Summary
Humans and animals use the classical five senses of sight, sound, touch, smell and taste to monitor their environment. The very survival of feral animals depends on these sensory perception systems, which is a central theme in scholarly research on comparative aspects of anatomy and physiology. But how do all of us sense and respond to an infection? We cannot see, hear, feel, smell or taste bacterial and viral pathogens, but humans and animals alike are fully aware of symptoms of sickness that are caused by these microbes. Pain, fatigue, altered sleep pattern, anorexia and fever are common symptoms in both sick animals and humans. Many of these physiological changes represent adaptive responses that are considered to promote animal survival, and this constellation of events results in sickness behavior. Infectious agents display a variety of pathogen-associated molecular patterns (PAMPs) that are recognized by pattern recognition receptors (PRRs). These PRR are expressed on both the surface [e.g. Toll-like receptor (TLR)-4] and in the cytoplasm [e.g. nucleotide-binding oligomerization domain (Nod)-like receptors] of cells of the innate immune system, primarily macrophages and dendritic cells. These cells initiate and propagate an inflammatory response by stimulating the synthesis and release of a variety of cytokines. Once an infection has occurred in the periphery, both cytokines and bacterial toxins deliver this information to the brain using both humoral and neuronal routes of communication. For example, binding of PRR can lead to activation of the afferent vagus nerve, which communicates neuronal signals via the lower brain stem (nucleus tractus solitarius) to higher brain centers such as the hypothalamus and amygdala. Blood-borne cytokines initiate a cytokine response from vascular endothelial cells that form the blood–brain barrier (BBB). Cytokines can also reach the brain directly by leakage through the BBB via circumventricular organs or by being synthesized within the brain, thus forming a mirror image of the cytokine milieu in the periphery. Although all cells within the brain are capable of initiating cytokine secretion, microglia have an early response to incoming neuronal and humoral stimuli. Inhibition of proinflammatory cytokines that are induced following bacterial infection blocks the appearance of sickness behaviors. Collectively, these data are consistent with the notion that the immune system communicates with the brain to regulate behavior in a way that is consistent with animal survival.
Key words: behavior, cytokine, immunology, sickness, depression, inflammation
Sickness and depression
Over the past 30 years, it has become clear that the immune system plays a critical role in animal behavior. This role is tightly linked to the obvious role that immune cell activation plays in the clearance of pathogenic organisms. Systemic or central infections elicit a group of symptoms that are necessary for the organism to conserve resources, reorganize priorities and limit the spread of the infection to other members of the community. This sickness behavior is a motivational state that is common to most pathogen-induced infections ranging from viruses to multicellular parasites, but, because of its ubiquitous nature, is frequently accepted as an unavoidable and non-specific consequence of infection. However, considering the broad spectrum of symptoms – fever, nausea, decreased appetite, malaise, fatigue and achiness – it seems clear that a highly organized, although not pathogen-specific, response is being manifested to aid in the fight against infection (Dantzer, 2001; Ericsson et al., 1995).
We are all familiar with the human symptoms of sickness but, to investigate changes in behavior associated with sickness, it is critical to have reliable animal measurements that relate to changes in the affective state. Using preclinical animal models, sickness behavior is best evaluated when the test involves a means to assess motivation. Sickness behavior is frequently assessed as social exploration/investigation (in rodent models, this response is frequently reported as a decrease in time actively seeking interaction with a novel animal as a result of diminished motivation for social exploration or neophobia). Social exploration is a naturalistic behavior and all animals have a strong motivation, whether driven by curiosity or sexual desire. With this strong motivational stimulus, infections cause a strong differential between time of exploration of healthy and sick animals, with less time of exploration being observed with sick animals. Another simpler but effective model is exploration of the environment [often referred to as locomotor activity (LMA), representing some level of physical movement in a novel environment such as rearing, quadrant entry, line crossings or total distance traveled; all of which are highly correlated]. LMA is best conducted in a novel environment, as the motivation to explore is stronger in this situation in healthy animals compared with sick animals. Often, LMA is assessed using animals in their home cage. Although decreased home cage activity is associated with sickness, its motivational component is less than that when exploration is assessed in a novel environment. Similarly, exploration of a novel object is a powerful test (this test has a motivational component intermediate between social exploration and LMA, especially when the object is presented in a novel environment). Consumption, of a sweetened liquid or food, is also a motivated behavior that provides a simple test, with adequate differences noted between healthy and sick animals, consumption being less for sick animals. Physiologically, the sickness response is often quantified using measures such as elevated body temperature, decreased unsweetened food consumption and loss of body mass of sick animals compared with controls. The loss of body mass is generally accredited to the decreased food and fluid intake. Behavior is defined as observable activities of humans or animals. Thus, physiological measures, such as fever or loss of body mass, are not behaviors per se although they are symptoms of sickness. Amazingly, considering the diverse symptoms attributed to sickness, all of these symptoms are commonly expressed by sick animals despite the broad spectrum of possible pathogens that may cause the sickness.
Another behavioral syndrome that has an inflammatory component is depression (Raedler, 2011; Raison and Miller, 2011). Symptoms of depression appear after pro-inflammatory cytokines are produced by the body or administered exogenously. The temporal progression of prior inflammation to later depression suggests a cause–effect relationship and indicates that immune activation can precipitate depression. Several symptoms of inflammation-induced depression overlap with sickness behaviors, including fatigue, changes in sleep pattern, lack of interest in daily or pleasurable activities (anhedonia), changes in appetite or body mass and unexplained aches and pains. These symptoms are readily assessable, but using animal models it is difficult to relate these symptoms with either sickness or depression. Other human symptoms of depression are directly associated with mood; suicidal thoughts/attempts, feelings of helplessness or despair, anhedonia, feelings of worthlessness or guilt, self-loathing, recklessness, changes in mood, irritability and short-temper. Mood assessment of patients is done by questionnaire and thus impossible to quantify with animal models. Tests that attempt to determine the state of despair or changes in mood of animals are therefore referred to as measuring ‘depressive-like’ behaviors. Helplessness and behavioral despair are frequently assessed using the forced swim and tail suspension tests (FST and TST, respectively). Mice or rats are placed in an inescapable situation (placement in a bucket or water with the rims of the bucket out of reach or suspension by the tail), and depressive-like behavior is evident as an increase in time of immobility; i.e. less ‘desire’ to escape an index of despair or helplessness. Immobility in the FST and TST is decreased by antidepressant treatment, and these tests were originally developed to screen drugs for antidepressant activity. Anhedonia is modeled by several tests; most commonly as preference to consume a sweetened ‘pleasurable’ solution over water; i.e. the sucrose or saccharine preference tests. Depressive-like behavior is often presented as a decrease in consumption of the sweetened solution or diet without a preference component. However, these measurements are susceptible to changes in thirst or hunger (more sickness related) in addition to hedonic behavior. As mentioned above, sickness is associated with decreased hunger and thirst even for sweetened foods or liquids. However, the choice to consume a pleasurable solution or sweetened food over water or normal diet provides a stronger discriminatory assessment of anhedonia and thus depressive-like behavior. It is not the goal of this review to discuss the strengths and weaknesses of behavioral assessment paradigms, but when interpreting experiments the test employed is just as critical as the treatment combinations. Importantly, all of the preclinical animal tests mentioned have been shown to respond to immune challenges.
Immune activation, body and brain
The body manages to respond to infectious agents, such as bacteria, yeast and viruses, with a common set of symptoms despite a lack of similarities between these types of pathogens. It does this by focusing the response through sentinel cells located throughout the body (Fig. 1). These first responders form the base of the innate immune system. Monocytes are considered critical first responders and monitor the circulating fluids whereas differentiated monocyte-derived cells monitor other fluids and are resident in all tissues (examples: peritoneal macrophages → peritoneal cavity; Kupffer cells → liver; giant cells and histiocytes → connective tissue; dust cells and alveolar macrophages → lungs; and osteoclasts → bone) (Douglas and Musson, 1986). These monocytic cells, along with resident dendritic cells, respond to a variety of signals including infectious agents and a variety of factors produced by the host organism that are released following trauma, autoimmune responses or abnormal accumulation of endogenous molecules (Magrone and Jirillo, 2012). In any case, the cells of the innate immune system then respond with the initiation of an inflammatory response that leads to a mirrored immune response within the central nervous system (CNS), often referred to as neuroinflammation. A bout of neuroinflammation results in behavioral consequences. Altered behavior is dependent on changes in neuronal activity, although specific loci within the CNS that mediate each of these responses have not been clearly defined. If the inflammatory response is fully resolved and does not involve death of cells within the brain, then behavior returns to normal. If neuroinflammation is extremely strong or prolonged, cell death within the CNS results in irreversible loss of function: functio laesa, identified as the fifth sign of acute inflammation.
Fig. 1.
Focusing the innate immune response. Insults to the body, from the outside or from the inside, activate cells of the innate immune system. The immune response transmits this information to the brain to cause physiological and behavioral responses. A mild inflammatory response – such as a low-grade infection, trauma (such as dropping a weight on one’s foot) or even strenuous exercise – results in reversible consequences as they are a result of altered cellular (neuron) function. A severe response induces often irreversible consequences as a result of cell death. In either case, the causal event is initiated by monocytic and dendritic cells with the initiation of an inflammatory response.
Recognition of infection is a first and most critical step in the development of an appropriate physiological response to fight infection and to initiate appropriate changes in behavior. Recognition of pathogens by monocytes and dendritic cells is mediated by several classes of receptors collectively referred to as pattern-recognition receptors (PRRs). Unlike receptors for cytokines, growth factors or hormones, which each recognize a specific moiety present only on a small subset of highly conserved ligands, PRRs recognize classes of molecules termed pathogen-associated molecular patterns (PAMPs). These patterns are not normally present on endogenous extracellular molecules derived from the host, although DAMPs (damage-associated molecular patterns) are found on molecules released from dying host cells that can activate PRRs (Jeannin et al., 2008). Thus, PAMPs are recognized by PRRs as non-self-molecules and DAMPs as self-molecules, both of which elicit activation of the innate immune system; one in an attempt to remove infectious materials and the other to remove damaged tissue.
