Abstract
Background:
It is uncertain whether the effort and expense of performing a second walk for the 6-min walk test improves test performance. Hence, we attempted to quantify the improvement in 6-min walk distance if an additional walk were to be performed.
Methods:
We studied patients consecutively enrolled into the National Emphysema Treatment Trial who prior to randomization and after 6 to 10 weeks of pulmonary rehabilitation performed two 6-min walks on consecutive days (N = 396). Patients also performed two 6-min walks at 6-month follow-up after randomization to lung volume reduction surgery (n = 74) or optimal medical therapy (n = 64). We compared change in the first walk distance to change in the second, average-of-two, and best-of-two walk distances.
Results:
Compared with the change in the first walk distance, change in the average-of-two and best-of-two walk distances had better validity and precision. Specifically, 6 months after randomization to lung volume reduction surgery, changes in the average-of-two (r = 0.66 vs r = 0.58, P = .01) and best-of-two walk distances (r = 0.67 vs r = 0.58, P = .04) better correlated with the change in maximal exercise capacity (ie, better validity). Additionally, the variance of change was 14% to 25% less for the average-of-two walk distances and 14% to 33% less for the best-of-two walk distances than the variance of change in the single walk distance, indicating better precision.
Conclusions:
Adding a second walk to the 6-min walk test significantly improves its performance in measuring response to a therapeutic intervention, improves the validity of COPD clinical trials, and would result in a 14% to 33% reduction in sample size requirements. Hence, it should be strongly considered by clinicians and researchers as an outcome measure for therapeutic interventions in patients with COPD.
The 6-min walk test (6MWT) is a simple and inexpensive test that is increasingly used to measure impairment in exercise capacity because of respiratory, cardiovascular, and neurologic disease.1‐9 Unlike some other effort-dependent tests, such as spirometry, where performing multiple efforts significantly improves the quality of test results and is standard practice, there is no consensus regarding the use of multiple or practice walks during the 6MWT.9‐15 Currently, the standard 6MWT includes only one walk per test,16‐20 and the most recent American Thoracic Society recommendations state that “a practice walk is not needed in most clinical settings but should be considered.”2 Notably, the investigators who first developed the 6MWT recommended—without providing concrete data—that patients should perform multiple or practice walks because walk distances became reproducible only after the second or third effort.21‐23
Performing more than one walk per 6MWT has potential advantages and disadvantages. Unlike the first walk, subsequent walks are less likely to contain random error because patients are better able to follow instructions, can better pace themselves, and have less anxiety.2,24,25 A more significant advantage is that changes in walk distance after clinical interventions may more closely represent the true change in the patients’ exercise capacity; that is, it would be more valid and contain less random error (be more precise).23,26 The disadvantage would be the increased cost and effort to perform an additional walk.
Therefore, we compared the validity and precision of changes in 6MWT results using a single walk vs two walks per walk test. We hypothesized that adding a walk would substantially improve the validity and precision of test results.
Materials and Methods
National Emphysema Treatment Trial
The National Emphysema Treatment Trial (NETT) was a randomized clinical trial comparing lung volume reduction surgery (LVRS) with optimal medical therapy (OMT) for the treatment of advanced emphysema. A detailed design of this trial has been published previously.27 The study was approved by the institutional review boards of all the individual study sites.3,27 The major enrollment criteria were bilateral emphysema judged suitable for LVRS, FEV1 ≤ 45% predicted, residual volume ≥ 150% predicted, Paco2 ≤ 60 mm Hg (≤ 55 mm Hg in Denver, Colorado), and absence of clinical pulmonary hypertension.
At enrollment (baseline), patients completed a 6MWT, a symptom-limited incremental cardiopulmonary exercise test (CPET) using a cycle ergometer, spirometry, an assessment of lung volumes using body plethysmography, and two quality-of-life instruments (St. George’s Respiratory Questionnaire and University of California, San Diego, Shortness of Breath Questionnaire). These tests were repeated after all patients underwent 6 to 10 weeks of pulmonary rehabilitation and subsequently at 6 months, 12 months, and yearly after they were randomized to LVRS or OMT.
Patient Cohort
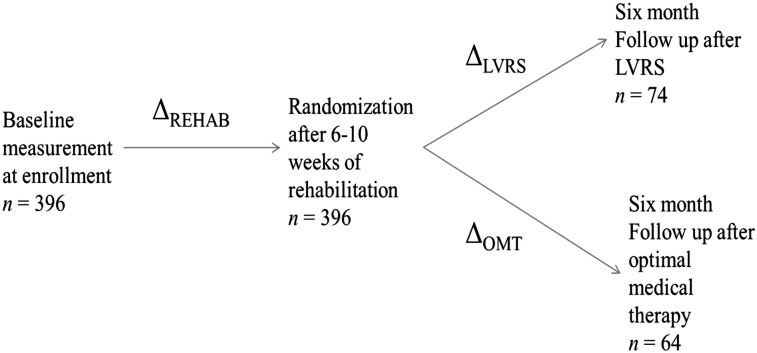
Of the 1,218 patients randomized in NETT, the first 396 performed two walks on consecutive days for the 6MWT, once at baseline and once after 6 to 10 weeks of pulmonary rehabilitation (Fig 1). Of the 396 patients, 138 also performed two 6-min walks for the 6MWT 6 months postrandomization (74 randomized to LVRS and 64 to OMT) (Fig 1). The remaining 822 of the 1,218 patients did a single walk for each 6MWT because of a protocol modification during the trial that eliminated the additional walk to reduce protocol burden; hence, these patients were not included in the analysis. The number of patients with two 6-min walks for the 6MWT at 12 months or beyond was insufficient for inclusion in this analysis.
