Abstract
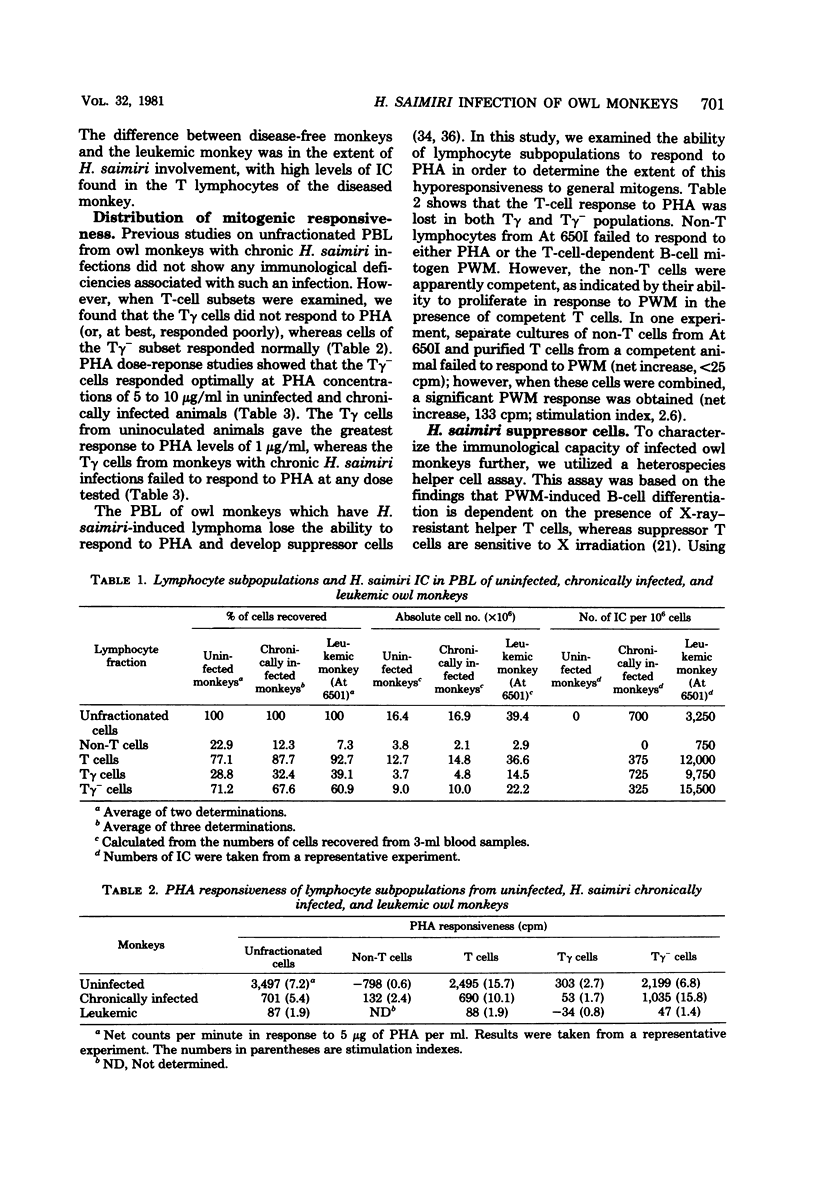
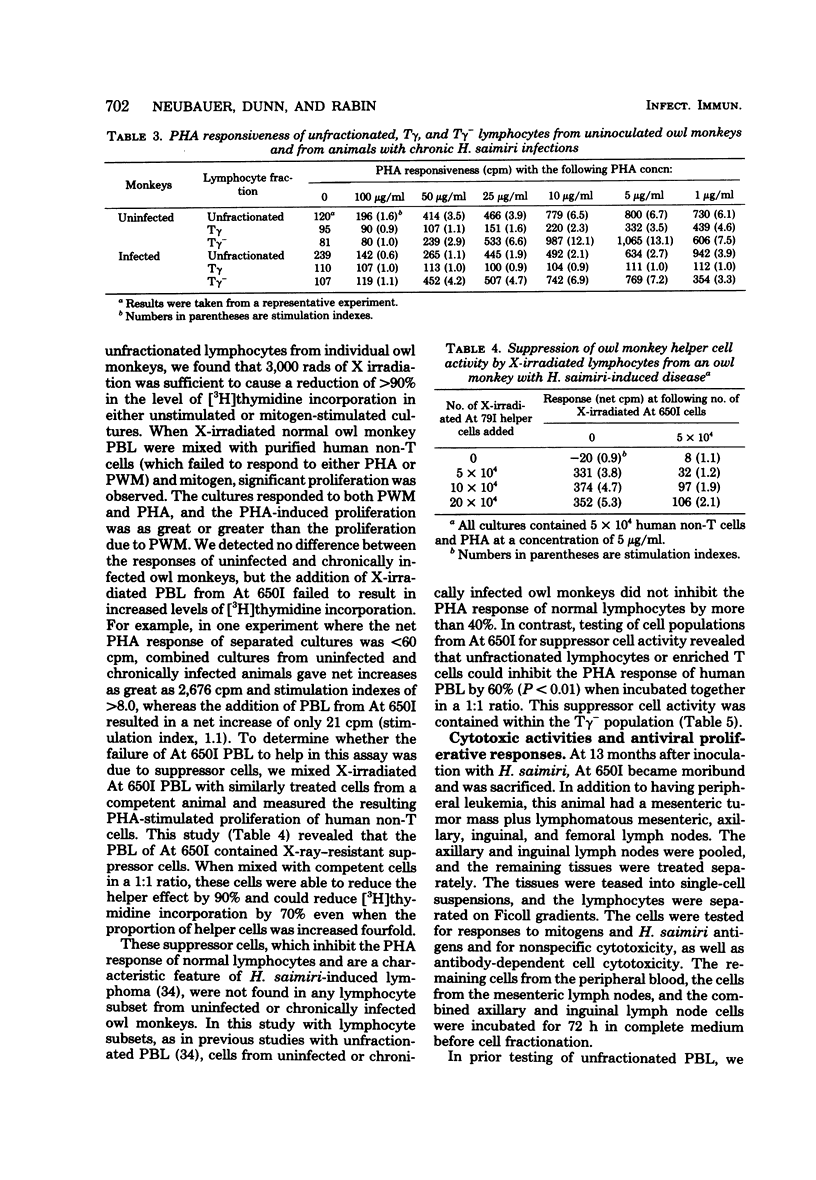
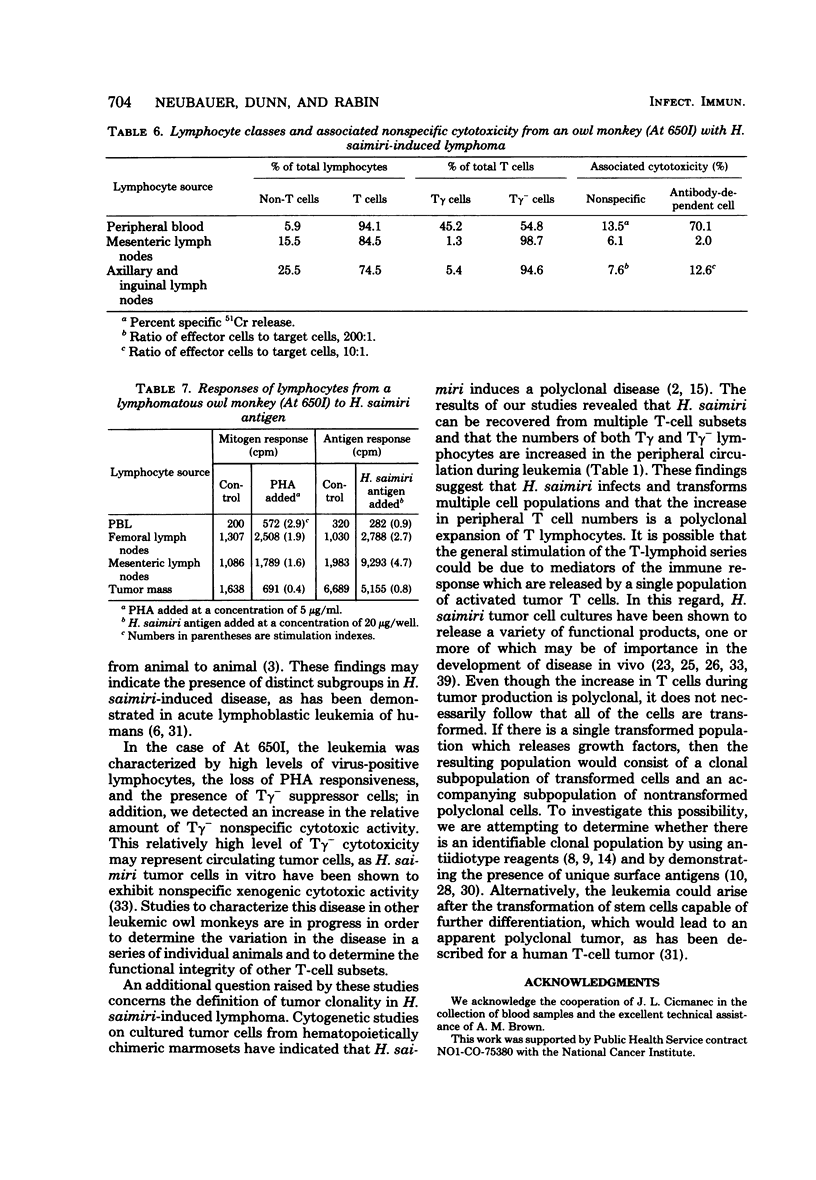
We examined the association of Herpesvirus saimiri with lymphocyte subsets and the functional integrity and distribution of these populations in owl monkeys with chronic, disease-free infections, in uninoculated, control animals, and in one monkey with H. saimiri-induced lymphoma. The lymphocyte subpopulations examined included total T cells, T cells with receptors for the Fc portion of immunoglobulin G (the Tγ cells), T cells lacking this receptor (the Tγ− population), and non-T cells. These studies showed that in chronically infected monkeys, H. saimiri was found in both Tγ and Tγ− populations and that the relative distribution of lymphocyte subsets was not different than the relative distribution in normal animals. The peripheral blood of the one leukemic animal studied showed an increase in total T cells, and both Tγ and Tγ− cells were increased in number and contained recoverable H. saimiri. In animals with chronic infections, which previously were thought to be immunologically normal, we showed that the Tγ cells had lost the ability to respond to phytohemagglutinin. When the level of nonspecific cytotoxic activity was examined, we found that the lymphocytes from infected animals were as active as those from uninfected monkeys and that this activity was maintained at normal levels during disease. In the leukemic blood there was a relative increase in the cytotoxic activity of the Tγ− cells. The Tγ− cells obtained from leukemic blood lacked the ability to respond to phytohemagglutinin and could suppress the phytohemagglutinin response of normal cells. This suppressor cell activity was resistant to 3,000 rads of X irradiation. We also found that cells reactive to H. saimiri antigens could be demonstrated in the lymph nodes but not in the peripheral circulation of the lymphomatous monkey.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. R., Orr T., Ablashi D. V., Pearson G. R., Rabin H., Luetzeler J., Loeb W. F., Valerio M. G. Chronic Herpesvirus saimiri infection in an owl monkey. J Natl Cancer Inst. 1976 May;56(5):1069–1071. doi: 10.1093/jnci/56.5.1069. [DOI] [PubMed] [Google Scholar]
