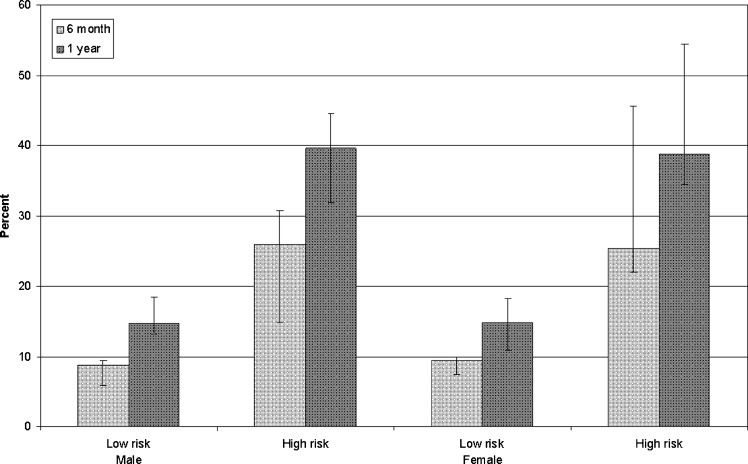
Table 2 illustrates average life spans and baseline risk scores across age-categories, gender, and baseline predicted 30-day mortality risk. Using the Kaplan–DEALE method, the average life-span of the discharged heart failure population was 5.5 years (SD: 3.8), with life-expectancy similar between men and women, but ranging approximately 4.5 years between low and high-risk individuals. On average, 9 % and 14.7 % of low-risk patients had average life-spans of less than 6 months and one year respectively, while 25.7 % and 39.2 % of high-risk patients had average life-spans of less than 6 months and one year respectively (Fig. 1).
Table 2.
The Average Life-Span and Predicted 30-day Mortality of Heart Failure Patients at the Time of Hospital Discharge According to Gender, Age, and Risk Group
| Age group (years) | Risk group* | Number of patients | Number of patients who died (%) | Average life-span† (yrs) mean +/− sd |
|---|---|---|---|---|
| Males | Low risk | 1,999 | 1,551 (77.6 %) | 8.37 +/− 4.32 |
| High risk | 1,917 | 1,831 (95.5 %) | 3.35 +/− 1.23 | |
| < 50 | Low risk | 139 | 66 (47.5 %) | 18.17 +/− 6.99 |
| High risk | 8 | 8 (100.0 %) | 7.10 +/− 2.26 | |
| 50-60 | Low risk | 335 | 208 (62.1 %) | 11.74 +/− 3.07 |
| High risk | 47 | 40 (85.1 %) | 5.85 +/− 1.43 | |
| 61-70 | Low risk | 608 | 471 (77.5 %) | 8.17 +/− 1.99 |
| High risk | 223 | 199 (89.2 %) | 4.46 +/− 1.13 | |
| 71-80 | Low risk | 694 | 595 (85.7 %) | 6.11 +/− 1.32 |
| High risk | 791 | 759 (96.0 %) | 3.50 +/− 0.99 | |
| > 80 | Low risk | 223 | 211 (94.6 %) | 4.77 +/− 1.05 |
| High risk | 848 | 825 (97.3 %) | 2.74 +/− 0.92 | |
| Females | Low risk | 1,933 | 1,516 (78.4 %) | 7.30 +/− 3.79 |
| High risk | 2,016 | 1,941 (96.3 %) | 3.12 +/− 1.13 | |
| < 50 | Low risk | 83 | 34 (41.0 %) | 18.08 +/− 6.75 |
| High risk | <=5 | <=5 (100.0 %) | 6.24 +/− 1.67 | |
| 50-60 | Low risk | 216 | 127 (58.8 %) | 11.63 +/− 3.51 |
| High risk | 22 | 21 (95.5 %) | 5.84 +/− 1.48 | |
| 61-70 | Low risk | 406 | 296 (72.9 %) | 8.05 +/− 1.93 |
| High risk | 126 | 117 (92.9 %) | 4.45 +/− 1.28 | |
| 71-80 | Low risk | 757 | 620 (81.9 %) | 6.07 +/− 1.30 |
| High risk | 591 | 569 (96.3 %) | 3.53 +/− 1.00 | |
| > 80 | Low risk | 471 | 439 (93.2 %) | 4.76 +/− 0.91 |
| High risk | 1,273 | 1,230 (96.6 %) | 2.74 +/− 0.89 | |
| Total | Any | 7,865 | 6,839 (87.0 %) | 5.54 +/− 3.80 |
| Low risk | 3,932 | 3,067 (78.0 %) | 7.85 +/− 4.10 | |
| High risk | 3,933 | 3,772 (95.9 %) | 3.23 +/− 1.19 |
*Low risk = predicted risk of 30 day mortality less than the heart failure median (3.05 %); high-risk = predicted risk of 30 day mortality higher than or equal to the median; predicted 30-day mortality was derived using the EFFECT Heart Failure 30 day risk score.