Figure 1.
Layout of the National Emphysema Treatment Trial showing timing of measurements, sample sizes for the current analysis, and differences that were calculated using data from different time points. Beyond the 6-mo follow-up visit, there were too few patients with two walks per 6-min walk test for a meaningful analysis. LVRS = lung volume reduction surgery; OMT = optimal medical therapy; REHAB = rehabilitation.
6MWT Procedure
Participants underwent CPET on the day before the first of two walk tests. Prior to the walk, a simple (1-2 miles/h) treadmill study was done to determine supplemental oxygen requirement for the 6MWT.27 This was done by adjusting the oxygen concentration of inspired air to maintain the oxygen saturation of arterial blood at > 90% while the patient walked on a treadmill, on a level grade, at 1 mph.27
During the walk, research staff walked behind the participant, carried the oxygen delivery system if required (different from current American Thoracic Society guidelines), and provided scripted instructions at 1-min intervals. After 6 min, the participant was told to stop, and the walk distance was recorded. The walk was then repeated the following day.
Statistical Analysis
The difference from baseline to postrehabilitation for each of the parameters (6MWT, CPET, pulmonary function studies, and quality-of-life scores) was calculated for each patient as follows: Δrehab = (postrehabilitation) − (baseline) (Fig 1). Similarly, the difference in these parameters between the prerandomization and the 6-month postrandomization visits was calculated for patients randomized to LVRS (Δlvrs) and OMT (Δomt). The prerandomization values for the LVRS and OMT analyses were the 6MWT distances at the postrehabilitation visit, not the distances at baseline.
We analyzed all the different ways of interpreting change in walk distance when an additional walk was performed, including changes in the second, average-of-two, and best-of-two walk distances. These were compared against change in the first walk distance, that is, the walk distance that would have been obtained if the single walk 6MWT had been performed. For example, if a patient walked 1,000 and 1,200 ft on the first and second walks of the baseline 6MWT, respectively, and 1,400 and 1,300 ft for the first and second walks on the postrehabilitation 6MWT, respectively, the first-walk Δ6MWTrehab would be 1,400 − 1,000 = 400 ft, the second-walk Δ6MWTrehab would be 1,300 − 1,200 = 100 ft, the average-of-two Δ6MWTrehab would be 1,350 − 1,100 = 250 ft, and the best-of-two Δ6MWTrehab would be 1,400 − 1,200 = 200 ft.
To compare the precision of the first-walk Δ6MWT with the second-walk, average-of-two, and best-of-two Δ6MWTs, we compared the variance (square of the SD) of these Δ6MWTs with one another after each intervention in NETT (Δrehab, Δlvrs, and Δomt). To understand how changes in precision would affect the feasibility of future clinical trials that use Δ6MWT as the end point, the variances were used for sample size calculations of a future clinical trial, assuming typical design parameters (to detect a Δ6MWT of 131.2 ft [40 m] after a therapeutic intervention in a cohort of patients with a two-tailed α of .05).28 Power curves were plotted to summarize the results.
To examine validity of the Δ6MWT, the change in maximal watts on CPET postintervention (ΔCPET) was used as the gold standard measure of change in exercise capacity.29‐31 We compared the strength of the correlation between the first-walk Δ6MWTlvrs and ΔCPETlvrs, with the strength of each correlation among the second-walk, best-of-two, and average-of-two Δ6MWTlvrs and ΔCPETlvrs using Steiger Z statistic.32 Scatterplots with fitted regression lines were used to display the results.
All analyses were performed using STATA version 10.0 (StataCorp LP) statistical software. For comparing continuous variables, the t test was used. Statistical significance was defined as a two-tailed P < .05.
Results
Table 1 summarizes the baseline characteristics of all 396 patients included in the study. E-Table 1 (408.6KB, pdf) compares the 396 patients who performed the 6MWT at baseline and after rehabilitation and a subset of 138 patients who were followed for 6 months after randomization to LVRS or OMT (Fig 1). The baseline characteristics of these two groups were comparable (P > .05). E-Table 2 (408.6KB, pdf) describes the number of patients who needed supplemental oxygen during the walk and the mean oxygen flow rate provided. At the baseline visit, the second walk distance was longer than the first walk distance for 70% of the participants, with a median difference of 65 ft [interquartile range, 3-138 ft; P = .002] between the two walks.
Table 1.
