Case notes
Support of the uterus and upper vagina is one of the most critical factors in the etiology of pelvic organ prolapse.1,2 This apical or level-1 support is provided by the cardinal and uterosacral ligaments.3 Cadaver dissection has provided a rich body of useful information about these structures, but anatomical observations are limited by distortions due to embalming artifacts and the advanced age of most specimens.4–6
Modern magnetic resonance (MR) imaging has made it possible to study these structures in living women and perform comprehensive in situ analyses in women with and without pelvic floor disorders. The identification of these structures and details of the uterosacral ligament have been described.7,8
However, important structural specifics of the cardinal ligament’s attachments and its relationship with the uterosacral ligament, as seen in MR imaging of living women, have not been fully established. We used MR cross-sectional imaging and 3-dimensional (3D) modeling to study characteristic features of the cardinal ligament. At the same time, we examined anatomic distinctions and structural relationships between the cardinal and uterosacral ligaments relevant to their role in apical support.
This study was actually a secondary analysis of MR images made by the Pelvic Floor Research Group at the University of Michigan. Twenty pelvic high-resolution MR scans were examined to identify the cardinal and uterosacral ligaments, respectively, and the associations between them. Participants were women with normal pelvic organ support who volunteered as controls for a study on pelvic floor anatomy. They had to be asymptomatic and 18 years of age or older with an intact uterus and cervix. In addition, they had to have no history of surgery for pelvic organ prolapse or incontinence.
We selected the 20 most recent scans that contained the full extent of the cardinal and uterosacral ligaments and were without motion artifact. This sample size proved adequate to reveal typical structural features and relationships in normal women.9, 10 All subjects gave informed written consent in accordance with the Institutional Review Board’s guidelines.
Static proton-density MR images were obtained in the supine position in axial, coronal, and sagittal planes with a body coil. Multiplanar 2-dimensional proton-density pelvic images (echo time, 15 ms; repetition time, 4000 ms) were acquired with a 3 Tesla superconducting magnet (General Electric Signa Horizon LX, GE Healthcare, Waukesha, WI). The fields of view in axial and coronal images were 16 × 16 cm; the sagittal images, 20 × 20 cm. Each image had a slice thickness of 4 mm with a 1 mm gap between slices.
Detailed slice-by-slice examination of axial, sagittal, and coronal images of the subjects was conducted using the original DICOM (Digital Imaging and Communications in Medicine) through the RadPix software interface (Weadock Software, LLC, Ann Arbor, MI). Typical features of the apical support components in each plane were identified. Next, the visibility of the characteristic features was tallied and a plane-specific percentage visibility was obtained.
One exemplar MR data set was selected for 3D reconstruction. Axial, coronal, and sagittal images for this particular subject were downloaded in the Slicer 3.4 software (Brigham and Women’s Hospital, Boston, MA and Massachusetts Institute of Technology, Cambridge, MA). Each image plane was aligned automatically with the other 2, and resulting image transforms were registered and hardened. The tracings on the MR scans were strictly based on anatomical definitions of the cardinal and uterosacral ligaments obtained from a literature review, from our senior author’s anatomical dissection experience, and the MR appearance of these ligaments in the literature.4,5,7,8,11,12
Given that the cardinal ligament is the perivascular sheath of the internal iliac artery, which goes to the genital tract, the origin of the anterior trunk of the internal iliac vessels, located at the upper margin of the greater sciatic foramen, was assumed to be the pelvic attachment for the cardinal ligament.5,11–13 This attachment was considered a regional anatomical landmark rather than a fixed one. Thus, on the coronal MR scans, we first searched for the anterior trunk of the internal iliac artery to pinpoint the proximal attachment of the cardinal ligament. If this vessel was not visualized, we used the upper margin of the greater sciatic foramen, visible on all MR scans, as our reference region for the proximal attachment. Slicer 3.4 software generated 3D models from the tracings of the cardinal and uterosacral ligaments in the plane in which each structure was best visualized. The goal was to have a visual 3D appreciation of the orientation and relationships of the apical support elements.