†Extrapolation used the Declining Exponential Approximation of Life Expectancy (DEALE) which assumes the mortality rates are constant. Cox Proportional Hazards models were used in conjunction with DEALE extrapolation, adjusting for age, sex, predicted risk of 30-day mortality, left ventricular function, and comorbidity. Life-expectancies were derived from the areas under the respective survival curves.
Figure 1.
Percent of patients with average life-spans of less than 6 months and less than 1 year by gender and predicted mortality risk score. Error bars correspond to % variations in age from <50 to >80 years old. Predicted mortality risk were derived using the EFFECT Heart Failure 30-day risk score (high-risk = predicted risk of 30 day mortality higher than or equal than the median; low-risk = predicted risk of 30 day mortality lower than the median; the median 30-day mortality rate was 3.05 %).
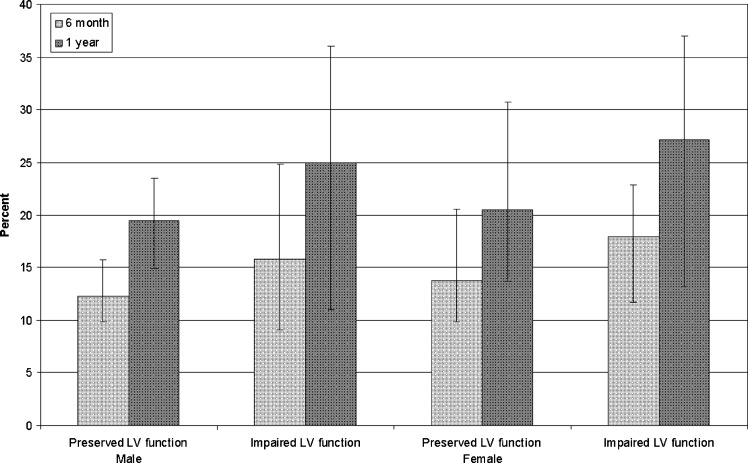
Table 3 illustrates average life-spans across age, gender, and left ventricular function. For each age and gender subgroup, patients with preserved left ventricular function had very similar life-spans as those with impaired left ventricular function. In total, 13.2 % and 16.6 % of patients with preserved left ventricular function had average life-spans of less than 6 months and one year respectively, and 20.1 % and 25.8 % of patients with impaired left ventricular function had average life-spans of less than 6 months and one year respectively (Fig. 2).
Table 3.
The Average Life-Span and Predicted 30-day Mortality of Heart Failure Patients at the Time of Hospital Discharge According to Gender, Age, and Left Ventricular Function
| Age group (years) | Left ventricular function* | Number of patients | Number of patients who died (%) | Average life span† (yrs) mean +/− sd |
|---|---|---|---|---|
| Males | Preserved | 608 | 507 (83.4 %) | 5.89 +/− 4.15 |
| Impaired | 1,354 | 1,132 (83.6 %) | 6.64 +/− 4.53 | |
| < 50 | Preserved | 15 | <=5 (26.7 %) | 20.79 +/− 11.09 |
| Impaired | 82 | 37 (45.1 %) | 17.82 +/− 6.21 | |
| 50-60 | Preserved | 52 | 28 (53.8 %) | 11.43 +/− 3.65 |
| Impaired | 165 | 106 (64.2 %) | 11.26 +/− 3.32 | |
| 61-70 | Preserved | 121 | 88 (72.7 %) | 7.15 +/− 2.32 |
| Impaired | 332 | 267 (80.4 %) | 7.27 +/− 2.51 | |
| 71-80 | Preserved | 254 | 227 (89.4 %) | 4.89 +/− 1.73 |
| Impaired | 533 | 486 (91.2 %) | 4.68 +/− 1.71 | |
| > 80 | Preserved | 166 | 160 (96.4 %) | 3.43 +/− 1.27 |
| Impaired | 242 | 236 (97.5 %) | 3.16 +/− 1.11 | |
| Females | Preserved | 932 | 774 (83.0 %) | 5.47 +/− 3.58 |
| Impaired | 801 | 674 (84.1 %) | 5.84 +/− 3.89 | |
| < 50 | Preserved | 34 | 12 (35.3 %) | 15.62 +/− 5.97 |
| Impaired | 22 | 9 (40.9 %) | 18.51 +/− 6.69 | |
| 50-60 | Preserved | 65 | 34 (52.3 %) | 10.92 +/− 4.00 |
| Impaired | 77 | 49 (63.6 %) | 11.45 +/− 3.56 | |
| 61-70 | Preserved | 117 | 87 (74.4 %) | 7.16 +/− 2.39 |
| Impaired | 144 | 104 (72.2 %) | 7.57 +/− 2.29 | |
| 71-80 | Preserved | 342 | 288 (84.2 %) | 5.06 +/− 1.79 |
| Impaired | 296 | 264 (89.2 %) | 4.81 +/− 1.60 | |
| > 80 | Preserved | 374 | 353 (94.4 %) | 3.44 +/− 1.29 |
| Impaired | 262 | 248 (94.7 %) | 3.35 +/− 1.21 | |
| Total | Preserved | 1,540 | 1,281 (83.2 %) | 5.64 +/− 3.82 |
| Impaired | 2,155 | 1,806 (83.8 %) | 6.34 +/− 4.32 |
*Left ventricular function: Impaired = Reported left ventricular function of < =40 %; Preserved = Reported left ventricular function of >40 %; Missing = No echocardiogram was done or the left ventricular function was not reported.