—Baseline Characteristics of Participants Included in the Current Analysis
| Characteristic | Full Cohort | Female Participants | Male Participants |
| No. (%) | 396 | 152 (38.3) | 244 (61.6) |
| Age, y | 68.0 (65.0-71.0) | 67.7 (65.0-70.4) | 68.1 (64.4-71.0) |
| BMI, kg/m2 | 24.4 (21.9-27.1) | 23.7 (21.2-26.5) | 24.7 (22.2-27.4) |
| FEV1, % predicted | 26.0 (21.0-31.0) | 28.0 (24.0- 34.0) | 24.0 (20.0- 30.0) |
| FVC, % predicted | 66.0 (55.0-76.5) | 68.0 (56.0-77.5) | 64.0 (54.5-74.5) |
| FEV1/FVC | 31.2 (26.4-35.9) | 31.2 (26.4-35.9) | 31.2 (26.4-35.9) |
| TLC, % predicted | 129.0 (121.0-139.0) | 130.0 (121.5-138.5) | 128.5 (120.5-139.0) |
| RV, % predicted | 225.0 (197.5-254.0) | 210.0 (187.0-235.0) | 236.0 (207.5-269.0) |
| RV/TLC | 65.6 (60.5-72.0) | 68.8 (63.0-74.2) | 63.7 (59.1-70.3) |
| Dlco, % predicted | 28.0 (21.0-34.0) | 28.0 (22.0-33.0) | 27.0 (21.0-34.0) |
| SGRQ total score | 55.1 (45.5-66.3) | 54.3 (43.5-64.4) | 56.7 (46.9-66.4) |
| SOBQ score | 67.0 (55.0-80.0) | 66.0 (54.0-80.0) | 67.0 (55.0-79.0) |
| Exercise capacity, W | 35.0 (22.0-48.0) | 28.0 (16.0-35.0) | 40.0 (26.0-57.0) |
| 6MWT distance, fta | 1,166 (930-1346) | 1,089 (874-1257) | 1,199 (974-1416) |
Data are presented as median (interquartile range), unless otherwise indicated. 6MWT = 6-min walk test; Dlco = diffusing capacity of the lung for carbon monoxide; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; SOBQ = University of California, San Diego, Shortness of Breath Questionnaire; TLC = total lung capacity.
Obtained from the first walk.
Validity of the Δ6MWT
The Δ6MWT and other tests after each intervention in NETT are summarized in Table 2. Compared with rehabilitation, LVRS produced much-larger improvements in exercise capacity, spirometry, and quality-of-life scores (P < .0001 when comparing Δrehab and Δlvrs) (Table 2). These changes were best reflected by the best-of-two walk distance, which significantly increased after LVRS compared with after rehabilitation (P = .02), unlike the change in the first walk distance (P > .05).
Table 2.
—Changes in 6MWT, Cycle Ergometry, Pulmonary Function Tests, and Quality-of-Life Scores Following Various Interventions Within the National Emphysema Treatment Trial
| Characteristic | Δrehaba | Δlvrsb | Δomtc |
| No. participants | 396 | 74 | 64 |
| First-walk 6MWT, ft | 97.0 ± 180.2 | 89.4 ± 252.9 | −82.2 ± 179.1 |
| Second-walk 6MWT, ft | 63.2 ± 181.2 | 104.5 ± 240.8 | −95.8 ± 156.3 |
| Average-of-two 6MWT, ft | 80.1 ± 157.3 | 96.9 ± 233.8 | −89.0 ± 154.7 |
| Best-of-two 6MWT, ft | 64.9 ± 167.7 | 97.9 ± 234.8d | −82.0 ± 147.6 |
| Exercise capacity, W | 3.1 ± 10.2 | 9.5 ± 14.9e | −4.5 ± 11.0 |
| FEV1, % predicted | −0.4 ± 3.6 | 9.9 ± 9.1e | −0.6 ± 5.0 |
| FVC, % predicted | −0.8 ± 9.5 | 13.6 ± 15.6e | −1.2 ± 10.1 |
| MVV, L/min | −0.3 ± 4.6 | 10.3 ± 9.5e | −0.7 ± 6.4 |
| RV, % predicted | −0.3 ± 28.7 | −57.4 ± 51.3e | 3.6 ± 29.7 |
| SGRQ total score | −3.0 ± 10.1 | −12.8 ± 14.4e | 1.5 ± 10.5 |
| SOBQ score | −3.4 ± 13.8 | −21.1 ± 21.9e | 3.6 ± 17.7 |
Data are presented as mean ± SD, unless otherwise indicated. LVRS = lung volume reduction surgery; MVV = minute ventilation volume; OMT = optimal medical therapy; REHAB = rehabilitation. See Table 1 legend for expansion of other abbreviations.
Δrehab = postrehabilitation − baseline.
Δlvrs = 6 mo after randomization to LVRS − postrehabilitation.
Δomt = 6 mo after randomization to OMT − postrehabilitation.
P < .0001 by two-tailed t test when comparing Δrehab and Δlvrs.
P < .05 by two-tailed t test when comparing Δrehab and Δlvrs.
The correlations between change in walk distance and other parameters with LVRS are summarized in Table 3. These correlations were moderately, but consistently stronger for the second-walk, average-of-two, and best-of-two Δ6MWT vs the first-walk Δ6MWT, except for quality-of-life scores where they were comparable (Table 3). The best-of-two and average-of-two Δ6MWTlvrs correlated significantly better than the first-walk Δ6MWTlvrs with ΔCPETlvrs (P = .04 for best of two and P = .01 for average of two) (Table 3). The average-of-two Δ6MWTlvrs also correlated significantly better with ΔFEV1lvrs than the first-walk Δ6MWTlvrs (P = .03).
Table 3.