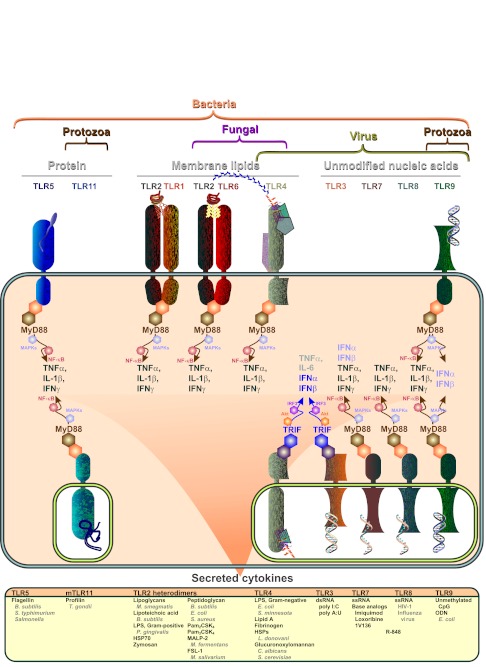
Fig. 2 illustrates the best-characterized members of the Toll-like receptors (TLRs), the most widely studied PRRs. In order to assure recognition of pathogens, TLRs have evolved to recognize proteins, lipids and unmodified nucleic acid molecules found on infectious pathogens. Extracellular pathogens, for example many bacteria, are recognized by trans-membrane TLRs that have their PAMP recognition moieties on the outside of the plasma membrane. TLRs 5, 11, 2/1 heterodimers, 2/6 heterodimers, 4 and 9 fall under this category. Intracellular pathogens, for example viruses or bacterial components released from extracellular pathogens that enter cells, are recognized by TLRs localized within the responsive cell. These TLRs are localized to endosomes and lysosomes within the cells. PAMP association with TLRs induces intracellular signaling cascades through two major pathways. Most of the TLRs associate with myeloid differentiation primary response gene 88 (MyD88), which is a universal adapter protein designed to recruit intracellular enzymes that initiate a cascade to eventually activate NF-κB (Fig. 2). Translocation of NF-κB to the cell nucleus directly activates gene transcription of, among other things, pro-inflammatory cytokines such as TNFα, IL-1β and type II interferon (IFNγ). Of the well-characterized TLRs, only TLR3, which responds to dsRNA, strictly associates with TRIF to activate IRF3 and directly induce the expression of type I interferons. TLR4 activates both pathways, and TLR9 induces type I interferon (IFNα) expression through NF-κB. Although there is considerable overlap and varying crosstalk across the MyD88 and TRIF pathways, the MyD88 response is more strongly keyed to fight bacterial infections whereas the induction of type I IFN plays a key role in fighting viral infections. Expression of cytokines by monocytic and dendritic cells then recruits and activates other cells of the immune system to fight infections.
Fig. 2.
Classification of Toll-like receptors (TLRs). All TLRs recognize bacteria pathogen-associated molecular patterns (PAMPs) of protein, lipid or nucleotide composition. Approximately half recognize viral PAMPs, either lipids or nucleotides. TLR2/6 and TLR4 recognize fungal PAMPs whereas TLR9 and TLR11 recognize protozoan PAMPs. Several of the TLRs respond to extracellular ligands (1, 2, 4, 5, 6, 9 and 10 not shown) whereas others localize to cellular vesicles and respond to PAMPs that have been internalized by the cell (3, 7, 8, 9 and murine 11; human TLR11 is a pseudogene). Although some of the TLRs also activate proliferation of immune cells through an Akt-dependent pathway (not shown), they all induce the expression and secretion of cytokines. Cytokine production is largely responsible for behavioral changes induced by infection. All TLRs shown (TLR10 cooperates with TLR2 to recognize triacylated lipoproteins but does not activate typical TLR signaling) (Guan et al., 2010), except TLR3, directly induce the expression of TNFα, IL-1β and IFNγ whereas TLR3, 4, 7 and 9 activation results primarily in IFNα and IFNβ expression (Hanke and Kielian, 2011). A brief list of PAMPs or active analogs is shown for each TLR. For definitions, see List of abbreviations.
Similar to TLRs, nucleotide-binding oligomerization domain (Nod) proteins initiate an inflammatory response following activation by peptidoglycans derived from bacteria (Fig. 3). Activation of Nod1 or Nod2 increases association of Nod proteins with RIPK or RICK. This association leads to eventual NF-κB activation and, like TLR activation, cytokine and type II interferon expression.
Fig. 3.
Classification of nucleotide-binding oligomerization domain proteins (Nods). Similar to TLRs, Nod1 and Nod2 are pattern recognition receptors (PRRs) responding to pathogen-associated molecular patterns (PAMPs) of bacterial origin (Newton and Dixit, 2012). Both Nods are localized to the cytoplasm, requiring either phagocytosis of bacteria and subsequent peptidoglycan entry into the cytoplasm or uptake of peptidoglycan by endocytosis, peptide transporters or pore-forming toxins. Nod1 is distributed across tissues and cell types whereas Nod2 is localized principally to leucocytes but can be induced in epithelium (Clarke and Weiser, 2011; Newton and Dixit, 2012). The primary difference between TLRs and Nods (and Nod-like receptors, NLRs) is the identity of the ligand and intracellular pathway. RICK or RIPK/RIP-2 initiate the eventual activation of NF-κB, as compared to MyD88 or TRIF. Similar to TLRs, Nods induce the expression and secretion of cytokines. For definitions, see List of abbreviations.
Pathways that mediate inflammation-induced behavior
After recognition of the infectious agent, a signal must be received by the brain for behavioral changes to ensue. There are two major routes by which infections influence behavior. The neural and humoral routes both provide input to the brain (Fig. 4). When activated, both pathways elicit behavioral responses and, as described below, the importance of each pathway is dependent on the site of infection. The existence of a neural component is supported by early observations that sensory processing is necessary for development of heat and for the sensation of pain at the site of infection (these are two classic inflammation signs: calor and dolor). With the discovery of the blood–brain barrier (BBB), which was originally believed to exclude proteinaceous signals from the brain, afferent input was thought to be the major signaling pathway from the periphery to the brain that was responsible for behavioral changes.
Fig. 4.
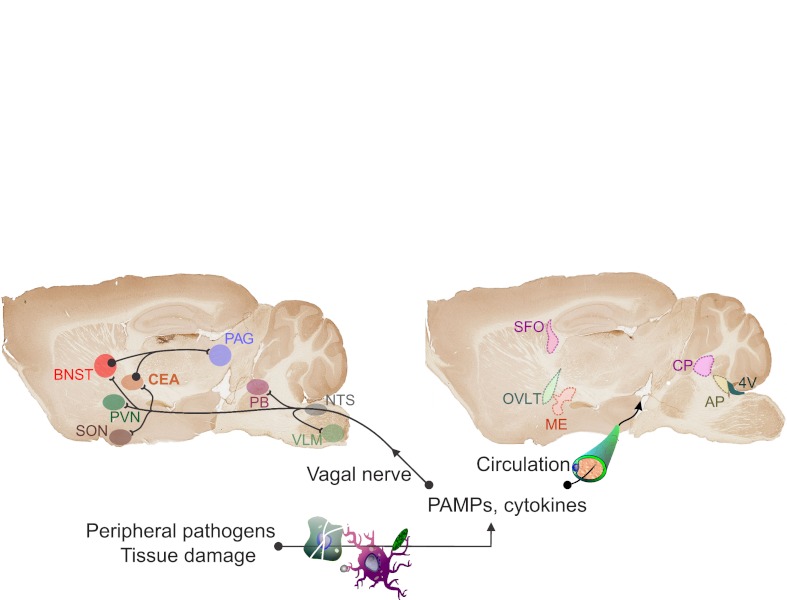
Neural and humoral activation of the brain by the periphery. Peripheral infections alter behavior by communicating with the brain via neural and humoral pathways. The neural pathway occurs via afferent nerves. As an example, the vagal nerve has a proven role in mediating infection-induced behavior. The afferent vagus projects to the nucleus tractus solitaries (NTS) → parabrachial nucleus (PB) → ventrolateral medulla (VLM) before proceeding to the paraventricular nucleus of the hypothalamus (PVN), supraoptic nucleus of the hypothalamus (SON), central amygdala (CEA) and bed nucleus of the stria terminalis (BNST). The CEA and BNST, which are part of the extended amygdala, then project to the periaqueductal gray (PAG). By these pathways, activation of the vagus by abdominal or visceral infections influences activity of several brain regions implicated in motivation and mood. The humoral pathway involves delivery of PAMPs or cytokines from the peripheral site of infection directly to the brain. Active transport into the brain across the blood–brain barrier (BBB), volume diffusion into the brain or direct contact with brain parenchymal cells at the choroid plexus (CP) and circumventricular organs [median eminence (ME), organum vasculosum of the laminae terminalis (OVLT, i.e. supraoptic crest), area postrema (AP) and suprafornical organ (SFO)] that lie outside the BBB all transpose the peripheral signal into a central neuroinflammatory response that mirrors the response at the periphery (Dantzer et al., 2008).
Indeed, early studies found that lipopolysaccharide (LPS) given intraperitoneally (i.p.) caused a rapid increase in c-fos immunoreactivity within the nucleus tractus solitaries (NTS) (Wan et al., 1993). This marker of neuron activation localized to primary and secondary areas of projection of the vagus (Fig. 4). Similarly, the trigeminal nerve activates neurons within the hypothalamus known to control feeding behavior (Malick et al., 2001). Subdiaphragmatic vagotomy drastically reduces the sickness response to i.p. LPS, clearly illustrating that neural input to the brain is directly responsible for a significant part of the early behavioral changes associated with some infections (Bluthé et al., 1996a; Bluthé et al., 1996b; Bretdibat et al., 1995; Watkins et al., 1994). In contrast to these findings, vagotomy does not block the pyrogenic action of LPS when LPS is administered i.p. (Hansen et al., 2000; Luheshi et al., 2000). Vagotomy also does not block the induction of sickness behavior by i.v. (intravenous) or s.c. (subcutaneous) LPS (Bluthé et al., 1996a; Bluthé et al., 1996b). These later findings suggest that additional, humoral pathways are also able to mediate the ability of infections to modulate behavior. Even after vagotomy, i.p. LPS increases IL-1β levels within the brain (Van Dam et al., 2000) and vagotomy does not attenuate the ability of LPS to increase circulating cytokine levels (Gaykema et al., 2000; Hansen et al., 2000). When it was found that circulating cytokines could enter the brain by active transport, that cytokines could be produced at the BBB in response to circulating PAMPs and that cytokines could enter the brain by volume diffusion at the circumventricular organs (Fig. 4) (Quan and Banks, 2007), it was clear that behavioral responses that occur in response to i.v. and s.c. PAMPs or cytokines are transcribed by the brain in response to humoral signals. Similarly, some of the behaviors that occur in response to i.p. challenges have a humoral component even after vagotomy (Gaykema et al., 2000; Hansen et al., 2000). It is clear, however, that all behavioral responses to infection have a cytokine basis, as even i.p. LPS induces a CNS inflammatory response corresponding to the sites of c-fos activation by the vagal nerve afferent projections (Konsman et al., 2008). Thus, intraperitoneal or meningeal infections induce behavioral changes that are partially mediated by neural afferents through the vagal and trigeminal nerves, respectively. These afferent nerves induce an inflammatory response and cytokine expression in the brain, thereby providing the cytokine component of the neural pathway. In contrast, other peripheral sites of infection have a stronger dependence on the humoral pathway with the induction of local cytokines or release of PAMPs, which enter the circulation and then act directly at the level of the CNS. The level of infection is roughly proportionate to the level of CNS cytokine production and is related to the behavioral changes. One of the early issues that arose from these associations was the identity of the cytokines responsible for behavioral responses.
