Abstract
DNA methyltransferase 3B (DNMT3B) is critically involved in de novo DNA methylation and genomic stability, while the regulatory mechanism in liver is largely unknown. We previously reported that diurnal variation occurs in the mRNA expression of Dnmt3b in adult mouse liver. The aim of this study was to determine the mechanism underlying the diurnal expression pattern. The highest level and the lowest level of Dnmt3b mRNA expression were confirmed to occur at dawn and in the afternoon, respectively, and the expression pattern of Dnmt3b closely coincided with that of Bmal1. Since the diurnal pattern of Dnmt3b mRNA expression developed at weaning and scheduled feeding to separate the feeding cycle from the light/dark cycle led to a phase-shift in the expression, it could be assumed that feeding plays a critical role as an entrainment signal. In liver-specific Bmal1 knockout (L-Bmal1 KO) mice, L-Bmal1 deficiency resulted in significantly higher levels of Dnmt3b at all measured time points, and the time when the expression was the lowest in wild-type mice was shifted to earlier. Investigation of global DNA methylation revealed a temporal decrease of 5-methyl-cytosine percentage in the genome of wild-type mice in late afternoon. By contrast, no such decrease in 5-methyl-cytosine percentage was detected in L-Bmal1 KO mice, suggesting that altered Dnmt3b expression affects the DNA methylation state. Taken together, the results suggest that the feeding and hepatic clockwork generated by the clock genes, including Bmal1, regulate the diurnal variation in Dnmt3b mRNA expression and the consequent dynamic changes in global DNA methylation.
Keywords: Dnmt3b, liver, Bmal1, feeding, DNA methylation
Introduction
DNA methylation is an essential epigenetic machinery that suppresses gene expression via transcription silencing at gene promoters and repetitive sequences and is considered to be important not only in early development1,2 but also in the pathogenesis of diseases, such as cancer, diabetes and stress disorder in adults.1,3,4 The cytosine that is present in abundance in CpG islands of genomic DNA can be converted to 5-methyl-cytosine (5-mC) by DNA methyltransferase (DNMT). Three subtypes of DNMT, DNMT1, DNMT3A and DNMT3B, have been found to be indispensable for conversion of the cytosine in genomic DNA to 5-mC.2 Dnmt1 plays a central role in the transmission of methylation pattern from mother strand to daughter strand, whereas DNMT3A and DNMT3B are thought to be responsible for de novo DNA methylation. Overexpression of DNMT3B, but not of DNMT3A, in adult ApcMin/+ mice, a murine model of intestinal neoplasia, causes methylation and transcriptional silencing of tumor suppressor genes and promotes tumorigenesis.5 In humans, mutations in Dnmt3b account for 60–70% of the cases of ICF (immunodeficiency, centromeric instability and facial anomalies) syndrome, a rare autosomal recessive disease.6,7 Marked hypomethylation and decondensation of pericentromeric heterochromatin on specific chromosomes have been reported in adult patients with ICF syndrome,2,6,7 suggesting that Dnmt3b plays a critical role in DNA methylation and genomic stability in adults as well as in embryos.
The balance between methylation and demethylation determines DNA methylation status.8 Recent studies demonstrated the ten-eleven translocation (TET) family proteins, TET1–3, as the key molecules involved in active DNA demethylation.9 The growth arrest and DNA damage (Gadd) 45 family proteins have also been reported to be associated with active demethylation.10,11 The DNA methylation state at specific loci in the brain, such as the Bdnf and Fgf1 promoter loci, can be changed within 4 h by active demethylation catalyzed by TET1 and/or GADD45B.11,12 Although the methylation state of genomic DNA was generally regarded as capable of changing over time but not in a very flexible manner, the results of these studies clearly indicated that the machinery of active demethylation allows cells to change the DNA methylation state of their genome rather easily.
In the liver, DNA hyper- and hypomethylation are often associated with the onset of adult-onset diseases1,3,4 and life events that adversely affect DNA methylation, such as exposure of environmental pollutants, dietary habits and drug intakes, may increase susceptibility to adult-onset diseases.3 Some agents are known to alter the DNA methylation state by changing the level of expression of Dnmt and/or by modifying the enzymatic activity of DNMT.3,13 Transcription of Dnmt3a and Dnmt3b is reported to be regulated by the ubiquitous transcription factors specific protein 1 (Sp1) and Sp3.14 However, the regulatory mechanisms of expression of these enzymes are not fully elucidated. We previously reported that Dnmts mRNA expression in adult mouse liver can vary with diet and according to gender, in combination with chronic arsenic exposure.15 We have further reported a dynamic change in Dnmt3b mRNA expression in mouse liver between the morning and the afternoon.15 These findings suggest a novel regulatory mechanism of Dnmt3b linked to clockwork.
Clockwork seems to be driven by a limited number of core molecules.16,17 Two of the core molecules, CLOCK and BMAL1 form a heterodimer, which binds to E-box motifs and activates transcriptional expression of two other core circadian pacemakers, Period (Per) and Cryptochrome (Cry),18 by means of the histone acetyltransferase activity of CLOCK protein.19 The newly formed heterodimer complex of PER and CRY negatively regulates transcriptional activation of the CLOCK/BMAL1 heterodimer by recruiting histone deacetylase to the Per1 E-box site.20 In addition to these molecules, several other circadian oscillators, including Rev-erbα,21 Rorα22-24 and Dbp,25 have been demonstrated to play an important role in modifying the feedback loop formed by CLOCK/BMAL1 and PER/CRY.
This study attempted to determine the mechanism that underlies generation of the diurnal expression of Dnmt3b mRNA in mouse liver. To investigate the mechanism, we (1) compared the Dnmt3b transcription pattern to the transcription patterns of circadian pacemaker genes, (2) conducted an experiment to analyze the ontogeny of diurnal Dnmt3b expression, (3) conducted an experiment to determine whether feeding is responsible for the entrainment of Dnmt3b expression, (4) conducted an experiment to test whether intrinsic clock genes in liver control the expression pattern of Dnmt3b mRNA using liver-specific Bmal1 knockout (L-Bmal1 KO) mice and (5) measured 5-mC in L-Bmal1 KO and wild-type mice to determine whether the diurnal Dnmt3b change affects the DNA methylation state in adult liver.