CONCLUSIONS
The study population had a mean age of 56.3 ± 7.2 (standard deviation) years, a mean body mass index of 27.9 ± 4.3 kg/m2, and a mean parity of 2.1 ± 1.0. No subject had any urinary or fecal incontinence. Bulging symptoms of pelvic organ prolapse were not present in the study group. The lowest Pelvic Organ Prolapse-Quantification (POPQ) vaginal points were at least 1 cm above the hymenal ring for every woman.
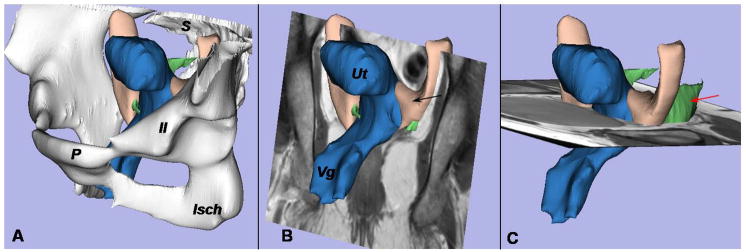
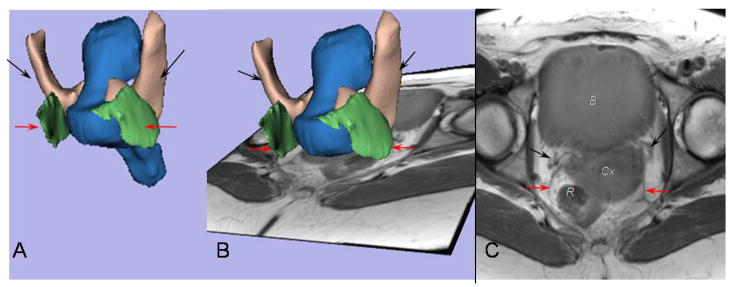
The general orientation, origins, and insertions of the ligaments are seen in the 3D model shown in Figure 1A, where the bony pelvis is in place with the pubis anteriorly and the sacrum posteriorly. The 2 apical supporting ligaments lie in perpendicular planes. The coronal plane (Figure 1B), used for cardinal ligament tracing, and the axial one (Figure 1C), used for the uterosacral ligament tracing, have been integrated in the model to show the relative positions of the scan planes and ligaments in 3D space.
Figure 1.
A 3- dimensional (3D) model of the cardinal ligament and uterosacral ligament is oriented relative to the coronal and axial planes. A, The bony pelvis, including the pubis (P), ilium (Il), ischium (Isch), and sacrum (S), is displayed. B, Positioning of the uterus (Ut), cardinal ligament (black arrow), and vagina (Vg) can be seen in this coronal image. C, Note the location of the uterosacral ligament (red arrow) in this axial MR scan.
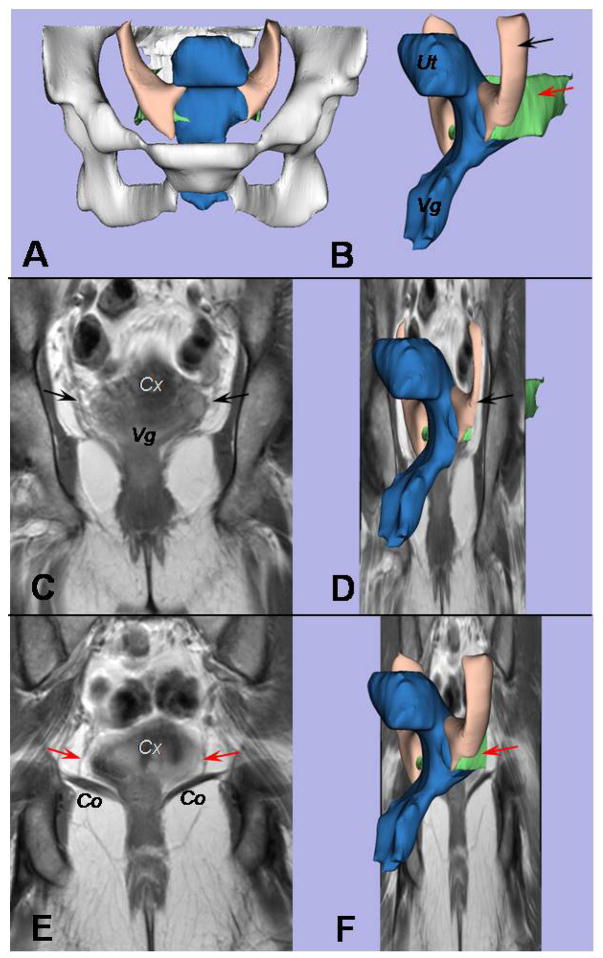
Despite diverging origins at the pelvic sidewalls, the cardinal and uterosacral ligaments have fibers that are adjacent and intermingled at the cervix and the upper vagina (Figure 2). The structures are continuous with no clear boundary between them, as displayed in Figures 2C and 2E; the uterosacral ligament can be visualized when the view is moved 10 mm dorsal in the posterior compartment from where the cardinal ligament is shown in Figure 2C (Figure 2E).