IL-1β and behavior
IL-1β delivered to cells within the CNS from the periphery via the humoral pathway (Anisman et al., 2008), expressed within the brain dependent on neural input (Layé et al., 1994; Marquette et al., 2003) or expressed by the CNS in response to humoral stimuli (Churchill et al., 2006) or exogenously added to the brain (Bluthé et al., 2006) induces sickness behavior. The behavioral effects of IL-1β are mediated through the interleukin 1 receptor (IL-1R1) (Fig. 5). Following receptor binding, IL-1R1 activates an intracellular signaling cascade dependent on MyD88 binding, similar to most of the TLRs. With the identification of IL-1R1 on several cell types within the CNS (Fig. 6) (including neurons, microglia, astrocytes and endothelial cells) (Ericsson et al., 1995; Katsuura et al., 1988), the identity of the cell type responsible for mediating behavioral changes has not been conclusively made. However, knock-out (KO) of IL-1R1, which removes the receptor from all cell types, has conclusively shown that this receptor, and not IL-1R2, mediates IL-1-dependent behaviors (Bluthé et al., 2000a). IL-1R1 KO mice do not respond with the typical sickness-associated decrease in social interaction, loss of body mass or depressed food intake. However, these mice do respond to i.p. LPS or intracerebroventricular (i.c.v.) TNFα with normal sickness behavior. These data suggest that, in the absence of IL-1β, TNFα is able to compensate and play the lead role in the induction of sickness behavior. In fact, in this same study, LPS-induced sickness of IL-1R1 KO mice was blocked by a TNFα antibody (Bluthé et al., 2000a). Conversely, i.c.v. TNFα-induced sickness is markedly diminished by soluble IL-1 receptor antagonist (IL-1ra), which is a soluble form of IL-1R1, suggesting that TNFα sickness-inducing activity is at least partially mediated by the central induction of IL-1β (Bluthé et al., 1994). These data suggest that sickness behavior can be mediated by IL-1β or TNFα, but that at least one of these cytokines must be present.
Fig. 5.
Cytokine intracellular signaling pathways. Cytokines bind to transmembrane allosterically regulated proteins. Upon ligand binding, the intracellular signaling pathways that are activated correlate to their ability to alter behavior. Three classic proinflammatory cytokines – TNFα, IL-1β and IL-6 – activate cascades leading to NF-κB and MAPK (p38 and JNK) activation. The MAPK cascade is enhanced by parallel signaling pathways that produce ceramide. In contrast, IFNγ, IFNα/β and IL-6 signal primarily through the JAK/STAT pathway. The NF-κB, MAPK and JAK/STAT pathways are considered proinflammatory, inducing a feed-forward cytokine inflammatory response. The ceramide-generating and MAPK pathways have distinct enhancing and inhibitory effects on neuron excitation.
Fig. 6.
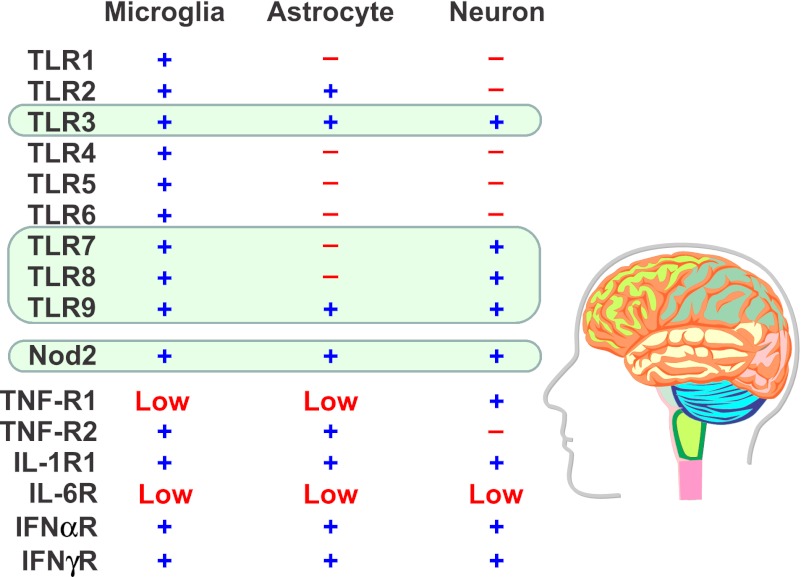
Expression of pattern recognition receptors (PRRs) and proinflammatory cytokine receptors in the brain. Although most infections occur at the periphery, the cells of the central nervous system (CNS) are the ultimate mediators of changes in behavior. Receptors within the CNS for pathogen-associated molecular patterns (PAMPs) and proinflammatory cytokines are divided into two categories, intracellular (green boxes) and those that span the plasma membrane. PAMPs reaching the CNS parenchyma can directly activate microglia, which, like other monocyte-derived cells, possess a full complement of TLRs. Thus, microglia are able to respond to PAMPs or peripherally derived cytokines with a central induction of proinflammatory cytokine expression. Astrocytes and neurons have a very limited ability to respond to PAMPs. Neurons only possess intracellular TLRs and Nod2. In contrast, neurons have cell surface receptors for proinflammatory cytokines, TNFα (Bette et al., 2003), IL-1β (French et al., 1999), low expression of IL-6 (Lehtimäki et al., 2003), type I IFNα/β (Paul et al., 2007) and limited (region-specific) expression of the type II IFNγ receptor (Chesler and Reiss, 2002). The absence of most of the bacterial recognition TLRs on neurons indicates that the effects of an extracellular bacterial infection on behavior are secondary to activation of other cells of the CNS, primarily microglia. In contrast, neurons are directly responsive to cytokines.
By far the most abundant literature related to IL-1β regards its action as a pro-inflammatory cytokine, i.e. induction of local inflammation, immune cell recruitment and necessity to rapidly clear infections. The IL-1β → IL-1R1 → NF-κB pathway is predominant in monocytes, including brain microglia (Srinivasan et al., 2004), and this pathway leads to elevated cytokine expression, further monocyte/microglia activation and astrocyte activation within the CNS. By themselves, these actions have no direct means to alter behavior as neuron function per se is not altered. In contrast, IL-1β interaction with IL-1R1 on neurons has a greater induction of the MAPK pathways and MyD88-dependent Src activation (Davis et al., 2006; Srinivasan et al., 2004) than it does with non-neuronal cells. Within the hippocampus, IL-1β acts through the MAPKs, p38 and JNK (Fig. 5) to inhibit neuron long-term potentiation (LTP) via an inhibition of calcium channels (Schäfers and Sorkin, 2008; Viviani et al., 2007). In contrast, IL-1β may also have a direct excitatory effect on neurons mediated by an increase in ceramide (a family of lipids that act as intracellular signaling molecules) synthesis and subsequent NMDA-mediated calcium influx (Viviani et al., 2003). Thus, the presence of IL-1β within the CNS directly alters neuron function. Despite these responses by neurons, it remains unknown if either inhibition of LTP, via MAPKs, or neuron excitation, via ceramide, is responsible for IL-1β’s ability to act within the CNS to induce sickness behavior. However, it is clear that IL-1β administered at very low levels induces a potent sickness response (Bluthé et al., 2006). Of note, to date there are no reports that IL-1β is necessary for the development of depressive-like behaviors.
TNFα and behavior
TNFα within the brain can derive from peripheral expression, expression within the brain dependent on neural input (Marquette et al., 2003), secretion within the brain in response to humoral stimuli by PAMPs or cytokines (Bluthé et al., 2002; Churchill et al., 2006; Park et al., 2011b) or exogenous addition to the brain (Bluthé et al., 2006). Current dogma suggests that all sources have the same behavioral effect: TNFα induces sickness behavior, reminiscent of the actions of IL-1β. TNFα administration to the periphery causes the entire spectrum of sickness, including fever, weight loss and changes in motivated behavior (Bluthé et al., 1994). There is a strong correlation between infection-related TNFα expression in the periphery and the degree of sickness behavior, as blocking cytokine expression during inflammation attenuates sickness behavior (O’Connor et al., 2009b). TNFα was shown to act through TNF-R1 to induce sickness (Palin et al., 2007). Mice lacking TNF-R2 respond to TNFα with a full spectrum of sickness whereas TNF-R1 KO mice are refractory to TNFα. This finding supported earlier work using human recombinant TNFα, which binds murine TNF-R1 but not murine TNF-R2 and induces sickness behavior (Bluthé et al., 1991; Bluthé et al., 1994). Unlike the IL-1R2, TNF-R2 is a fully functional trans-membrane receptor that signals similar to TNF-R1 except for an inability to activate ceramide synthesis (MacEwan, 2002). Importantly, within the brain, TNF-R1 is localized primarily to neurons and TNF-R2 is localized primarily to glia (Fig. 6). This finding, together with the KO experiments, suggests that TNFα induces behavioral changes by interacting with neuronal TNF-R1.
TNFα changes NMDA-R processing through ceramide via TNR-R1, in one case increasing NR1 phosphorylation and clustering via activation of ceramide production (Wheeler et al., 2009). Through this mechanism, TNFα increases hippocampal neuron calcium flux and excitatory postsynaptic currents (EPSCs). In separate studies, the effect of TNFα was found to be related to time of exposure. A short exposure to TNFα enhances synaptic transmission, EPSCs and AMPA-R insertion into neuronal membranes whereas a longer exposure, more than 50 min, inhibits LTP (Beattie et al., 2002; Tancredi et al., 1992). Whether AMPA-R insertion or decreased LTP is ceramide dependent in these later examples is unknown; however, prolonged exposure to TNFα decreases Ca2+ currents in response to glutamate and this action was mimicked by added ceramide (Furukawa and Mattson, 1998). Thus, as for IL-1β, TNFα directly acts on neurons to alter excitation through the stimulation of ceramide synthesis. Importantly, ceramide production by TNFα occurs through the activation of neutral-sphingomyelinase (N-SMase) (Fig. 5). N-SMase activation requires the activation of factor-associated with N-SMase (FAN). We used FAN-deficient mice to show that this pathway is required for TNFα-, but not LPS-, induced sickness behavior (Palin et al., 2009). The induction of sickness by TNFα also required TNF-R1 and not TNF-R2, only the former activating ceramide synthesis through FAN (Palin et al., 2009). LPS was still able to induce sickness in the absence of FAN, suggesting that the induction of other cytokines, such as IL-1β, is adequate to induce sickness and that their actions are not FAN dependent. These data, however, do show that TNFα-induced sickness behaviors require ceramide production via TNF-R1 on neurons. These data also strongly suggest that IL-1β-induced ceramide production and subsequent changes in neuron activity may mediate its behavior-modifying activity.
Although data pertaining to ceramide production suggest a mechanism of action for both IL-1β- and TNFα-induced sickness, the MAPK pathway is also required for TNFα to induce sickness. An inhibitor of JNK activation, D-JNKI-1, blocks TNFα-induced sickness (Palin et al., 2008). As mentioned above, TNF-R1 is primarily localized to neurons within the CNS (Bette et al., 2003). It is not known if the activation of JNK by TNFα, as a prerequisite for sickness, occurs within neurons or within glia through TNF-R2 (Fig. 5). Attenuation of glia activation by the inhibition of JNK could act to decrease the net inflammatory response (Relja et al., 2009) and thus decrease the ability of the brain to express cytokines, which could then act on neurons. In any case, it is clear that proinflammatory cytokines act by at least two pathways to fully induce sickness.
