Abstract
Live imaging of animals infected with pathogenic microbes poses a contamination risk to equipment, personnel and other animals. A Caliper animal isolation chamber designed for the IVIS® Spectrum imaging system was tested as a containment device for mice infected with microbes assigned to animal biosafety level-3 (ABSL-3). A testing protocol was developed by adapting two published standards to test other equipment in high containment environments. The protocol included quantitative leak-testing of the high efficiency particulate air (HEPA) filters, soap bubble testing of the animal isolation chamber, and pressure decay testing of the complete containment system. HEPA filters were > 99.999% efficient (< 0.001% leakage). When attached to the Spectrum at the normal flow rate of oxygen/anesthetic mix (0.25 L/min), the chamber was positively pressurized at 0.11 inches of water (in H2O). No leaks were detected by soap bubble testing at flow rates of 0.25 L/min to 2.0 L/min, generating pressures up to 2.90 in H2O. (26-fold increase over normal operating pressure). The complete containment system passed pressure decay testing at 2.0 in H2O by sustaining 95% of the initial pressure over a 30 minute period.
The Caliper animal isolation chamber provides appropriate isolation for the IVIS® Spectrum imaging system. When used as a containment device, it must undergo periodic performance testing, as described here, since it operates under positive pressure. The chamber is an appropriate component of ABSL-3 containment when combined with proper administrative controls and work practices. The testing protocol described here can be used to validate containment devices for other imaging systems or animal species.
Keywords: animal biocontainment, biosafety, biosafety level-3
Introduction and Risk Assessment
The Regional Biocontainment Laboratory (RBL) at Duke University Medical Center was primarily funded by the National Institute of Allergy and Infectious Diseases. The facility was built to support basic and translational research for the development of drugs, vaccines, and diagnostics to protect society from emerging infections and biothreats. Many of the infectious microbes studied in the building require animal biosafety level-3 (ABSL-3) containment, as described by the NIH/CDC publication Biosafety in Microbiological and Biomedical Laboratories (CDC, 2007). This level of containment is typically reserved for indigenous or exotic agents that may cause serious or potentially lethal disease due to exposure by the inhalation route. Any physical containment device used in ABSL-3, like the animal chamber being evaluated as part of this study, must be capable of preventing the release of microorganisms into the laboratory environment under normal use conditions.
To minimize the number of animals needed for a particular study and to efficiently track disease progression, cell trafficking, and gene expression patterns in vivo, an IVIS® Spectrum (Caliper Life Sciences, Hopkinton, MA) whole animal imaging system was purchased and installed in an animal holding module in the RBL at Duke (Figure 1). The Spectrum uses patented optical imaging technology to facilitate non-invasive in vivo monitoring through an optimized set of high efficiency filters and spectral unmixing algorithms, which allow bioluminescent and fluorescent reporters across the blue to near-infrared wavelength region (Caliper, 2009). The imaging unit is calibrated to enable absolute quantification of the bioluminescent signal by measuring dark charge during down-time and running a self-calibration during start-up (Lim, 2009). In addition, this imaging technology allows single-view 3D tomography for both fluorescent and bioluminescent reporters that can be analyzed in an anatomical context.
Figure 1.
IVIS® Spectrum imaging system in an animal containment suite in the Regional Biocontainment Laboratory at Duke University Medical Center.
Animals infected with microbes assigned to ABSL-3 pose a potential risk to personnel, other animals, and the laboratory environment. Aerosol exposures by either whole body or nose-only routes contaminate the fur of experimental animals (data not shown). Infected animals may shed microbes through saliva, urine, or feces (CDC, 2007; Gomes-Keller, M.A., et al., 2005; Kruse & Wedum, 1965).