- Chu E. W., Rabson A. S. Chimerism in lymphoid cell culture line derived from lymph node of marmoset infected with Herpesvirus saimiri. J Natl Cancer Inst. 1972 Mar;48(3):771–775. [PubMed] [Google Scholar]
- Cicmanec J. L., Loeb W. F., Valerio M. G. Lymphoma in owl monkeys (Aotus trivirgatus) inoculated with Herpesvirus saimiri: clinical, hematologic and pathologic findings. J Med Primatol. 1974;3(1):8–17. doi: 10.1159/000459960. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta. 1978 Oct 27;516(2):193–230. doi: 10.1016/0304-419x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Jondal M., Svedmyr E., Klein E., Singh S. Killer T cells in a Burkitt's lymphoma biopsy. Nature. 1975 May 29;255(5507):405–407. doi: 10.1038/255405a0. [DOI] [PubMed] [Google Scholar]
- Jones V. E., Graves H. E., Orlans E. The detection of F(ab')2-related surface antigens on the thymocytes of children. Immunology. 1976 Feb;30(2):281–288. [PMC free article] [PubMed] [Google Scholar]
- Lea T., Førre O. T., Michaelsen T. E., Natvig J. B. Shared idiotypes of human peripheral blood B and T lymphocytes. J Immunol. 1979 Jun;122(6):2413–2417. [PubMed] [Google Scholar]
- Levy R., Dilley J., Fox R. I., Warnke R. A human thymus-leukemia antigen defined by hybridoma monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6552–6556. doi: 10.1073/pnas.76.12.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Lum L. G., Muchmore A. V., Keren D., Decker J., Koski I., Strober W., Blaese R. M. A receptor for IgA on human T lymphocytes. J Immunol. 1979 Jan;122(1):65–69. [PubMed] [Google Scholar]
- Ma N. S., Jones T. C., Miller A. C., Morgan L. M., Adams E. A. Chromosome polymorphism and banding patterns in the owl monkey (Aotus). Lab Anim Sci. 1976 Dec;26(6 Pt 2):1022–1036. [PubMed] [Google Scholar]