There is new direct evidence that TNFα may be involved in depressive-like behavior. A very recent study (Kaster et al., 2012) used extremely low doses of TNFα administered i.c.v. to show that TNFα within the brain causes depressive-like behavior. Depressive-like behavior was assessed as increased time of immobility during the FST and TST. This low dose of TNFα did not change locomotor activity (an index of sickness behavior), thus dissociating the two types of behavior as TNFα sensitive and TNFα insensitive (Kaster et al., 2012). They also showed that TNF-R1-deficient mice and mice treated with a neutralizing antibody to TNFα had a decreased time of immobility during the FST, an anti-depressant response. No such direct evidence is available wherein IL-1R1 mediates depressive-like behavior. This study supports earlier work showing that TNF-R1- or TNF-R2-deficient mice have a lower immobility during the FST, indicative of attenuated helplessness/despair. These mice also have increased consumption of a sucrose solution, indicative of a hedonic response being mediated through TNF-Rs. These mice have normal LMA, indicative of the absence of sickness behavior, and unchanged performance in an elevated plus maze; thus, no evidence for changes in anxiety (Simen et al., 2006). Thus, the loss of either neuronal TNF-R1 or glial TNF-R2 elicits an anti-depressant response. Taken together, these data indicate that both TNF-R1 and TNF-R2 are involved in depressive-like behavior.
IL-6 and behavior
Unlike IL-1β and TNFα, IL-6 does not, by itself, elicit behavioral changes despite the induction of fever and activation of the hypothalamic-pituitary-adrenal (HPA) axis (Lenczowski et al., 1999). These data can be interpreted in several ways but they suggest that induction of fever and an HPA response are not directly responsible for behavioral changes and are indeed distinct responses. These data do not suggest that IL-6 has no effect on behavior. In contrast, IL-6 is necessary for a full sickness response. Soluble gp130, a natural inhibitor of interleukin-6 receptor trans-signaling responses, administered i.c.v. prior to LPS enhances recovery from sickness. Soluble gp130 in both in vivo and in vitro models decreases IL-6 signaling, STAT phosphorylation, and the expression of the pro-inflammatory cytokines IL-6 and TNFα but not IL-1β (Burton et al., 2011). Using a genetic KO model, IL-6 deficiency decreases the sickness response to i.p. administration of LPS or IL-1β and the sickness response to i.c.v. LPS or IL-1β (Bluthé et al., 2000b). Thus, normal IL-6 is required for sickness behaviors, but IL-6 alone is insufficient to directly induce sickness.
Major depression in patients has been correlated to circulating IL-6 levels. These data provided some of the early evidence that depression may be related to a tonic state of immune activation (Dantzer, 2006). Although there is no evidence that IL-6 induces depression, similar to sickness behavior, mice deficient for IL-6 have diminished depressive-like behavior, illustrated by decreased time of immobility in the FST and TST and a greater preference for a sucrose solution, suggesting lower despair and diminished anhedonia (Chourbaji et al., 2006). Following s.c. LPS, Sprague-Dawley rats elicit typical sickness with a fever and decreased LMA, assessed as running wheel activity (Harden et al., 2006; Harden et al., 2011). Interestingly, treatment with anti-IL-6 blocked the LPS-induced decrease in LMA; however, treatment with anti-TNFα or anti-IL-1β were without effect. These data support the hypothesis that IL-6 is necessary for sickness behavior. Inactivation of either TNFα or IL-1β is insufficient to prevent sickness behavior, in agreement with the aforementioned need for only one of these two cytokines for sickness behavior (Bluthé et al., 2000a).
There are two possible explanations by which IL-6 is ineffective in itself as an inducer of changes in behavior. One likely candidate is the low level of IL-6 receptors within the brain (Fig. 6). It is possible that the inflammatory response to IL-6 alone is weaker than that of other pro-inflammatory cytokines such as TNFα and IL-1β. However, a stronger candidate is the type of intracellular signaling that occurs post-IL-6R activation (Fig. 5). IL-6 activates the same MAPK and NF-κB pathways as do TNFα and IL-1β and, in addition, activates the JAK → STAT pathway. All three of these pathways lead to an inflammatory response and, in particular, the induction of pro-inflammatory cytokines. However, there is no evidence that IL-6 stimulates ceramide synthesis, which we have implicated in neuron-mediated cytokine-dependent sickness behavior (Palin et al., 2009).
Thus, IL-6 is required for a feed-forward loop that amplifies neuroinflammation and CNS cytokine levels, probably by glial expression. In the absence of IL-6, central cytokines do not reach critical levels to induce full-blown sickness behaviors. On the other hand, either TNFα or IL-1β (but not necessarily both) are also needed to induce CNS cytokine expression primarily by glia, but at least one of these is needed to generate ceramide production by neurons and thereby alter neuronal activity. Ceramide production further enhances the MAPK pathways (Kyriakis and Avruch, 2001), leading to an accentuation of the inflammatory pathway in response to cytokines (Fig. 5). Ceramide itself does not signal pro-inflammatory cytokine expression but is a specific MAPK and maybe NF-κB pathway accentuator (Medvedev et al., 1999; Sakata et al., 2007). In brief, low IL-6 results in inadequate neuroinflammation whereas low TNFα + IL-1β results in inadequate neuronal dysfunction. In either case, no sickness behavior occurs. Whether this combination of pathways is involved in depressive-like behaviors has not been directly addressed.
Interferons (IFNs) and behavior
IFNα has been used to activate the innate immune response to treat patients with viral infections (for example hepatitis C) or cancer. At the onset of treatment, patients develop full-blown sickness behavior. Patients experience fatigue, pain, anorexia and fever. After several weeks of cytokine therapy, approximately one-third of the patients elicit behavioral symptoms of depression (Capuron et al., 2004; Raison et al., 2009). Despite this strong effect with human subjects, the preclinical evidence that IFNs directly induce sickness behavior is lacking. However, O’Connor studied IFNγR-deficient mice infected with Bacillus Calmette-Guérin (BCG) and found that BCG induced the expression of IFNγ within brains and lungs of IFNγR-deficient and wild-type mice (O’Connor et al., 2009a). Even in the absence of IFNγR, mice developed a normal sickness response, suggesting that IFNγ is not required for a sickness response. Similarly, treatment of rats with IFNα does not induce sickness behavior (Kentner et al., 2007). Polyinosinic–polycytidylic acid (Poly I:C) injection into mice induces sickness behavior and IFNβ expression, but sickness was not altered by treatment with an anti-IFNβ neutralizing antibody (Matsumoto et al., 2008b). In this same report, rats were directly treated with IFNβ and failed to elicit sickness behavior assessed by wheel-running activity. Similarly, pegylated IFNα-2a or IFNα-2b does not induce sickness in Lewis rats (Loftis et al., 2006) nor does IFNα treatment of Sprague-Dawley rats or C57BL/6J mice (Kentner et al., 2006; Wang et al., 2009). IFN-stimulated genes (ISGs) are expressed at very low levels in the naive brain (Ida-Hosonuma et al., 2005). ISGs are induced in a positive feedback loop, but low initial expression may limit the initial inflammatory response to IFN treatment. This low-level initial response may prevent a strong acute sickness response to IFN treatment. These preclinical data strongly suggest that the IFNs are not directly responsible or required for sickness behavior.
The studies with patients suggest that, in a preexisting immune activation (for example hepatitis C infection), IFNα treatment elicits a behavioral response. Prolonged treatment with IFNα results in psychiatric side effects including confusion, manic condition, sleep disturbance and a syndrome characteristic of depression (Paul et al., 2007; Raison et al., 2005). This behavioral response is possibly elicited by an amplification of the actions of other existing pro-inflammatory cytokines, much as described above for IL-6. If IFNs act in a similar way to IL-6 to amplify behaviors elicited by other cytokines, it would be expected that IL-6 and IFNs share a common intracellular signaling mechanism. Indeed, that is the case as illustrated in Fig. 5. All IFNs signal through the JAK → STAT pathway, as does IL-6. After a careful literature search, we could not find evidence that IFNs activate ceramide synthesis within neurons despite the presence of IFN receptors on neurons (Fig. 5). Thus, IFNs alone do not directly alter behavior but instead alter behavior on a background of pre-existent immune activation.
Unlike sickness behaviors, there is a probable role of IFNs in the induction of depression. Mice lacking IFNγRs do not develop depressive-like behavior when infected with BCG (O’Connor et al., 2009a). The lack of a depressive-like response may again be analogous to IL-6 action. In the absence of IFNγRs, brain and lung cytokine expression at the time of depressive-like behaviors was less than that of wild-type controls. Similarly, IFNγ-deficient mice have an attenuated cytokine response (Litteljohn et al., 2010). Thus, IFNγ action may be necessary to maintain the expression of other cytokines or elicit a separate but parallel signal that is insufficient alone but is needed to drive depressive-like behaviors. This hypothesis is supported by the lack of depressive-like behaviors of naive mice treated with IFNα (Kosel et al., 2011; Wang et al., 2009). Therein, IFNα treatment alone has no depressive-like effect because there is no pre-existing pro-inflammatory response to amplify.
Cytokines and behavior summary
The mediating role of cytokines on behavior can be summarized by saying that TNFα (sickness and depression) and IL-1β (sickness) alter behavior by direct actions on neurons probably mediated by ceramide synthesis. In contrast, IL-6 and IFNs play little, if any, direct role in modulating behavior in the absence of other cytokines but amplify the behavior effects induced by TNFα and IL-1β. The direct behavior-altering actions of TNFα and IL-1β on neurons does not preclude a lack of input by other cells within the brain. TNFα and IL-1β regulate glia activity to control uptake and release of neurotransmitters. Indeed, a low level of pro-inflammatory cytokine activity within the brain is necessary for normal cognition via maintenance of proper neurotransmitter levels (Yirmiya and Goshen, 2011). It is only when the neuroinflammatory response and input on neurons is at an imbalance that behavior shifts to a sickness or depressive-like state. We believe that inhibition of JNK blocks TNFα-induced sickness because it acts to suppress the feed-forward cytokine loop mediated by the MAPK pathways within glia whereas FAN deficiency illustrates that cytokines cannot induce behavioral changes unless neuron activity is altered by ceramide (Fig. 5).
TLRs and behavior
The penultimate question that is to be addressed below is: what is the mechanism by which infections induce behavioral changes? An even cursory literature review would indicate that infection causes sickness. Every person experiences multiple bouts of sickness throughout life and many people experience some form of depression so we all know that behavior is modified by infections. Clearly, bacterial, fungal, viral or parasitic infection will induce malaise, social withdrawal and fatigue in addition to fever and depressed appetite; this is indisputable. However, to develop new therapies to treat behavioral changes associated with infection, the pathways involved in eliciting these changes must be identified. Identifying these pathways should permit the alleviation of behavioral changes associated with inflammation, without ameliorating the inflammatory response needed to fight the infection. From the above discussion, we have described that infections cause inflammation, inflammation elicits cytokine expression, and cytokine expression changes behavior. Below, we will examine whether all TLRs are involved in this cascade.