†Extrapolation used the Declining Exponential Approximation of Life Expectancy (DEALE) which assumes the mortality rates are constant. Cox Proportional Hazards models were used in conjunction with DEALE extrapolation, adjusting for age, sex, predicted risk of 30-day mortality, left ventricular function, and comorbidity. Life-expectancies were derived from the areas under the respective survival curves.
Figure 2.
Percent of patients with average life-spans of less than 6 months and one year across gender and LV function subgroups. Error bars correspond to % variations in age from <50 to >80 years old.
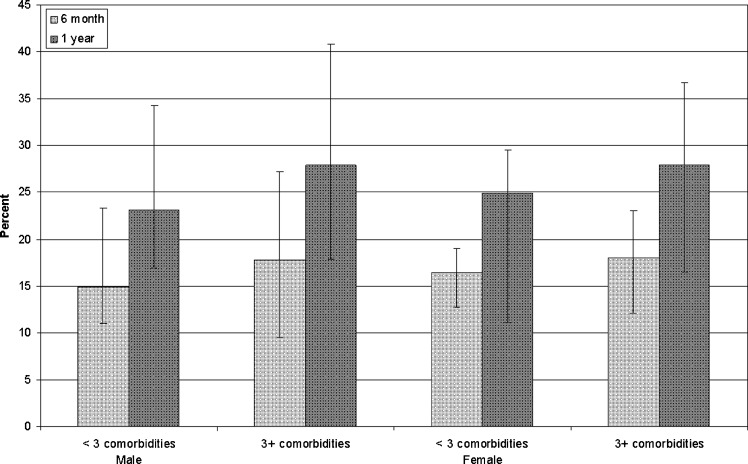
Table 4 illustrates average life-spans across age, gender, and comorbidities. Patients with multiple comorbidities at baseline generally had a reduction in their average life-spans by approximately one year, depending on the age gender subgroup and the survival analytic methods employed. The adverse effects of comorbidity on life-expectancy were more pronounced in younger than in older ages. In total, 15.7 % and 24.1.7 % of patients with fewer than three comorbidities had average life-spans of less than 6 months and one year, respectively, and 17.9 % and 27.9 % of patients with three or more comorbidities had average life-spans of less than 6 months and one year respectively (Fig. 3).
Table 4.
The Average Life-Span and Predicted 30-day Mortality of Heart Failure Patients at the Time of Hospital Discharge According to Gender, Age, and the Number of Comorbidities at Baseline
| Age group (years) | Number of comorbidities at baseline* | Number of patients | Number of patients who died (%) | Average life span† (yrs) +/− mean +/− sd |
|---|---|---|---|---|
| Males | 0 | 92 | 70 (76.1 %) | 8.21 +/− 7.79 |
| 1, 2 | 725 | 573 (79.0 %) | 6.57 +/− 4.92 | |
| 3+ | 3,099 | 2,739 (88.4 %) | 5.69 +/− 3.63 | |
| < 50 | 0 | 12 | <=5 (16.7 %) | 24.53 +/− 10.16 |
| 1, 2 | 40 | 14 (35.0 %) | 20.68 +/− 6.88 | |
| 3+ | 95 | 58 (61.1 %) | 15.38 +/− 5.93 | |
| 50-60 | 0 | 7 | <=5 (57.1 %) | 10.31 +/− 3.78 |
| 1, 2 | 65 | 31 (47.7 %) | 12.10 +/− 3.74 | |
| 3+ | 310 | 213 (68.7 %) | 10.80 +/− 3.40 | |
| 61-70 | 0 | 15 | 11 (73.3 %) | 8.72 +/− 2.34 |
| 1, 2 | 123 | 88 (71.5 %) | 7.94 +/− 2.35 | |
| 3+ | 693 | 571 (82.4 %) | 7.00 +/− 2.42 | |
| 71-80 | 0 | 19 | 17 (89.5 %) | 6.26 +/− 1.16 |
| 1, 2 | 247 | 203 (82.2 %) | 5.34 +/− 1.67 | |
| 3+ | 1,219 | 1,134 (93.0 %) | 4.57 +/− 1.73 | |
| > 80 | 0 | 39 | 36 (92.3 %) | 3.57 +/− 1.22 |
| 1, 2 | 250 | 237 (94.8 %) | 3.43 +/− 1.24 | |
| 3+ | 782 | 763 (97.6 %) | 3.06 +/− 1.25 | |
| Females | 0 | 115 | 100 (87.0 %) | 5.91 +/− 4.73 |
| 1, 2 | 955 | 804 (84.2 %) | 5.54 +/− 3.88 | |
| 3+ | 2,879 | 2,553 (88.7 %) | 5.01 +/− 3.25 | |
| < 50 | 0 | <=5 | 0 (0.0 %) | 27.31 +/− 4.57 |
| 1, 2 | 34 | 12 (35.3 %) | 17.42 +/− 5.68 | |
| 3+ | 49 | 26 (53.1 %) | 16.81 +/− 7.56 | |
| 50-60 | 0 | <=5 | <=5 (100.0 %) | 10.27 +/− 2.62 |
| 1, 2 | 54 | 26 (48.1 %) | 12.70 +/− 3.58 | |
| 3+ | 182 | 120 (65.9 %) | 10.63 +/− 3.72 | |
| 61-70 | 0 | 15 | 10 (66.7 %) | 8.36 +/− 2.38 |
| 1, 2 | 98 | 69 (70.4 %) | 8.33 +/− 2.43 | |
| 3+ | 419 | 334 (79.7 %) | 6.89 +/− 2.26 | |
| 71-80 | 0 | 30 | 27 (90.0 %) | 6.30 +/− 1.13 |