Figure 2.
A coronal view of the cardinal and uterosacral ligaments is provided. A, The bony pelvis can be seen in this front view of the 3D model. B, An oblique left-side view displays the uterus (Ut) and vagina (Vg) in blue, the uterosacral ligament in green (red arrow), and the cardinal ligament in cream (black arrow). C, A coronal scan is at the level of the cardinal ligament; the cervix (Cx) and vagina (Vg) are marked. D, This is where the structures in the 3D model shown in Figure 2B would be situated in the plane. E, The uterosacral ligament (red arrows) can be seen when the view is moved 10 mm dorsal from where the cardinal ligament is seen in Figure 2C. The cervix (Cx) and coccygeus muscle (Co) are shown. F, Again, the 3D model shown in Figure 2B is shown within the coronal plane.
From the 20 MR scans analyzed, the cardinal ligament, as its definition indicates, proved to be a mesentery-like structure in 100% of cases. Its distal endpoint consisted of fibers going to the cervix and upper vagina, and this was visible in all 20 cases. In 6 cases (30%), fibers going to the bladder were identified as well (Figure 3). The characteristic that best allowed identification of the cardinal ligament was its web-like aspect, which surrounds the axis of the internal iliac artery’s anterior trunk and its divisions. These vessels—and thus, the ligament—go in a cranial-to-caudal direction to the genital tract from their origin on the pelvic sidewall at the level of the upper margin of the greater sciatic foramen. The region of the cardinal ligament’s proximal attachment was the origin of the internal iliac artery’s anterior trunk in 7 subjects (35%) (Figure 4). In contrast, the upper margin of the greater sciatic foramen served as the proximal attachment in the 13 remaining subjects (65%).
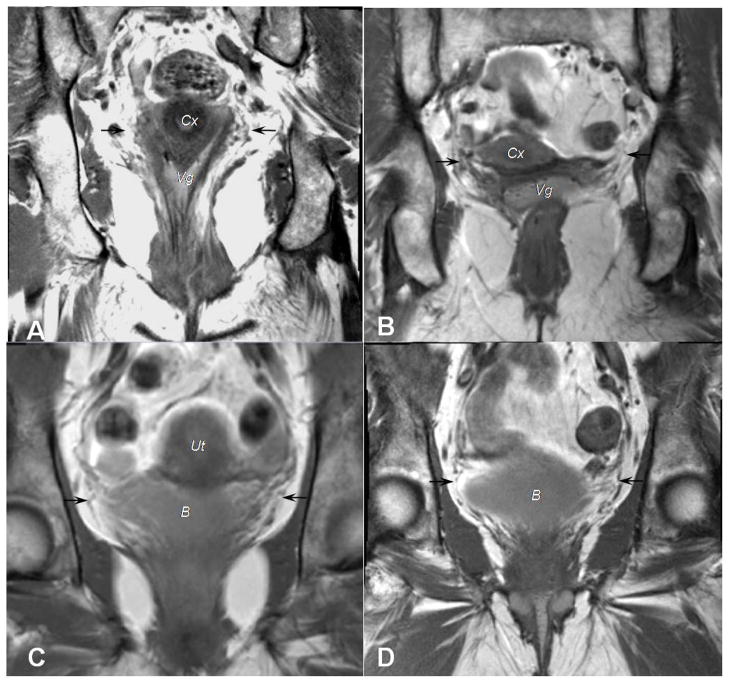
Figure 3.