TLR5 and TLR11: protein-activated PRRs
A study examining TLR5 activation and sickness illustrates a well-designed approach to the validation of TLR specificity. Flagellin activates TLR5, but infectious agents such as flagellate bacteria also contain other TLR agonists; in this case, Gram-negative bacterial LPS could also activate TLR4 to induce behavioral changes. In a study by Matsumoto, sickness behavior, which was quantified as decreased LMA (wheel-running activity) was induced by the injection of live Salmonella (Matsumoto et al., 2008a). To confirm that this flagellate was acting through TLR5, Salmonella was injected into C3H/HeJ mice, which lack functional TLR4. An almost identical LMA response was found between C3H/HeJ and control (C3H/HeN) mice. In the same study, gentamicin-treated Salmonella, which have reduced flagellin content, have a markedly diminished sickness response compared with non-treated Salmonella. In addition, flagellin-treated mice also respond with diminished LMA, showing that the purified ligand itself induces a sickness behavior. This study, by itself, confirmed that live bacteria elicit sickness through a TLR-specific mechanism that can be mimicked by direct administration of the ligand and can be attenuated by loss of the ligand. Flagellin injection also elicits a systemic inflammatory response (Eaves-Pyles et al., 2001), which is the likely mechanism for the behavioral response as described above. The Matsumoto research also indicates that Salmonella initiates an inflammatory response largely independent of TLR4 and that heat-killed Salmonella, with denatured flagellin, was less potent than live bacteria (Matsumoto et al., 2008a). It was hypothesized that TLR5 activation by flagellin initiated the immune response. Only after this initiation and attack on live bacteria by the host would LPS be released to activate TLR4 and synergize with the initial response to clear the body of the bacteria. Thus, it is likely that Salmonella elicit full-blown sickness behavior by activating at least two TLRs.
Although profilin is necessary for Toxoplasma recognition and activation of cytokines (Plattner et al., 2008; Yarovinsky et al., 2005), the role of this ligand, the subsequent activation of TLR11, the release of IL-12 and the expression of IFNγ in behavioral changes has not been investigated. The human TLR11 analog is a nonfunctional pseudogene and thus does not play a role in the immune response or subsequent behavioral changes (Pifer and Yarovinsky, 2011). Thus, it is not known if TLR11 activation is directly responsible for behavioral changes.
TLR3, TLR7, TLR8 and TLR9: nucleic acids
Although frequently studied relative to infection, activation of the TLRs that recognize nucleotides – TLR3, TLR7, TLR8 and TLR9 – has been given relatively little attention as direct modifiers of animal behavior, with the exception of studies with TLR3. Poly I:C has proven to be an effective activator of TLR3 and inducer of transient sickness. Systemic administration of poly I:C induces weight loss and diminished food intake. This physiological response is associated with neuroinflammation, especially type I IFNγ/β expression, within the brain. This neuroinflammatory response mimics the peripheral inflammatory response (Field et al., 2010). Poly I:C administration to mice induces a transient slight increase in core body temperature but a strong sickness behavioral response, assessed as a decrease in LMA and burrowing activity (Cunningham et al., 2007). This sickness response is accompanied by a marked increase in circulating IFNβ, IL-6 and TNFα followed by CNS mRNA expression of the same cytokines and, to a lesser extent, IL-1β albeit IFNγ is not increased (Cunningham et al., 2007; Gandhi et al., 2007; Konat et al., 2009). This cytokine profile is similar to that shown in Fig. 2.
In addition to a sickness response, poly I:C administered i.p. has been shown to induce chronic fatigue syndrome, evidenced as a prolonged decrease in voluntary wheel-running (LMA) (Katafuchi et al., 2005; Katafuchi et al., 2003). Fatigue was present while CNS IFNα mRNA level was still elevated, but after central IL-1β expression, it had returned to control levels. This TLR3-mediated behavior appears to be a form of central fatigue or depressive-like behavior as it was ameliorated by the anti-depressant imipramine, which is a nonselective serotonin reuptake inhibitor. Although poly I:C induces a prolonged fatigue response and a rapid rise in circulating IFNβ, an acute injection of IFNβ does not mimic this behavioral effect (Matsumoto et al., 2008b). These data suggest that other cytokines are necessary for the behavioral response, as discussed earlier in the IFN section. In addition to prolonged fatigue, prenatal exposure of dams to poly I:C has been used as a model for inflammation-induced schizophrenia-like behaviors that are expressed by the offspring (Macêdo et al., 2012; Piontkewitz et al., 2012). Thus, age-at-exposure to an immune challenge alters the phenotypic expression pattern. With adult mice, poly I:C also decreases swim time (increases immobility) in the FST for up to 1 week post i.p. administration (Sheng et al., 2009). Although referred to as a fatigue response within the manuscript, an increase in immobility in the FST is used to assess despair and diagnose depressive-like behavior in preclinical rodent models. The diminished performance in the FST continued for several days after spontaneous cage LMA had returned to normal (an index of sickness); thus distinguishing fatigue/depressive-like behavior from sickness.
Exposure to imiquimod, a TLR7 agonist, induces only a modest cytokine response within the brain that is associated with the induction of fever. Similarly, only a modest sickness response was evidenced as a slight decrease in food and water intake but no change in overall LMA of rats (Damm et al., 2012). I have confirmed this and found a modest sickness response to imiquimod with mice (R.H.M., unpublished observations). In contrast, the TLR7 agonist 1V136 induces a potent sickness/anorexic response when administered i.p. but a more potent response when administered intranasally (i.n.) (Hayashi et al., 2008). Intranasal administration elicited a greater behavioral response despite a similar peripheral cytokine response, suggesting that neuroinflammation was responsible for anorexia. These data are consistent with probable transport of the TLR7 agonist directly to the brain via the trigeminal or olfactory pathway when administered i.n. and suggest that activation of TLR3 and TLR7 elicits a behavioral response. These data with TLR3 implicate cytokine production as the mediator of the behavioral changes.
TLR2 heterodimers and TLR4: membrane lipids
As illustrated in Fig. 2, TLR2 forms heterodimers with TLR1 and TLR6, thereby changing the ligand recognition pattern. Both heterodimer receptors are localized to the plasma membrane to recognize extracellular PAMPs. TLR2 is not considered to be endogenously expressed TLR on naive neurons. However, activation of TLR2/6 heterodimers with macrophage-activating lipopeptide (MALP)-2 derived from Mycoplasma fermentans induced sickness behavior and an accompanying loss of body mass and decrease in food consumption in rats (Knorr et al., 2008). When given s.c., a local inflammatory response, involving elevated expression of TNFα and IL-6, resulted in elevated circulating IL-6 and activation of STAT3 in the organum vasculosum of the laminae terminalis (OVLT), suprafornical organ (SFO) and area postrema (AP) (see Fig. 1). TNFα was not detectable in the circulation (IL-1β was not quantified). This IL-6-dependent activation of cytokine signaling within the circumventricular organs of the brain was accompanied by sickness behavior assessed as decreased home cage activity; i.e. LMA.
In a previous study using i.p. injections, MALP-2 and fibroblast-stimulating lipopeptide (FSL)-1, a TLR2/6 synthetic activator based on the structure from Mycoplasma salivarium, induced a transient fever as well as a prolonged decrease in home cage activity, low LMA, and elevated circulating levels of both TNFα and IL-6 (Hübschle et al., 2006). The comparative strength of the immune response between these two studies paralleled sickness behavior, supporting the role of cytokines as mediators of sickness behaviors following TLR2/6 activation. This relationship was confirmed by the use of TNF binding protein. TNFbp blocked the pyrogenic effect of FSL-1 and its ability to induce IL-6 expression (Greis et al., 2007). Zymosan, a yeast particulate, given to rats induced a fever and diminished a motivated behavior: decreased consumption of sweetened cereal (Cremeans-Smith and Newberry, 2003). Neither fever nor food disappearance are behaviors per se but, together with behavioral assessment in the previous studies, these physiological responses indicate that zymosan activation of TLR2 signaling is probably a behavior-modifying event. Indeed, zymosan given i.p. to several strains of mice induces full blown sickness behavior including diminished locomotor activity, body writhes (pain) and sedation. The sickness response was attenuated by morphine, which has anti-inflammatory activity (Natorska and Plytycz, 2005).
Clearly, TLR4 activation by LPS is the model most prevalent in the literature that is used to induce inflammatory-dependent behavioral changes. Numerous investigators have contributed to this literature and it would be impossible to acknowledge all the important work in a single review. Our recent work has added to the understanding of the sickness response by showing that an i.c.v. dosage as low as 10 ng of LPS induces a central immune response, including elevated expression of TNFα, IL-1β and IL-6 in the brain of mice. Even this low dose of LPS causes full-blown sickness behavior, including depressed LMA and decreased social exploration, together with expected physiological responses such as loss of body mass and reduced food intake (Park et al., 2011a). In contrast, a higher dosage of LPS is required when administered i.p. (Park et al., 2011b). At 330 or 830μg kg–1 body mass i.p. (∼10,000 and 25,000 ng mouse–1, respectively), LPS induces an inflammatory response within the brain and a full spectrum of sickness behaviors. Unlike a low i.c.v. dose, i.p. LPS induces a peripheral immune response, including the induction of circulating IL-6, IL-1β, TNFα and IFNγ (Finney et al., 2012; Gibb et al., 2008).
Similar to the well-characterized sickness response, LPS was shown almost 20 years ago to induce behaviors that relate to depression of humans. Systemic injection of LPS in rats causes a typical sickness response and an anhedonic phenotype, assessed as a decreased preference for consumption of a saccharin solution (Yirmiya, 1996). Our recent data support this early literature by showing that central (i.c.v.) or peripheral (i.p.) LPS induces a depressive-like phenotype when quantified as increased time of immobility in the TST and FST (Park et al., 2011a; Park et al., 2011b). The increased time of immobility in these tests is frequently used as an index of despair. More importantly, the depressive-like behavior is still evident after food intake and LMA have returned to normal, indicating that sickness behavior had waned (O’Connor et al., 2009b). This later point is critical to the interpretation of depressive-like behavior. Within the acute-phase immune response to LPS (<24 h for i.p. dosage of 830μg kg–1 body mass), mice have decreased immobility in the LMA test, FST and TST. However, by waiting until LMA activity is back to control levels (>24 h), it is easier to defend the increase in time of immobility as a depressive-like behavior and distinct from sickness behavior. In this same study, minocycline, which decreases cytokine production, prevents both sickness and depressive-like behavior, illustrating that cytokines mediate the behavioral changes. Similarly, the anti-inflammatory COX inhibitors indomethacin and nimesulide and the anti-inflammatory glucocorticoid analog dexamethasone, attenuated i.p. LPS-induced sickness, depressive-like behavior and anxiety of mice (de Paiva et al., 2010). In this study, sickness was evident following LPS treatment; decreased food disappearance and loss of body mass. Sickness behavior was evident as a decrease in LMA and number of rearings. Depressive-like behavior was quantified as an increase time of immobility in the FST and TST. Anxiogenic-like behavior was evident following LPS treatment using the light–dark box test wherein LPS caused a reduction in number of transitions between light and dark regions of the box. This extensive behavioral evaluation clearly indicates the global action of LPS on a variety of behaviors and the role of inflammation in a variety of behaviors.