Results
Diurnal variations in Dnmt mRNA expression and comparison to the patterns of circadian pacemaker genes
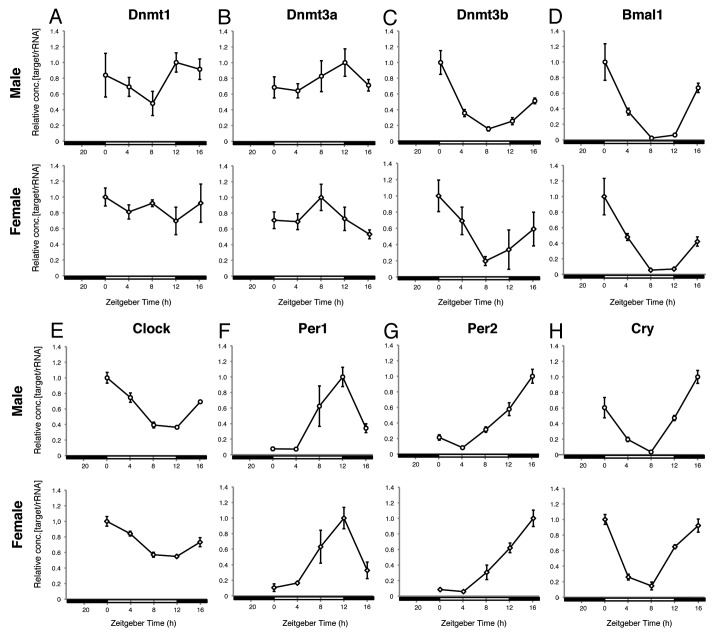
At zeitgeber times (ZTs) of 0, 4, 8, 12 and 16 h, 7-week-old mice of both sexes were sacrificed, and the gene expression patterns of Dnmt1, Dnmt3a, Dnmt3b, Bmal1, Clock, Per1, Per2 and Cry were examined. Although there was no significant diurnal variation in the Dnmt1 or 3a mRNA levels (Fig. 1A and B), a clear diurnal variation was observed in Dnmt3b mRNA expression in both sexes. The highest and the lowest Dnmt3b mRNA expression levels were confirmed at ZT0 and ZT8, respectively, and the lowest level was less than 20% of the peak level (Fig. 1C). Comparison with typical circadian clock genes revealed an obvious similarity between the diurnal patterns of Dnmt3b and Bmal1 expression (Fig. 1C and D), whereas the daily patterns of expression of other circadian pacemakers, e.g.., Clock, Per1, Per2 and Cry, did not seem to be very similar to the pattern of Dnmt3b expression (Fig. 1E‒H).
Figure 1. Diurnal mRNA expression of Dnmts and circadian clock-related genes in the liver. The mRNA expressions of Dnmt1 (A), 3a (B), 3b (C) and circadian clock-related genes such as Bmal1 (D), Clock (E), Per1 (F), Per2 (G) and Cry1 (H) were examined in livers of male and female mice sacrificed at ZT 0, 4, 8, 12 and 16. n = 3 at each time point. Highest mRNA level in each figure was set as 1 and relative concentrations are shown.
To investigate possible sex differences in Dnmt3b expression, gonad-intact 11-week-old males and females were sacrificed when the Dnmt3b mRNA level was the highest (ZT0) or the lowest (ZT8). There were differences between the Dnmt3b mRNA level at ZT0 and ZT8 in both sexes, and at ZT0 the Dnmt3b mRNA level was about 1.6 times higher, and significantly higher in the females than in the males (Fig. S1). Gonadectomized groups were established by performing orchiectomy and ovariectomy at 7 weeks of age, and after waiting 4 weeks for their sex steroid hormones to become deleted, at 11 weeks of age they were sacrificed at ZT 0 or ZT8. The patterns observed in the gonadectomized mice were very similar to the patterns observed in gonad-intact mice (Fig. S1), indicating that the differences in hormonal milieu created by different gonads in adults is unrelated either to the diurnal pattern of Dnmt3b transcription or to the sex difference in Dnmt3b mRNA level observed at ZT0.
Differences between Dnmt and Bmal1 mRNA expression at ZT0 and ZT8 during development
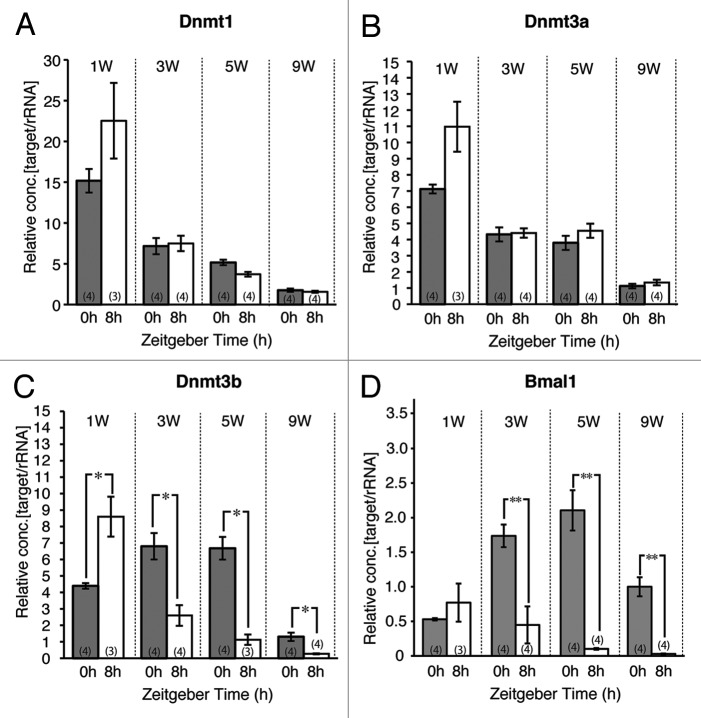
Male mice at 1, 3, 5 and 9 weeks of age were sacrificed at either ZT0 or ZT8 and mRNA levels in the liver were examined. The Dnmt1 and Dnmt3a mRNA levels decreased with age, but there was no significant difference between levels at ZT 0 and 8 at any age (Fig. 2A and B). The pattern of Dnmt3b mRNA expression being high at ZT0 and low at ZT8 that was observed in adults was also observed at 3, 5 and 9 weeks of age (Fig. 2C), but the pattern of expression at 1 week of age was the opposite, i.e., high at ZT8 and low at ZT0 (Fig. 2C). Similar to Dnmt3b mRNA expression, Bmal1 mRNA expression was found to be high at ZT 0 and low at ZT 8 at 3, 5 and 9 weeks of age (Fig. 2D), but at 1 week of age, there was no difference between expression of ZT0 and ZT8. These results indicate that adult-like patterns of diurnal variation in the expression of Dnmt3b and Bmal1 mRNA emerge sometime between 1 week and 3 weeks of age.
Figure 2.Dnmts and Bmal1 mRNA expression at ZT 0 and 8 in the liver during postnatal development. There were no significant differences between ZT 0 and 8 through postnatal development in mRNA expressions of Dnmt1 (A) and Dnmt3a (B). Significant decrease of Dnmt3b mRNA in postnatal 3, 5, and 9 weeks at ZT 8 vs. ZT 0, although significant increase was found in postnatal 1 week (C). Significant increase of Bmal1 mRNA in postnatal 3, 5, and 9 weeks at ZT 8 vs. ZT 0 (D). The expression level in ZT 0 at postnatal 9 weeks was set as 1 and relative concentrations are shown. Numbers used in each group is shown in parentheses. *p < 0.05, **p < 0.01 (Student’s t-test).