Coronal scans from 4 different women illustrate attachments of the cardinal ligament to the pelvic organs. A, B, Black arrows point to the cardinal ligament’s insertions at both the cervix (Cx) and upper vagina (Vg). C,D, Fibers of the cardinal ligament, again identified with black arrows, continue to the bladder (B).
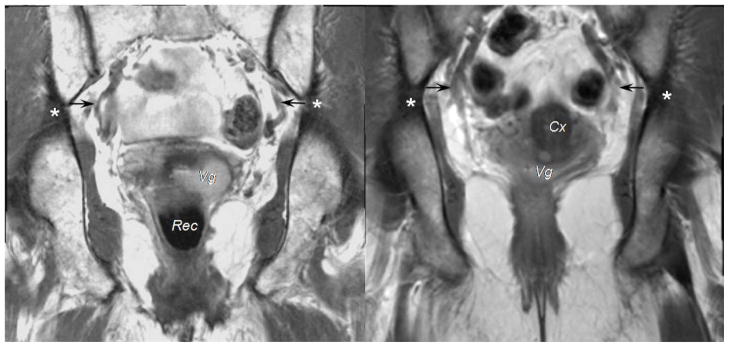
Figure 4.
On coronal scans of 2 different subjects, you can see the region of the cardinal ligament’s attachment to the pelvic wall (black arrow) near the origin of the internal iliac artery’s anterior trunk. This was seen in 7 patients. For 13 patients, the region of attachment was located at the upper level of the great sciatic foramen, as identified with asterisks. The vagina (Vg), rectum (Rec), and cervix (Cx) are labeled.
The geometry and structural relationships of the ligaments were clearly seen in the axial plane (Figure 5). The uterosacral and cardinal ligaments share a common region of insertion at the cervix and/or upper part of the vagina. Cardinal ligament fibers coming from the coronal (cranial-to-caudal) plane merge with the uterosacral ligament fibers traveling from the axial (ventral-to-dorsal) plane. The different pelvic sidewall origins and common distal insertions lead to divergent projections. Because the cardinal ligament’s fixation point is near the greater sciatic foramen, it has a more vertical trajectory from the cervix and vagina; the uterosacral ligament follows a dorsal course. Differences in texture between the cardinal ligament’s vascular sheath and the uterosacral ligament’s thin band-like structure allow their separate locations to be identified, but no distinct boundary exists between them.
Figure 5.
The cardinal and uterosacral ligaments mingle near the cervix and upper vagina. A, This is the back of the 3D model. The red arrows identify the uterosacral ligament, and the black arrows show the cardinal ligament. B, This MR scan is at the level of the cervix. C, Fibers of both ligaments converge towards the cervical-vaginal junction, where they are in close continuity. The bladder (B), the cervix (Cx), and the rectum (R) are noted.
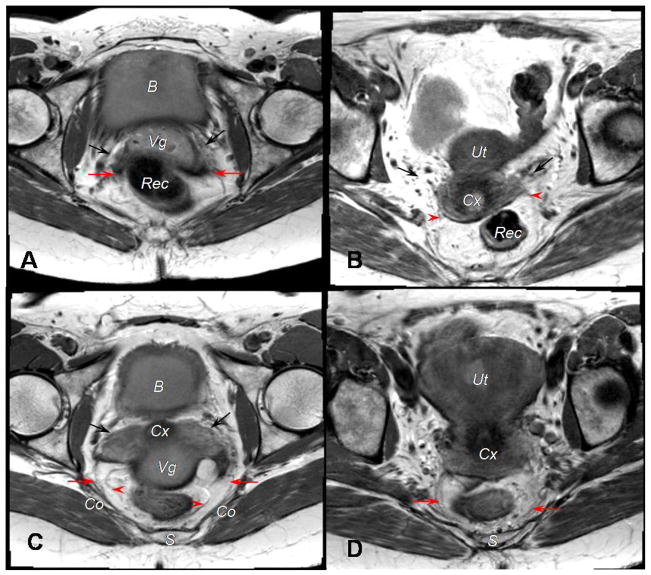
We were able to identify 19 (95%) ventral and dorsal attachments of the uterosacral ligament from the 20 MR scans analyzed. The uterosacral ligament was inserted at the upper vagina in 8 patients (42%); at the cervix in 2 patients (10.5%), and at both the cervix and vagina in 9 patients (47%) (Figures 6A and 6B). Its origins were at the coccygeus muscle/sacrospinous ligament in 14 subjects (73%), the presacral fascia in 4 women (21%), and the ischial spine in 1 (5%) patient (Figures 6C and 6D).