Prostaglandin involvement in TLR-mediated behaviors
As discussed above within the cytokine section, where either TNFα or IL-1β are required for sickness behaviors, these cytokines are not the only factors involved in LPS-induced behavioral changes. In another study, inhibition of COX-1 alleviates sickness behaviors without changing peripheral or central expression of proinflammatory cytokines (Teeling et al., 2010). In this study, the selective COX-1 inhibitor piroxicam was effective at attenuating the LPS-induced decrease of burrowing activity but not in attenuating LPS-induced LMA. Thus, despite the importance of cytokines in LPS activity, a separate pathway involving prostaglandin production via COX-1 may be requisite for certain behaviors such as species-specific burrowing. Using other inhibitors, Teeling et al. also showed that COX-2 activity, thromboxane production and PPAR-γ activity did not appear to be requisite for LPS activity (Teeling et al., 2010). The mechanism by which COX-1 acts to modulate behavior may involve neuroinflammation, although this was not revealed by the Teeling study. Inhibition of COX-1 activity with SC-560 or COX-1 deficiency attenuates i.c.v. LPS-induced IL-1β and TNFα, but not IL-6, expression within the brain (Choi et al., 2008), suggesting that COX-1 is necessary for the inflammatory activity of LPS. Whether prostaglandins accentuate cytokine-dependent sickness behaviors, playing an amplifying role as described for IL-6 and the IFNs, or have other distinct modus operandi awaits further studies. A study performed 30 years ago, however, does indicate that PGD2 decreases LMA, and this finding supports a direct sickness effect for prostaglandins on sickness behavior (Förstermann et al., 1983). Prostaglandin synthesis is clearly implicated in the febrile response elicited by LPS (Pecchi et al., 2009), but its mediating effect on inflammation-dependent behavior is poorly understood. However, the bulk of the literature implicates cytokines as required initiators and sustainers of both inflammation-induced sickness and depressive-like behaviors. Indeed, inhibition of neuroinflammation, as occurs with i.c.v. administration of IGF-I, results in attenuated depressive-like behaviors, indicating that a naturally occurring neurotrophin feeds back within the CNS to regulate inflammation-induced depression (Park et al., 2011a).
Being the most exploited model, some very important aspects of inflammatory-dependent behaviors have been made using LPS as an inducer. Of most importance to this review is the dependence of cytokines in behavior changes. Surprisingly, mice respond to LPS with behavioral changes even when lacking TNF-R1 (Palin et al., 2009) or IL-1R1 (Bluthé et al., 2000a) but require TNFα if the IL-1R1 is absent (Bluthé et al., 2000a). Similarly, treatment with neutralizing antibodies to either IL-1β or TNFα does not attenuate LPS-induced changes in behavior, with sickness behavior assessed as burrowing activity (Teeling et al., 2007), because neutralizing both is necessary to block LPS activity. These data indicate that either TNFα or IL-1β alone are able to mediate the sickness response associated with LPS but at least one of these cytokines must be present within the brain to induce sickness. These data strongly suggest that, in the absence of TNFα and IL-1β, the remaining cytokines, including IL-6 and IFNs, and prostaglandins are not sufficient to alter behavior. As described earlier, TNFα or IL-1β administered alone initiate full-blown sickness behaviors, whereas IL-6 and IFNs administered alone are insufficient. IFNγ signaling is needed for LPS to induce depressive-like behaviors (see IFN section), but IFNs administered alone do not cause these behavioral changes. It appears that LPS-induced IL-6 and IFNγ are needed to amplify the actions of TNFα or IL-1β and thus their behavioral response.
Nod1 and Nod2: bacterial peptidoglycans
Nod1 and Nod2 activation has not been extensively studied with regard to animal behavior. After an extensive search, direct evidence that Nod1 activation induces sickness or depressive-like behavior has been elusive. However, there are reports that bacterial peptidoglycans are direct mediators of sickness via Nod2. The minimally active subunit of bacterial peptidoglycan, muramyl dipeptide (MDP), is able to elicit a decrease in food intake of rats (Fosset et al., 2003). In addition to food disappearance, MDP was shown to change the eating behavior. MDP caused a decrease in eating bout frequency that corresponded with an increase in eating bout duration. This change in eating behavior was accompanied by a greater time resting and less time grooming for MDP-treated rats compared with controls. The increased resting and diminished grooming are considered sickness behaviors. Interestingly, one of only a handful of studies that compare the behavioral effects of various TLR ligands was performed with MDP, LPS and poly I:C (Baillie and Prendergast, 2008). In this study, i.p. administration of LPS caused a loss of body mass, diminution of food disappearance, decrease in consumption of a saccharin solution and decrease in nesting behavior in Siberian hamsters. LPS and poly I:C had similar effects on changes in body mass, food intake and saccharin consumption, but poly I:C did not affect nesting behavior. In direct contrast to poly I:C, MDP did not alter body mass, food intake or saccharin intake but did decrease nesting behavior compared with controls. Reverting to our TLR signaling pathways (Fig. 2), it is possible to propose that the MyD88 pathways activated by TLR2/6 and TLR4 mediate the change in nesting behavior induced by LPS and MDP, respectively, whereas the TRIF-dependent pathways activated by TLR3 and TLR4 via poly I:C and LPS, respectively, regulate feeding and drinking activity and subsequent change in body mass. This, of course, is an oversimplification of the intricacies of the TLR immune response. However, the results do suggest that specific TLR agonists, by themselves, may not fully activate all aspects of sickness. This hypothesis suggests that different symptoms may be related to the induction of a specific combination of cytokines. In a separate study, LPS was a more potent anorexic agent than MDP, and this action correlated to the greater ability of LPS to induce TNFα and IL-1β expression in the cerebellum, hippocampus and hypothalamus (Plata-Salamán et al., 1998). Thus, anorexia was related to specific cytokines being expressed in specific brain regions. These data indicate that Nod2 activation can induce behavioral changes, but taken together they clearly indicate that LPS is the most potent inducer of sickness behavior, possibly because it directly induces both of the inflammatory signaling pathways (NF-κB and TRIF) and thus induces the most complete array of cytokine expression.
In conclusion
This Review in no way encompasses all the mechanisms by which infection alters behavior but instead aims to bring several critical issues to light. (1) Independent of the type of infection, the inflammatory response is critical to the induction of behavioral changes. These behavioral changes, albeit accompanied by physiological changes such as fever, are not a direct response to these physiological responses. (2) Pro-inflammatory cytokine expression, in contrast, is requisite for behavioral alterations. Cytokine-dependent sickness behaviors are perhaps the best-characterized model for immune-mediated changes in behavior. This phenomenon has been studied intensely, as these behaviors are easily and repeatedly demonstrable with rodent models. Symptoms of sickness are elicited by a multitude of inflammatory agents, clearly demonstrating the universality of cytokine-dependent sickness. Where available, other types of behaviors that are elicited by immune activation are mentioned throughout the text and include (but are not restricted to) fatigue, depression and species-specific behaviors such as burrowing. Many species-specific behaviors are also cytokine-dependent and some of these species-specific changes in behavior are mentioned in other reviews within this issue. (3) As behavior is controlled by neuronal function, behavioral changes associated with infection are a result of pro-inflammatory cytokine direct interaction with neurons. Of the cytokines discussed, TNFα, IL-1β or both are required for the development of sickness and depressive-like behaviors. Other cytokines, including IFNγ, IFNα, IFNβ and IL-6 (and possibly prostaglandins), are necessary for behavioral changes but may not directly elicit these behavioral responses. These later pro-inflammatory cytokines appear to play a role as amplifiers of the central responses initiated by TNFα or IL-1β. The TLRs and Nods all elicit behavioral changes, but being able to assign a definitive role for each receptor to specific behaviors is in its infancy. Clearly, infectious agents are able to activate more than one TLR or Nod and, thus, the omnipresence of all sickness behaviors following an infection reflect this multi-hit approach to immune activation.
Supplementary Material
List of abbreviations
- AMPA-R
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BBB
blood–brain barrier
- BCG
Bacillus Calmette-Guérin
- CNS
central nervous system
- COX
cyclooxygenase
- DAMPs
damage-associated molecular patterns
- EPSCs
excitatory postsynaptic currents
- FAN
factor-associated with N-SMase
- FST
forced swim test
- HPA
hypothalamic-pituitary-adrenal
- i.c.v.
intracerebroventricular
- i.n.
intranasal administration
- i.p.
intraperitoneal administration
- i.v.
intravenous administration
- IFNα
type I interferon
- IFNγ
type II interferon
- IL-1R1
interleukin 1 receptor
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- IRF3
interferon regulatory factor 3
- ISGs
interferon-stimulated genes
- JNK
c-Jun N-terminal kinase
- KO
knock-out
- LMA
locomotor activity
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- MALP
macrophage-activating lipopeptide
- MAPKs
mitogen-activated protein kinases
- MDP
muramyl dipeptide
- NF-κB
nuclear factor κB
- NMDA
n-methyl-d-aspartate
- NMDA-R
NMDA receptor
- Nod
nucleotide-binding oligomerization domain
- N-SMase
neutral-sphingomyelinase
- NTS
nucleus tractus solitaries
- PAMPs
pathogen-associated molecular patterns
- PGD2
prostaglandin D2
- Poly I:C
polyinosinic–polycytidylic acid
- PPAR
peroxisome proliferator-activated receptor
- PRRs
pattern recognition receptors
- RICK
receptor-interacting serine–threonine kinase 2, aka RIPK
- RIPK
receptor-interacting serine–threonine kinase 2, aka RICK
- s.c.
subcutaneous administration
- STAT
signal transducer and activator of transcription
- TLRs
Toll-like receptor
- TNF-R
tumor necrosis factor receptor
- TNFα
tumor necrosis factor α
- TRIF
TIR (Toll/interleukin-1 receptor-like) domain-containing adapter-inducing interferon-β
- TST
tail suspension test
Footnotes
Funding
This work was supported by National Institutes of Health [MH083767 to R.H.M.; AG029573 to K.W.K.]. Deposited in PMC for release after 12 months.