—Correlation Between Change in Walk Distance and Change in Exercise Capacity, Pulmonary Function Tests, and Quality-of-Life Scores From Randomization to 6 Months After LVRS (n = 74)
| Variable | ΔFirst Walk | ΔSecond Walk (P Value vs ΔFirst Walk) | ΔAverage-of-Two Walks (P Value vs ΔFirst Walk) | ΔBest-of-Two Walks (P Value vs ΔFirst Walk) |
| ΔCPET, W | 0.58 | 0.69 (.06) | 0.66 (.01)a | 0.67 (.04)a |
| ΔFEV1, % predicted | 0.45 | 0.52 (.30) | 0.50 (.03)a | 0.50 (.33) |
| ΔFVC, % predicted | 0.49 | 0.55 (.23) | 0.54 (.10) | 0.54 (.15) |
| ΔSGRQ scoreb | −0.43 | −0.35 (.27) | −0.42 (.82) | −0.38 (.35) |
| ΔSOBQ score | −0.59 | −0.51 (.22) | −0.58 (.80) | −0.57 (.68) |
The P values compare the strength of the correlation of the change in first walk distance to that of the change in walk distance mentioned in the respective column. CPET = cardiopulmonary exercise test (using cycle ergometry). See Table 1 and 2 legends for expansion of other abbreviations.
Significant at P < .05.
SGRQ is expressed as the total score of the symptoms, activity, and impact domains.
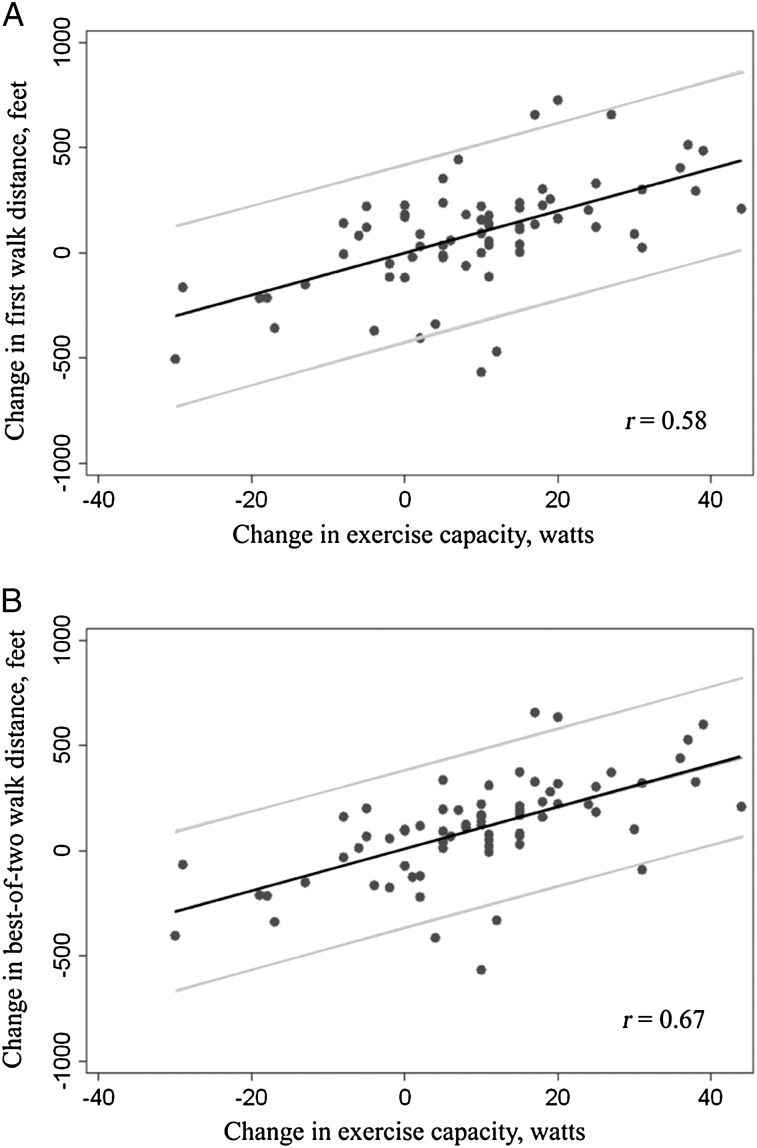
The correlations are visually depicted in Figure 2. The scatter was closer to the regression line, and the confidence bands were narrower for predicted changes when using the best-of-two Δ6MWTlvrs compared with first-walk Δ6MWTlvrs. None of the changes in walk distances correlated with changes in exercise capacity, pulmonary function tests, or quality-of-life scores during rehabilitation or in the medical therapy arm of NETT (all r < 0.2).
Figure 2.
A, B, Scatterplots for change in walk distance vs change in exercise capacity measured on cardiopulmonary exercise testing in patients undergoing lung volume reduction surgery (n = 74). Each plot has a fitted regression line with 95% confidence bands. The scatter was closer to the line, the 95% confidence bands were narrower, and the correlation (r) was stronger for best-of-two walk distances compared with change in the first walk distance. The increase in the correlation was significant for the best-of-two-walk distances vs that for the first walk distance (r = 0.58 vs r = 0.67, respectively, P = .04).