Figure 6.
Attachments of the uterosacral ligament on an axial MR image are viewed in scans from 4 different subjects. A, Ventral attachments are displayed. The black arrows indicate the cardinal ligament. Deep uterosacral fibers, as denoted by the red arrows, are inserted at the upper vagina. B, Again, ventral attachments are shown. The black arrows point to the cardinal ligament. Superficial uterosacral fibers, at the red arrowheads, are inserted at the cervix. C, Dorsal attachments are shown. The sacrospinous ligament-coccygeus muscle insertion of the deep uterosacral fibers are designated by red arrows; superficial uterosacral fibers, the red arrowheads. D, Another view of dorsal attachments is displayed. Presacral insertion of the uterosacral ligament is identified with red arrows. The bladder (B), vagina (Vg), rectum (Rec), cervix (Cx), uterus (Ut), sacrum (S), and coccygeus muscle (Co) are identified in different views.
No direct attachment to the sacrum was identified. Superficial uterosacral fibers corresponding to the peritoneal fold that covers the ligament were not consistently visible in all subjects. The uterosacral ligament was not seen in 1 subject because a large leiomyoma filled the posterior compartment, squeezing all lateral and posterior elements.
Our MR study of apical support in living women showed that the cardinal and uterosacral ligaments have distinct anatomical features. As noted, the web-like appearance of the cardinal ligament is provided by the blood vessels to the genital tract, a prominent component of its configuration, while the uterosacral ligament has a more distinct band-like aspect.8,12 The cardinal ligament is positioned in a cranial-to-caudal direction, whereas the uterosacral ligament is located in a ventral-to-dorsal direction. Yet, cardinal and uterosacral fibers merge together distally at the cervix and/or upper part of the vagina, creating the apical support. An angle is created between the 2 ligaments at the cervix, as each of them has a different 3D geometry within the pelvis.
Unlike the deep component of the uterosacral ligament, which is easily seen on MR imaging, the superficial uterosacral ligament, consisting mainly of the peritoneal endopelvic fascia, is seldom observed. While the cardinal ligament begins at the pelvic sidewall near the origin of the anterior trunk of the internal iliac artery, the deep uterosacral ligament comes from the coccygeus muscle/sacrospinous ligament junction in most patients.
The terminologies, anatomy, histology—even the existence—of the cardinal ligament are subjects of controversy. For the purpose of this study, we used the most current widely-accepted definition for identification of the cardinal ligament on MR imaging and for its modeling.12 Yabuki and colleagues showed that this entity was a mesentery-like structure covered by endopelvic fascia that was an extension of the internal iliac vessels’ perivascular sheath.12 In this way, it is a “visceral” ligament, the regional name for tissues that support an organ. Skeletal ligaments have a separate, dissimilar structure.
In order to identify and trace the cardinal ligament, the visceral branches of the anterior trunk of the internal iliac vessels, which course towards the cervix and vagina, were designated as the axis of the ligament, since the enveloping areolar tissue was not distinctly characterized (Figures 2–4). The functional origin of the cardinal ligament at the pelvic sidewall corresponds to the region where these vessels originate—at the upper border of the greater sciatic foramen.13
We saw the cardinal ligament in 100% of women in the study group, from its origin at either the anterior trunk of the internal iliac artery (35%) or the upper border of the greater sciatic foramen (65%) to its conclusion at the cervix and vagina. In 30% of cases, we identified fibers going to the bladder as well. Cardinal ligament fibers going to the cervix, vagina, and the bladder have been reported previously in cadaver-based dissection studies.12, 14 After analyzing the relationship between the pelvic organs and connective tissue bands, Peham and Amreich classified the cardinal ligament into independent bladder, vaginal-cervical, and rectal septa that did not cross each other.14 Thus, the fibers going to the bladder that we visualized on coronal MR scans probably relate to the bladder septa. Nevertheless, we could not find clear boundaries between the fibers going to different organs, and this might be because our MR study was not complicated by any dissection artifact.