References
- Anisman H., Gibb J., Hayley S. (2008). Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology (Berl.) 199, 231–244 [DOI] [PubMed] [Google Scholar]
- Baillie S. R., Prendergast B. J. (2008). Photoperiodic regulation of behavioral responses to bacterial and viral mimetics: a test of the winter immunoenhancement hypothesis. J. Biol. Rhythms 23, 81–90 [DOI] [PubMed] [Google Scholar]
- Beattie E. C., Stellwagen D., Morishita W., Bresnahan J. C., Ha B. K., Von Zastrow M., Beattie M. S., Malenka R. C. (2002). Control of synaptic strength by glial TNFα. Science 295, 2282–2285 [DOI] [PubMed] [Google Scholar]
- Bette M., Kaut O., Schäfer M. K., Weihe E. (2003). Constitutive expression of p55TNFR mRNA and mitogen-specific up-regulation of TNF alpha and p75TNFR mRNA in mouse brain. J. Comp. Neurol. 465, 417–430 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Dantzer R., Kelley K. W. (1991). Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur. J. Pharmacol. 209, 281–283 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Pawlowski M., Suarez S., Parnet P., Pittman Q., Kelley K. W., Dantzer R. (1994). Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 19, 197–207 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Michaud B., Kelley K. W., Dantzer R. (1996a). Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. Neuroreport 7, 1485–1488 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Michaud B., Kelley K. W., Dantzer R. (1996b). Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport 7, 2823–2827 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Layé S., Michaud B., Combe C., Dantzer R., Parnet P. (2000a). Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 12, 4447–4456 [PubMed] [Google Scholar]
- Bluthé R. M., Michaud B., Poli V., Dantzer R. (2000b). Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol. Behav. 70, 367–373 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Lestage J., Rees G., Bristow A., Dantzer R. (2002). Dual effect of central injection of recombinant rat interleukin-4 on lipopolysaccharide-induced sickness behavior in rats. Neuropsychopharmacology 26, 86–93 [DOI] [PubMed] [Google Scholar]
- Bluthé R. M., Kelley K. W., Dantzer R. (2006). Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav. Immun. 20, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretdibat J. L., Bluthé R. M., Kent S., Kelley K. W., Dantzer R. (1995). Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav. Immun. 9, 242–246 [DOI] [PubMed] [Google Scholar]
- Burton M. D., Sparkman N. L., Johnson R. W. (2011). Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J. Neuroinflammation 8, 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Ravaud A., Miller A. H., Dantzer R. (2004). Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav. Immun. 18, 205–213 [DOI] [PubMed] [Google Scholar]
- Chesler D. A., Reiss C. S. (2002). The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13, 441–454 [DOI] [PubMed] [Google Scholar]
- Choi S. H., Langenbach R., Bosetti F. (2008). Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 22, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S., Urani A., Inta I., Sanchis-Segura C., Brandwein C., Zink M., Schwaninger M., Gass P. (2006). IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 23, 587–594 [DOI] [PubMed] [Google Scholar]
- Churchill L., Taishi P., Wang M., Brandt J., Cearley C., Rehman A., Krueger J. M. (2006). Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res. 1120, 64–73 [DOI] [PubMed] [Google Scholar]
- Clarke T. B., Weiser J. N. (2011). Intracellular sensors of extracellular bacteria. Immunol. Rev. 243, 9–25 [DOI] [PubMed] [Google Scholar]
- Cremeans-Smith J. K., Newberry B. H. (2003). Zymosan: induction of sickness behavior and interaction with lipopolysaccharide. Physiol. Behav. 80, 177–184 [DOI] [PubMed] [Google Scholar]
- Cunningham C., Campion S., Teeling J., Felton L., Perry V. H. (2007). The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav. Immun. 21, 490–502 [DOI] [PubMed] [Google Scholar]
- Damm J., Wiegand F., Harden L. M., Gerstberger R., Rummel C., Roth J. (2012). Fever, sickness behavior, and expression of inflammatory genes in the hypothalamus after systemic and localized subcutaneous stimulation of rats with the Toll-like receptor 7 agonist imiquimod. Neuroscience 201, 166–183 [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2001). Cytokine-induced sickness behavior: where do we stand? BrainBehav. Immun. 15, 7–24 [DOI] [PubMed] [Google Scholar]
- Dantzer R. (2006). Cytokine, sickness behavior, and depression. Neurol. Clin. 24, 441–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. N., Tabarean I., Gaidarova S., Behrens M. M., Bartfai T. (2006). IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J. Neurochem. 98, 1379–1389 [DOI] [PubMed] [Google Scholar]
- de Paiva V. N., Lima S. N., Fernandes M. M., Soncini R., Andrade C. A., Giusti-Paiva A. (2010). Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav. Brain Res. 215, 146–151 [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Musson R. A. (1986). Phagocytic defects – monocytes/macrophages. Clin. Immunol. Immunopathol. 40, 62–68 [DOI] [PubMed] [Google Scholar]
- Eaves-Pyles T., Murthy K., Liaudet L., Virág L., Ross G., Soriano F. G., Szabó C., Salzman A. L. (2001). Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I κ B α degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166, 1248–1260 [DOI] [PubMed] [Google Scholar]
- Ericsson A., Liu C., Hart R. P., Sawchenko P. E. (1995). Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J. Comp. Neurol. 361, 681–698 [DOI] [PubMed] [Google Scholar]
- Field R., Campion S., Warren C., Murray C., Cunningham C. (2010). Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav. Immun. 24, 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney S. J., Leaver S. K., Evans T. W., Burke-Gaffney A. (2012). Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med. 38, 324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Heldt R., Hertting G. (1983). Effects of intracerebroventricular administration of prostaglandin D2 on behaviour, blood pressure and body temperature as compared to prostaglandins E2 and F2 α. Psychopharmacology (Berl.) 80, 365–370 [DOI] [PubMed] [Google Scholar]
- Fosset S., Fromentin G., Rampin O., Lang V., Mathieu F., Tomé D. (2003). Pharmacokinetics and feeding responses to muramyl dipeptide in rats. Physiol. Behav. 79, 173–182 [DOI] [PubMed] [Google Scholar]
- French R. A., VanHoy R. W., Chizzonite R., Zachary J. F., Dantzer R., Parnet P., Bluthé R. M., Kelley K. W. (1999). Expression and localization of p80 and p68 interleukin-1 receptor proteins in the brain of adult mice. J. Neuroimmunol. 93, 194–202 [DOI] [PubMed] [Google Scholar]
- Furukawa K., Mattson M. P. (1998). The transcription factor NF-κB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J. Neurochem. 70, 1876–1886 [DOI] [PubMed] [Google Scholar]
- Gandhi R., Hayley S., Gibb J., Merali Z., Anisman H. (2007). Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav. Immun. 21, 477–489 [DOI] [PubMed] [Google Scholar]
- Gaykema R. P., Goehler L. E., Hansen M. K., Maier S. F., Watkins L. R. (2000). Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce IL-1β levels in the circulation. Auton. Neurosci. 85, 72–77 [DOI] [PubMed] [Google Scholar]
- Gibb J., Hayley S., Gandhi R., Poulter M. O., Anisman H. (2008). Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav. Immun. 22, 573–589 [DOI] [PubMed] [Google Scholar]
- Greis A., Murgott J., Rafalzik S., Gerstberger R., Hübschle T., Roth J. (2007). Characterization of the febrile response induced by fibroblast-stimulating lipopeptide-1 in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R152–R161 [DOI] [PubMed] [Google Scholar]
- Guan Y., Ranoa D. R., Jiang S., Mutha S. K., Li X., Baudry J., Tapping R. I. (2010). Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J. Immunol. 184, 5094–5103 [DOI] [PubMed] [Google Scholar]
- Hanke M. L., Kielian T. (2011). Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. (Lond.) 121, 367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. K., Daniels S., Goehler L. E., Gaykema R. P., Maier S. F., Watkins L. R. (2000). Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever. Auton. Neurosci. 85, 83–87 [DOI] [PubMed] [Google Scholar]
- Harden L. M., du Plessis I., Poole S., Laburn H. P. (2006). Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol. Behav. 89, 146–155 [DOI] [PubMed] [Google Scholar]
- Harden L. M., du Plessis I., Roth J., Loram L. C., Poole S., Laburn H. P. (2011). Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1β and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior. Psychoneuroendocrinology 36, 608–622 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Cottam H. B., Chan M., Jin G., Tawatao R. I., Crain B., Ronacher L., Messer K., Carson D. A., Corr M. (2008). Mast cell-dependent anorexia and hypothermia induced by mucosal activation of Toll-like receptor 7. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R123–R132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübschle T., Mütze J., Mühlradt P. F., Korte S., Gerstberger R., Roth J. (2006). Pyrexia, anorexia, adipsia, and depressed motor activity in rats during systemic inflammation induced by the Toll-like receptors-2 and -6 agonists MALP-2 and FSL-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R180–R187 [DOI] [PubMed] [Google Scholar]
- Ida-Hosonuma M., Iwasaki T., Yoshikawa T., Nagata N., Sato Y., Sata T., Yoneyama M., Fujita T., Taya C., Yonekawa H., et al. (2005). The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 79, 4460–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannin P., Jaillon S., Delneste Y. (2008). Pattern recognition receptors in the immune response against dying cells. Curr. Opin. Immunol. 20, 530–537 [DOI] [PubMed] [Google Scholar]
- Kaster M. P., Gadotti V. M., Calixto J. B., Santos A. R., Rodrigues A. L. (2012). Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology 62, 419–426 [DOI] [PubMed] [Google Scholar]
- Katafuchi T., Kondo T., Yasaka T., Kubo K., Take S., Yoshimura M. (2003). Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: a model for immunologically induced fatigue. Neuroscience 120, 837–845 [DOI] [PubMed] [Google Scholar]
- Katafuchi T., Kondo T., Take S., Yoshimura M. (2005). Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur. J. Neurosci. 22, 2817–2826 [DOI] [PubMed] [Google Scholar]
- Katsuura G., Gottschall P. E., Arimura A. (1988). Identification of a high-affinity receptor for interleukin-1β in rat brain. Biochem. Biophys. Res. Commun. 156, 61–67 [DOI] [PubMed] [Google Scholar]
- Kentner A. C., Miguelez M., James J. S., Bielajew C. (2006). Behavioral and physiological effects of a single injection of rat interferon-alpha on male Sprague-Dawley rats: a long-term evaluation. Brain Res. 1095, 96–106 [DOI] [PubMed] [Google Scholar]
- Kentner A. C., James J. S., Miguelez M., Bielajew C. (2007). Investigating the hedonic effects of interferon-alpha on female rats using brain-stimulation reward. Behav. Brain Res. 177, 90–99 [DOI] [PubMed] [Google Scholar]
- Knorr C., Hübschle T., Murgott J., Mühlradt P., Gerstberger R., Roth J. (2008). Macrophage-activating lipopeptide-2 (MALP-2) induces a localized inflammatory response in rats resulting in activation of brain sites implicated in fever. Brain Res. 1205, 36–46 [DOI] [PubMed] [Google Scholar]
- Konat G. W., Borysiewicz E., Fil D., James I. (2009). Peripheral challenge with double-stranded RNA elicits global up-regulation of cytokine gene expression in the brain. J. Neurosci. Res. 87, 1381–1388 [DOI] [PubMed] [Google Scholar]