| 1, 2 | 266 | 219 (82.3 %) | 5.46 +/− 1.66 | |
| 3+ | 1,052 | 943 (89.6 %) | 4.79 +/− 1.72 | |
| > 80 | 0 | 64 | 61 (95.3 %) | 3.68 +/− 1.14 |
| 1, 2 | 503 | 478 (95.0 %) | 3.47 +/− 1.27 | |
| 3+ | 1,177 | 1,130 (96.0 %) | 3.19 +/− 1.26 | |
| Total | 0 | 207 | 170 (82.1 %) | 6.93 +/− 6.36 |
| 1, 2 | 1,680 | 1,377 (82.0 %) | 5.99 +/− 4.39 | |
| 3+ | 5,978 | 5,292 (88.5 %) | 5.36 +/− 3.47 |
*Comorbidities at baseline included: hypertension, diabetes, family history of heart disease, hyperlipidemia, smoking currently, angina, CAD, CVA/TIA, previous PTCA or stent, previous CABG, previous MI, atrial fibrillation, deep vein thrombosis, aortic valve disease, peripheral vascular disease, mitral valve disease, valve replaced or repaired, cardiomyopathy, alcohol consumption >2 drinks/day, asthma, cancer, COPD, coronary pulmonale, cirrhosis, dementia/Alzheimer’s disease, depression, GI ulcer disease, hepatitis, HIV/AIDS, hyperthyroid, nephritic syndrome, renal dialysis, renal artery stenosis, uncontrolled seizure, syncope in the past year.
†Extrapolation used the Declining Exponential Approximation of Life Expectancy (DEALE) which assumes the mortality rates are constant. Cox Proportional Hazards models were used in conjunction with DEALE extrapolation, adjusting for age, sex, predicted risk of 30-day mortality, left ventricular function, and comorbidity. Life-expectancies were derived from the areas under the respective survival curves.
Figure 3.
Percent of patients with average life-spans of less than 6 months or 1 year according to gender and comorbidity burden. Error bars correspond to % variations in age from <50 to >80 years old.
Sensitivity Analyses
Results using the Cox Proportional Hazards DEALE methodology yielded comparable life-expectancies to those generated from Kaplan–Meier-DEALE techniques with a 0.01 year difference in overall life-expectancies between the two methods (Life-expectancy +/− SD = 5.54 +/− 3.80 and 5.53 +/− 10.02 for Cox Proportional Hazards-DEALE and Kaplan–Meier-DEALE). Among lower risk patients, Cox Proportional Hazards-DEALE yielded modestly longer life-expectancies than the Kaplan–Meier-DEALE method (Life-expectancy +/− SD: 7.85 +/− 4.10 and 7.31 +/− 13.33, Cox-Proportional Hazards DEALE vs. Kaplan–Meier–DEALE respectively), whereas the converse was true for high-risk populations (Life-expectancy +/− SD: 3.23 +/− 1.19 and 3.53 +/− 6.94; Cox-Proportional Hazards-DEALE vs. Kaplan–Meier-DEALE, respectively). In contrast, the age-based left truncated right censored method yielded life-expectancies that were 2 to 4 years shorter than the other two methods (Life-expectancy +/− SD: 3.81 +/− 2.13 and 2.11 +/− 0.49 and 3.02+/− 1.81 for low-risk, high-risk, and the total population respectively). Similar patterns were observed across left ventricular and comorbidity strata. When examining the subgroup of 6839 patients (87 % of the entire cohort) who had died during follow-up, the correlation between observed and predicted life-expectancies were highest for Kaplan–Meier (r = 0.92) followed by Cox Proportional Hazards (r = 0.72). However, age-based truncation yielded poor correlation between observed and predicted life-expectancies (r = 0.66).