- Marchalonis J. J., Warr G. W., Wang A. C., Burns W. H., Burton R. C. An Fab-related surface component of some normal and neoplastic human and marmoset T cells. Demonstration, functional analysis and partial characterization. Mol Immunol. 1980 Jul;17(7):877–891. doi: 10.1016/0161-5890(80)90036-x. [DOI] [PubMed] [Google Scholar]
- Marczynska B., Falk L., Wolfe L., Deinhardt F. Transplantation and cytogenetic studies of Herpesvirus saimiri-induced disease in Marmoset monkeys. J Natl Cancer Inst. 1973 Feb;50(2):331–337. doi: 10.1093/jnci/50.2.331. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., Daniel M. D., Blake B. J., Garcia F. G. Acute lymphocytic leukemia in owl monkeys inoculated with herpesvirus saimiri. Science. 1971 Mar 19;171(3976):1161–1163. doi: 10.1126/science.171.3976.1161. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A. Hunan T cell subpopulations in normal and pathologic conditions. Immunol Rev. 1979;45:163–193. doi: 10.1111/j.1600-065x.1979.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Webb S. R., Pearl E. R., Lydyard P. M., Grossi C. E., Lawton A. R., Cooper M. D. Imbalances in T cell subpopulations associated with immunodeficiency and autoimmune syndromes. Eur J Immunol. 1977 Oct;7(10):696–700. doi: 10.1002/eji.1830071009. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Poplack D. G., Holiman B. J., Henkart P. A. ADCC against human erythrocyte target cells: role of the anti-target cell antibodies in determining lymphocyte killer activity. Clin Exp Immunol. 1979 Mar;35(3):447–453. [PMC free article] [PubMed] [Google Scholar]
- Neubauer R. H., Rabin H., Dunn F. E. Antiviral cell-mediated immune responses and effect of chromosome polymorphism in Herpesvirus saimiri-infected monkeys. Infect Immun. 1980 Feb;27(2):549–555. doi: 10.1128/iai.27.2.549-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer R. H., Wallen W. C., Rabin H. Inhibition of the mitogenic response of normal peripheral lymphocytes by extracts or supernatant fluids of a Herpesvirus saimiri lymphoid tumor cell line. Infect Immun. 1975 Nov;12(5):1021–1028. doi: 10.1128/iai.12.5.1021-1028.