- Konsman J. P., Veeneman J., Combe C., Poole S., Luheshi G. N., Dantzer R. (2008). Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur. J. Neurosci. 28, 2499–2510 [DOI] [PubMed] [Google Scholar]
- Kosel M., Bilkei-Gorzo A., Zawatzky R., Zimmer A., Schlaepfer T. E. (2011). Pegylated human interferon alpha 2a does not induce depression-associated changes in mice. Psychiatry Res. 185, 243–247 [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Avruch J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 [DOI] [PubMed] [Google Scholar]
- Layé S., Parnet P., Goujon E., Dantzer R. (1994). Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. Brain Res. 27, 157–162 [DOI] [PubMed] [Google Scholar]
- Lehtimäki K. A., Peltola J., Koskikallio E., Keränen T., Honkaniemi J. (2003). Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res. Mol. Brain Res. 110, 253–260 [DOI] [PubMed] [Google Scholar]
- Lenczowski M. J., Bluthé R. M., Roth J., Rees G. S., Rushforth D. A., van Dam A. M., Tilders F. J., Dantzer R., Rothwell N. J., Luheshi G. N. (1999). Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am. J. Physiol. 276, R652–R658 [DOI] [PubMed] [Google Scholar]
- Litteljohn D., Cummings A., Brennan A., Gill A., Chunduri S., Anisman H., Hayley S. (2010). Interferon-gamma deficiency modifies the effects of a chronic stressor in mice: Implications for psychological pathology. Brain Behav. Immun. 24, 462–473 [DOI] [PubMed] [Google Scholar]
- Loftis J. M., Wall J. M., Pagel R. L., Hauser P. (2006). Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology 31, 1289–1294 [DOI] [PubMed] [Google Scholar]
- Luheshi G. N., Bluthé R. M., Rushforth D., Mulcahy N., Konsman J. P., Goldbach M., Dantzer R. (2000). Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton. Neurosci. 85, 127–132 [DOI] [PubMed] [Google Scholar]
- Macêdo D. S., Araújo D. P., Sampaio L. R., Vasconcelos S. M., Sales P. M., Sousa F. C., Hallak J. E., Crippa J. A., Carvalho A. F. (2012). Animal models of prenatal immune challenge and their contribution to the study of schizophrenia: a systematic review. Braz. J. Med. Biol. Res. 45, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan D. J. (2002). TNF receptor subtype signalling: differences and cellular consequences. Cell. Signal. 14, 477–492 [DOI] [PubMed] [Google Scholar]
- Magrone T., Jirillo E. (2012). Mechanisms of neutrophil-mediated disease: innovative therapeutic interventions. Curr. Pharm. Des. 18, 1609–1619 [DOI] [PubMed] [Google Scholar]
- Malick A., Jakubowski M., Elmquist J. K., Saper C. B., Burstein R. (2001). A neurohistochemical blueprint for pain-induced loss of appetite. Proc. Natl. Acad. Sci. USA 98, 9930–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquette C., Linard C., Galonnier M., Van Uye A., Mathieu J., Gourmelon P., Clarençon D. (2003). IL-1β, TNFα and IL-6 induction in the rat brain after partial-body irradiation: role of vagal afferents. Int. J. Radiat. Biol. 79, 777–785 [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Shiva D., Kawanishi N., Kato Y., Woods J. A., Yano H. (2008a). Salmonella administration induces a reduction of wheel-running activity via a TLR5-, but not a TLR4, dependent pathway in mice. Exerc. Immunol. Rev. 14, 38–50 [PubMed] [Google Scholar]
- Matsumoto T., Takahashi H., Shiva D., Kawanishi N., Kremenik M. J., Kato Y., Yano H. (2008b). The reduction of voluntary physical activity after poly I:C injection is independent of the effect of poly I:C-induced interferon-beta in mice. Physiol. Behav. 93, 835–841 [DOI] [PubMed] [Google Scholar]
- Medvedev A. E., Blanco J. C., Qureshi N., Vogel S. N. (1999). Limited role of ceramide in lipopolysaccharide-mediated mitogen-activated protein kinase activation, transcription factor induction, and cytokine release. J. Biol. Chem. 274, 9342–9350 [DOI] [PubMed] [Google Scholar]
- Natorska J., Plytycz B. (2005). Strain-specific differences in modulatory effects of morphine on peritoneal inflammation in mice. Folia Biol. (Krakow) 53, 189–195 [DOI] [PubMed] [Google Scholar]
- Newton K., Dixit V. M. (2012). Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J. C., André C., Wang Y., Lawson M. A., Szegedi S. S., Lestage J., Castanon N., Kelley K. W., Dantzer R. (2009a). Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 29, 4200–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J. C., Lawson M. A., André C., Moreau M., Lestage J., Castanon N., Kelley K. W., Dantzer R. (2009b). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K., Bluthé R. M., McCusker R. H., Moos F., Dantzer R., Kelley K. W. (2007). TNFα-induced sickness behavior in mice with functional 55 kD TNF receptors is blocked by central IGF-I. J. Neuroimmunol. 187, 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K., McCusker R. H., Strle K., Moos F., Dantzer R., Kelley K. W. (2008). Tumor necrosis factor-alpha-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology (Berl.) 197, 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin K., Bluthé R. M., McCusker R. H., Levade T., Moos F., Dantzer R., Kelley K. W. (2009). The type 1 TNF receptor and its associated adapter protein, FAN, are required for TNFalpha-induced sickness behavior. Psychopharmacology (Berl.) 201, 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. E., Dantzer R., Kelley K. W., McCusker R. H. (2011a). Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflammation 8, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. E., Lawson M., Dantzer R., Kelley K. W., McCusker R. H. (2011b). Insulin-like growth factor-I peptides act centrally to decrease depression-like behavior of mice treated intraperitoneally with lipopolysaccharide. J. Neuroinflammation 8, 179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Ricour C., Sommereyns C., Sorgeloos F., Michiels T. (2007). Type I interferon response in the central nervous system. Biochimie 89, 770–778 [DOI] [PubMed] [Google Scholar]
- Pecchi E., Dallaporta M., Jean A., Thirion S., Troadec J. D. (2009). Prostaglandins and sickness behavior: old story, new insights. Physiol. Behav. 97, 279–292 [DOI] [PubMed] [Google Scholar]
- Pifer R., Yarovinsky F. (2011). Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 27, 388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y., Arad M., Weiner I. (2012). Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology 62, 1273–1289 [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R., Ilyin S. E., Gayle D., Flynn M. C. (1998). Gram-negative and gram-positive bacterial products induce differential cytokine profiles in the brain: analysis using an integrative molecular-behavioral in vivo model. Int. J. Mol. Med. 1, 387–397 [DOI] [PubMed] [Google Scholar]
- Plattner F., Yarovinsky F., Romero S., Didry D., Carlier M. F., Sher A., Soldati-Favre D. (2008). Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3, 77–87 [DOI] [PubMed] [Google Scholar]
- Quan N., Banks W. A. (2007). Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735 [DOI] [PubMed] [Google Scholar]
- Raedler T. J. (2011). Inflammatory mechanisms in major depressive disorder. Curr. Opin. Psychiatry 24, 519–525 [DOI] [PubMed] [Google Scholar]
- Raison C. L., Miller A. H. (2011). Is depression an inflammatory disorder? Curr. Psychiatry Rep. 13, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C. L., Demetrashvili M., Capuron L., Miller A. H. (2005). Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 19, 105–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C. L., Borisov A. S., Majer M., Drake D. F., Pagnoni G., Woolwine B. J., Vogt G. J., Massung B., Miller A. H. (2009). Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol. Psychiatry 65, 296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relja B., Schwestka B., Lee V. S., Henrich D., Czerny C., Borsello T., Marzi I., Lehnert M. (2009). Inhibition of c-Jun N-terminal kinase after hemorrhage but before resuscitation mitigates hepatic damage and inflammatory response in male rats. Shock 32, 509–516 [DOI] [PubMed] [Google Scholar]
- Sakata A., Ochiai T., Shimeno H., Hikishima S., Yokomatsu T., Shibuya S., Toda A., Eyanagi R., Soeda S. (2007). Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology 122, 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfers M., Sorkin L. (2008). Effect of cytokines on neuronal excitability. Neurosci. Lett. 437, 188–193 [DOI] [PubMed] [Google Scholar]
- Sheng R., Xu X., Tang Q., Bian D., Li Y., Qian C., He X., Gao X., Pan R., Wang C., et al. (2009). Polysaccharide of radix Pseudostellariae improves chronic fatigue syndrome induced by poly I:C in mice. Evid Based Complement. Alternat. Med. 2011, 840516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen B. B., Duman C. H., Simen A. A., Duman R. S. (2006). TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol. Psychiatry 59, 775–785 [DOI] [PubMed] [Google Scholar]
- Srinivasan D., Yen J. H., Joseph D. J., Friedman W. (2004). Cell type-specific interleukin-1beta signaling in the CNS. J. Neurosci. 24, 6482–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V., D’Arcangelo G., Grassi F., Tarroni P., Palmieri G., Santoni A., Eusebi F. (1992). Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci. Lett. 146, 176–178 [DOI] [PubMed] [Google Scholar]
- Teeling J. L., Felton L. M., Deacon R. M., Cunningham C., Rawlins J. N., Perry V. H. (2007). Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav. Immun. 21, 836–850 [DOI] [PubMed] [Google Scholar]
- Teeling J. L., Cunningham C., Newman T. A., Perry V. H. (2010). The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav. Immun. 24, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam A. M., Bol J. G., Gaykema R. P., Goehler L. E., Maier S. F., Watkins L. R., Tilders F. J. (2000). Vagotomy does not inhibit high dose lipopolysaccharide-induced interleukin-1β immunoreactivity in rat brain and pituitary gland. Neurosci. Lett. 285, 169–172 [DOI] [PubMed] [Google Scholar]
- Viviani B., Bartesaghi S., Gardoni F., Vezzani A., Behrens M. M., Bartfai T., Binaglia M., Corsini E., Di Luca M., Galli C. L., et al. (2003). Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 23, 8692–8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B., Gardoni F., Marinovich M. (2007). Cytokines and neuronal ion channels in health and disease. Int. Rev. Neurobiol. 82, 247–263 [DOI] [PubMed] [Google Scholar]
- Wan W., Janz L., Vriend C. Y., Sorensen C. M., Greenberg A. H., Nance D. M. (1993). Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res. Bull. 32, 581–587 [DOI] [PubMed] [Google Scholar]
- Wang J., Dunn A. J., Roberts A. J., Zhang H. (2009). Decreased immobility in swimming test by homologous interferon-alpha in mice accompanied with increased cerebral tryptophan level and serotonin turnover. Neurosci. Lett. 452, 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins L. R., Wiertelak E. P., Goehler L. E., Mooney-Heiberger K., Martinez J., Furness L., Smith K. P., Maier S. F. (1994). Neurocircuitry of illness-induced hyperalgesia. Brain Res. 639, 283–299 [DOI] [PubMed] [Google Scholar]
- Wheeler D., Knapp E., Bandaru V. V., Wang Y., Knorr D., Poirier C., Mattson M. P., Geiger J. D., Haughey N. J. (2009). Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J. Neurochem. 109, 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky F., Zhang D., Andersen J. F., Bannenberg G. L., Serhan C. N., Hayden M. S., Hieny S., Sutterwala F. S., Flavell R. A., Ghosh S., et al. (2005). TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 [DOI] [PubMed] [Google Scholar]
- Yirmiya R. (1996). Endotoxin produces a depressive-like episode in rats. Brain Res. 711, 163–174 [DOI] [PubMed] [Google Scholar]
- Yirmiya R., Goshen I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 25, 181–213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.