The Spectrum has an open imaging chamber with a moveable stage on which anesthetized animals are placed. The Spectrum was not constructed to provide physical containment of infectious materials. Ease of cleanability is an essential element of an ABSL-3 containment environment. Unfortunately, the surfaces within the chamber are difficult to decontaminate thoroughly because of configuration and composition. Some surfaces are essentially unreachable and are considered noncleanable. The RBL Safety Team concluded in its initial risk assessment that animals infected with microbes assigned to ABSL-3 could not be safely imaged on the Spectrum without the addition of a validated physical containment system. The system would be required to transmit light, provide gas anesthetic, be cleanable, and prevent contamination of the Spectrum unit, personnel, and laboratory environment.
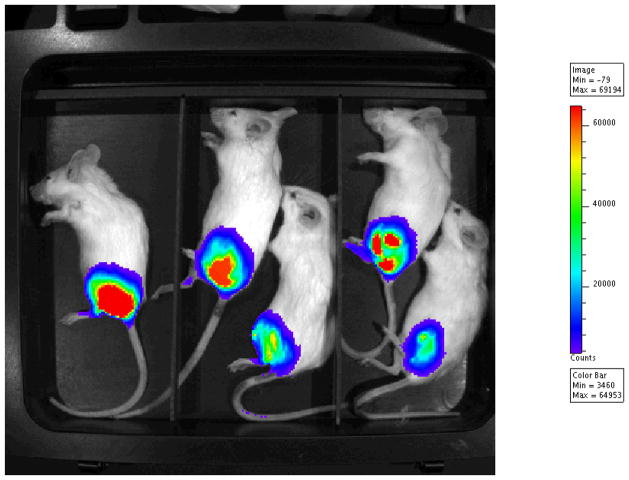
Based on these requirements, a Caliper XIC-3 Animal Isolation Imaging Chamber (AIIC, Caliper Life Sciences, Hopkinton, MA), which was designed to protect immune deficient mice from environmental pathogens, was evaluated. It was hypothesized that the chamber design features would provide simultaneous isolation and containment. The relatively simple design includes a rubber gasket between the chamber lid and base and anesthesia supply and exhaust lines that are protected by ISOGARD® HEPA filters (Figure 2). The chamber lid is made of Plexiglas® and transmits light in the visible spectrum. Figure 3 shows mice contained in the AIIC during bioluminescent imaging. The chamber shown in Figure 3 is segmented in five sections by the use of plastic dividers provided by the manufacturer to minimize signal interference from animal to animal.
Figure 2.
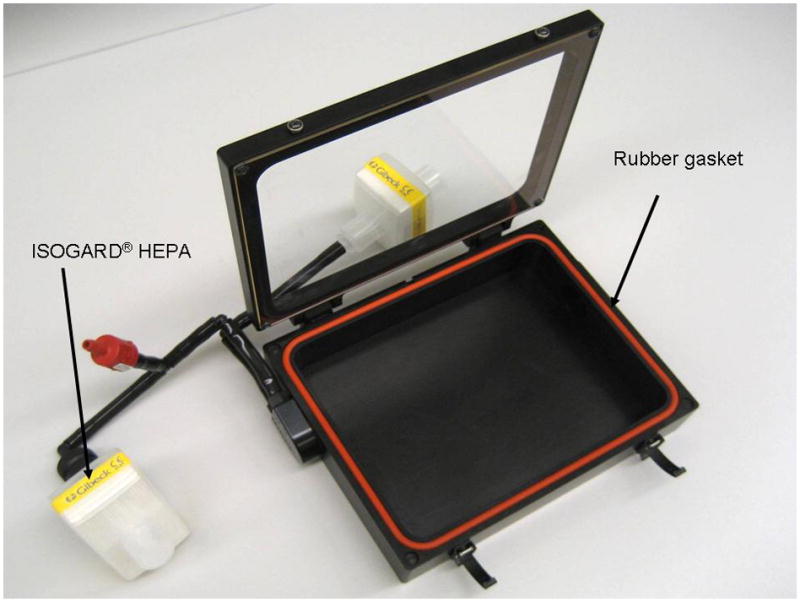
Animal isolation imaging chamber with attached ISOGARD® HEPA filters.
Figure 3.