Alteration of the patterns of Dnmt3b and Bmal1 expression by scheduled feeding
Based on the result showing that the adult-like diurnal variation in Dnmt3b mRNA expression was first detected at 3 weeks of age, we hypothesized that the ontogeny of the adult-like pattern of Dnmt3b mRNA expression is associated with the start of weaning, which, in mice, is generally between 2 and 3 weeks after birth.26,27 If eating chow acts as an entrainment signal for diurnal variation in Dnmt3b mRNA expression in the liver, scheduled feeding (SF) during daytime alone, which is intended to separate the feeding cycle from the light/dark cycle, should lead to a phase-shift in the Dnmt3b mRNA expression pattern. We therefore compared Dnmt3b mRNA expressions at ZT0, 4, 8, 12 and 16 in an ad-lib fed group and SF group. In the SF group, the male mice were allowed to adapt for 6 d to a feeding schedule in which they were given access to food only from ZT5 to ZT9. In the SF group, the lowest Dnmt3b mRNA level and the highest Dnmt3b mRNA level shifted to ZT4 and ZT8, respectively, which were just before the start of feeding and just after the start of feeding, respectively (Fig. 3A). Thus, the diurnal pattern of Dnmt3b expression undergoes a phase-shift in response to SF. Similar to the results reported in a previous study,28 SF induced a phase-shift in the diurnal pattern of Bmal1 expression, with the lowest expression occurring at ZT4 and the highest expression at ZT16 (Fig. 3B). There were significant differences between the groups regarding Dnmt3b and Bmal1 expression at all time points (Figs. 3A and B). These results demonstrate that feeding is a key factor in entraining the diurnal expression pattern of both Dnmt3b and Bmal1.
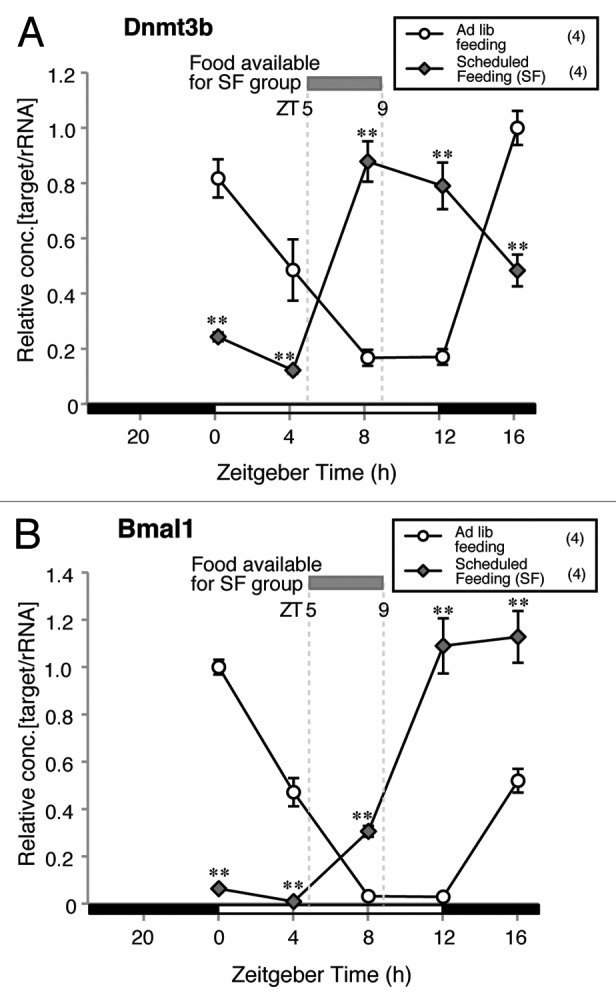
Figure 3. Effect of scheduled feeding on Dnmt3b and Bmal1 mRNA expression patterns in the liver. Significant differences at all time points were found between groups in both Dnmt3b and Bmal1 mRNA expressions (**p < 0.01 vs. ad lib fed group, Student’s t-test). The expression level in ZT 16 for Dnmt3b and ZT 0 for Bmal1 of ad lib fed group was set as 1 and relative concentrations are shown. Numbers used in each group is shown in parentheses.
Effect of L-Bmal1 KO on the level and pattern of Dnmt3b mRNA expression
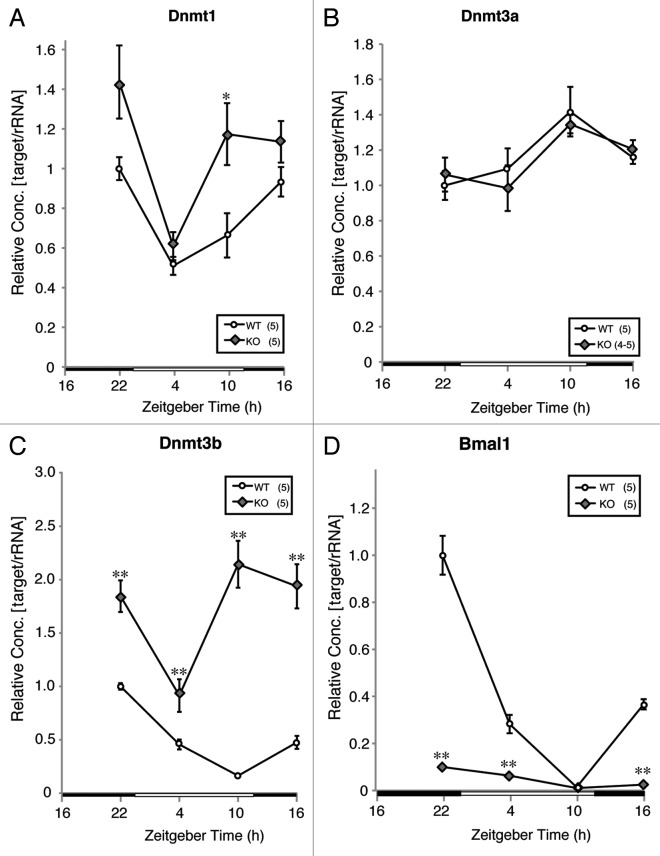
Since BMAL1 is one of the core circadian oscillators, gene targeting destruction of tissue-specific Bmal1 expressions by the Cre-loxP system induces circadian dysfunction in several tissues.29-31 To determine whether L-Bmal1 KO affects the diurnal expression of Dnmt3b mRNA, Dnmt expression levels at ZTs 4, 10, 16, and 22 were compared in L-Bmal1 KO mice and wild-type mice. A slight but significant increase in Dnmt1 mRNA expression was seen at ZT10 as a result of L-Bmal1 KO (Fig. 4A), but there were no significant differences between the groups in Dnmt3a mRNA expressions at any time point (Fig. 4B). The most dramatic change as a result of L-Bmal1 KO was in Dnmt3b expression (Fig. 4C). Dnmt3b mRNA levels of the L-Bmal1 KO group were significantly higher than in the wild-type group at all time points and, at ZT10, the Dnmt3b mRNA level in the L-Bmal1 KO mice reached more than 10 times the level in the wild-type mice. The time point when the level of expression was lowest had shifted in the L-Bmal1 KO mice. Although the lowest level occurred at ZT10 in wild-type mice, it occurred at ZT4 in the L-Bmal1 KO mice, indicating that not only increased mRNA level but also the phase-shift in expression pattern in Dnmt3b were induced by L-Bmal1 KO. Finally, Bmal1 mRNA levels were measured to confirm Bmal1 deficiency in the liver of L-Bmal1 KO mice, and they were found to be extremely low at ZT4, 16 and 22 (Fig. 4D).