DISCUSSION
Our study confirms the feasibility of generating adjusted life-tables for post-hospitalized heart failure populations. The average life-spans of heart failure patients at the time of hospital discharge was 5.5 years, with over 17 % and 25 % of patients having average life-spans of less than 6 months and 1 year respectively. Average life-spans markedly varied according to age, gender, baseline predicted mortality risk, left ventricular function, and comorbidity burden.
While there have been many longitudinal studies which have calculated median survival among heart failure patients, ours is the first to have derived life-expectancies in subgroups defined by key clinical and comorbid factors.36 Given that survival data are skewed, median survival will under-estimate the average life-span of heart failure populations. Few heart failure population-based studies have tracked all patients until death. Our study provided the longest follow-up of any contemporary natural history heart failure study, and did so from a key transitional inception point (i.e., hospital discharge) among unselected cases. Our results yielded comparable one-year mortality rates and median survival rates as other population-based heart failure cohorts. For example, the median survival after the onset of heart failure in the Framingham study was 1.7 years in men and 3.2 years for women.37
There have been several statistical approaches proposed when estimating life-expectancies among disease-specific populations, where follow-up periods are insufficient for the longitudinal tracking until all patients have not died. Most such approaches, including Cox Proportional Hazards, require assumptions about survival distributions and/or hazards of risk over time, which may or may not be valid.34 Our study was less dependent on data extrapolation and predictive modeling, given that nearly 90 % of our sample had died during follow-up.
While no gold-standard existed in our study, the risk-adjusted average life-spans derived using Cox-Proportional Hazards models were similar to the risk-stratified average life-spans generated from Kaplan–Meier survival techniques. Both methods incorporated the DEALE extrapolation which assumes a simple exponential declining survival function over time. In contrast, the age-based left-censoring adjusted survival analytic methods yielded life-expectancies markedly shorter than the other two methods, for reasons that are not entirely clear. The age-based left truncated right-censoring analysis has the theoretical advantage of not requiring specific parametric assumptions about the form of the survival function. While previous studies have also demonstrated that age-based left censoring methods compare favourably against other traditionally applied survival analytic techniques (e.g., exponential distribution) which performed poorly in complex populations, our results suggested otherwise.33
Our study also yielded important observations when interpreted within the context of other populations. For example, as compared with otherwise healthy non-diseased individuals, we demonstrated that patients with heart failure had life expectancies in keeping with many advanced malignant diseases.38–41 However, sex-specific differences in average life-spans that are known to exist among otherwise healthy populations were observed to have been only modest or negligible in our study. Specifically, as abstracted from life-table data, the average life-expectancies of healthy men and women ages 50–60 years are 24.7 and 32.7 years respectively.42 However, as demonstrated in our study, the average life-spans among otherwise low-risk heart failure patients of similar ages was 5.65 years for men, and 6.28 years for women. These results suggest that gender-specific life-expectancy differences may be markedly attenuated or abolished in patients discharged from hospitalization as a result of heart failure.
Several studies have suggested that prognostication using life-expectancy can theoretically play an important role in clinical decision-making particularly among patients at or near their end-of-life.6,8,16,17,33,43 For example, eligibility for hospice care usually necessitates that patients have average life-spans of less than six months.20,44 Conversely, eligibility for cardiac defibrillator therapy generally necessitates minimum life-expectancies of greater than one-year.10,11 In our study, 20 % of heart failure patients died within 6 months, and 30 % died within one-year. One might have accordingly concluded that up to 20 % of individuals would have been potentially eligible to receive hospice care and 30 % ineligible to receive cardiac defibrillator therapy, based on mortality data alone. However, the average life-spans for all subgroups examined in our study actually exceeded one-year, suggesting that few, if any patients would have been identified apriori as being eligible for hospice care or ineligible for cardiac defibrillator therapy from point of hospital discharge based on best available life-expectancy methodologies. Such observations underscore a challenge when implementing life-expectancy eligibility requirements within clinical guideline criteria. Despite the incorporation of detailed clinical data and risk-adjustment methodology, life-expectancies for heart failure populations lacked precision especially for those at or near their end-of-life—a finding consistent with other studies.45
Notwithstanding imprecision in life-expectancy estimates at or near the end of life, available evidence suggests that explicitly derived life-expectancy data which incorporate statistical approaches such as the ones used in this study may be superior to implicit, best-judgement estimates of average life-spans at the bed-side,8,16 as evidenced by markedly lower than expected mortality rates among heart failure patients referred to hospice care following hospitalization.44 In order for life-expectancy data to become more clinically applicable, future research must improve predictive risk-assessment criteria and/or risk-assessment methodology to more accurately identify those individuals with limited life-expectancies following heart failure hospitalization. That said, our study serves as the first in a series of risk-specific life-tables for heart failure populations, against which other life-expectancies can be compared.