Animal imaging using the AIIC with the IVIS® Spectrum. Mice were imaged 72 hours after infection with an infectious agent expressing light-producing luciferase.
The goal of the present study was to demonstrate that the AIIC could be used as a containment device for mice during in vivo imaging experiments. If validated, the device could be used as a primary barrier in high-containment to protect workers and the environment from accidental exposure to infectious microbes. In the absence of a published standard for testing the isolation chamber, a test protocol was developed based on a combination of test standards that are commonly used in high-containment environments. This report describes three tests that were conducted to evaluate the effectiveness of the ISOGARD® HEPA filters: 1) a quantitative check of the HEPA filters; 2) a leak test of the animal isolation chamber while connected to the Spectrum’s anesthesia equipment under normal and enhanced air pressures; and 3) a pressure decay test of the complete containment system at 2.0 inches of water (in H2O).
Methods and Results
HEPA Filter Leak Testing
Separate supply and exhaust hoses allow the chamber to be connected to an anesthesia machine. The hoses are protected by in-line HEPA filters that protect the animals from environmental contaminants and the environment from pathogenic microbes associated with the animals.
Poly-alpha olefin (PAO) smoke, brand name Emery 3004 (Air Techniques International, Owings Mills, MD), was used as a surrogate for “infectious aerosols” for testing the ISOGARD® HEPA filters (Hudson RCI, Research Triangle Park, NC). A ¼ Laskin nozzle cold smoke generator (PAT Model MM250, Raleigh, NC) was used to produce polydispersed smoke, with an expected size range of 0.1 – 1.0 μm. The filters were tested in accordance with EST-RP-CC034.2: Testing HEPA and ULPA Filters (IEST, 2005) by constructing an apparatus consisting of a 65 cfm blower, six feet of four-inch flex duct with test port, and a reducer. The HEPA filters to be tested were attached, one at a time, to the outlet of the test apparatus. While the blower was running, PAO smoke was introduced into the apparatus by the aerosol generator. An upstream aerosol concentration (80 μg/L) was measured using an aerosol photometer (ATI Model TDA-2E, Air Techniques International, Owins Mills, MD) and confirmed to be on-scale, meaning the instrument was capable of indicating 100% of the upstream concentration. A downstream sample was then read and compared to the upstream measurement to determine percent leakage through the test filter. A leak allowance criterion of 0.01% was selected; thus, the ISOGARD® HEPA filters had to be at least 99.99% efficient in order to pass. Traditionally, HEPA filtration has been defined as removal of 0.3 μm particles at an efficiency of at least 99.97% (CDC, 2000). Three filters demonstrated leakage < 0.001%, documenting an overall efficiency of 99.999%.
Pressure and Bubble-Testing of the AIIC While Connected to the IVIS® Spectrum
Animals must be immobilized by anesthesia during imaging studies. When connected to the Spectrum’s gas anesthesia system, the AICC is positively pressurized. To measure pressure within the containment system at different flow rates, a Magnehelic gauge was attached (Dwyer Instruments, Inc., Michigan City, IN) between the AIIC and the supply HEPA. Pressures were recorded at the normal operating flow rate of 0.25 L/min and at higher flow rates of 1.0 L/min, 1.5 L/min, and 2.0 L/min (Table 1). To perform a leak-test of the complete containment system under normal operating conditions (i.e. AIIC plus filters), the Magnehelic gauge was removed and a soap bubble method was employed, similar to that described in NSF/ANSI 49 Standard for leak-testing biological safety cabinets with positive pressure plenums (NSF/ANSI, 2009). Party Bubbles (Target Corporation, Minneapolis, MN) were carefully spread across all seams, penetrations, and connections using a small paint brush. Small leaks would be indicated by bubble formation. Large leaks would be identified when bubble fluid was blown from a hole without forming a bubble or by hearing a hissing or crackling noise when wiping fluid over a hole. A successful positive control test was conducted by slowly pulling the tubing off of a barbed connector, resulting in visible bubble formation. Testing was conducted at each of the four flow rates listed above. Successful soap bubble testing was defined by the absence of detectable leaks.