Figure 4. Effect of L-Bmal1 KO on diurnal expressions of Dnmts and Bmal1 mRNA in the liver. Slight but significant increase of Dnmt1 mRNA expression was demonstrated in L-Bmal1 knockout at ZT 10 (A, *p < 0.05, Student’s t-test). There was no significant difference between groups in Dnmt3a mRNA expressions (B). Significant increases of Dnmt3b mRNA expressions at ZT 4, 10, 16 and 22 by L-Bmal1 knockout was revealed [(C) **p < 0.01 at ZT 22, 10, 16 and *p < 0.05 at ZT4, Student’s t-test]. Significant reductions of Bmal1 mRNA expressions at ZT 4, 16 and 22 by L-Bmal1 knockout were confirmed [(D) **p < 0.01, Student’s t-test]. The expression level in ZT 22 of wild-type was set as 1 and relative concentrations are shown. Numbers used in each group is shown in parentheses.
Binding of circadian oscillators on the regulatory regions of Dnmt3b gene
The results of experiments using L-Bmal1 KO mice clearly showed the linkage between BMAL1 and the expression of Dnmt3b. Since the Dnmt3b promoter region contains putative E-box consensus sequences, which mediate transactivation by CLOCK/BMAL1 heterodimer, we investigated whether BMAL1 binds to these E-boxes by ChIP assay. When the DNA sequence containing E-box in Per1 promoter was examined as a positive control, we found that the efficiency of DNA recovery by anti-BMAL1 antibody was significantly increased at ZT 8 (Fig. S2A), as previously reported.20 Then, we tested whether BMAL1 can bind to six different putative E-box consensus sequences localized in the Dnmt3b promoter. However, we detected no significant increase in DNA recovery, indicating that BMAL1 does not bind to the consensus sequences having a homology to E-box in Dnmt3b promoter. We also examined the involvement of REV-ERB/ROR binding elements (RREs) in genomic loci associated with Dnmt3b. RRE is another oscillator sequence: binding of REV-ERB α/β suppress and binding of ROR α/β/γ enhance transcription.21-24 As for ChIP assay using anti-REV-ERBα antibody, we used a known RRE sequence in Bmal1 promoter as a positive control (Fig. S2B). The efficiency of DNA recovery was significantly increased at ZT 8, as previously reported.32 For Dnmt3b, we examined the DNA recovery of two RREs localized in the promoter region and five RREs in gene body (Fig. S2B). However, we did not find time-dependent changes of DNA recovery between ZTs (Fig. S2B), indicating the possibility that REV-ERBα does not play a critical role in diurnal variation of Dnmt3b mRNA. Further arguments about this notion are considered in the Discussion section.
Diurnal variation in 5-mC as a percentage of the total cytosine in liver genomic DNA and the effect of L-Bmal1 KO on the 5-mC percentage
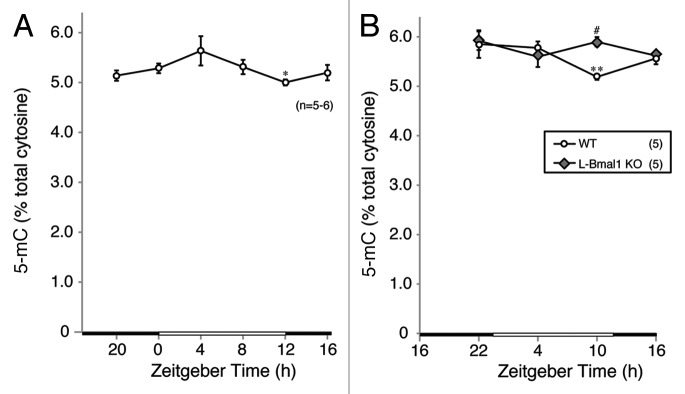
In order to investigate whether the diurnal changes in Dnmt3b expression affects global DNA methylation, we accurately measured non-methylated cytidine and 5-methyl-deoxycytidine (5-medC) levels in liver genomic DNA by a highly reliable method that we established previously15 and calculated 5-mC as a percentage of total cytosine. Comparing the 5-mC percentages at ZTs 0, 4, 8, 12, 16 and 20 showed that the lowest level that was significantly different from the level at ZT0 occurred at ZT12 (Fig. 5A). In addition, the 5-mC percentage at ZT12 tended to be lower than at ZT4 and at ZT8 (p = 0.07 and 0.08, respectively). Thus, the 5-mC level seemed to follow the variation of Dnmt3b expression with a certain time lag. The 5-mC level was changed by 11.2% between the highest and lowest time points in a day. We also analyzed 5-mC percentages in wild-type and L-Bmal1 KO mice sacrificed at ZTs 4, 10, 16, and 22. In the wild-type mice, 5-mC as a percentage of total cytosine was significantly lower at ZT10 than at the other time points (Fig. 5B). By contrast, the time point-specific decline in 5-mC percentage seen in wild-type mice at ZT10 was not observed in L-Bmal1 KO mice (Fig. 5B). Recent studies have discovered an active DNA demethylation pathway starting with hydroxylation of 5-mC by TET family proteins and enabling fast DNA demethylation.9 The analyses of mRNA levels of Tet1, 2 and 3 showed no significant diurnal variation (Fig. S3). These results support the notion that the changes in 5-mC level are attributable to diurnal changes in methylation by DNMT3B but not to diurnal changes in demethylation.