Beyond end-of-life prognostication, average life-span data for heart failure populations have theoretical advantages in providing more accurate life-table information. Unlike median survival, life-expectancy data can be used to more accurately quantity of number of months gained or lost as a result of therapy—measures which can better inform the risk-benefit tradeoffs and cost-effectiveness of therapies, which may imply that the withholding of selected therapies.46–48 Life-expectancy data may be used to motivate the participation in, the referral to, and/or the provision of access for, prevention activities and/or health care-related services.49,50 Life-expectancy data can also be applied for other purposes, such as population health surveillance and health outcomes research,51,52 as well as policy and system-planning.43,53–56
There are several limitations with our study. First, while our life-expectancy data adjusted for several important clinical variables at baseline, others such as ethnicity, socio-economic status, psychosocial factors, and lifestyle behaviours were not included in our predictive models. Moreover, data regarding changes in clinical severity over time were not available and could not be adjusted for in our analyses. Second, a minority of patients remained alive at the end of the study follow-up period, which necessitated DEALE extrapolation methods to project future mortality. While we do advocate interpretative caution, we believe that such extrapolation will have had only modest impact on our overall results, given that nearly 90 % of our cohort had died - -an observed event rate far higher than most other population-based heart failure studies. Finally, not all patients were incident heart failure cases. While all patients had been free of heart failure hospitalizations for at least 3 years prior to inception, some patients may have had previous diagnoses of heart failure in ambulatory care settings or through other remote hospitalizations.
In conclusion, the determination of risk-adjusted life-expectancies for heart failure populations is feasible. The average life-span of patients discharged from hospital with heart failure varies widely across clinical risk strata and comorbidity burden, and are comparable to many advanced stages of cancer. While the clinical applicability of such data necessitates further research, such data could have utility for clinical decision-making, system-planning and prioritization, particularly among those with limited life-expectancies.
Acknowledgments
Funders
This project was supported by CIHR operating grant number MOP 79514. Dr. Tu is Canada Research Chair in Health Services Research and a Career Investigator with the Heart and Stroke Foundation of Ontario. Drs. Alter and Austin are supported in part by Career Investigator Awards from Heart and Stroke Foundation of Ontario. Dr. Ko is supported in part by a Canadian Institutes of Health Research (CIHR) New Investigator Award. Dr. Lee is a clinician-scientist of the Canadian Institutes of Health Research (CIHR). The Institute for Clinical Evaluative Sciences is supported in part by a grant from the Ontario Ministry of Health. The results, conclusions, and opinions are those of the authors, and no endorsement by the Ministry, the Institute, or the CIHR is intended or should be inferred.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Heron M, Tejada-Vera B. Deaths: leading causes for 2005. Natl Vital Stat Rep. 2009;58:1–97. [PubMed] [Google Scholar]
- 2.Sin DD, Man SF. Impact of cancers and cardiovascular diseases in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:115–21. doi: 10.1097/MCP.0b013e3282f45ffb. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S. Prognosis of patients with heart failure compared with common types of cancer. Heart Fail Monit. 2003;3:87–94. [PubMed] [Google Scholar]
- 4.Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, et al. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CP, Lambert PC, Squire IB, Jones DR. Relative survival: what can cardiovascular disease learn from cancer? Eur Heart J. 2008;29:941–47. doi: 10.1093/eurheartj/ehn079. [DOI] [PubMed] [Google Scholar]
- 6.Lai DJ, Tarwater PM, Hardy RJ. Measuring the impact of HIV/AIDS, heart disease and malignant neoplasms on life expectancy in the USA from 1987 to 2000. Public Health. 2006;120:486–92. doi: 10.1016/j.puhe.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Goldraich L, Beck-da-Silva L, Clausell N. Are scores useful in advanced heart failure? Expert Rev Cardiovasc Ther. 2009;7:985–97. doi: 10.1586/erc.09.68. [DOI] [PubMed] [Google Scholar]