Table 1.
Pressure and bubble-test data
| Flow Rate (L/min) | Pressure (in H2O) | Leaks/Bubbles? |
|---|---|---|
| 0.25 | 0.11 | None detected |
| 1.00 | 1.00 | None detected |
| 1.50 | 1.90 | None detected |
| 2.00 | 2.90 | None detected |
No leaks were detected at the normal flow rate or at increased flow rates up to 2.0 L/min (Table 1). The highest flow rate tested was eight times higher than normal and generated a pressure more than 26 times the normal operating pressure of the containment system.
Pressure-Decay Testing of the Complete Containment System
The third test method was a pressure decay test of the complete containment system (AIIC plus filters) at 2.0 in H2O. A successful test was defined as a pressure loss of no more than 10% (0.2 in H2O) over 30 minutes. This approach is similar to the NSF/ANSI 49 Standard for leak-testing biological safety cabinets with positive pressure plenums (NSF/ANSI, 2009).
The complete containment system with an attached Magnehelic gauge was connected to a compressed air line (Figure 4). Air was added to the system until the pressure gauge indicated an internal pressure of 2.0 in H2O and a timer was started. At three different times during the test period, the system was lifted off the work surface and placed back down to simulate “stress” on seams and connections, as would be expected when transporting infected contained animals from a biological safety cabinet to the IVIS® imaging chamber. After 30 minutes had elapsed, the internal pressure of the containment system was 1.9 in H2O (a total pressure loss of only 5%), indicating a successful pressure decay testing.
Figure 4.
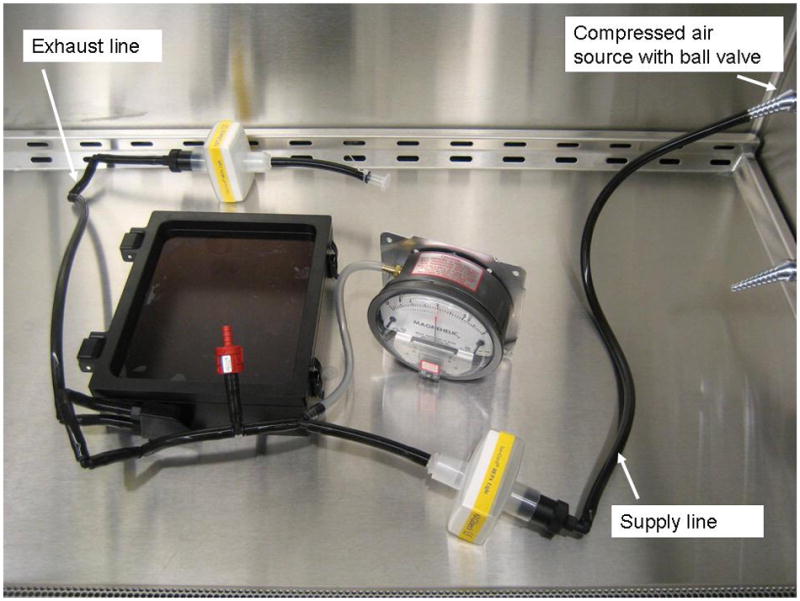
Pressure decay testing set-up for the complete containment system.
Discussion
This study has demonstrated that the AIIC with ISOGARD® HEPA filters attached to separate supply and exhaust hoses provides effective physical isolation and containment. The AIIC is an appropriate component of ABSL-3 containment when combined with proper administrative controls and work practices.