Precision of Δ6MWT
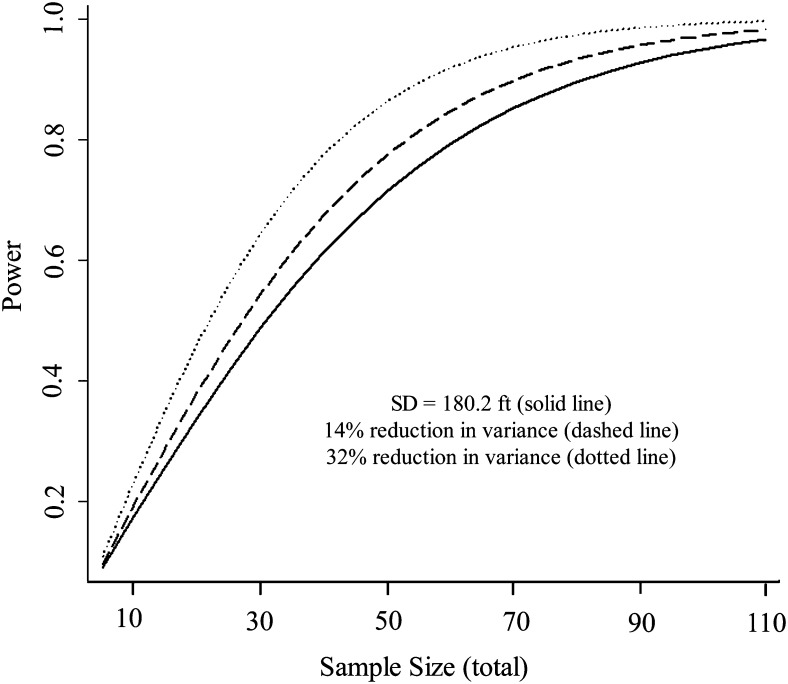
The mean and SDs for change in first, second, average-of-two and best-of-two walk distances after each intervention in NETT are summarized in Table 2. The SD change in the second, average-of-two, and best-of-two walk distances were consistently lower compared with change in first walk distance. This reduction was the largest for the best-of-two walk distance, the variance of which was lower by 14% after rehabilitation, 14% after LVRS, and 33% after OMT compared with the variance of the first walk distance. Hence, replacing the first walk distance with the best-of-two walk distance would reduce the required sample size by 14% to 33% for clinical trials that use change in walk distance as the end point (Fig 3) because the sample size required for a clinical trial is directly proportional to the variance of the end point. This reduction in sample size would apply to trials that compare before and after 6MWT results in a single cohort of patients or that compare Δ6MWT in patients randomized one-to-one to two arms (ie, a parallel-arm randomized clinical trial). Sample size requirements would also be reduced for trials that use more-complex designs and can be calculated using the relevant formulas.
Figure 3.
Power curves for a parallel-arm clinical trial designed to identify an improvement in walk distance of 131.2 ft (40 m) with a two-tailed α of .05. The solid curve uses an SD of 180.2 ft that was observed using the first walk distance during rehabilitation in the National Emphysema Treatment Trial. The dashed and dotted curves represent the left shift in the power curve due to a 14% and 33% reduction in variance, respectively, with a resulting reduction in sample size if patients in the trial performed two instead of one walk for the 6-min walk test and either change in average-of-two or in best-of-two walk distance was used as the final test result.
Discussion
The 6MWT is increasingly being used as a measure of exercise capacity to test the efficacy of therapeutic interventions for COPD and other chronic diseases.3‐9,11,12,16‐20,29 Although previous studies and standardized guidelines recommend multiple efforts for other common effort-dependent tests, such as spirometry, the merits of performing multiple walk distances have not been investigated. As a result, there continues to be inconsistency in the use of one vs two walks per 6MWT in clinical trials and in practice and lack of clarity regarding the optimal method of interpreting results from two walk distances when they are obtained.33,34 Using data from the NETT, we report for the first time to our knowledge that performing two instead of one walk per 6MWT and using the best-of-two or average-of-two walk distances as the test result significantly improve the precision and validity of the test.
Prior studies, including that by Hernandes et al,35 reported that when patients repeat a 6MWT on consecutive days, the distance increases by an average of 27 to 35 m.24 However, an increase in walk distance by itself does not constitute enough evidence that a second walk should be performed. Such evidence should come from a relative comparison of the different walk distances in terms of their validity and precision. Dolmage et al36 recently argued that if a person’s ability to perform the 6-min walk is artificially made stable by assuming that the walk distance does not improve on a second walk or that the person does not fatigue on repeat walks, then the average may be better than the best of three to seven consecutive walk distances. However, because some patients clearly demonstrate an improvement in the second walk distance and others often develop fatigue on repetitive walks, and because they used a simulated data set, the results of Dolmage et al36 are difficult to apply in actual practice and to compare with our findings.24,37
The present study clearly shows that using the average-of-two or best-of-two walk distance resulted in a lower SD from the mean walk distance compared with a single walk distance, thus increasing precision. Spencer et al38 also reported lower SDs for change in best-of-two (160.7 ft) vs first (183.7 ft) walk distances in patients with COPD after 8 weeks of pulmonary rehabilitation. However, studying the properties of the various walk distances was not the aim of their study, and their patients had milder disease (FEV1, 56% predicted). Interestingly, these SDs (160.7 vs 183.7 ft) are consistent with the present results (Table 2), and per our calculation, they correspond to a 23.4% reduction in variance, thus confirming our findings related to precision in an unrelated cohort of patients.