- 8.Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–42. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–73. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 10.Sanders GD, Kong MH. Al Khatib SM, Peterson ED. Cost-effectiveness of implantable cardioverter defibrillators in patients > or = 65 years of age. Am Heart J. 2010;160:122–31. doi: 10.1016/j.ahj.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 11.You JJ, Woo A, Ko DT, Cameron DA, Mihailovic A, Krahn M. Life expectancy gains and cost-effectiveness of implantable cardioverter/defibrillators for the primary prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy. Am Heart J. 2007;154:899–907. doi: 10.1016/j.ahj.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Jeldres C, Latouff JB, Saad F. Predicting life expectancy in prostate cancer patients. Curr Opin Support Palliat Care. 2009;3:166–69. doi: 10.1097/SPC.0b013e32832e9c80. [DOI] [PubMed] [Google Scholar]
- 13.Parks SM, Winter L. End of life decision-making for cancer patients. Prim Care. 2009;36:811–23. doi: 10.1016/j.pop.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Glare PA, Sinclair CT. Palliative medicine review: prognostication. J Palliat Med. 2008;11:84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 15.Jeldres C, Latouff JB, Saad F. Predicting life expectancy in prostate cancer patients. Curr Opin Support Palliat Care. 2009;3:166–69. doi: 10.1097/SPC.0b013e32832e9c80. [DOI] [PubMed] [Google Scholar]
- 16.Kellett J. Prognostication–the lost skill of medicine. Eur J Intern Med. 2008;19:155–64. doi: 10.1016/j.ejim.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Walz J, Gallina A, Hutterer G, Perrotte P, Shariat SF, Graefen M, et al. Accuracy of life tables in predicting overall survival in candidates for radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69:88–94. doi: 10.1016/j.ijrobp.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Mazur DJ, Hickam DH. The effect of physician’s explanations on patients’ treatment preferences: five-year survival data. Med Decis Making. 1994;14:255–58. doi: 10.1177/0272989X9401400307. [DOI] [PubMed] [Google Scholar]
- 19.Axente L, Sinescu C, Bazacliu G. Heart failure prognostic model. J Med Life. 2011;4:210–225. [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas JM, O’Leary JR, Fried TR. Understanding their options: determinants of hospice discussion for older persons with advanced illness. J Gen Intern Med. 2009;24:923–28. doi: 10.1007/s11606-009-1030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried TR, Ness PH, Byers AL, Towle VR, O’Leary JR, Dubin JA. Changes in preferences for life-sustaining treatment among older persons with advanced illness. J Gen Intern Med. 2007;22:495–501. doi: 10.1007/s11606-007-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein NE, Lynn J. Trajectory of end-stage heart failure: the influence of technology and implications for policy change. Perspect Biol Med. 2006;49:10–18. doi: 10.1353/pbm.2006.0008. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman SR. Making longevity in an aging society: linking ethical sensibility and Medicare spending. Med Anthropol. 2009;28:317–25. doi: 10.1080/01459740903303852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko DT, Alter DA, Austin PC, You JJ, Lee DS, Qiu F, et al. Life expectancy after an index hospitalization for patients with heart failure: a population-based study. Am Heart J. 2008;155:324–31. doi: 10.1016/j.ahj.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, et al. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA. 2009;302:2330–2337. doi: 10.1001/jama.2009.1731. [DOI] [PubMed] [Google Scholar]
- 26.Tu JV, Naylor CD, Austin P. Temporal changes in the outcomes of acute myocardial infarction in Ontario, 1992–1996. CMAJ. 1999;161:1257–61. [PMC free article] [PubMed] [Google Scholar]
- 27.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–46. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 28.Bradshaw PJ, Ko DT, Newman AM, Donovan LR, Tu JV. Validity of the GRACE (Global Registry of Acute Coronary Events) acute coronary syndrome prediction model for six month post-discharge death in an independent data set. Heart. 2006;92:905–9. doi: 10.1136/hrt.2005.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–87. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 30.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the "DEALE"). I. Validation of the method. Am J Med. 1982;73:883–88. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 31.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the "DEALE"). II. Use in medical decision-making. Am J Med. 1982;73:889–97. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 32.Mark DB, Harrington RA, Lincoff AM, Califf RM, Nelson CL, Tsiatis AA, et al. Cost-effectiveness of platelet glycoprotein IIb/IIIa inhibition with eptifibatide in patients with non-ST-elevation acute coronary syndromes. Circulation. 2000;101:366–71. doi: 10.1161/01.CIR.101.4.366. [DOI] [PubMed] [Google Scholar]