Figure 5. Diurnal change in global 5-mC in liver genomic DNA from L-Bmal1 KO and wild-type mice. Comparison of 5-mC percentage at ZT0, 4, 8, 12, 16 and 20. ZT12 showed the lowest 5-mC percent value, being significantly different from that at ZT0 [(A) *p < 0.05, Student’s t-test]. In addition, 5-mC percent at ZT12 showed decreased tendency when compared with ZT4 and 8 (p = 0.07 and 0.08, respectively, Student’s t-test). In (B) we compared 5-mC percentage in wild-type and L-Bmal1 KO mice at ZT4, 10, 16, 22. In wild-type mice, significantly lower 5-mC percent at ZT10 was found, compared with other time points (**p < 0.01 vs. ZT4, p < 0.05 vs. ZT16 and 22, Student’s t-test). In L-Bmal1 KO mice, the time point-specific decline of 5-mC percentage (found in wild-type mice at ZT10) was not observed; instead, a significant increase in 5-mC percentage was found (#p < 0.001 vs. wild-type, Student’s t-test).
Discussion
In this study, we showed that expression of one of the Dnmt subtypes, Dnmt3b, undergoes dynamic changes closely coinciding with those of Bmal1 during the day in the adult liver. The diurnal pattern of Dnmt3b mRNA expression was shown to be common to both sexes and to be maintained in mice that had been subjected to ovariectomy and orchiectomy, to create models of menopause and andropause, respectively.33,34 Although the sex of the animals affected the expression level of Dnmt3b at ZT0, the difference between sexes was relatively small. These results indicate that the diurnal pattern of Dnmt3b mRNA expression in the liver is not specifically influenced by the hormonal milieu originated from the difference in gonads between the sexes and might be maintained stable in mature mice.
Comparison between Dnmt3b and Bmal1 mRNA expression at ZT0 and 8 at 1, 3, 5 and 9 weeks of age showed that the adult-like diurnal expression pattern of both genes, in which expression was high at ZT0 and low at ZT8, started at 3 weeks of age. The previous study on the ontogeny of circadian oscillations revealing that the adult-like diurnal expression pattern of Bmal1 in rat liver starts 20 d after birth35 coincides with our present result. The hepatic circadian clock has been found to be entrained by central signals36-38 and peripheral signals of ingested nutrients.39-42 In view of the similarities between the expression patterns of Bmal1 and Dnmt3b observed in our developmental experiment and SF experiment, the entrainment signals that reset the diurnal expression of Bmal1 may also regulate Dnmt3b mRNA expression in the liver, although the hierarchical relationships between central and peripheral signals for entrainment of Dnmt3b remains unknown at present.
To determine definitively whether endogenous clockwork in the liver regulates the diurnal expression pattern of Dnmt3b, we compared Dnmt3b mRNA expression in wild-type and L-Bmal1 KO mice. The Dnmt3b mRNA levels of the L-Bmal1 KO mice were significantly higher at all time points examined. The time of the lowest Dnmt3b mRNA level shifted from ZT10 to ZT4. These results demonstrate that the endogenous hepatic clockwork controlled by core clock genes including Bmal1 is the driving force behind the diurnal rhythm of Dnmt3b. On the other hand, the results of ChIP assay using anti-BMAL1 and anti-REV-ERBα antibodies did not show binding of these proteins to their binding sites around Dnmt3b. We also could not find any BMAL1-binding site around Dnmt3b gene by searching the database of BMAL1-binding sites (CircaClock, http://circaclock.epfl.ch) and no specific reference of Dnmt3b in the gene lists appeared in the recent ChIP-seq analyses using anti-REV-ERB antibodies.32,43,44 Since it is not feasible that Dnmt3b transcription is regulated by E-box and RREs, we are now assuming that Dnmt3b transcription is not controlled directly by core-clock genes such as Bmal1 and Rev-erbs but, instead, by the secondary and higher-order clock genes. For example, DBP and E4BP4 bind to D-site and B-site, respectively, and work as intermediaries of clockwork.25,45 The transcription of Dnmt3b might be affected by their transcriptional regulations. In addition, as it has been reported recently that Per2 mRNA circadian oscillation is modulated by mRNA rhythmic degradation,46 there is growing evidence that posttranscriptional regulation is important in the circadian oscillation of mRNA. Since it has been reported that Dnmt3b mRNA stabilization is regulated by RNA-binding proteins such as HuR47 and microRNAs,48,49 there is a possibility that Dnmt3b mRNA receives posttranscriptional regulation along with the time of day. Further study based on these hypotheses will be required to unveil the mechanism of diurnal variation of Dnmt3b transcription.
By measuring the amounts of cytidine and 5-medC by high-performance liquid chromatography/electrospray ionizing mass spectrometry (LC/ESI-MS) using stable-isotope-labeled surrogates as internal standards,15 this study revealed that the level of 5-mC in liver genomic DNA varies with the time of day. A previous study that assessed global DNA methylation by fluorescently labeled cytosine extension assay reported diurnal changes in global DNA methylation in humal blood.50 The present study is the first to demonstrate a diurnal rhythm of global DNA methylation in the liver. Furthermore, the variation appeared to be coordinated with the diurnal variation in Dnmt3b expression. mRNA expressions of Tet1–3, the enzymes regulating active DNA demethylation, were not altered with time of day (Fig. S3), suggesting the possibility that the active methylation process contributes greatly to diurnal variation of global DNA methylation. In comparing 5-mC level between wild-type mice and L-Bmal1 KO mice, wild-type mice showed the lowest 5-mC level at ZT10, when Dnmt3b mRNA level was also the lowest. On the other hand, L-Bmal1 KO mice showed higher degree of DNA methylation in parallel with increased Dnmt3b expression at ZT10. These results suggest the possibility that DNMT3B in the adult liver dominantly controls the diurnal change in global DNA methylation state. In our previous study, we measured the 5-mC level of the liver of mice fed a methyl-deficient diet, which has been reported to induce DNA hypomethylation,51 by the precise LC/ESI-MS method, and found that 5 mo of feeding with methyl-deficient diet reduced the 5-mC level by 3.6% in the liver genome of male mice.15 This study demonstrated that the 5-mC level is changed by up to 11.2% in a day and the deviation is thought to be substantial. On the other hand, we could not find the quantitative difference of DNMT3B protein between ZT 0 and 8 by western blotting analysis (Fig. S4). The development of methods with better accuracy to detect subtle difference in DNMT3b expression levels, such as radioimmunoassay or enzyme-linked immunoassay, might be required to clarify whether DNMT3B protein level in liver varies within a day.
The biological significance of diurnal variation in global DNA methylation is yet unclear. In the present study we found higher Dnmt3b expression around the age younger than 5-weeks-old, when pup’s organs rapidly grow with extensive cellular proliferation. Cell cycle progression is regulated by core clock molecules such as BMAL1/CLOCK complex and shows a diurnal pattern.52 DNMT3B is reported to localize to centromeric/pericentromeric satellite repeats and play a role in maintaining chromosomal function, which is essential for accurate chromosome segregation during mitosis, through DNA methylation as well as histone modifications.53 Although it has not yet been clarified whether DNA methylation and histone modifications show diurnal changes, the diurnal changes in Dnmt3b expression and level of 5-mC might be involved in centromeric/pericentromeric chromatin organization in clock-regulated cell cycle progression.