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R., Orr T., Rabin H., Cicmanec J., Ablashi D., Armstrong G. Antibody patterns to Herpesvirus saimiri-induced antigens in owl monkeys. J Natl Cancer Inst. 1973 Dec;51(6):1939–1943. doi: 10.1093/jnci/51.6.1939. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Ritz J., Lazarus H., Costello S. B., Sallan S., Schlossman S. F. Leukemia-associated antigens in ALL. Blood. 1979 Dec;54(6):1240–1248. [PubMed] [Google Scholar]
- Prevost J. M., Pearson G. R., Wallen W. C., Rabin H., Qualtiere L. F. Antibody responses to membrane antigens in monkeys infected with Herpesvirus saimiri. Int J Cancer. 1976 Nov 15;18(5):679–686. doi: 10.1002/ijc.2910180517. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Golde D. W. Helper and suppressor t-lymphocyte leukemia in ataxia telangiectasia. N Engl J Med. 1979 Mar 29;300(13):700–704. doi: 10.1056/NEJM197903293001303. [DOI] [PubMed] [Google Scholar]
- Shaw S., Pichler W. J., Nelson D. L. Fc receptors on human T-lymphocytes. III. Characterization of subpopulations involved in cell-mediated lympholysis and antibody-dependent cellular cytotoxicity. J Immunol. 1979 Feb;122(2):599–604. [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H., Cicmanec J. L. Nonimmune rosette formation by lymphoma and leukemia cells from Herpesvirus saimiri-infected owl monkeys. J Natl Cancer Inst. 1973 Sep;51(3):967–975. doi: 10.1093/jnci/51.3.967. [DOI] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H. Evidence for suppressor cell activity associated with induction of Herpesvirus saimiri-induced lymphoma. Clin Exp Immunol. 1975 Dec;22(3):468–472. [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H. In vitro immunological characteristics of lymphoid cells derived from owl monkeys infected with Herpesvirus saimiri. J Med Primatol. 1974;3(1):41–53. doi: 10.1159/000459963. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Wright J., Falk L. A., Deinhardt F. Interferon production by simian lymphoblastoid cell lines. J Natl Cancer Inst. 1974 Jul;53(1):271–275. doi: 10.1093/jnci/53.1.271. [DOI] [PubMed] [Google Scholar]