The present study also shows that change in the best-of-two walk distance correlated better with ΔCPET and that change in the average-of-two walk distance correlated better with ΔCPET and ΔFEV1 than change in the first walk distance, thus improving validity. Despite these differences, there was no improvement in correlation with quality-of-life scores. We believe that unlike pulmonary function tests or CPET, these quality-of-life instruments are less representative of small changes in exercise capacity.
The present findings are relevant for future clinical trials that use the 6MWT as the end point. The minimal clinically important distance is similar between one walk and the best of two walks39,40; therefore, a lower SD obtained by replacing a single walk by the best of two walks will reduce sample size requirements by up to one-third. This will lead to a significant reduction in enrollment time, study duration, and cost. Additionally, trial results will be more valid because changes in the best-of-two walk distance better correlate with CPET. On the other hand, some investigators may still choose to compromise on the cost and validity of their trial when the trial protocol is already burdensome or in settings where an additional walk is simply not feasible. Therefore, instead of providing a universal recommendation that only the best-of-two walk distance be used in clinical trials, we recommend that investigators use the present data to objectively weigh the pros and cons of adding a walk to their 6MWTs when designing future individual clinical trials.
The present results are also applicable for the clinical use of the 6MWT, where validity, unlike precision, is most relevant. Using the change in best-of-two or average-of-two walk distances will help the clinician to better assess response to COPD treatments over time. However, performing a second walk increases cost and time, and some patients may not be willing or able to return the next day. Therefore, clinicians should determine whether an additional walk is feasible in their exercise laboratory and patient population.
Potential limitations of the present study must be considered when interpreting the results. First, we used CPET as the gold standard measure of exercise capacity to validate the 6MWT, which may not be ideal because these tests measure slightly different aspects of exercise capacity; whereas CPET measures maximal effort, the 6MWT measures submaximal exercise ability. Despite this difference, we believe that it is meaningful to use CPET as the gold standard for the 6MWT. CPET is a more robust predictor of mortality and functional status than the 6MWT, which mainly is a simpler replacement for the more advanced testing offered by CPET. Additionally, we believe that CPET represents the best among the alternative gold standard tests available for measuring exercise capacity.31,41 Second, we have described the 6MWT characteristics in a relatively homogeneous cohort with advanced emphysema and low comorbidity. Although it is possible that the test characteristics may vary in patients with milder emphysema or with other diseases, the improvement in characteristics of effort-dependent tests with multiple efforts appears universal.9,12,14,23,42 Further, Spencer et al38 studied patients with milder COPD, and we calculated the same reduction in variance using the data they published. Therefore, we expect the results to be generalizable to patients with COPD with varying disease severity who did the two walks on 2 consecutive days. Finally, because participants did an additional walk only in the early part of NETT, the sample size in the LVRS arm in the present analysis was relatively small. This likely diminished statistical power to detect significant improvements in the correlation between Δ6MWT and changes in spirometry results.
Future research on the 6MWT should include development of protocols that take advantage of improved test characteristics associated with the addition of a walk as demonstrated in the present cohort in order to minimize the increase in test burden associated with doing walks on consecutive days. One way of achieving this may be to use a short, perhaps 2-min practice walk followed by a brief rest prior to a formal 6-min walk on the same day.43 Further improvements in walk distance and possibly precision may be achieved with three or more walks9,14,44; however, this would probably make the test too burdensome for most applications.
Conclusion
Adding a walk to the standard single-walk 6MWT and using the average-of-two or best-of-two walk distance as the test result improve the precision and validity of the test. If the best-of-two walk distance were to replace the first walk distance as the end point in clinical trials, the sample size requirements would reduce by 14% to 33%, and the trial would become more valid. Investigators performing clinical trials that use 6MWT as the end point should strongly consider the use of an additional walk for the 6MWT. Clinicians should use the present findings to objectively assess whether their patients should perform an additional walk during the 6MWT. Future research on the 6MWT should be aimed at developing test protocols that include an additional walk while minimizing the extra time and effort.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Drs Chandra and Sciurba had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Chandra: contributed to the study conceptualization, data analysis, and manuscript preparation.
Dr Wise: contributed to the study conceptualization, design, and manuscript preparation.
Dr Kulkarni: contributed to the data analysis, data interpretation, and manuscript preparation.
Dr Benzo: contributed to the data collection, data interpretation, and manuscript revision.
Dr Criner: contributed to study conceptualization, data collection, manuscript revision for intellectual content, and final approval of the version to be published.
Dr Make: contributed to study conceptualization, data collection, manuscript revision for intellectual content, and final approval of the version to be published.
Mr Slivka: contributed to study conceptualization, data interpretation, and manuscript preparation.
Dr Ries: contributed to the study design, manuscript preparation, and final approval of the version to be published.
Dr Reilly: contributed to study conceptualization, data collection, data interpretation, manuscript revision for intellectual content, and final approval of the version to be published.
Dr Martinez: contributed to study conceptualization, data collection, manuscript revision for intellectual content, and final approval of the version to be published.