It is also not elucidated how the diurnal variation in Dnmt3b expression is involved in gene specific DNA methylation and hepatic function. Glucose homeostasis and lipid homeostasis in the liver exhibit a circadian pattern, which is known to at least in part be dependent on the diurnal variation in expression of metabolism-related genes.54,55 The circadian rhythm of metabolism-related genes has been reported to be regulated by epigenetic histone modifications.43 Since there is still no clear evidence linking the diurnal change in DNA methylation to the circadian gene expression and subsequent hepatic functions associated with metabolism, future studies measuring precise promoter methylation states in individual glucose and lipid homeostasis-related genes along with the time of day may lead to a better understanding of the link between the diurnal Dnmt3b variation and liver physiology. The results of the present study may also help to elucidate the mechanism by which clock disorders lead to metabolic abnormalities that have attracted attention as a result of epidemiological studies of shift workers.56 Aberrant DNA methylation levels have been found to be associated with the onset and progression of adult-onset metabolic diseases.3,4,13,56,57 Thus, we can speculate that the higher Dnmt3b expression and the temporarily higher DNA methylation state, as a result of L-Bmal1 KO, increase susceptibility to such diseases. Indeed, L-Bmal1 KO mice have been reported to have abnormal glucose tolerance, possibly due to the impaired expression of glucokinase and glucose transporter-2, which regulate hepatic glucose homeostasis.29 Importantly, the expression of these genes is known to be controlled by the methylation state of their promoters.58,59 Although further study is required, the results of the present study suggest that overexpression of Dnmt3b has some role in the pathogenesis of metabolic diseases triggered by molecular clock dysfunction.
Materials and Methods
Mice
Except for L-Bmal1 KO experiments, male and female C57BL/6J mice were purchased from CLEA Japan and were bred in National Institute for Environmental Studies (NIES). They were acclimatized to the environment for about 1 week prior to use. Throughout the experiment, the animals were maintained in a controlled environment at a temperature of 24 ± 1°C and humidity of 50 ± 10% and under a 12/12 h light/dark cycle (light, ZT0–12; dark, ZT12–24). Food (CE-2, CLEA) and water were available ad libitum unless otherwise indicated.
For L-Bmal1 KO experiments, C57BL/6J mice with or without genetic modification were maintained at the School of Pharmacy, Nihon University at 23 ± 1°C with 50 ± 10% humidity and under a 12/12 h light dark cycle (light, ZT0–12; dark, ZT12–24). The Cre-loxP system was applied for creating L-Bmal1 KO mice as described elsewhere.31 Briefly, we constructed a conditional Bmal1 (flox/flox) mice, which carry the conditional Bmal1 allele containing exons 6–8 flanked by loxP sites using ES cells derived from C57BL/6J mice. To create the liver-specific Bmal1 excision, the mice expressing Bmal1 (flox/flox) allele were crossed to the mice expressing a Cre transgene driven by the albumin promoter (Jackson Laboratories). Mice homozygous for the floxed allele and hemizygous for the Cre transgene [Bmal1(flox/flox/CreAlb) were obtained by crossing Bmal1 (flox/+/CreAlb) mice to Bmal1(flox/flox) mice. Littermates that were negative for Cre transgenes (Bmal1(flox/flox)] were used as controls.
Ethical statement
Except for L-Bmal1 KO experiments, the mice were handled in a humane manner in accordance with the NIES guidelines. For L-Bmal1 KO experiments, the experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Nihon University.
Sampling of liver along with time points from adult mice
Seven weeks-old male and female mice (n = 3 at each time point) were killed by decapitation either at ZT 0, 4, 8, 12, and 16. For obtaining total RNA, ten mg of liver was collected and homogenized with Qiagen RLT buffer containing 1% β-mercaptoethanol by a microhomogenizing system (Micro Smash MS-100, TOMY, JAPAN) at 3,000 rpm for 30 sec immediately after sampling. For obtaining genomic DNA, 100 mg of liver was collected from each individual and stored at -80°C until extraction.
Gonadectomy and sampling
Twelve males and 12 females at 7 weeks of age were orchidectomized and ovariectomized under ether anesthesia, respectively. They were raised for additional 4 weeks until autopsy in order to establish lower gonadal sex hormone levels. These mice were decapitated at ZT 0 and 8 (n = 6 at each time point) and livers were collected. Male and female mice at 11 weeks of age were used as non-manipulated gonad-intact controls. Homogenate of liver was made as the same method as mentioned above.
Sampling from developing mice
Adult male and female were cohabitated as 1 by 1 to obtain newborn pups. Vaginal plug and delivery was checked every day to estimate and confirm the birthday of pups. One, 3, 5, 9 weeks after birth, mice were sacrificed at either ZT 0 or 8 (n = 3–4 at each time point) and homogenate of liver was made as the same method as mentioned above.
Sampling from adult mice under scheduled feeding
In the SF group, food was available only during ZT 5 to 9 for 6 d without any alteration of light regimen. On the other hand, in ad lib feeding group, feeding availability was not limited. Mice in each group were killed by decapitation either at ZT 0, 4, 8, 12 and 16. Homogenate of liver was made as the same method as mentioned above.
RNA extraction and measurement of mRNA expressions
Total RNA was extracted by RNeasy Mini Kit (QIAGEN KK) following the manufacturer’s instruction. Using 100 ng total RNA as template, cDNA was synthesized by reverse transcriptase XL (Takara Bio Inc.). Expression of target genes and rRNA (rRNA) was quantified by real-time PCR on LightCycler instrument (Roche Diagnostics) as described previously.60 Amplification in experimental samples during the log linear phase was compared with the standard curve from the dilution series of a control cDNA using LightCycler quantification software (Version 3.5). The control cDNA to make a standard curve was prepared from the livers of C57BL/6 mice. The primer sequences and annealing temperatures used for real-time PCR are shown in Table 1.