A formal risk assessment process is essential prior to all laboratory and animal research with hazardous materials (CDC, 2007). For imaging studies with infected animals, this process should consider the risks associated with the specific microbe and the degree of contamination expected with the experimental animal. Risk assessment will determine the proper physical containment, administrative controls, and work practices to conduct a specific project safely. The RBL at Duke has Standard Operating Procedures (SOPs) for ABSL-3 work with the IVIS® Spectrum which include induction of anesthesia in a class II, type A2 biological safety cabinet (BSC) that is thimble-connected to the exhaust system, containment of mice inside the AIIC for transfer and imaging, surface decontamination of the exterior of the AIIC prior to removal from the BSC, and surface decontamination of all potentially contaminated work surfaces after each procedure. This includes a thorough decontamination of the interior and exterior of the AIIC by spraying with an appropriate disinfectant while in the BSC, wiping all reachable surfaces, and then transporting it to a tub of disinfectant solution in the laboratory sink. The AIIC is immersed in the disinfectant for an appropriate time as outlined in the relevant agent-specific SOP. Personal protective equipment includes dedicated clothing, double-gloves, a Tyvek® coverall, Tyvek® sleeve-covers, and a powered air purifying respirator. A risk assessment is repeated for each new microbe.
When used as a physical containment device in an ABSL-3 environment, the AIIC must undergo periodic performance testing, as described here, since it operates under positive pressure. During each use, the chamber is opened, closed, and transported. Each of these movements creates stress on seams, connections and other potential leak-points. Pressure decay testing, as described here, is recommended prior to each experiment using a microbe assigned to ABSL-3.
This study details a procedure for validation of animal isolation chambers. The approach to performance testing described here is generally applicable to other containment devices designed for other imaging systems or different animal species; however, results are directly applicable only to the product tested. Performance testing of animal containment devices will improve safety and expand the scope of studies that can be completed at ABSL-3.
Acknowledgments
The authors would like to acknowledge Mr. Mike Rose for his technical expertise and help with filter testing, Dr. Wayne Thomann for his thoughtful feedback on this manuscript, Ms. Kris Riebe for sharing her animal expertise and assisting with the project, and Drs. David Pickup and Elizabeth Ramburg for allowing access to their research mice expressing luciferase.
This publication was supported in part by NIH grants to the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (AI057157), the Regional Biocontainment Laboratory at Duke (AI058607), and the Duke Center for Translational Research (AI051445).
Footnotes
Disclaimer
The authors have no financial conflicts of interest.
References
- Caliper Life Sciences, Inc. (Caliper) [Accessed online 2009];IVIS® Spectrum product brochure. 2009 Available at: www.caliperls.com/assets/011/6708.pdf.
- Centers for Disease Control and Prevention (CDC) [Accessed online 2009];Biosafety in Microbiological and Biomedical Laboratories. (5). 2007 Available at: www.cdc.gov/OD/ohs/biosfty/bmbl5/bmbl5toc.htm.
- Centers for Disease Control and Prevention (CDC) [Accessed online 2009];Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets. (2). 2000 Available at: www.cdc.gov/od/ohs/biosfty/bsc/bsc.htm.
- Gomes-Keller MA, Tandon R, Gonczi E, Meli ML, Hofmann-Lehmann R, Lutz H. Shedding of Feline Leukemia Virus RNA in Saliva is a Consistent Feature in Viremic Cats. Veterinary Microbiology. 2006;112:11–21. doi: 10.1016/j.vetmic.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Institute of Environmental Sciences and Technology (IEST) [Accessed online 2009];Testing HEPA and ULPA Filters. 2005 Available for purchase at: www.iest.org.
- Kruse RL, Wedum AG. United States Army Biological Laboratories Fort Detrick, Miscellaneous Publication 12. [Accessed online 2010];Recovery of Specific Microorganisms from Urine and Feces of Infected Animals. 1965 Available at: www.dtic.mil/cgi-bin/GetTRDoc?AD=AD625256&Location=U2&doc=GetTRDoc.pdf.
- Lim E, Modi K, Kim J. In vivo bioluminescent imaging of mammary tumor using IVIS spectrum. Journal of Visualized Experiments. 2009:26. doi: 10.3791/1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSF International Standard/American National Standard 49. (NSF/ANSI) Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. 2009. [Google Scholar]