Dr Sciurba: contributed to the study conceptualization and design, data collection, data interpretation, manuscript preparation, and final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Make has participated in advisory boards, speaker bureaus, consultations, and multicenter clinical trials with funding from the National Heart, Lung, and Blood Institute; Abbott Laboratories; Astellas Pharma US, Inc; AstraZeneca; Boehringer Ingelheim GmbH; Dey Pharma LP; Forest Laboratories, Inc; GlaxoSmithKline plc; Merck & Co, Inc; MedImmune, LLC; Nabi Biopharmaceuticals; Novartis Pharmaceuticals Corporation; Takeda Pharmaceuticals International GmbH; Pfizer, Inc; Philips Respironics; Sunovion Pharmaceuticals Inc; Sequel Pharmaceuticals, Inc; and Talecris Biotherapeutics. Dr Martinez has participated in advisory boards in COPD development for Actelion Pharmaceuticals Ltd; AstraZeneca; Bayer AG; BoomComm; Fb Communications; Forest Laboratories, Inc; Almirall, SA; GlaxoSmithKline plc; Ikaria, Inc; MedImmune LLC; Merck & Co, Inc; Novartis Pharmaceuticals Corporation; Takeda Pharmaceuticals International GmbH; Pearl Therapeutics Inc; Pfizer, Inc; Hoffmann-La Roche Inc; Bayer Schering Pharma AG; and Talecris Biotherapeutics. He has been a member of steering committees for COPD studies sponsored by Actelion Pharmaceuticals, Inc; GlaxoSmithKline plc; Forest Laboratories, Inc; Mpex Pharmaceuticals, Inc; and Takeda Pharmaceuticals International GmbH. He has participated in Food and Drug Administration mock panels for Boehringer Ingelheim GmbH and Forest Laboratories, Inc. The University of Michigan received funds from Boehringer Ingelheim GmbH for a COPD study. Dr Martinez has served on speaker’s bureaus or in continuing medical education activities sponsored by the American Lung Association; Almirall, SA; Altana; AstraZeneca; Boehringer Ingelheim GmbH; CME Incite; ePocrates, Inc; Forest Laboratories, Inc; The France Foundation; GlaxoSmithKline plc; Med Ed, Inc; NACE International; Pfizer, Inc; Potomac Center for Medical Education; Prescott Pharmaceuticals; Sanofi-Aventis US LLC; Vox Medica, Inc; WebMD, LLC; and UpToDate, Inc. He has received royalties from Associates in Medical Marketing and Castle Connolly Medical Ltd. Dr Sciurba has received research funds from the National Institutes of Health; GlaxoSmithKline plc; Pfizer, Inc; Boehringer Ingelheim GmbH; and Forest Laboratories, Inc. He has received consulting funds from GlaxoSmithKline plc; AstraZeneca plc; and PnuemRX. Drs Chandra, Wise, Kulkarni, Benzo, Criner, Ries, and Reilly and Mr Slivka have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsor: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The NETT Credit Roster is provided in e-Appendix 1 (408.6KB, pdf) . We also acknowledge Arthur Gelb, MD, of Lakewood Regional Medical Center, Lakewood, California.
Additional information: The e-Appendix and e-Tables can be found in the “Supplemental Materials” section of the online article.
Abbreviations
- 6MWT
6-min walk test
- CPET
cardiopulmonary exercise testing
- LVRS
lung volume reduction surgery
- NETT
National Emphysema Treatment Trial
- OMT
optimal medical therapy
Footnotes
Funding/Support: The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute [N01HR76101 through N01HR76119, P50HL084948]; the Centers for Medicare & Medicaid Services; and the Agency for Healthcare Research and Quality. This article is subject to the National Institutes of Health public access policy (http://www.nhlbi.nih.gov/funding/policies/public-access.htm).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Salzman SH. The 6-min walk test: clinical and research role, technique, coding, and reimbursement. Chest. 2009;135(5):1345-1352 [DOI] [PubMed] [Google Scholar]
- 2.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117 [DOI] [PubMed] [Google Scholar]
- 3.National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073 [DOI] [PubMed] [Google Scholar]
- 4.Patel SA, Sciurba FC. Emerging concepts in outcome assessment for COPD clinical trials. Semin Respir Crit Care Med. 2005;26(2):253-262 [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75-81 [DOI] [PubMed] [Google Scholar]
- 7.Peurala SH, Tarkka IM, Pitkänen K, Sivenius J. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1557-1564 [DOI] [PubMed] [Google Scholar]
- 8.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34(9):2173-2180 [DOI] [PubMed] [Google Scholar]
- 9.Andersson C, Asztalos L, Mattsson E. Six-minute walk test in adults with cerebral palsy. A study of reliability. Clin Rehabil. 2006;20(6):488-495 [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984;39(11):818-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Drutz C, Kumar R, et al. Use of the six-minute walk test poststroke: is there a practice effect?. Arch Phys Med Rehabil. 2008;89(9):1686-1692 [DOI] [PubMed] [Google Scholar]
- 12.Pankoff BA, Overend TJ, Lucy SD, White KP. Reliability of the six-minute walk test in people with fibromyalgia. Arthritis Care Res. 2000;13(5):291-295 [DOI] [PubMed] [Google Scholar]
- 13.Solway S, Brooks D, Lau L, Goldstein R. The short-term effect of a rollator on functional exercise capacity among individuals with severe COPD. Chest. 2002;122(1):56-65 [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Sanderson B, Bittner V. The 6-minute walk test: how important is the learning effect?. Am Heart J. 2003;146(1):129-133 [DOI] [PubMed] [Google Scholar]