Table 1. Primer sequences and PCR conditions for amplification.
| Targets | Primer sequences (5′- 3′) | Annealing temperature (°C) |
|---|---|---|
| Dnmt1 |
CCAAGCTCCGGACCCTGGATGTGT |
64 |
| |
CGAGGCCGGTAGTAGTCACAGTAG |
|
| Dnmt3a |
GCACCTATGGGCTGCTGCGAAGACG |
64 |
| |
CTGCCTCCAATCACCAGGTCGAATG |
|
| Dnmt3b |
GTCTGCACACCAGAGACCAGAG |
64 |
| |
TCAGAGCCATTCCCATCATCTAC |
|
| Bmal1 |
ATGCAGAACACCAAGGAAGG |
64 |
| |
CCATCCTTAGCACGGTGAGT |
|
| Clock |
CAAAATGTCACGAGCACTTAATGC |
64 |
| |
ATATCCACTGCTGGCCTTTGG |
|
| Per1 |
ACAGCAGCCACGGTTCTC |
64 |
| |
GCTGCCACAGTCCACACA |
|
| Per2 |
CAACACAGACGACAGATCA |
64 |
| |
TCCTGGTCCTCCTTCAACAC |
|
| Cry1 |
CTCGGGTGAGGAGGTTTTCTT |
64 |
| |
GACTTCCTCTACCGAGAGCTTCAA |
|
| 18S rRNA |
TACCACATCCAAGGAAGGCAG |
64 |
| TGCCCTCCAATGGATCCTC |
Measurements of global DNA methylation
For 5-medC measurements, genomic DNA was prepared from the liver tissue by phenol/chloroform extraction. DNA hydrolysis to nucleosides was performed according to the previous report.15 Briefly, 1 μg of DNA was denatured by heating at 98°C for 3 min. The solution was mixed with 2 units of nuclease P1 (Wako) and incubated at 45°C for 2 h in 10 mM ammonium acetate. Next, the solution was supplemented with 0.002 units of phosphodiesterase I (Worthington Biochemical Corp.) and incubated at 37°C for 2 h in 100mM ammonium bicarbonate, and then 0.5 units of alkaline phosphatase (Promega) was added and incubation was continued at 37°C for an additional 1 h.
LC–MS analyses were performed by using LC/MS-2010A mass spectrometer with an ESI ionization probe (Shimadzu) according to the previous report15 with minor modification. Deoxycytidine (dC) and 5-medC were separated by using a reversed-phase column (Atlantis dC18, 2.1 X 150 mm, 5 μm, Waters) in gradient mode with methanol (2–20%) -10 mM ammonium acetate (v/v) at a total flow rate of 0.2 ml/min. The internal standards (13C9, 15N3-2’-deoxycytidine, 13C10, 15N2-5-methyl-2'-deoxycytidine, 100 ng each) were added to 15 μl of the hydrolyzed sample, and the mixture was diluted to 500 μl with H2O. A 10 μl volume of the mixture was injected into LC–MS, and dC and 5-medC were analyzed on a selected-ion monitoring (SIM) mode. The SIM m/z of dC and 5-medC was 228.1 and 242.1, respectively. The SIM m/z of the stable-isotope labeled dC and 5-medC was 240.1 and 254.1, respectively. Quantification of 5-mC percent in total cytosine was calculated from integration peak areas of 5-medC relative to global cytidine (5-medC+dC).
Statistical analyses
Data was expressed as mean ± SEM. Samples number used was indicated in parentheses. In Figure 2 A‒D, the difference between ZTs was analyzed by unpaired Student’s t-test by Microsoft Excel (MS-Excel 2008 for Mac, Microsoft). In Figures 3, 4 and 5, the differences between groups and among time points were analyzed by unpaired Student’s t-test.
Supplementary Material
Acknowledgments
The authors gratefully thank Drs. Yasutomi Kamei (Tokyo Medical and Dental University), Rika Numano (Toyohashi University of Technology), Akihito Adachi (Saitama University) and Satoshi Yamashita (National Cancer Center Research Institute) for their kind comments on this work. The authors thank Drs. Tadashi Matsuura and Nobuhito Masuda (Perseus Proteomics Inc.) for providing us anti-REV-ERBα antibody. We also thank Ms. Hikari Murai and Michiyo Matsumoto for their superior technical assistance and Ms. Sayuri Itaki for her excellent secretarial assistance.
Glossary
Abbreviations:
- 5-mC
5-methyl-cytosine
- 5-medC
5-methyl-deoxycytidine
- ANOVA
analysis of variance
- BER
base excision repair
- BMAL1
Brain and Muscle Arnt-Like Protein-1
- cDNA
complementary DNA
- CLOCK
Circadian Locomotor Output Cycles Kaput
- CRY
Cryptochrome
- DBP
D site of albumin promoter (albumin D-box) binding protein
- DEC
differentiation of human embryo chondrocytes
- Dnmt
DNA methyltransferase
- dC
deoxycytidine
- Gadd
growth arrest and DNA damage
- ICF
immunodeficiency, centromeric instability and facial anomalies
- L-Bmal1 KO
liver-specific Bmal1 knockout
- LC/ESI-MS
liquid chromatography/electrospray ionizing mass spectrometry
- Per
Period
- Rev-erb
Reverse-erb receptor
- Ror
RAR-related orphan receptor
- RRE
Rev-Erb/ROR binding element
- rRNA
ribosomal RNA
- S.E.M
standard error of the mean
- SF
scheduled feeding
- TET
ten-eleven translocation
- ZT
zeitgeber time
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Disclosures
This work was supported by the National Institute for Environmental Studies (0710AG333 to KN, 115AA082), the Ministry of the Environment Japan (Environment Research and Technology Development Fund, S-01 to KN), the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grants-in-Aid for Scientific Research (C) 24590307 and Grant-in-Aid for Scientific Research (B) 23310043 to FM, Grant-in-Aid for Scientific Research (B) 23390166 and Banyu Foundation Research Grant to KN], a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the “High-Tech Research Center” Project for Private Universities, a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology of Japan 2007 to SS.
Author Contributions
Conceived and designed the experiments: F.M. and K.N. Performed the experiments: F.M., S.S., S.T., T.S., T.S., M.O., J.B., T.E. and K.N. Analyzed the data: F.M., S.T., M.O. and J.B. Contributed reagents/materials/analysis tools: F.M., S.S., T.S. (Sano), Y.O. and K.N. Wrote the paper: F.M. and K.N.