- 33.Nelson CL, Sun JL, Tsiatis AA, Mark DB. Empirical estimation of life expectancy from large clinical trials: use of left-truncated, right-censored survival analysis methodology. Stat Med. 2008;27:5525–55. doi: 10.1002/sim.3355. [DOI] [PubMed] [Google Scholar]
- 34.Klein JP, Moeschberger ML. Survival Analysis: Techniques for censored and truncated data. New York, NY: Springer-Verlag; 1997. [Google Scholar]
- 35.Alter DA, Naylor CD, Austin PC, Tu JV. Long-term MI outcomes at hospitals with or without on-site revascularization. JAMA. 2001;285:2101–8. doi: 10.1001/jama.285.16.2101. [DOI] [PubMed] [Google Scholar]
- 36.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 37.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.CIR.88.1.107. [DOI] [PubMed] [Google Scholar]
- 38.Mohan R, Beydoun HA, Beydoun MA, Barnes-Eley M, Davis J, Lance R et al. Self-rated health as a tool for estimating health-adjusted life expectancy among patients newly diagnosed with localized prostate cancer: a preliminary study. Qual Life Res. 2010. [DOI] [PMC free article] [PubMed]
- 39.Otten JD, Broeders MJ, Heeten GJ, Holland R, Fracheboud J, Koning HJ, et al. Life expectancy of screen-detected invasive breast cancer patients compared with women invited to the Nijmegen screening program. Cancer. 2010;116:586–91. doi: 10.1002/cncr.24771. [DOI] [PubMed] [Google Scholar]
- 40.Straatman H, Verbeek AL, Peer PG, Borm G. Estimating life expectancy and related probabilities in screen-detected breast cancer patients with restricted follow-up information. Stat Med. 2004;23:431–48. doi: 10.1002/sim.1539. [DOI] [PubMed] [Google Scholar]
- 41.Kao SC, Butow P, Bray V, Clarke SJ, Vardy J. Patient and oncologist estimates of survival in advanced cancer patients. Psychooncology. 2010. [DOI] [PubMed]
- 42.Social Security Online Actuarial Publications. Social Security Online . 12-12-2010. Ref Type: Electronic Citation
- 43.Glare PA, Sinclair CT. Palliative medicine review: prognostication. J Palliat Med. 2008;11:84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 44.Bain KT, Maxwell TL, Strassels SA, Whellan DJ. Hospice use among patients with heart failure. Am Heart J. 2009;158:118–25. doi: 10.1016/j.ahj.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 45.David AS, Mary R, Neil S, James G. Deaths from heart failure in general practice: implications for palliative care. Palliat Med. 2002;16:495–98. doi: 10.1191/0269216302pm596oa. [DOI] [PubMed] [Google Scholar]
- 46.Kelley AS, Morrison RS, Wenger NS, Ettner SL, Sarkisian CA. Determinants of treatment intensity for patients with serious illness: a new conceptual framework. J Palliat Med. 2010;13:807–13. doi: 10.1089/jpm.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lytsy P, Burell G, Westerling R. How do prescribing doctors anticipate the effect of statins? J Eval Clin Pract. 2010. [DOI] [PubMed]
- 48.Stewart GC, Brooks K, Pratibhu PP, Tsang SW, Semigran MJ, Smith CM, et al. Thresholds of physical activity and life expectancy for patients considering destination ventricular assist devices. J Heart Lung Transplant. 2009;28:863–69. doi: 10.1016/j.healun.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Harbottle EJ, Birmingham CL, Sayani F. Anorexia nervosa: a survival analysis. Eat Weight Disord. 2008;13:e32–e34. [PubMed] [Google Scholar]
- 50.Hellstedt LF. Transitional care issues influencing access to health care: employability and insurability. Nurs Clin North Am. 2004;39:741–53. doi: 10.1016/j.cnur.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Harper S, Lynch J, Burris S, Davey SG. Trends in the black-white life expectancy gap in the United States, 1983–2003. JAMA. 2007;297:1224–32. doi: 10.1001/jama.297.11.1224. [DOI] [PubMed] [Google Scholar]
- 52.Na’amnih W, Muhsen K, Tarabeia J, Saabneh A, Green MS. Trends in the gap in life expectancy between Arabs and Jews in Israel between 1975 and 2004. Int J Epidemiol. 2010;39:1324–32. doi: 10.1093/ije/dyq073. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein MC. Economic assessments of medical practices and technologies. Med Decis Making. 1981;1:309–30. doi: 10.1177/0272989X8100100403. [DOI] [PubMed] [Google Scholar]
- 54.Stone P, Kelly L, Head R, White S. Development and validation of a prognostic scale for use in patients with advanced cancer. Palliat Med. 2008;22:711–17. doi: 10.1177/0269216308095200. [DOI] [PubMed] [Google Scholar]
- 55.Maltoni M, Amadori D. Prognosis in advanced cancer. Hematol Oncol Clin North Am. 2002;16:715–29. doi: 10.1016/S0889-8588(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 56.Zanetti O, Solerte SB, Cantoni F. Life expectancy in Alzheimer’s disease (AD) Arch Gerontol Geriatr. 2009;49(Suppl 1):237–43. doi: 10.1016/j.archger.2009.09.035. [DOI] [PubMed] [Google Scholar]