Our inventory of anatomic findings, and the frequency with which these features can be seen in MR imaging, are consistent with data from the dissection-based anatomical literature, providing reassurance that our MR investigative method was accurate. We saw that the uterosacral ligament insertions are predominantly attached to the sacrospinous ligament-coccygeus junction proximally and to the cervix and vagina distally, as also observed by Umek in MR imaging.8
Anatomically, the uterosacral ligament’s consistency, thickness, and texture change as it progresses into the deep pelvis. The superficial component, made up of peritoneum and subperitoneum connective tissue, is the structure observed by surgeons during laparoscopy and laparotomy. On MR imaging, this section is difficult to visualize since, as opposed to its deep counterpart, it contains fewer nerve fibers and vessels; thus it is rarely seen.15 Indeed, investigators found that the deep uterosacral ligament was a major conduit of nerve fibers from the pelvic plexus to the pelvic organs.4,15 Interestingly, as compared to classical cadaver dissection, we noted that the uterosacral ligament was more commonly attached to the sacrospinous ligament—coccygeus muscle junction in our subjects than it was to the presacral fascia and the attachments were a shorter length; then again, MR investigations of the uterosacral ligament in living women examine tissues that have tone, while information originally gathered from cadavers is based on the study of tissues without tone.4, 6, 16
From the serial MR images, we were able to construct 3D models of the cardinal and uterosacral ligaments in a living woman to help display the directions in which they travel from the pelvic sidewalls to the genital tracts. Oriented in the standing posture (Figures 1 and 2), the ligaments diverge from their origins at the pelvic sidewalls, but they share a common region of insertion at the cervix and/or upper part of the vagina. The differing directions of the ligaments are seen clearly in views that have been undisturbed by dissection and embalming artifacts. The cardinal ligament’s cranial-to-caudal geometry in living women contrasts with the long-held concept of the “transverse” orientation of this ligament; it was formerly known as the transverse cervical ligament.5
Previous anatomic studies were carried out on cadavers or during surgery when the patients were in the decubitus position and the uterus and cardinal ligament were under upward tension, which altered the ligament, giving it a transverse appearance. Likewise, vaginal surgery suggested the same transverse positioning because thickened intermingling fibers of the cardinal and uterosacral ligaments were noted at the cervix, as shown in our 3D models. The present study shows that it is arranged in a cranial-to-caudal direction in a living woman who is standing.
The major strength of our study is that it is a dissection-free technique on living women. False fascial planes and anatomical spaces are often created by forceps and scissors, causing discrepancies between the findings of anatomists and surgeons in the past. Our findings, based on anatomic definitions, can be easily reproduced by other investigators without any dissection or tissue-fixing artifact. Until now, the fascial structures were identified as obscure with no clear definition on MRI.7 Our MR images were acquired with a 3 Tesla magnet, which improved discrimination between different tissue constituents. Indeed, by avoiding dissection artifacts, this MR study unraveled the real architecture of apical supporting ligaments as continuous pelvic connective structures with different textures and geometrical orientation. The 3D model constructed renders a visual representation of the relationships between the 2 components of apical support.
Nevertheless, in interpreting the results, several limitations must be kept in mind. The 5 mm gap between each image slice decreases the accuracy of the constructed 3D model, as compensation for this lack of data is accomplished artificially by smoothing. Slice intervals of 2-mm should improve this situation in the future. In addition, anatomical variations cannot be fully established from this sample size and differences between different population groups remain to be evaluated.