- 15.Troosters T, Gosselink R, Decramer M. Six-minute walk test: a valuable test, when properly standardized. Phys Ther. 2002;82(8):826-827 [PubMed] [Google Scholar]
- 16.Ulrich S, Speich R, Domenighetti G, et al. Bosentan therapy for chronic thromboembolic pulmonary hypertension. A national open label study assessing the effect of Bosentan on haemodynamics, exercise capacity, quality of life, safety and tolerability in patients with chronic thromboembolic pulmonary hypertension (BOCTEPH-Study). Swiss Med Wkly. 2007;137(41-42):573-580 [DOI] [PubMed] [Google Scholar]
- 17.Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JD, Malthaner RA, Goldsmith CH, et al. ; Canadian Lung Volume Reduction Surgery Study A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg. 2006;81(1):314-320 [DOI] [PubMed] [Google Scholar]
- 19.Gomberg-Maitland M, Tapson VF, Benza RL, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172(12):1586-1589 [DOI] [PubMed] [Google Scholar]
- 20.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91(6):749-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982;284(6329):1607-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGavin CR, Gupta SP, McHardy GJ. Twelve-minute walking test for assessing disability in chronic bronchitis. BMJ. 1976;1(6013):822-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins S, Cecins NM. Six-minute walk test in pulmonary rehabilitation: do all patients need a practice test?. Respirology. 2010;15(8):1192-1196 [DOI] [PubMed] [Google Scholar]
- 24.Sciurba F, Criner GJ, Lee SM, et al. ; National Emphysema Treatment Trial Research Group Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167(11):1522-1527 [DOI] [PubMed] [Google Scholar]
- 25.Larsson UE, Reynisdottir S. The six-minute walk test in outpatients with obesity: reproducibility and known group validity. Physiother Res Int. 2008;13(2):84-93 [DOI] [PubMed] [Google Scholar]
- 26.Eisen EA, Letz RA, Wegman DH, Baker EL, Jr, Pothier L. Defining measurement precision for effort dependent tests: the case of three neurobehavioural tests. Int J Epidemiol. 1988;17(4):920-926 [DOI] [PubMed] [Google Scholar]
- 27.The National Emphysema Treatment Trial Research Group Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest. 1999;116(6):1750-1761 [DOI] [PubMed] [Google Scholar]
- 28.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155(4):1278-1282 [DOI] [PubMed] [Google Scholar]
- 29.Rostagno C, Gensini GF. Six minute walk test: a simple and useful test to evaluate functional capacity in patients with heart failure. Intern Emerg Med. 2008;3(3):205-212 [DOI] [PubMed] [Google Scholar]
- 30.Starobin D, Kramer MR, Yarmolovsky A, et al. Assessment of functional capacity in patients with chronic obstructive pulmonary disease: correlation between cardiopulmonary exercise, 6 minute walk and 15 step exercise oximetry test. Isr Med Assoc J. 2006;8(7):460-463 [PubMed] [Google Scholar]
- 31.Cote CG, Pinto-Plata VM, Marin JM, Nekach H, Dordelly LJ, Celli BR. The modified BODE index: validation with mortality in COPD. Eur Respir J. 2008;32(5):1269-1274 [DOI] [PubMed] [Google Scholar]
- 32.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87(2):245-251 [Google Scholar]
- 33.Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci. 2011;53(2):81-85 [PubMed] [Google Scholar]
- 34.Theander K, Jakobsson P, Jörgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil. 2009;23(2):125-136 [DOI] [PubMed] [Google Scholar]
- 35.Hernandes NA, Wouters EF, Meijer K, Annegarn J, Pitta F, Spruit MA. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J. 2011;38(2):261-267 [DOI] [PubMed] [Google Scholar]
- 36.Dolmage TE, Hill K, Evans RA, Goldstein RS. Has my patient responded? Interpreting clinical measurements such as the 6-minute-walk test. Am J Respir Crit Care Med. 2011;184(6):642-646 [DOI] [PubMed] [Google Scholar]
- 37.Chandra D, Kulkarni HS, Sciurba FC. Learning from the learning effect in the six-minute-walk test [letter]. Am J Respir Crit Care Med. 2012;185(6):684. [DOI] [PubMed] [Google Scholar]
- 38.Spencer LM, Alison JA, McKeough ZJ. Six-minute walk test as an outcome measure: are two six-minute walk tests necessary immediately after pulmonary rehabilitation and at three-month follow-up?. Am J Phys Med Rehabil. 2008;87(3):224-228 [DOI] [PubMed] [Google Scholar]
- 39.Puhan MA, Chandra D, Mosenifar Z, et al. ; National Emphysema Treatment Trial (NETT) Research Group The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37(4):784-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225 [DOI] [PubMed] [Google Scholar]
- 41.Martinez FJ, Foster G, Curtis JL, et al. ; NETT Research Group Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-923 [PMC free article] [PubMed] [Google Scholar]
- 43.Eiser N, Willsher D, Doré CJ. Reliability, repeatability and sensitivity to change of externally and self-paced walking tests in COPD patients. Respir Med. 2003;97(4):407-414 [DOI] [PubMed] [Google Scholar]
- 44.Swinburn CR, Wakefield JM, Jones PW. Performance, ventilation, and oxygen consumption in three different types of exercise test in patients with chronic obstructive lung disease. Thorax. 1985;40(8):581-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement