Abstract
Reported here is a water soluble Rh(I)-based catalyst for performing parahydrogen induced polarization (PHIP). The [Rh(I)(norbornadiene)(THP)2]+[BF4]- catalyst utilizes the monodentate phosphine ligand tris(hydroxymethyl)phosphine (THP). The monodentate PHIP catalyst is less susceptible to oxygenation by air and THP ligand and is significantly less expensive than bidentate water-soluble PHIP ligands. In situ PHIP detection with this monodentate Rh(I) based catalyst in water yielded 12% 13C polarization for the parahydrogen addition product, 2-hydroxyethyl 1-13C-propionate-d2,3,3 (HEP), with a 13C T1 relaxation of 108 seconds at 0.0475 T. PHIP polarization yields were high, reflecting efficient hydrogenation even under conditions of high content of the oxidized phosphine form of the THP ligand.
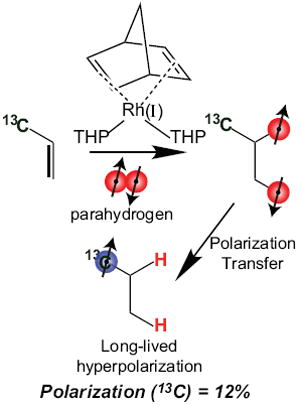
Keywords: Parahydrogen, NMR, PASADENA, hyperpolarization, 13C, PHIP, catalysis, molecular hydrogenation, tris(hydroxymethyl)phosphine, rhodium
Hyperpolarization using spin order of parahydrogen was demonstrated more than 25 years ago by Bowers and Weitekamp.1 The resulting polarization approaching unity vastly exceeds nuclear spin polarization achievable under equilibrium conditions in any currently available high field magnet by several orders of magnitude. This polarization method requires using a molecular mechanism to chemically add parahydrogen to an unsaturated chemical bond. The technique found practical application in the early 2000s when the Malmö group successfully demonstrated fast molecular quantitative addition of parahydrogen on a time scale of several seconds.2 The latter allows for inducing Zeeman polarization on nearby 13C spin sites in hydrogenated molecules in a relatively large sample. The resulting 13C polarization3 of such samples, up to 50%, can be used for bolus injection in living organisms to interrogate metabolic processes with hyperpolarized molecular contrast agents whose in vivo lifetime exceeds one minute.4-6 Preparation of hyperpolarized states using such a process is often termed parahydrogen-induced polarization (PHIP).7
If the PHIP requirement for fast molecular addition is not fulfilled, the singlet state of nascent protons obtained during the hydrogenation reaction decays on the time scale of seconds with the net result of poorly polarized 13C sites. Rh(I) based catalyst such as Wilkinson catalyst accelerates the hydrogenation reaction. While water-soluble catalysts are not mandatory in principle, typical solvents such as chloroform severely limit biological applications,8-11 which is the main driver behind hyperpolarized technologies’ recent revival.12
The only successful PHIP water-soluble Rh(I) based catalyst utilizes 1, 4-Bis[(phenyl-3-propanesulfonate) phosphine] butane disodium salt (717347, Sigma-Aldrich-Isotec, Miamisburg, OH)13 and its close variant,14 where Rh(I) is chelated by norbornadiene (nbd) and two phosphines connected by a four carbon bridge, Scheme 1.15,16 The latter is critical to the catalyst activity as norbornadiene is replaced by hydrogen molecules during hydrogenation after nbd is hydrogenated and removed. The active catalytic complex features Rh(I) chelated by a bidentate phosphine ligand, 1, 4-Bis[(phenyl-3-propanesulfonate) phosphine]. This bidendate ligand is relatively expensive due to an elaborate chemical structure and it is prone to oxidation by oxygen. The latter cannot be mitigated by balancing the Rh(I) and ligand ratio, because if one chelating phosphine arm is dysfunctional, the other phosphine arm can no longer properly chelate and is disabled. As a result, even a small % of oxidized form leads to undesirable polarization loss.
Scheme 1.
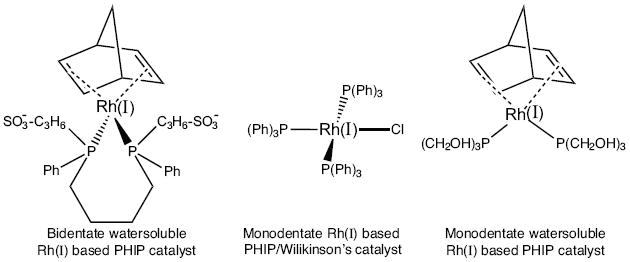
Chemical structures of three PHIP compatible catalysts.
The alternative solution presented here, is to use the monodentate phosphine ligand, Tris(hydroxymethyl)phosphine, P(CH2OH)3, (THP). While the compound has been known for more than 50 years, its coordination chemistry has only been recently studied.17 Besides excellent Rh(I) chelation, it is highly soluble and stable in water.17,18 Moreover, hydroxyl groups of the significantly simpler and less expensive THP ligand offer an intrinsic advantage that can be exploited by preparation of surface tethered Rh(I)-THP complexes paving the road to heterogeneous PHIP (HET-PHIP) catalysis of aqueous contrast agents. HET-PHIP is potentially more biologically compatible, because it avoids the necessity of purifying PHIP hyperpolarized contrast agents’ mixtures, as Rh(I) is retained in a solid phase and it does not enter living organisms. Although [Rh(I)-THP] complexes have been recently reported and characterized,19 the goals of this study were to investigate their application to PHIP and to simplify their preparation using mildly water soluble [Rh(I)(nbd)2]+[BF4]- and THP.
[RhCl(cod)]2 is insoluble in water as well as many organic solvents such as acetone and this results in tedious preparation protocols, whereas [Rh(I)(nbd)2]+[BF4]- as used here is soluble in water and the catalyst preparation procedure is relatively straightforward, Fig. 1.
Figure 1.

Molecular diagrams of catalyst preparation in aqueous medium. The presence of THP oxide is not shown.
Proton decoupled 31P spectroscopy was used to validate these complexes by comparison to prior literature,19 Fig. 2. Free THP in water, THP oxide and [Rh(I)-THP] complexes notably have different 31P chemical shifts. Phosphine resonances of [Rh(I)-THP] are distinctive doublets due to 103Rh-31P spin-spin coupling of 100 Hz or more.19
Figure 2.
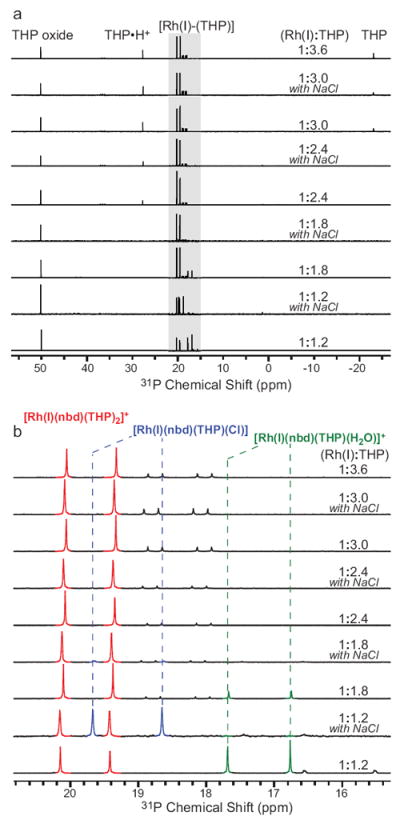
High resolution 31P{1H} NMR spectra of Rh(I):THP mixtures in 99.8% D2O acquired at 11.7 T with proton decoupling: a) entire spectral bandwidth showing all 31P species, and b) zoomed region corresponding to [Rh(I)-THP] complexes (blue trace). The Rh(I):THP ratio and NaCl presence are shown for each spectrum.
Titration of [Rh(I)(nbd)2]+[BF4]- with THP and excess NaCl was performed to assign 31P doublets of three different [Rh(I)-THP] complexes: [Rh(I)(nbd)(THP)2]+[BF4]-, [Rh(I)(nbd)(THP)(H2O)]+[BF4]-, and [Rh(I)(nbd)(THP)(Cl)], Figs. 1 and 2. Moreover, because commercial samples of THP have up to 40% oxide form, these titration studies guided our efforts for choosing optimal conditions for preparation of [Rh(I)(nbd)(THP)2]+[BF4]- and other [Rh(I)-THP] mixtures for the purpose of PHIP studies, Fig. 3.
Figure 3.
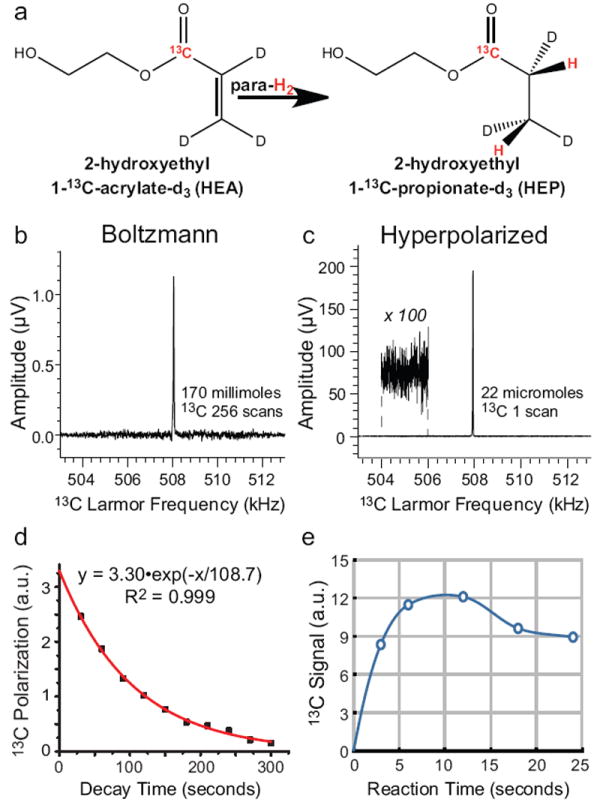
13C PHIP hyperpolarization of HEP using [Rh(I)(nbd)(THP)2]+[BF4]- catalyst in D2O with PASADENA20 (parahydrogen and synthesis allow dramatically enhanced nuclear alignment). a) The diagram of molecular hydrogenation of 2-hydroxyethyl 1-13C-acrylate-d2,3,3(HEA) yielding 2-hydroxyethyl 1-13C-propionate-d2,3,3(HEP). 13C NMR spectroscopy conducted at 0.0475 T: b) 13C spectrum of 170 millimoles (14 g in 50 mL D2O) of sodium 1-13C-acetate, Boltzmann polarization at 35 °C, 256 averages, c) 13C spectrum of 22 micromoles (2.6 mg in 4 mL 99.8% D2O at pH = 4.0) hyperpolarized HEP, polarization P = 12% corresponding to signal enhancement by 3,000,000 fold. d) Decay of 13C hyperpolarized signal was measured with 10° excitation pulses. The T1 relaxation was modeled (red trace) as an exponential decay taking into account the effect of RF pulses on 13C magnetization, e) In situ detected 13C PHIP signal versus reaction time was carried out under conditions of proton decoupling. One reaction was performed for each data point in Fig. 3e with an estimated polarizer reproducibility of approximately 5%. The detected 13C PHIP signal in 3e is proportional to the product of hydrogenated HEP and decay of the proton singlet state during proton decoupling/reaction time. Hyperpolarized 13C decay is negligible, because 13C PHIP signal is induced from nascent parahydrogen protons at the end of the proton decoupling period during approximately 0.1 second polarization transfer step of the pulse sequence and detected immediately.
Briefly, THP (1.0 mmol, 0.124 g) containing 40% of the oxide form was dissolved in 99.8% D2O, Isotec, Miamisburg, OH, (20 mL) resulting in solution containing 0.050 mmol (1 eq) in 1 mL. [Rh(I)(nbd)2]+[BF4]-, Strem Chemicals, Inc., Newburyport, MA, (0.25 mmol, 94 mg) was dissolved in high purity acetone (1 mL) to create a solution containing 0.050 mmol (1 eq) in 0.2 mL. NaCl (0.40 g, 6.8 mmol) was dissolved in D2O (5 mL) resulting in solution containing 1.4 mmol (27 eq) in 1 mL. THP solution was divided between five vials as follows: 2, 3, 4, 5, and 6 mL respectively followed by addition of 0.2 mL (1 eq) of [Rh(I)(nbd)2]+[BF4]- solution to each vial producing solutions corresponding to Rh(I):non-oxidized THP ratios of 1:1.2, 1:1.8, 1:2.4, 1:3.0, and 1:3.6 respectively. 0.5 mL aliquot was extracted from each vial for 31P spectroscopy, Fig. 2. Following extraction, 1.0 mL of NaCl solution (27 eq) was added to each vial to prepare samples with excess NaCl, Fig. 2.
When sufficient quantity of THP is present in aqueous solution, [Rh(I)(nbd)(THP)2]+[BF4]- is formed with excess THP residing in a form of free THP or its protonated form THP•H+, Fig. 2. The excess of THP does not lead to the formation of [Rh(I)(nbd)(THP)4]+[BF4]- and/or [Rh(I)(nbd)(THP)4(Cl)] as expected from previously reported studies in non-aqueous conditions and with cyclooctadiene (cod) moiety.19 The addition of excess NaCl to a mixture of [Rh(I)(nbd)(THP)2]+[BF4]- and [Rh(I)(nbd)(THP)(H2O)]+[BF4]-, obtained using a Rh(I):THP ratio of < 1:2.0 leads to conversion of [Rh(I)(nbd)(THP)(H2O)]+[BF4]- to [Rh(I)(nbd)(THP)(Cl)], Figs. 1 and 2.
Because [Rh(I)(nbd)(THP)2]+[BF4]- was a dominant complex based on the above titration study, the PHIP study was primarily focused on this complex. [Rh(I)(nbd)(THP)2]+[BF4]- was formed using THP and 2.7 mM [Rh(I)(nbd)2]+[BF4]- with a 1:2 Rh(I):THP molar ratio for starting materials in 99.8% D2O. Approximately 2 equivalents of PHIP substrate HEA (5-6 mM solution) were added to the catalyst solution. The PHIP procedure was performed identically to previously described hardware and protocols,21 and 13C hyperpolarization was detected in situ22 immediately after 13C hyperpolarization. 97% parahydrogen for PHIP was produced using a semi-automated parahydrogen generator.23
The scheme of molecular addition of parahydrogen to HEA leading to 13C hyperpolarized HEP is shown in Fig. 3a. 13C hyperpolarized signal exemplified in Fig. 3c was compared to a reference signal from sodium 1-13C-acetate under conditions of equilibrium Boltzmann polarization at 0.0475 T, Fig. 3b. Maximum achieved 13C polarization was ~12% corresponding to signal enhancement of 3,000,000 fold at this magnetic field strength. 13CT1 of hyperpolarized HEP was measured in a separate experiment by monitoring signal decay using a series of NMR single scan acquisitions with 10° excitation RF pulses. The exponential decay was simulated, taking into account polarization loss due to RF pulses, and the 13C T1 was determined to be 108.7±1.7 seconds, Fig. 3d. This value is somewhat larger than the one previously reported for H2O22, which is likely due to the very low concentration of protons in 99.8% D2O. In situ detection also allows for convenient monitoring of percent 13C hyperpolarization as a function of reaction time, Fig. 3e. Reactions are carried out under 1H RF decoupling, during which (i) the product forms corresponding to the rise shown in Fig. 3e and (ii) nascent proton spin order decays due to unfavorable relaxation processes especially notable after >12 second long hydrogenation. The somewhat slower kinetics of molecular hydrogenation with this catalyst explains why only 12% HEP hyperpolarization was achieved compared to 20%22 reported by us earlier with bidentate ligand with observed maximum at 4 s of reaction time. The potential reasons for the slower reaction rate besides the different catalyst nature include significant amounts of THP oxide and more importantly small quantities of free THP. The latter was reported to have reducing properties in the absence of Rh(I).17 If such reduction was indeed the case, THP rather than parahydrogen was a source of reducing material. Any HEA reduced using THP leads to a pool of non-polarized HEP, therefore decreasing the effective percentage of 13C hyperpolarization. The PHIP using [Rh(I)(nbd)(THP)2]+[BF4]- without NaCl resulted in 2 fold lower 13C hyperpolarization with similar kinetics. Excess of THP leading to a pool of free THP, Fig. 2, also resulted in decreased 13C hyperpolarization. When the THP:Rh(I) ratio was < 2 leading to formation of mono-THP Rh(I) complex, 13C hyperpolarization was also lower indicating that [Rh(I)(nbd)(THP)2]+[BF4]- is the most efficient among the ones studied here.
The [Rh(I)(nbd)(THP)2]+[BF4]- based catalyst solution ejected from the polarizer after performing PHIP hydrogenation was tested for stability when exposed to air. Specifically, 31P{1H} NMR spectra of starting THP material, PHIP mixtures for hydrogenation before and after PHIP were recorded, Fig. 4. Briefly, NaCl (0.10 g, 1.7 mmol) and THP (0.050 g, 0.40 mmol) were dissolved in 99.8% D2O (50 mL) and degassed. The 31P{1H} NMR spectrum of THP ligand was recorded using this solution. Rhodium catalyst [Rh(I)(nbd)2]+[BF4]- (0.050 g, 0.134 mmol) was dissolved in 3 mL of acetone and added to the degassed THP solution. HEA (0.032 g, 0.268 mmol) was added under nitrogen atmosphere. 31P{1H} NMR spectra were recorded before and after PHIP, Fig. 4. After ejection of the PHIP polarized sample from the polarizer, it was exposed to air. The 31P{1H} spectrum showed only trace amounts of the [Rh(I)(nbd)(THP)2]+[BF4]- complex and significantly more THP oxide. A likely explanation for this finding is the loss of nbd ligand during PHIP procedure results in rapid oxidation of active Rh(I)-containing species upon exposure to air after PHIP experiments. Therefore, the catalyst solution cannot be re-used. This feature is similar to the single use Rh(I)-based catalyst with bidentate ligand.14 This is not a limitation as it is not necessary to expose the catalyst to atmosphere with this type of polarizer. This new aqueous catalyst is suitable for PHIP hyperpolarization of biomolecules useful as hyperpolarized metabolic contrast agents for molecular imaging of cancer and other metabolically altering diseases. These molecules include 13C-succinate in ethylsuccinate form,6 13C-tetrafluoropropylpropionate,4 13C-phospholactate24 and others that were demonstrated to be amenable to molecular hydrogenation with similar ease as the PHIP polarized HEP presented here.
Figure 4.
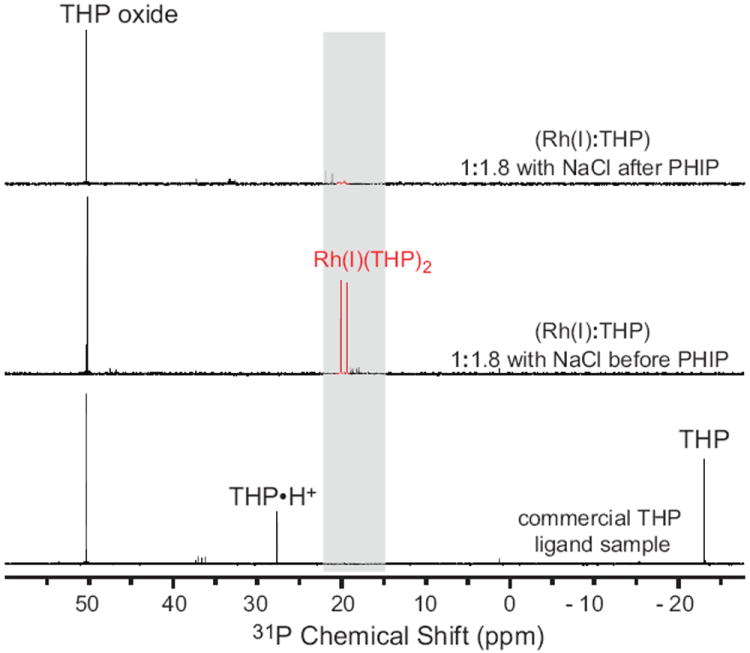
High-resolution 31P{1H} NMR spectra of Rh(I):THP mixtures in 99.8% D2O acquired at 11.7 T with proton decoupling: (bottom) THP without Rh(I) (middle) Rh(I):THP:HEA:NaCl (1:1.8:2:12) before PHIP hydrogenation, (top) Rh(I):THP:HEP:NaCl (1:1.8:2:12) after PHIP hydrogenation.
This work reports on the first use of monodentate Rh(I) based hydrogenation catalyst for PHIP in aqueous medium. Moreover, [Rh(I)(nbd)(THP)2]+[BF4]- catalyst was prepared in aqueous medium unlike in previously reports, where organic solvents were used. The catalyst is air stable for hours18 before it enters PHIP hydrogenation and PHIP is efficient even with THP containing high quantities of the oxidized form. The rate of catalytic reaction appears lower than the reaction rate of the catalyst with the bidentate ligand based on percentage polarization achieved. This can be remedied by the use of higher purity THP or potentially by the use of other THP based Rh(I) complexes such as [Rh(I)(THP)4]. The latter is very attractive as nbd does not enter the PHIP device and consequently living organisms after injection of hyperpolarized contrast agents. More importantly, however, THP is significantly simpler and less expensive compared to the water-soluble bidentate ligand. Moreover, hydroxyl groups offer a moiety that can be exploited to potentially prepare surface tethered Rh(I)-THP complexes paving the road to heterogeneous PHIP catalysis of aqueous contrast agents. This has already been demonstrated in seminal work by Koptyug and Kovtunov for gas phase PHIP.25,26 Heterogeneous aqueous PHIP catalysis is a ‘holy grail’ as it would allow for (i) preparation of pure hyperpolarized contrast agents for in vivo and human use, (ii) significant size reduction of the reaction chamber and other MR hardware components thereby reducing cost and increasing portability of PHIP polarizers, and (iii) promoting catalyst recycling. This work significantly simplifies the chemistry necessary for such heterogeneous catalysis.
Supplementary Material
Acknowledgments
We thank for funding support NIH ICMIC 5P50 CA128323-03, 5R00 CA134749-03, R25 CA136440, 3R00CA134749-02S1.
ABBREVIATIONS
- Cod
cyclooctadiene
- HEA
2-hydroxyethyl 1-13C-acrylate-d2,3,3
- HEP
2-hydroxyethyl 1-13C-propionate-d2,3,3
- nbd
norbornadiene
- PASADENA
parahydrogen and synthesis allow dramatically enhanced nuclear alignment
- PHIP
parahydrogen induced polarization
- THP
tris(hydroxymethyl)phosphine
Footnotes
Detailed experimental methods are provided in Supplementary Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bowers CR, Weitekamp DP. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical-Reaction and Nuclear-Magnetic-Resonance. Phys Rev Lett. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- 2.Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Parahydrogen-Induced Polarization in Imaging: Subsecond C-13 Angiography. Magn Reson Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 3.Goldman M, Johannesson H, Axelsson O, Karlsson M. Design and Implementation of C-13 Hyperpolarization from Para-Hydrogen, for New Mri Contrast Agents. C R Chimie. 2006;9:357–363. [Google Scholar]
- 4.Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bünger R, Ross BD. Parahydrogen-Induced Polarization (Phip) Hyperpolarized Mr Receptor Imaging in Vivo: A Pilot Study of 13c Imaging of Atheroma in Mice. NMR Biomed. 2011;24:1023–1028. doi: 10.1002/nbm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman M, Johannesson H, Axelsson O, Karlsson M. Hyperpolarization of C-13 through Order Transfer from Parahydrogen: A New Contrast Agent for Mfi. Magn Reson Imaging. 2005;23:153–157. doi: 10.1016/j.mri.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. Real-Time Molecular Imaging of Tricarboxylic Acid Cycle Metabolism in Vivo by Hyperpolarized 1-C-13 Diethyl Succinate. J Am Chem Soc. 2012;134:934–943. doi: 10.1021/ja2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J Am Chem Soc. 1987;109:8089–8091. [Google Scholar]
- 8.Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. Towards Hyperpolarized 13c-Succinate Imaging of Brain Cancer. J Magn Reson. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hövener J-B, Chekmenev E, Harris K, Perman W, Robertson L, Ross B, Bhattacharya P. Pasadena Hyperpolarization of 13c Biomolecules: Equipment Design and Installation. Magn Reson Mat Phys Biol Med. 2009;22:111–121. doi: 10.1007/s10334-008-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Kindervater P, Raich H-P, Bargon J, Spiess HW, Muennemann K. Continuous H-1 and C-13 Signal Enhancement in Nmr Spectroscopy and Mri Using Parahydrogen and Hollow-Fiber Membranes. Angew Chem Int Ed. 2010;49:8358–8362. doi: 10.1002/anie.201002725. [DOI] [PubMed] [Google Scholar]
- 11.Roth M, Koch A, Kindervater P, Bargon J, Spiess HW, Muennemann K. C-13 Hyperpolarization of a Barbituric Acid Derivative Via Parahydrogen Induced Polarization. J Magn Reson. 2010;204:50–55. doi: 10.1016/j.jmr.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Ultra-Fast Three Dimensional Imaging of Hyperpolarized C-13 in Vivo. Magn Reson Mat Phys Biol Med. 2005;18:245–256. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 14.Kadlecek S, Vahdat V, Nakayama T, Ng D, Emami K, Rizi R. A Simple and Low-Cost Device for Generating Hyperpolarized Contrast Agents Using Parahydrogen. NMR Biomed. 2011;24:933–942. doi: 10.1002/nbm.1757. [DOI] [PubMed] [Google Scholar]
- 15.Gridnev ID, Higashi N, Asakura K, Imamoto T. Mechanism of Asymmetric Hydrogenation Catalyzed by a Rhodium Complex of (S,S)-1,2-Bis(Tert-Butylmethylphosphino)Ethane. Dihydride Mechanism of Asymmetric Hydrogenation. J Am Chem Soc. 2000;122:7183–7194. [Google Scholar]
- 16.Gridnev ID, Imamoto T. On the Mechanism of Stereoselection in Rh-Catalyzed Asymmetric Hydrogenation: A General Approach for Predicting the Sense of Enantioselectivity. Acc Chem Res. 2004;37:633–644. doi: 10.1021/ar030156e. [DOI] [PubMed] [Google Scholar]
- 17.James BR, Lorenzini F. Developments in the Chemistry of Tris(Hydroxymethyl)Phosphine. Coord Chem Rev. 2010;254:420–430. [Google Scholar]
- 18.Moiseev DV, James BR. Air-Stability of Aqueous Solutions of (Hoch2)(3)P and (Hoch2ch2ch2)(3)P. Inorg Chim Acta. 2011;379:23–27. [Google Scholar]
- 19.Lorenzini F, Patrick BO, James BR. Synthesis and X-Ray Structures of Water-Soluble Tris(Hydroxymethyl)Phosphine Complexes of Rhodium(I) Dalton Trans. 2007:3224–3226. doi: 10.1039/b706691k. [DOI] [PubMed] [Google Scholar]
- 20.Bowers CR, Weitekamp DP. Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- 21.Coffey AM, Shchepin RV, Wilkens K, Waddell KW, Chekmenev EY. A Large Volume Double Channel 1h-X Rf Probe for Hyperpolarized Magnetic Resonance at 0.0475 Tesla. J Magn Reson. 2012;220:94–101. doi: 10.1016/j.jmr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waddell KW, Coffey AM, Chekmenev EY. In Situ Detection of Phip at 48 Mt: Demonstration Using a Centrally Controlled Polarizer. J Am Chem Soc. 2011;133:97–101. doi: 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW. A Pulsed Injection Parahydrogen Generator and Techniques for Quantifying Enrichment. J Magn Reson. 2012;214:258–262. doi: 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. Pasadena Hyperpolarized 13c Phospholactate. J Am Chem Soc. 2012;134:3957–3960. doi: 10.1021/ja210639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchard LS, Burt SR, Anwar MS, Kovtunov KV, Koptyug IV, Pines A. Nmr Imaging of Catalytic Hydrogenation in Microreactors with the Use of Para-Hydrogen. Science. 2008;319:442–445. doi: 10.1126/science.1151787. [DOI] [PubMed] [Google Scholar]
- 26.Kovtunov KV, Beck IE, Bukhtiyarov VI, Koptyug IV. Observation of Parahydrogen-Induced Polarization in Heterogeneous Hydrogenation on Supported Metal Catalysts. Angew Chem Int Ed. 2008;47:1492–1495. doi: 10.1002/anie.200704881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