Our results demonstrate the feasibility of examining the structural interrelationships between the 2 important elements of apical support in normal living women. Interestingly, the 2 different surgical approaches to apical support repair, sacral colpopexy and sacrospinous ligament suspension, seem to correspond to the 2 different vectors of the ligaments; vertical for the cardinal ligament and dorsally-directed for the uterosacral ligament. Perhaps sacrospinous ligament suspension is prone to anterior failures because it corrects only the uterosacral component of apical support and does not accommodate for the cardinal ligament’s vertical vector.17
As our knowledge of the geometry of these ligaments grows, biomechanical models can become more accurate, helping us to better understand the complex biological system that is the pelvic floor. Surgical strategies for pelvic organ prolapse repair can then be tailored to meet the normal orientations of the apical support, perhaps minimizing recurrence and allowing us to recreate as much of the normal pelvic anatomy as possible. Our MR-based investigative technique, used in living women, extends previous cadaver-based anatomical work.
Footnotes
This study was a podium presentation at: The Third Joint International Continence Society/International Urogynecological Association Conference; August 2010; Toronto, Canada.
All other authors report no conflict of interest.
Disclsoure: Luyun Chen: research support from American Medical Systems, John DeLancey: research support from American Medical Systems, Johnson & Johnson, Kimberly Clark.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194:1438–43. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195:1837–40. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6Pt1):1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RM. The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol. 1950;59:1–12. doi: 10.1016/0002-9378(50)90334-6. [DOI] [PubMed] [Google Scholar]
- 5.Range RL, Woodburne RT. The gross and microscopic anatomy of the transverse cervical ligament. Am J Obstet Gynecol. 1964;90:460–7. doi: 10.1016/0002-9378(64)90802-6. [DOI] [PubMed] [Google Scholar]
- 6.Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schön Ybarra MA, Bent AE. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873–9. doi: 10.1016/s0029-7844(01)01346-1. [DOI] [PubMed] [Google Scholar]
- 7.Tunn R, DeLancey JO, Quint EE. Visibility of pelvic organ support system structures in magnetic resonance images without an endovaginal coil. Am J Obstet Gynecol. 2001;184:1156–63. doi: 10.1067/mob.2001.112972. [DOI] [PubMed] [Google Scholar]
- 8.Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JO. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;103:447–51. doi: 10.1097/01.AOG.0000113104.22887.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu Y, Lewicky-Gaupp C, DeLancey JO. Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol. 2008;198:651.e1–7. doi: 10.1016/j.ajog.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon CJ, Lewicky-Gaupp C, Larson KA, Delancey JO. Anatomy of the perineal membrane as seen in magnetic resonance images of nulliparous women. Am J Obstet Gynecol. 2009;200:583.e1–6. doi: 10.1016/j.ajog.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenrodt A. Ueber die ursachen der normalen und pathologischen lagen des uterus. Arch F Gynak. 1895;48:393–421. [PubMed] [Google Scholar]
- 12.Yabuki Y, Sasaki H, Hatakeyama N, Murakami G. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: Harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005 Jul;193:7–15. doi: 10.1016/j.ajog.2005.02.108. [DOI] [PubMed] [Google Scholar]
- 13.Standring S, editor. Gray’s Anatomy. 39. London: Elsevier Churchill Livingstone; 2005. p. 1360. [Google Scholar]
- 14.Peham HV, Amreich J. In: Operative Gynecology. Ferguson LK, translator and editor. Philadelphia: JB Lippincott; 1934. [Google Scholar]
- 15.Butler-Manuel SA, Buttery LD, Polak JM, A’Hern R, Barton DP. Autonomic nerve trauma at radical hysterectomy: The nerve content and subtypes within the superficial and deep uterosacral ligaments. Reprod Sci. 2008;15:91–6. doi: 10.1177/1933719107309648. [DOI] [PubMed] [Google Scholar]
- 16.Vu D, Haylen BT, Tse K, Farnsworth A. Surgical anatomy of the uterosacral ligament. Int Urogynecol J. 2010;21:1123–8. doi: 10.1007/s00192-010-1147-8. [DOI] [PubMed] [Google Scholar]
- 17.Morgan DM, Rogers MA, Huebner M, Wei JT, Delancey JO. Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol. 2007;109:1424–33. doi: 10.1097/01.AOG.0000264066.89094.21. [DOI] [PubMed] [Google Scholar]