Supplemental Material
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/21539
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/21539
References
- 1.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 2.Geiman TM, Muegge K. DNA methylation in early development. Mol Reprod Dev. 2010;77:105–13. doi: 10.1002/mrd.21118. [DOI] [PubMed] [Google Scholar]
- 3.Szyf M. The implications of DNA methylation for toxicology: toward toxicomethylomics, the toxicology of DNA methylation. Toxicol Sci. 2011;120:235–55. doi: 10.1093/toxsci/kfr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–73. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linhart HG, Lin H, Yamada Y, Moran E, Steine EJ, Gokhale S, et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–22. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu GL, Bestor TH, Bourc’his D, Hsieh CL, Tommerup N, Bugge M, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–91. doi: 10.1038/46214. [DOI] [PubMed] [Google Scholar]
- 7.Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, et al. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1999;96:14412–7. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11:607–20. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto G, Schäfer A, Marhold J, Stach D, Swaminathan SK, Handa V, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–5. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 11.Ma DK, Jang M-H, Guo JU, Kitabatake Y, Chang M-L, Pow-Anpongkul N, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–7. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–34. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;119:11–9. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinawath A, Miyake S, Yanagisawa Y, Akiyama Y, Yuasa Y. Transcriptional regulation of the human DNA methyltransferase 3A and 3B genes by Sp3 and Sp1 zinc finger proteins. Biochem J. 2005;385:557–64. doi: 10.1042/BJ20040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nohara K, Baba T, Murai H, Kobayashi Y, Suzuki T, Tateishi Y, et al. Global DNA methylation in the mouse liver is affected by methyl deficiency and arsenic in a sex-dependent manner. Arch Toxicol. 2011;85:653–61. doi: 10.1007/s00204-010-0611-z. [DOI] [PubMed] [Google Scholar]
- 16.Storch K-F, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 17.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/S0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 18.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–82. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 19.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–9. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/S0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 22.Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–8. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 23.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–37. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–74. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 26.Terranova M, Laviola G. Individual differences in mouse behavioural development : effects of precocious weaning and ongoing sexual segregation. Anim Behav. 1995;50:1261–71. doi: 10.1016/0003-3472(95)80042-5. [DOI] [Google Scholar]
- 27.Sumová A, Bendová Z, Sládek M, El-Hennamy R, Laurinová K, Jindráková Z, et al. Setting the biological time in central and peripheral clocks during ontogenesis. FEBS Lett. 2006;580:2836–42. doi: 10.1016/j.febslet.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Oishi K, Kasamatsu M, Ishida N. Gene- and tissue-specific alterations of circadian clock gene expression in streptozotocin-induced diabetic mice under restricted feeding. Biochem Biophys Res Commun. 2004;317:330–4. doi: 10.1016/j.bbrc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 29.Lamia KA, Storch K-F, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–4. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6:e25231. doi: 10.1371/journal.pone.0025231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–67. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–91. doi: 10.1016/0169-6009(91)90124-I. [DOI] [PubMed] [Google Scholar]
- 34.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–20. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Sládek M, Jindráková Z, Bendová Z, Sumová A. Postnatal ontogenesis of the circadian clock within the rat liver. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1224–9. doi: 10.1152/ajpregu.00184.2006. [DOI] [PubMed] [Google Scholar]
- 36.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–50. doi: 10.1016/S0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 37.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, et al. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci U S A. 2003;100:6795–800. doi: 10.1073/pnas.0936797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, Ishida N. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 2005;12:191–202. doi: 10.1093/dnares/dsi003. [DOI] [PubMed] [Google Scholar]
- 39.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 41.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–78. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 42.Hirao A, Tahara Y, Kimura I, Shibata S. A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS One. 2009;4:e6909. doi: 10.1371/journal.pone.0006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–9. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–7. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohno T, Onishi Y, Ishida N. The negative transcription factor E4BP4 is associated with circadian clock protein PERIOD2. Biochem Biophys Res Commun. 2007;354:1010–5. doi: 10.1016/j.bbrc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 46.Woo KC, Kim TD, Lee KH, Kim DY, Kim W, Lee KY, et al. Mouse period 2 mRNA circadian oscillation is modulated by PTB-mediated rhythmic mRNA degradation. Nucleic Acids Res. 2009;37:26–37. doi: 10.1093/nar/gkn893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.López de Silanes I, Gorospe M, Taniguchi H, Abdelmohsen K, Srikantan S, Alaminos M, et al. The RNA-binding protein HuR regulates DNA methylation through stabilization of DNMT3b mRNA. Nucleic Acids Res. 2009;37:2658–71. doi: 10.1093/nar/gkp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duursma AM, Kedde M, Schrier M, le Sage C, Agami R. miR-148 targets human DNMT3b protein coding region. RNA. 2008;14:872–7. doi: 10.1261/rna.972008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takada S, Berezikov E, Choi YL, Yamashita Y, Mano H. Potential role of miR-29b in modulation of Dnmt3a and Dnmt3b expression in primordial germ cells of female mouse embryos. RNA. 2009;15:1507–14. doi: 10.1261/rna.1418309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bönsch D, Hothorn T, Krieglstein C, Koch M, Nehmer C, Lenz B, et al. Daily variations of homocysteine concentration may influence methylation of DNA in normal healthy individuals. Chronobiol Int. 2007;24:315–26. doi: 10.1080/07420520701290565. [DOI] [PubMed] [Google Scholar]
- 51.Okoji RS, Yu RC, Maronpot RR, Froines JR. Sodium arsenite administration via drinking water increases genome-wide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis. 2002;23:777–85. doi: 10.1093/carcin/23.5.777. [DOI] [PubMed] [Google Scholar]
- 52.Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8:832–7. doi: 10.4161/cc.8.6.7869. [DOI] [PubMed] [Google Scholar]
- 53.Gopalakrishnan S, Van Emburgh BO, Shan J, Su Z, Fields CR, Vieweg J, et al. A novel DNMT3B splice variant expressed in tumor and pluripotent cells modulates genomic DNA methylation patterns and displays altered DNA binding. Mol Cancer Res. 2009;7:1622–34. doi: 10.1158/1541-7786.MCR-09-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism - the epigenetic link. J Cell Sci. 2010;123:3837–48. doi: 10.1242/jcs.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudic RD, McNamara P, Curtis A-M, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 57.Ehara T, Kamei Y, Takahashi M, Yuan X, Kanai S, Tamura E, et al. Role of DNA Methylation in the Regulation of Lipogenic Glycerol-3-Phosphate Acyltransferase 1 Gene Expression in the Mouse Neonatal Liver. Diabetes. 2012 doi: 10.2337/db11-1834. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin B, Seong JK, Ryu D-Y. Tissue-specific and de novo promoter methylation of the mouse glucose transporter 2. Biol Pharm Bull. 2005;28:2054–7. doi: 10.1248/bpb.28.2054. [DOI] [PubMed] [Google Scholar]
- 59.Jiang M, Zhang Y, Liu M, Lan MS, Fei J, Fan W, et al. Hypermethylation of hepatic glucokinase and L-type pyruvate kinase promoters in high-fat diet-induced obese rats. Endocrinology. 2011;152:1284–9. doi: 10.1210/en.2010-1162. [DOI] [PubMed] [Google Scholar]
- 60.Nohara K, Ao K, Miyamoto Y, Ito T, Suzuki T, Toyoshiba H, et al. Comparison of the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced CYP1A1 gene expression profile in lymphocytes from mice, rats, and humans: most potent induction in humans. Toxicology. 2006;225:204–13. doi: 10.1016/j.tox.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.